Introduction
Atrial fibrillation (AF) is becoming more common in
patients with cardiovascular diseases, including rheumatic heart
disease (RHD), hypertension, coronary heart disease, congenital
heart disease, cardiomyopathy and pericardial disease (1). Atrial fibrillation can severely affect
human health; rapid ventricular rates may result in the development
of fatal malignant arrhythmia, such as ventricular fibrillation
(1). Another condition induced by AF
is mural thrombus, which may lead to the development of major
adverse cardiovascular events, including acute myocardial
infarction and stroke (2). Previous
studies have demonstrated that atrial structural remodeling occurs
during the onset and development of atrial fibrillation, which
leads to a dilated left atrium and a decline in left ventricular
ejection fraction (3–7). However, preventing or improving
myocardial fibrosis may significantly reduce the left atrial
diameter (LAD) and improve cardiac function in patients with AF
(4,5,8).
Therefore, blocking atrial fibrosis may be a better way of
preventing the development and deterioration of atrial
fibrillation.
Atrial fibrosis is mediated by cardiac fibroblasts,
which can be transferred from local static cardiac fibroblasts,
epithelial cells and bone marrow stem cells under pathological
conditions, such as ischemic injury, anoxic injury and the
stimulation of TGF-β (2,9). Previous studies have demonstrated that
epithelial and endothelial cells can be transformed into
fibroblasts in a process known as the epithelial mesenchymal
transition (EMT), which serves important roles in pulmonary
fibrosis, renal fibrosis, cardiac hypertrophy and cardiac fibrosis
(10–14). Snail1 is an important regulator and
specific marker of the EMT and contributes to the formation of many
tissues during embryonic development and to the acquisition of
invasive properties during epithelial carcinoma (15). It was determined that Snail1 may be
activated in the myocardium following myocardial infarction and
that it moves from the endothelium to the mesenchymal cells of the
coronary artery, reflecting the progression of the EMT (16). Therefore, it is hypothesized that the
EMT may be one of the mechanisms involved in the occurrence and
development of atrial fibrosis, and inhibiting the activity of
Snail1 may attenuate the EMT in patients with atrial fibrillation
and therefore improve the symptoms of atrial fibrosis. Wnt
signaling serves an important role in organ fibrosis, which is also
involved in renal fibrosis, lung fibrosis and liver fibrosis
(17–19). However, it remains unclear whether
Wnt signaling participates in the atrial fibrosis activated by EMT
that occurs in AF. Therefore, the current study aimed to
investigate the association between Snail1 and atrial fibrosis in
patients with AF and RHD.
Materials and methods
Patients
Patients with RHD aged 30–60 years old planning to
receive heart valve replacement surgery who were admitted to the
Department of Cardiovascular Surgery, Renmin Hospital of Wuhan
University (Wuhan, China) between June 2015 and April 2016 were
recruited in the current study. Cardiac function was ranked between
Class I and Class III using the New York Heart Association
Functional Classification (1).
Patients with coronary atherosclerotic heart disease, chronic
pulmonary heart disease, infective endocarditis, hyperthyroidism,
serious liver, kidney or lung dysfunction, or malignant tumor were
excluded from the current study. Patients taking drugs that could
attenuate ventricular remodeling, including angiotensin converting
enzyme inhibitors, angiotensin-receptor blockers, β-receptor
blockers and spironolactone were also excluded. Following an
initial evaluation, 19 patients (10 males and 9 females) were
enrolled in the current study. These patients were divided into two
groups: An AF group (n=10) and a sinus rhythm (SR) group (n=9). The
general clinical characteristics of the patients are presented in
Table I. The present study was
approved by the Ethics Committee of Renmin Hospital of Wuhan
University (Wuhan, China) and samples were obtained following the
regulations of Renmin Hospital of Wuhan University. All patients
provided written informed consent prior to their inclusion in the
current study.
 | Table I.Clinical characteristics of patients
in the SR and AF groups. |
Table I.
Clinical characteristics of patients
in the SR and AF groups.
| Characteristics | SR (n=9) | AF (n=10) |
|---|
| Sex ratio
(Male/female) | 5/4 | 5/5 |
| Age (years) | 48.44±7.38 | 48.40±7.96 |
| LVEF (%) | 54.56±6.65 | 52.70±3.56 |
| NYHA (II/III) | 4/5 | 4/6 |
| Smoker (yes/no) | 2/7 | 2/8 |
| LAD (mm) | 44.441±7.13 |
54.50±5.02a |
Reagents
Primary antibodies against Snail1 (cat. no. 125918)
were purchased from GeneTex, Inc. (Irvine, CA, USA). Primary
antibodies against Wnt1 (ab85060), Wnt8a (ab130930), phosphorylated
glycogen synthase kinase 3β (P-GSK3β; ab130937) and GAPDH (ab37168)
were obtained from Abcam (Cambridge, UK). Primary antibodies
against Wnt3a (bs1700R) and Wnt5a (bs1948R) were purchased from
BIOSS (Beijing, China). The primary antibody against Wnt11
(sc50360) was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Antibodies against β-catenin (BA0426) and
β-actin (BM0627) were obtained from Wuhan Boster Biological
Technology, Ltd. (Wuhan, China). The antibody against GSK3β
(22104-1-AP) was purchased from Wuhan Sanying Biotechnology (Wuhan,
China). The bicinchoninic acid (BCA) protein assay kit was
purchased from Beyotime Institute of Biotechnology (Haimen, China).
TRIzol (252250AX) was obtained from Aidlab Biotechnologies Co.,
Ltd. (Beijing, China). M-MLV Reverse Transcriptase (RNase H;
CO2010A) and ddH2O (DNase/RNase Free; C1D230A) were
purchased from GeneCopoeia, Inc. (Rockville, MD, USA) and RNase
Inhibitor (I21222) was obtained from TransGen Biotech, Inc.
(Beijing, China).
Human myocardium sample
collection
All patients underwent heart valve replacement
surgery, during which time ~200 mg right atrium tissue was
collected from each patient. Each sample was divided into two
sections. One section was rapidly placed into a liquid nitrogen
container and then placed in a refrigerator at −80°C; the other
section was washed with saline solution and subsequently soaked in
4% paraformaldehyde solution under the room temperature for
pathological analysis.
Hematoxylin and eosin (H&E)
staining
H&E staining was used to detect morphological
changes in atrial myocytes. Right atrium tissues were fixed in 4%
paraformaldehyde solution at room temperature for at least 24 h and
embedded in paraffin wax. Sections 4-µm thick were placed on clean,
positively-charged microscope slides. Following drying, the slides
were heated at 60°C. Following deparaffinization and rehydration,
the slides were washed, stained with hematoxylin for 7 min,
differentiated with 0.3% acid alcohol, rinsed in Scott's tap water
substitute, stained with eosin for 2 min and then dehydrated,
cleared and mounted. All the above experiments were performed at
room temperature. A light microscope was used to visualize
slides.
Masson's staining
Masson's staining was conducted to determine the
collagen deposition of interstitial and perivascular in atrial
myocardium. Following deparaffinization and rehydration, the slides
were washed and re-fixed in Bouin's solution for 1 h at 56°C to
improve staining quality. Slides were then stained with Weigert's
iron hematoxylin for 5 min, stained in Biebrich scarlet-acid
fuchsin solution for 8 min and then stained in phosphomolybdic acid
solution for 5 min. Slides were subsequently stained with aniline
blue solution and differentiated in 1% acetic acid, rapidly
dehydrated, cleared in xylene and mounted using resinous mounting
medium. All steps of Masson's staining were conducted at room
temperature. A blue stain indicated the presence of collagen and a
red stain indicated the presence of muscle and cytoplasm.
High-magnification light micrographs were captured using light
microscopy. Image-Pro Plus 6.0 (Media Cybernetics, Inc. Rockville,
MD, USA) was used to determine the collagen volume fraction
(CVF).
Immunohistochemistry
Immunohistochemistry was used to measure the
deposition of Snail1 in myocardium tissues. Following
deparaffinization and rehydration, EnVision™ two-step protocol, the
tissue specimens were fixed in 4% formaldehyde solution at room
temperature for at least 24 h, embedded in paraffin and sliced at 4
µm thickness. The paraffin was then removed. Bovine serum albumin
(BSA) solution (3%; Beijing Solarbio Science and Technology Co.,
Ltd., Beijing, China) was added to each slide until tissues were
covered with BSA solution, and incubated for 30 min at room
temperature. Subsequently, the slides were washed to get rid of the
blocking solution and the primary antibody against Snail1
(dilution, 1:50) was added for 50 min at room temperature.
Following washing with phosphate-buffered saline, horseradish
peroxidase-conjugated AffiniPure goat anti-rabbit antibody (cat.
no. K5007; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA)
was added to the slides for 50 min at room temperature.
3,3′-diaminobenzidine was subsequently added as color reagent until
the positive expression of brown-yellow appeared under the
microscope (maximum of 10 min). Then hematoxylin was used as a
counterstain for 3 min. Slides were then fixed with a neutral gum
mount. All experiments in this part were performed at room
temperature. A light microscope was used to observe the slides.
Brown-yellow expression in myocardium tissue indicated positive
expression of Snail1.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression of Snail1 was measured using RT-qPCR.
Total RNA was isolated from the right atrium tissue using TRIzol
reagent. A First Chain Synthesis kit for cDNA (Fermentas; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used for reverse
transcription, according to the manufacturer's protocol.
SYBR-Green/Fluorescein qPCR Master mix (2X) (Fermentas; Thermo
Fisher Scientific, Inc.) and Ex Taq™ (Takara Bio, Inc., Otsu,
Japan) were used for PCR. qPCR was performed on an Illumina-Eco
machine (Illumina, Inc., San Diego, CA, USA). The result was
normalized against β-actin gene expression. The sequences of all
primers used in the current study were as follows: Snail1, forward,
5′-GCACATCCGAAGCCACA-3′ and reverse, 5′-GAGAAGGTCCGAGCACA-3′;
β-actin, forward, 5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse,
5′-GGGCACGAAGGCTCATCATT-3′. The reaction conditions were as
follows: 50°C for 2 min, 95°C for 10 min, 95°C for 30 sec, 60°C for
30 sec. RT-PCR experiments were performed with 1 g of total RNA,
followed by 40 cycles of PCR amplification. The expression levels
were quantified using changes in the fluorescence signal through
the analysis of the Cq value and standard curve (20); the starting template was
quantitatively analyzed with RUO ViiA™ 7 software (Thermo Fisher
Scientific, Inc.).
Western blot analysis
Total protein was extracted from the right atrium
tissue using radioimmunoprecipitation assay lysis buffer (cat. no.
P0013B; Beyotime Institute of Biotechnology). The BCA Protein assay
kit was used to determine protein concentration. Denatured protein
was loaded and separated using 10% SDS-PAGE and then transferred to
a nitrocellulose membrane. Following blocking with 5% non-fat milk
for 60 min at room temperature, the membrane was incubated with
primary antibodies overnight at 4°C. Primary antibodies were as
follows: Snail1 (dilution, 1:3,000), Wnt1 (dilution, 1:1,000),
Wnt3a (dilution, 1:500), Wnt5a (dilution, 1:500), Wnt8a (dilution,
1:1,000), Wnt11 (dilution, 1:500), GAPDH (dilution, 1:10,000),
β-catenin (dilution, 1:200), GSK3β (dilution, 1:2,000), P-GSK3β
(dilution, 1:800), β-actin (dilution, 1:200). The following day,
the membrane was incubated with HRP-conjugated goat anti-rabbit
(cat. no. 074–1506, KPL, Inc., Gaithersburg, MD, USA; dilution,
1:10,000) as secondary antibodies for 2 h at room temperature. The
ChemiDoc Touch Imaging System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to detect the protein signals and
analysis was conducted using Bandscan 4.3; BioMarin Pharmaceutical,
Inc., San Rafearl, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences between the two groups were analyzed using an unpaired
Student's t-test. Linear correlation was applied to analyze the
relationship between levels of Snail1 mRNA and CVF by Pearson's
correlation coefficient. The value of r represents the correlation
between two variables; r>0.5 indicates a positive
correlation. Differences between the sex and heart function of two
groups were analyzed using Fisher's exact test. Differences between
the age, LVEF and LAD of two groups were analyzed using an unpaired
Student's t-test. SPSS 19.0 statistical software (IBM Corp.,
Armonk, NY, USA) was used to perform statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patients with AF and RHD exhibit an
expanded LAD
All patients participating in the present study
received preoperative routine testing, including a blood test,
chest X-ray, electrocardiogram and echocardiography. General
information about patients was collected and it was determined that
there were no significant differences in sex, age, left ventricular
ejection fraction and New York heart function classification
between the SR and AF groups (Table
I). However, compared with the SR group, the patients with AF
exhibited significantly expanded LAD (P<0.05; Table I). This indicates that patients with
AF and RHD have a larger LAD than patients with SR.
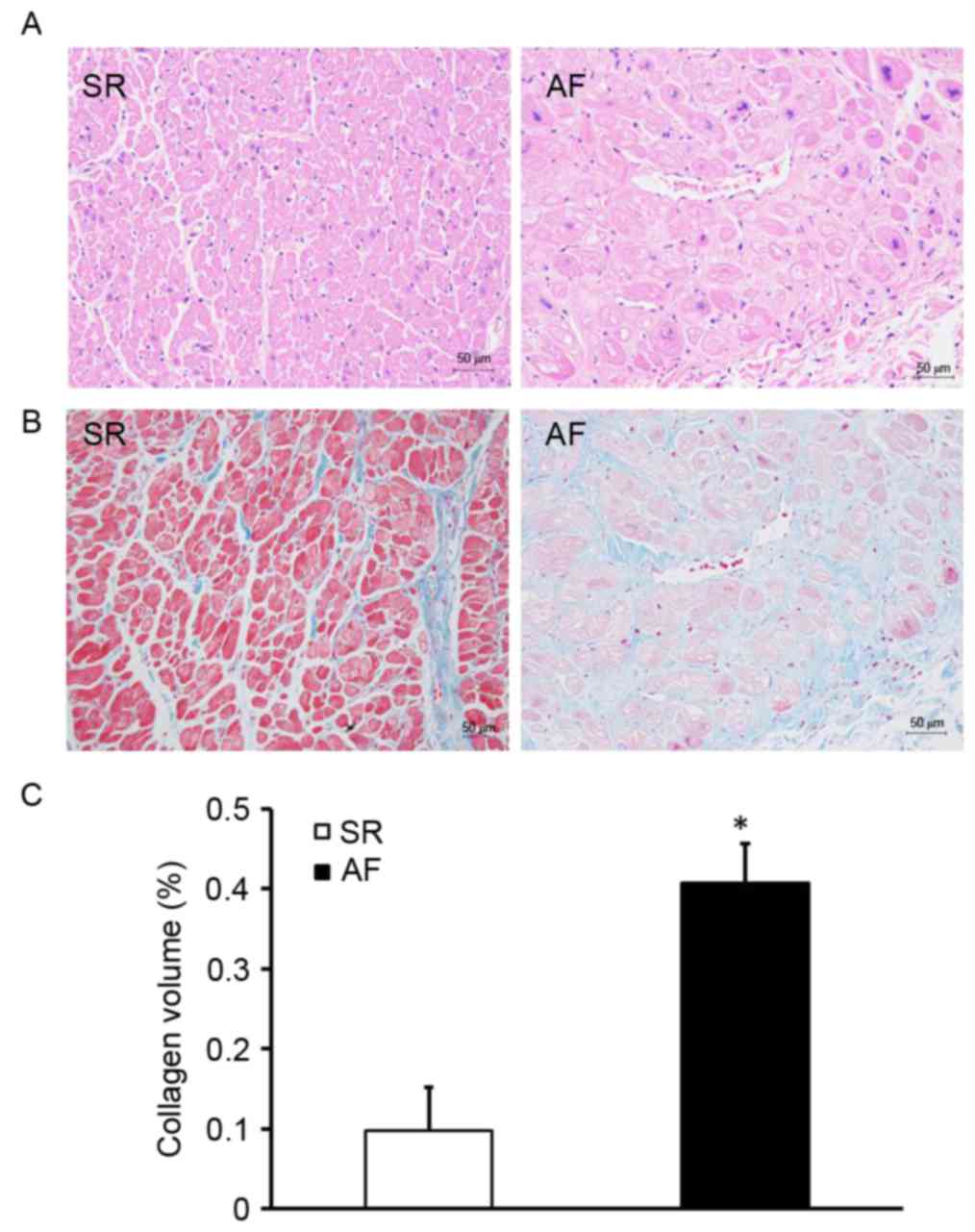
Patients with AF and RHD exhibit
expansive atrial myocytes and accumulated collagen
To investigate the effect of AF on atrial structural
remodeling, H&E and Masson staining were used to evaluate the
extent of myocardial fibrosis (Fig.
1). H&E staining indicated that the size of the myocardial
cells in the AF group was greater than those in the SR group
(Fig. 1A). Myocardial fibrosis was
evaluated by measuring the total amount of collagen in the
interstitial spaces of the myocardial tissue and determining the
CVF. Compared with the SR group, the AF group exhibited a disorder
in the arrangement of interstitial collagen fibers (Fig. 1B). Furthermore, the CVF was
significantly higher in the AF group compared with the SR group
(P<0.05; Fig. 1C). These results
indicate that patients with AF and RHD have expansive atrial
myocytes and exhibit extensive collagen deposition in the atrial
myocardium.
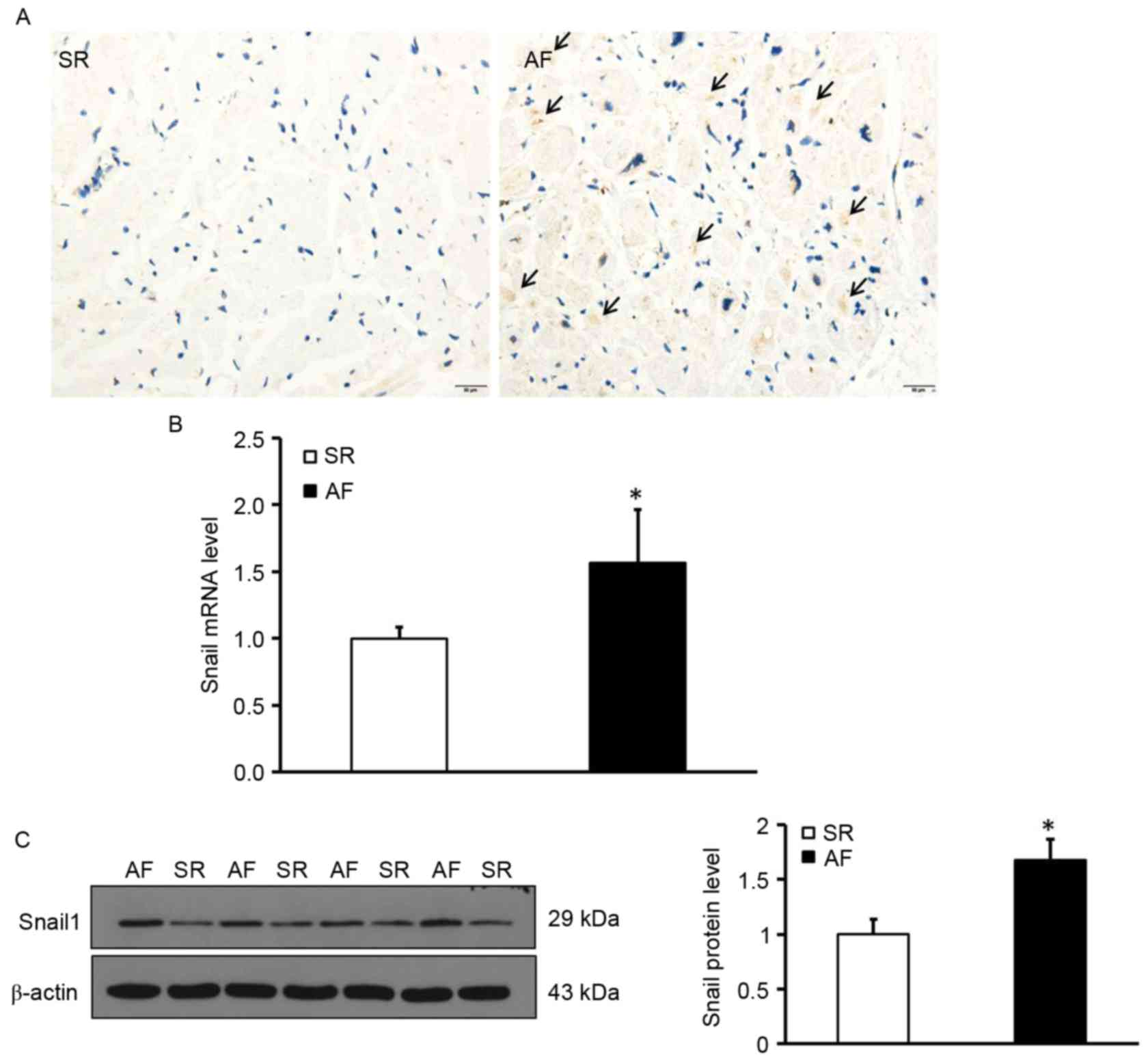
Elevated Snail1 expression in the
atrial myocardium of patients with AF and RHD
To determine the location and expression of Snail1,
deposition of Snail1 in the myocardial tissues was measured using
immunohistochemistry. The expression of Snail1 mRNA and protein was
measured using RT-qPCR and western blotting, respectively.
Immunohistochemical staining was performed in tissue sections to
determine the distribution of Snail1; the results indicated that
Snail1 was primarily distributed in the vascular endothelial and
interstitial cells and was almost undetectable in myocardial cells
in the AF group. (Fig. 2A).
Additionally, levels of Snail1 mRNA and protein in the atrial
tissues of patients with AF were significantly higher than those of
the SR group (P<0.05; Fig. 2B and
C). These data demonstrate that levels of Snail1 are increased
in the myocardium of patients with AF and indicate that Snail1 may
be involved in the development of atrial fibrosis.
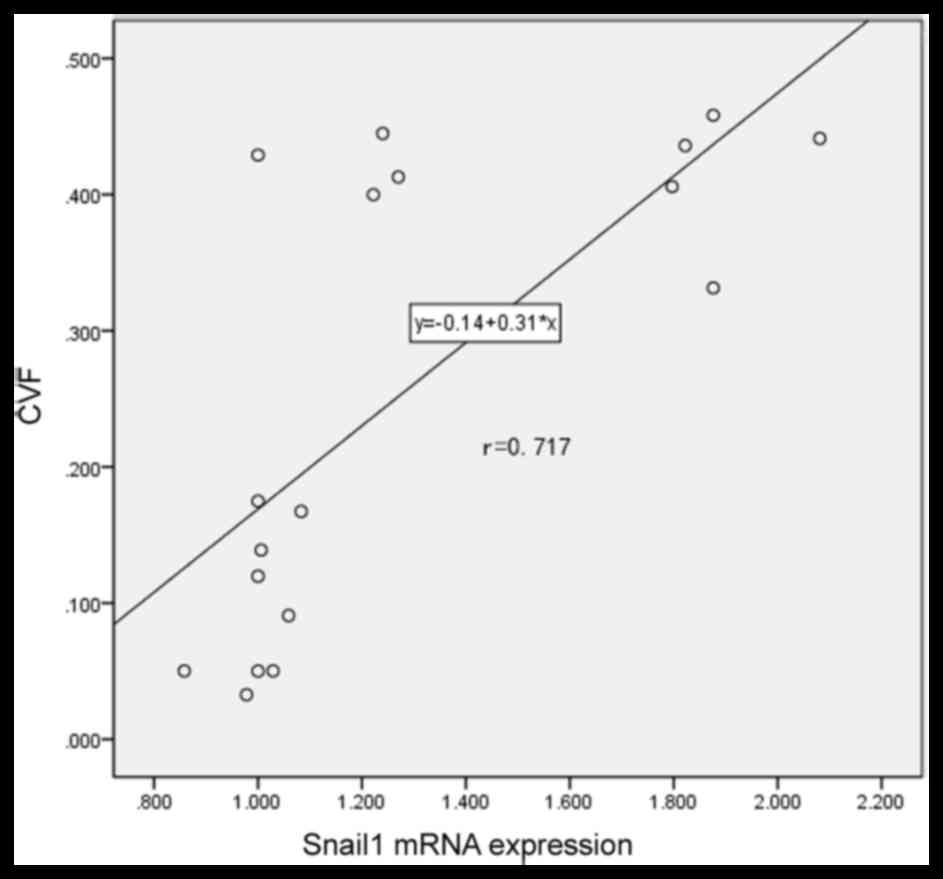
Snail1 is positively correlated with
atrial fibrosis in patients with AF and RHD
To determine the association between Snail1
expression and atrial fibrosis, a correlation analysis was
performed. A positive correlation was identified between levels of
Snail1 mRNA and CVF (r=0.717; P<0.001; Fig. 3). This confirms that there is a
positive association between Snail1 expression and atrial fibrosis
in patients with AF and suggests that Snail1 may be developed as a
novel biomarker to evaluate myocardial fibrosis for patients with
AF and RHD in the future.
The Wnt signaling pathway may
participate in the process of increased Snail1 expression and
atrial fibrosis in patients with AF and RHD
Wnt signaling serves an important role in organ
fibrosis, which is involved in renal, lung and liver fibrosis
(17,18,21). To
determine whether the activity of the Wnt signaling pathway
increased Snail1 expression and atrial fibrosis in patients with
AF, the expression of proteins involved in the Wnt signaling
pathway was investigated (Fig. 4).
It was determined by western blotting that, compared with the SR
group, the expression of Wnt1, 3a and 8a, which are involved in the
canonical Wnt signaling pathway, were significantly increased in
the AF group (P<0.05; Fig. 4B).
Furthermore, the levels of Wnt5a and Wnt11, which are involved in
the noncanonical Wnt signaling pathway, were also significantly
higher in the AF group compared with the SR group (P<0.05;
Fig. 4B). It was also observed that
the phosphorylation levels of GSK3β, as well as the levels of
β-catenin, were significantly elevated in the AF group (P<0.05;
Fig. 4C). These results indicate
that the Wnt signaling pathway, which is associated with the
development of EMT, may also contribute to increased Snail1
expression and the development of atrial fibrosis.
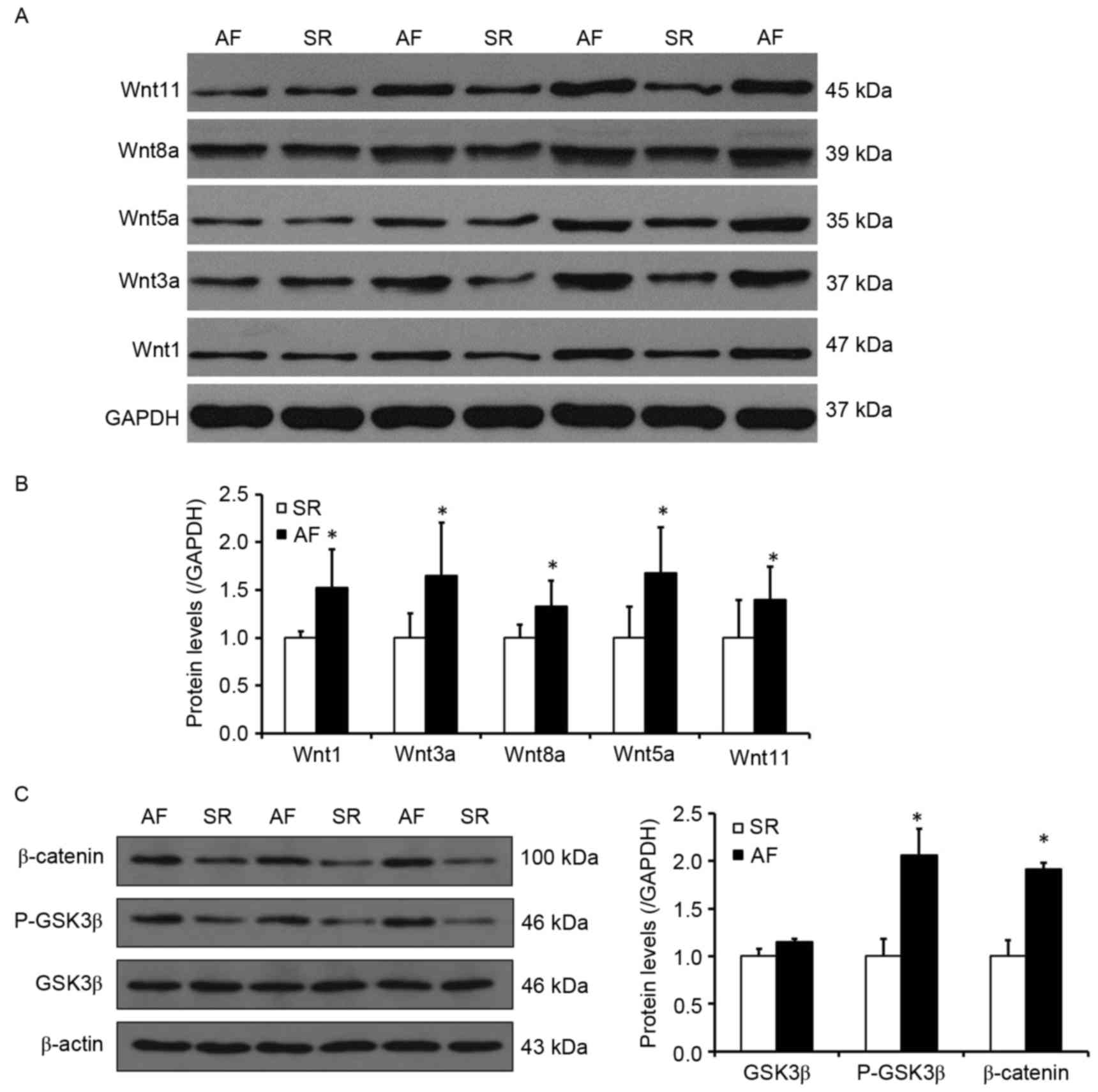 | Figure 4.The Wnt signaling pathway may
participate in the process of increased Snail1 expression and
atrial fibrosis in patients with AF and rheumatic heart disease.
(A) Representative western blot presenting the levels of Wnt1,
Wnt3a, Wnt5a, Wnt8a and Wnt11 protein. (B) Quantitative analysis of
Wnt1, Wnt3a, Wnt5a, Wnt8a and Wnt11 expression in the SR and AF
groups. (C) Left, representative western blot indicating the levels
of GSK3β, P-GSK3β and β-catenin expression. Right, quantitative
analysis of levels of GSK3β, P-GSK3β and β-catenin in the SR and AF
groups. *P<0.05 vs. SR group. SR, sinus rhythm; AF, atrial
fibrillation; GSK3β, glycogen synthase kinase 3β; P-GSK3β,
phosphorylated glycogen synthase kinase 3β. |
Discussion
In the present study, it was determined that the
size of myocardial cells was more expansive and the degree of
myocardial fibrosis was significantly increased in patients with
AF. Additionally, levels of Snail1 mRNA and protein were increased
in patients with AF and Snail1 was primarily deposited in vascular
endothelial and interstitial cells; expression in Snail1 was low in
myocardial cells. Correlation analysis determined that levels of
Snail1 mRNA were positively correlated with the degree of atrial
fibrosis in patients with AF and RHD. Finally, the expression of
factors involved in the canonical (Wnt1, 3a and 8a) and
noncanonical Wnt signaling pathways (Wnt5a and 11) were
significantly increased in the AF group and the phosphorylation
levels of GSK3β and β-catenin were also elevated in the AF group.
These results indicate that increased Snail1 expression is
positively associated with the degree of atrial fibrosis.
Furthermore, they suggest that the Wnt signaling pathway, which is
associated with the development of EMT, may also contribute to
increased Snail1 expression and atrial fibrosis in patients with AF
and RHD.
Atrial fibrosis is the primary process by which
atrial structure remodeling occurs and serves a key role in the
development and persistence of AF (3). Therefore, attenuating atrial fibrosis
to inhibit atrial structure remodeling is crucial to prevent the
onset and development of AF. Furthermore, atrial fibrosis is
mediated by cardiac fibroblasts and epithelial cells, and
endothelial cells can be transformed to fibroblasts during the EMT,
which performs important roles in pulmonary fibrosis, renal
fibrosis, cardiac hypertrophy and cardiac fibrosis (10–14).
Therefore, inhibiting the EMT to attenuate cardiac fibrosis may be
an important method of preventing the development of AF.
Snail1 is a member of the Snail family, which is a
specific marker of the EMT and serves an important role in tissue
fibrosis, including kidney and liver fibrosis (13,16,22–24). It
was demonstrated that during liver fibrosis, Snail1 expression is
upregulated and that it serves a key role in the progression of
liver fibrosis by upregulating the biosynthesis of the
extracellular matrix and promoting chronic inflammatory responses
(24). Additionally, it was
determined that the pattern of Snail1 expression is spatial and
temporal and may increase following injuries that occur during
fibrogenesis (16,25). In the present study, it was
demonstrated that Snail1 expression was markedly increased in the
myocardium and that Snail1 was primarily deposited in the vascular
endothelial and interstitial cells. Furthermore, it was determined
that Snail1 was positively correlated with the degree of atrial
fibrosis in patients with AF and RHD. These results indicate that
the EMT may serve an important role in the development of atrial
fibrosis in patients with AF and RHD.
Additionally, Wnt signaling is one of the most
important signaling pathways involved in tissue fibrosis and
regulates the adherence and migration of cells (15). It has been demonstrated that
Wnt/β-catenin signaling serves an important role in renal and
pulmonary fibrosis (17,18). Furthermore, it has been determined
that the EMT may be induced by Wnt signaling that is activated by
cardiac injury (26). Therefore, the
present study also measured changes in the levels of proteins
involved in Wnt signaling. It was demonstrated that, compared with
the SR group, levels of Wnt1, 3a and 8a involved in the canonical
Wnt signaling pathway and levels of Wnt5a and Wnt11 involved in the
noncanonical Wnt signaling pathway were significantly higher in the
AF group compared with the SR group. Additionally, the
phosphorylation level of GSK3β and level of β-catenin were
significantly increased in the AF group. It was thus determined
that the canonical and noncanonical Wnt signaling pathways were
activated in the myocardium of patients with AF. Previous studies
have demonstrated that the Snail1-induced EMT occurs, at least in
part, due to a decrease in E-cadherin transcription that stimulates
the development of epithelial phenotypic cells with adhesive and
polarity properties. These cells then gradually transform into
loose and activated mesenchymal cells and stimulate the development
of tissue fibrosis (22,27). These results indicate that the Wnt
signaling pathway is associated with the development of EMT and may
participate in the process of increased Snail1 and atrial fibrosis
in patients with AF and RHD.
In conclusion, the present study demonstrated that
Snail1 may be involved in the development and maintenance of atrial
fibrosis in patients with AF and RHD, and that Snail1 may be used
as a novel biomarker to evaluate atrial fibrosis in patients with
AF and RHD in the future. Furthermore, the Wnt signaling pathway
associated with the development of EMT may increase Snail1
expression and atrial fibrosis in patients with AF and RHD. Novel
drugs that inhibit the expression and/or function of Snail1, or
block the Wnt signaling pathway may prevent atrial fibrosis in
patients with AF and RHD. However the sample size of the current
study was relatively small and further studies with a larger sample
size or involving multiple centers are required to further
elucidate this mechanism of action. Future in vitro or in
vivo studies are also required to determine how the Wnt
signaling pathway regulates Snail1 expression and induces the
development of EMT.
Acknowledgements
The authors wish to thank Professors Zhiwei Wang and
Jun Xia, who helped out with the sample collection and all doctors
in the Department of Cardiology and the Cardiovascular Research
Institute of Renmin Hospital of Wuhan University for their expert
technical assistance and advice. The present study was supported by
grants from the National Natural Science Foundation of China (no.
81170085) and the Fundamental Research Funds for the Central
Universities (no. 2042016kf0074).
References
|
1
|
Li M, Yi X, Ma L and Zhou Y: Hepatocyte
growth factor and basic fibroblast growth factor regulate atrial
fibrosis in patients with atrial fibrillation and rheumatic heart
disease via the mitogen-activated protein kinase signaling pathway.
Exp Ther Med. 6:1121–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jalife J and Kaur K: Atrial remodeling,
fibrosis, and atrial fibrillation. Trends Cardiovasc Med.
25:475–484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan AY and Zimetbaum P: Atrial
fibrillation and atrial fibrosis. J Cardiovasc Pharmacol.
57:625–629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Huang ZY, Wang ZH, Li CP, Meng XL,
Zhang YJ, Su F and Ma N: TGF-β1 and TIMP-4 regulate atrial fibrosis
in atrial fibrillation secondary to rheumatic heart disease. Mol
Cell Biochem. 406:131–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang YJ, Ma N, Su F, Liu H and Mei J:
Increased TRPM6 expression in atrial fibrillation patients
contribute to atrial fibrosis. Exp Mol Pathol. 98:486–490. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyasato SK, Loeffler J, Shohet R, Zhang
J, Lindsey M and Le Saux CJ: Caveolin-1 modulates TGF-β1 signaling
in cardiac remodeling. Matrix Biol. 30:318–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Huang B, Scherlag BJ, Ritchey JW,
Embi AA, Hu J, Hou Y and Po SS: Structural changes in the
progression of atrial fibrillation: Potential role of glycogen and
fibrosis as perpetuating factors. Int J Clin Exp Pathol.
8:1712–1718. 2015.PubMed/NCBI
|
|
8
|
Kiryu M, Niwano S, Niwano H, Kishihara J,
Aoyama Y, Fukaya H, Masaki Y and Izumi T: Angiotensin II-mediated
up-regulation of connective tissue growth factor promotes atrial
tissue fibrosis in the canine atrial fibrillation model. Europace.
14:1206–1214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang R, Yi X, Li X and Jiang X: Fibroblast
growth factor-21 is positively associated with atrial fibrosis in
atrial fibrillation patients with rheumatic heart disease. Int J
Clin Exp Pathol. 8:14901–14908. 2015.PubMed/NCBI
|
|
10
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baum B, Settleman J and Quinlan MP:
Transitions between epithelial and mesenchymal states in
development and disease. Semin Cell Dev Biol. 19:294–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashimoto N, Phan SH, Imaizumi K, Matsuo
M, Nakashima H, Kawabe T, Shimokata K and Hasegawa Y:
Endothelial-mesenchymal transition in bleomycin-induced pulmonary
fibrosis. Am J Respir Cell Mol Biol. 43:161–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tennakoon AH, Izawa T, Kuwamura M and
Yamate J: Pathogenesis of Type 2 Epithelial to Mesenchymal
Transition (EMT) in renal and hepatic fibrosis. J Clin Med.
5:pii:E42015. View Article : Google Scholar
|
|
14
|
Widyantoro B, Emoto N, Nakayama K,
Anggrahini DW, Adiarto S, Iwasa N, Yagi K, Miyagawa K, Rikitake Y,
Suzuki T, et al: Endothelial cell-derived endothelin-1 promotes
cardiac fibrosis in diabetic hearts through stimulation of
endothelial-to-mesenchymal transition. Circulation. 121:2407–2418.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: Implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Du J, Zhang J, Weng M, Li X, Pu D,
Gao L, Deng S, Xia S and She Q: Snail1 is involved in de novo
cardiac fibrosis after myocardial infarction in mice. Acta Biochim
Biophys Sin (Shanghai). 44:902–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dang Y, Liu B, Xu P, Zhu P, Zhai Y, Liu M
and Ye X: Gpr48 deficiency induces polycystic kidney lesions and
renal fibrosis in mice by activating Wnt signal pathway. PLoS One.
9:e898352014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim TH, Kim SH, Seo JY, Chung H, Kwak HJ,
Lee SK, Yoon HJ, Shin DH, Park SS and Sohn JW: Blockade of the
Wnt/β-catenin pathway attenuates bleomycin-induced pulmonary
fibrosis. Tohoku J Exp Med. 223:45–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ge WS, Wang YJ, Wu JX, Fan JG, Chen YW and
Zhu L: β-catenin is overexpressed in hepatic fibrosis and blockage
of Wnt/β-catenin signaling inhibits hepatic stellate cell
activation. Mol Med Rep. 9:2145–2151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu F, Lu Z, Huang K, Wang X, Xu Z, Chen B,
Dong P and Zheng J: MicroRNA-17-5p-activated Wnt/β-catenin pathway
contributes to the progression of liver fibrosis. Oncotarget.
7:81–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boutet A, De Frutos CA, Maxwell PH, Mayol
MJ, Romero J and Nieto MA: Snail activation disrupts tissue
homeostasis and induces fibrosis in the adult kidney. EMBO J.
25:5603–5613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu-Dubois YC, Galichon P, Brocheriou I,
Baugey E, Morichon R, Jouanneau C, Ouali N, Rondeau E and Hertig A:
Expression of the transcriptional regulator snail1 in kidney
transplants displaying epithelial-to-mesenchymal transition
features. Nephrol Dial Transplant. 29:2136–2144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rowe RG, Lin Y, Shimizu-Hirota R, Hanada
S, Neilson EG, Greenson JK and Weiss SJ: Hepatocyte-derived Snail1
propagates liver fibrosis progression. Mol Cell Biol. 31:2392–2403.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou B, Honor LB, He H, Ma Q, Oh JH,
Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, et
al: Adult mouse epicardium modulates myocardial injury by secreting
paracrine factors. J Clin Invest. 121:1894–1904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duan J, Gherghe C, Liu D, Hamlett E,
Srikantha L, Rodgers L, Regan JN, Rojas M, Willis M, Leask A, et
al: Wnt1/βcatenin injury response activates the epicardium and
cardiac fibroblasts to promote cardiac repair. EMBO J. 31:429–442.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohnuki K, Umezono T, Abe M, Kobayashi T,
Kato M, Miyauchi M, Yamamoto N, Kimura M, Toyoda M and Suzuki D:
Expression of transcription factor Snai1 and tubulointerstitial
fibrosis in progressive nephropathy. J Nephrol. 25:233–239. 2012.
View Article : Google Scholar : PubMed/NCBI
|