Introduction
Endometrial carcinoma is one of the most prevalent
gynecological malignancies (1).
Despite advances in therapeutic approaches, many patients with
endometrial carcinoma develop localized, recurrent and/or distant
metastases (2). Therefore, it is
important to identify the key regulators of endometrial carcinoma
progression and metastasis to develop more effective therapies to
treat endometrial carcinoma.
Epithelial-mesenchymal transition (EMT) is a crucial
event in tumor invasion and metastasis, whereby epithelial cells
lose their polarity and cell-cell contacts and undergo cytoskeletal
remodeling (3). EMT is typically
associated with a downregulation of epithelial (E)-cadherin and
upregulation of neural (N)-cadherin (4), and decreases in E-cadherin expression
are associated with a more infiltrative growth pattern in a variety
of cancers, including non-small-cell lung cancer, prostate cancer
and endometrial cancer (5–7). The transcription factor Twist has been
identified as an inducer of EMT by its transcriptional repression
of E-cadherin (8,9). Twist is also involved in tumor cell
proliferation and survival (10) and
it has been documented that Twist mediates aggressive phenotypes in
human colorectal cancer cells, thus contributing to cell migration,
invasion and chemoresistance (11).
Twist also facilitates EMT and cell motility in breast cancer
through the integrin β1 subunit-focal adhesion
kinase/integrin-linked kinase signaling axis (12). Furthermore, during cervical
carcinogenesis, Twist has been implicated in the induction of EMT
by regulating the tumor growth factor-β/Smad3 signaling pathway
(13). Therefore, Twist may be a
promising target of anticancer therapies.
However, the biological functions of Twist in human
endometrial carcinoma remain unknown. Through loss-of-function
experiments, the present study aimed to elucidate the roles of
Twist in the proliferation, migration, invasion and EMT of
endometrial carcinoma cells.
Materials and methods
Cell culture
Human endometrial carcinoma Ishikawa cells were
provided by Professor XiaoPing Wan (Department of Gynecology,
Shanghai First People's Hospital, Shanghai, China). Cells were
cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with 10% fetal bovine serum (Invitrogen;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified incubator at 37°C and 5%
CO2 for 48 h.
Transfection with Twist small
interfering RNA (siRNA)
Three siRNAs targeting different sites in the mRNA
of Twist and one non-targeting control siRNA were designed
and synthesized by Shanghai Minghong Biotechnologies Co., Ltd.
(Shanghai, China). The sequences of the Twist siRNAs were as
follows: 5′-AAGCUGAGCAAGAUUCAGACC-3′, 5′-AGCGGGUCAUGGCUAACGUGC-3′
and 5′-AGGUACAUCGACUUCCUGUAC-3′. The Twist siRNAs (50 nM of each)
were transfected into Ishikawa cells, individually and in
combination to evaluated transfection efficiency compared with the
control and non-targeting control siRNA group, using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
mixture of three Twist siRNAs (1:1:1; 50 nM) was used to determine
the biological function of Twist. siRNA transfection efficiency was
determined by an in-parallel transfection with BLOCK-iT Alexa Fluor
red fluorescent oligonucleotide (Invitrogen; Thermo Fisher
Scientific, Inc.). The fluorescent signal was then observed under a
fluorescent microscope (Leica DFC550 Microsystems GmbH, Wetzlar,
Germany; magnification, ×400). When transfection efficiency was
>85%, transfected cells were used in the following
experiments.
Western blot analysis
Following transfection, cells were lysed in ice-cold
lysis buffer (50 mmol/l Tris-HCl, pH 7.4, 150 mmol/l NaCl, 1% NP-40
and 0.5% sodium deoxycholate) supplemented with protease inhibitors
(Roche Diagnostics, Indianapolis, IN, USA) for 30 min at 4°C. The
supernatant of cell lysates was obtained by centrifugation for 10
min at 12,000 × g at 4°C. A BCA Protein Assay kit (Beyotime
Institute of Biotechnology, Haimen, China) was used to determine
protein concentrations. Equal amounts of protein (20 µg per lane)
were separated by 8 or 12%SDS-PAGE and transferred onto
polyvinylidene difluoride membranes.
Following blocking with 5% skimmed milk for 2 h at
room temperature, membranes were probed with anti-Twist (cat. no.
46702), anti-E-cadherin (cat. no. 14472), anti-N-cadherin (cat. no.
14215) or anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
(cat. no. 2118; all 1:1,000; all Cell Signaling Technology, Inc.,
Danvers, MA, USA) antibodies overnight at 4°C, followed by
incubation with horseradish peroxidase-conjugated secondary
antibody (1:5,000; cat. nos. 7074 and 7076; Cell Signaling
Technology, Inc.) for 1 h at room temperature. Blots were developed
using an enhanced chemiluminescence kit (GE Healthcare Life
Sciences, Chalfont, UK). The intensities of immunoreactive bands
were measured by computerized image analysis using Quantity One
v4.62 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
normalized to GAPDH expression. A total of 3 replicates were
performed.
Cell counting kit (CCK8) assay
Ishikawa cells (1.0×104 cells/well) were
plated in 96-well plates (three wells per group) with RPMI-1640
medium with 10% fetal bovine serum, 100 U/ml penicillin and 100
µg/ml streptomycin in a humidified incubator at 37°C and 5%
CO2 and treated with honokiol (0.01 mg/ml) for 0–4 days.
On days 0–4 after seeding, CCK-8 solution (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was administered to cells,
according to the manufacturer's instructions, and cell
proliferation was measured at 490 nm using an ELISA reader
SpectraMax 190 microplate reader (Bio-Rad Laboratories, Inc.).
Quantification of apoptosis by flow
cytometry
Cell apoptosis was analyzed using an
Annexin-V/propidium iodide (PI) assay. Briefly, cells were digested
with 0.25% trypsin (Invitrogen; Thermo Fisher Scientific, Inc.),
washed and resuspended in binding buffer. Annexin V-FITC and PI
were then added (BD Pharmingen; BD Biosciences, Franklin Lakes, NJ,
USA) and apoptotic cells were detected using a flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA) washed and suspended in
staining buffer containing 1 µg/ml PI and 0.025 µg/ml Annexin
V-fluorescein isothiocyanate. Double labeling was performed at room
temperature for 10 min in the dark. Following incubation, the
percentage of apoptotic cells was measured by flow cytometry FC500
(Beckman Coulter, Inc., CA, USA). The software used for analysis is
Cytometer supplied by the instrument company.
Cell cycle analysis
Ishikawa cells (1×106 cells/well) were
plated with RPMI-1640 medium in 6-well plates (three wells per
group) and treated with vehicle or honokiol (5 or 10 µM) for 48 h.
For cell cycle assay, cells were washed twice with ice cold PBS,
fixed in 70% ethanol at −20°C for 4 h and incubated with 50 mg/ml
PI (Sigma-Aldrich; Merck KGaA). After fixation, cells were washed
by phosphate buffer 2 times and then stained by 50 ug/ml PI in 500
ul PBS containing 100 ug/ml RNase A for 30 min. Cell cycle
distribution was assessed by flow cytometry FC500 (Beckman Coulter,
Inc., CA, USA) and analyzed by the software provided.
Wound healing assay
Ishikawa cells (1×106 cells/well) were
plated in 6-well plates and grown to 70–80% confluence with
RMPI-1640 without fetal bovine serum for 24 h. Using a 200-µl
pipette tip, a scratch wound was made through the cell monolayer.
Cells were washed with PBS to remove any detached cells and
incubated in RMPI-1640 with 10% fetal bovine serum at 37°C for 48
h. The distance between the two sides of the wound was measured
with a light microscope before and after wound healing.
Statistical analysis
Data were expressed as the mean ± standard deviation
and analyzed by one-way analysis of variance followed by a Tukey's
multiple comparisons test. Statistical differences were determined
using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) and
P<0.05 was considered to indicate a statistically significant
difference. All experiments have been replicated three times.
Results
siRNA knockdown of endogenous Twist in
Ishikawa cells
To downregulate the expression of Twist in human
endometrial carcinoma Ishikawa cells, three different Twist siRNAs
were individually transfected into cells. Ishikawa cells without
siRNA transfection were used as control group and cells with
non-targeting control siRNA transfection were used as mock group to
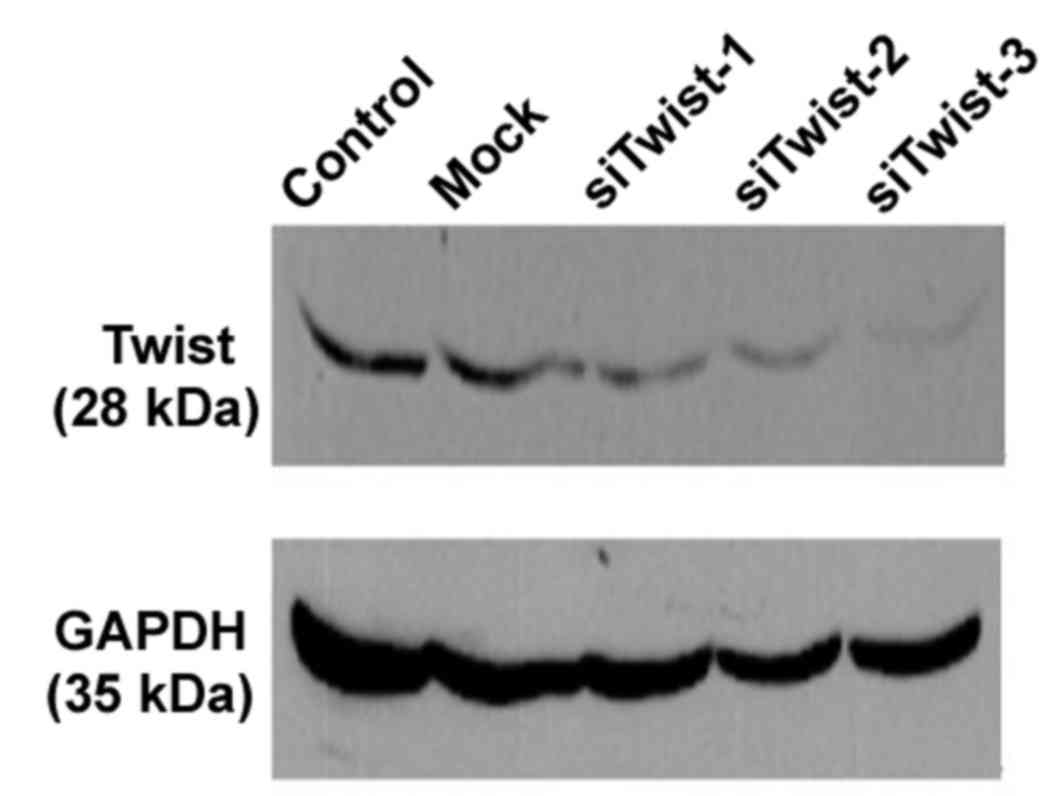
eliminate the effect of transfection treatment. As presented in
Fig. 1, transfection with each of
the Twist siRNAs lead to a marked reduction in the levels of Twist
protein levels by 48 h post-transfection, relative to mock and
control cells. As expected, expression of GAPDH was unaltered by
Twist siRNA transfection. These results indicate that the RNA
interference assay was specific and effective. Therefore, Ishikawa
cells were transfected with a mixture of all three Twist siRNAs in
subsequent experiments to determine the biological functions of
Twist.
Downregulation of Twist inhibits
Ishikawa cell proliferation
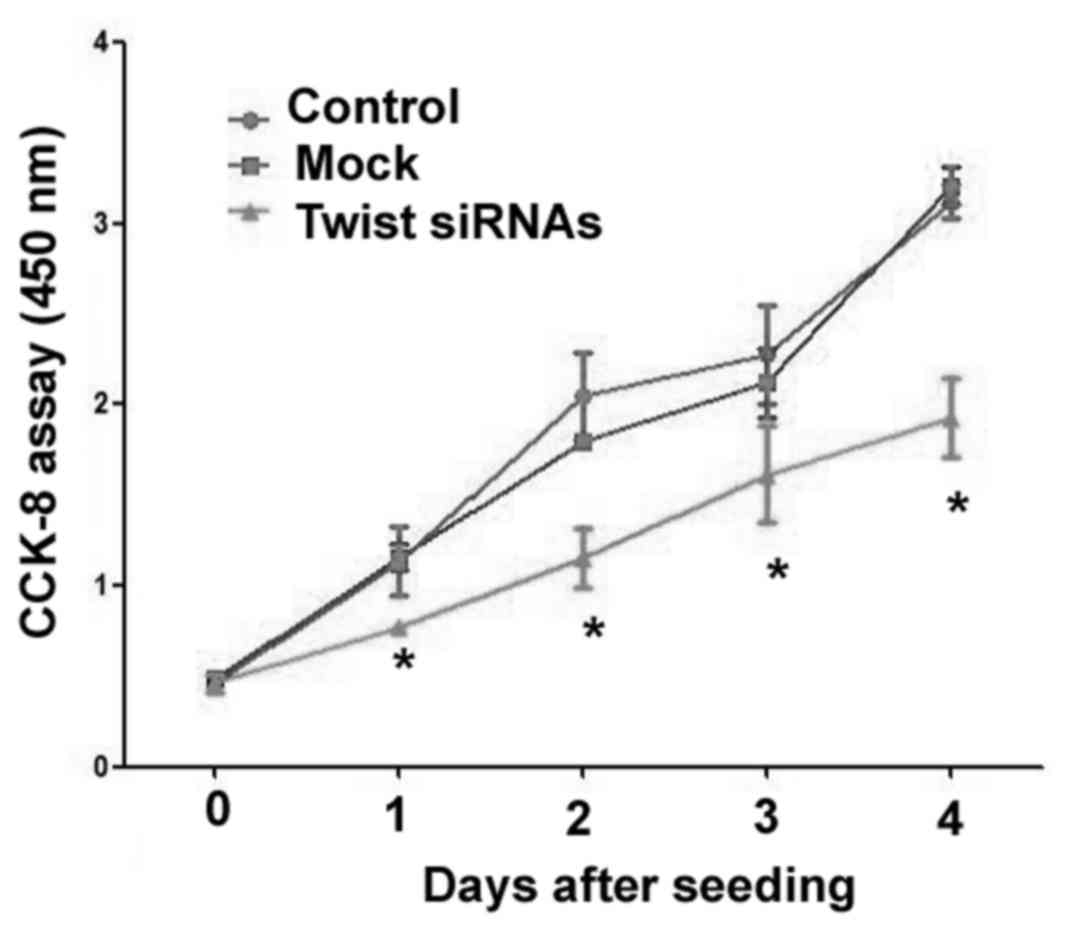
Results from the CCK-8 assay demonstrated that
downregulation of Twist led to a significant reduction in the
proliferation of Ishikawa cells, relative to mock cells transfected
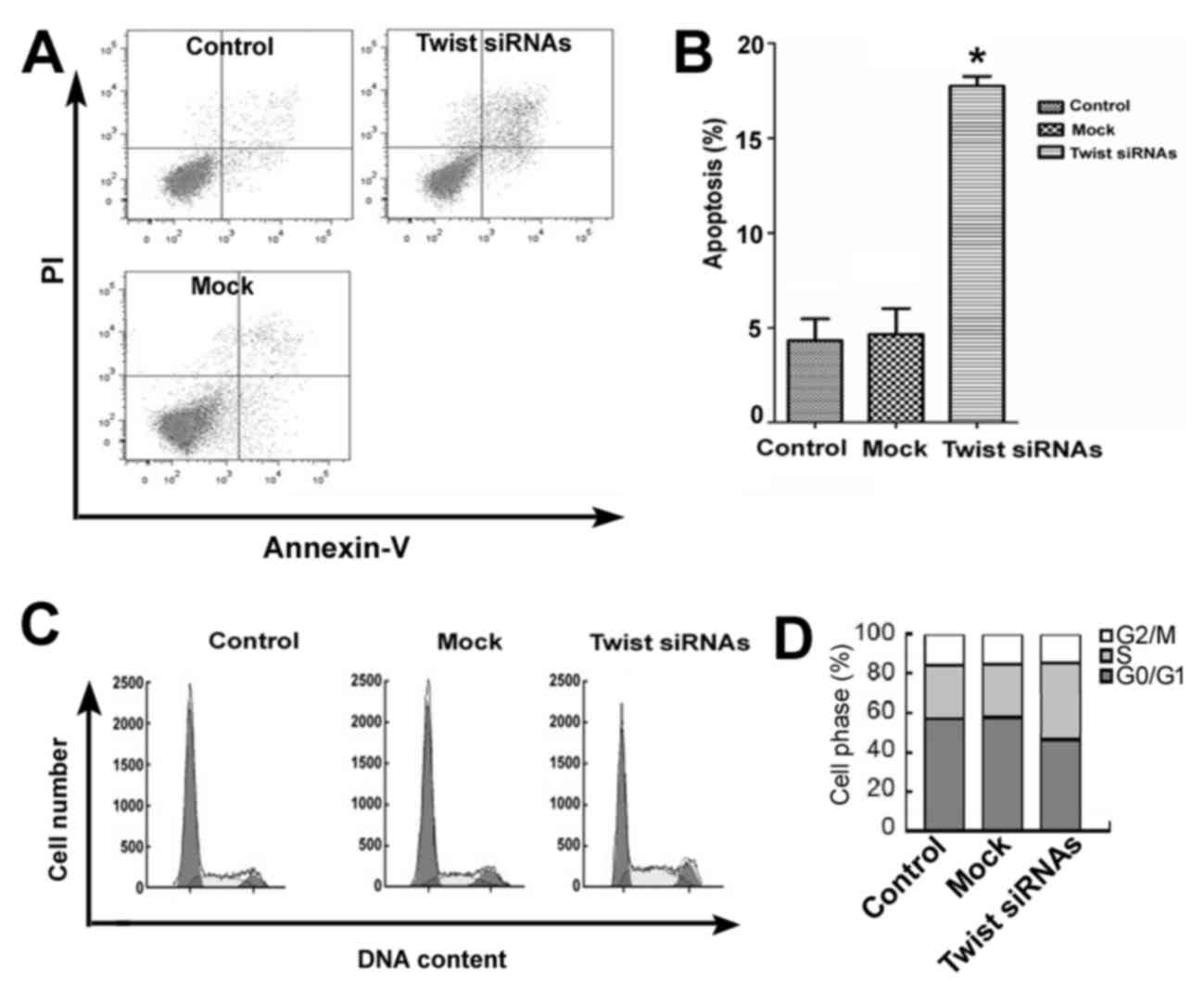
with non-targeting siRNA (P<0.05; Fig. 2). Flow cytometric analysis also
indicated that knockdown of Twist significantly induced apoptosis
(~4-fold) in Ishikawa cells compared with control and
mock-transfected cells (P<0.05; Fig.
3A and B). In addition, Twist downregulation led to a marked
decrease in the percentage of G0/G1 phase-cells and a concomitant
increase in the percentage of S-phase cells, indicating an arrest
of the cell cycle predominantly in the S-phase following Twist
knockdown (Fig. 3C and D).
Twist downregulation impairs Ishikawa
cell migration
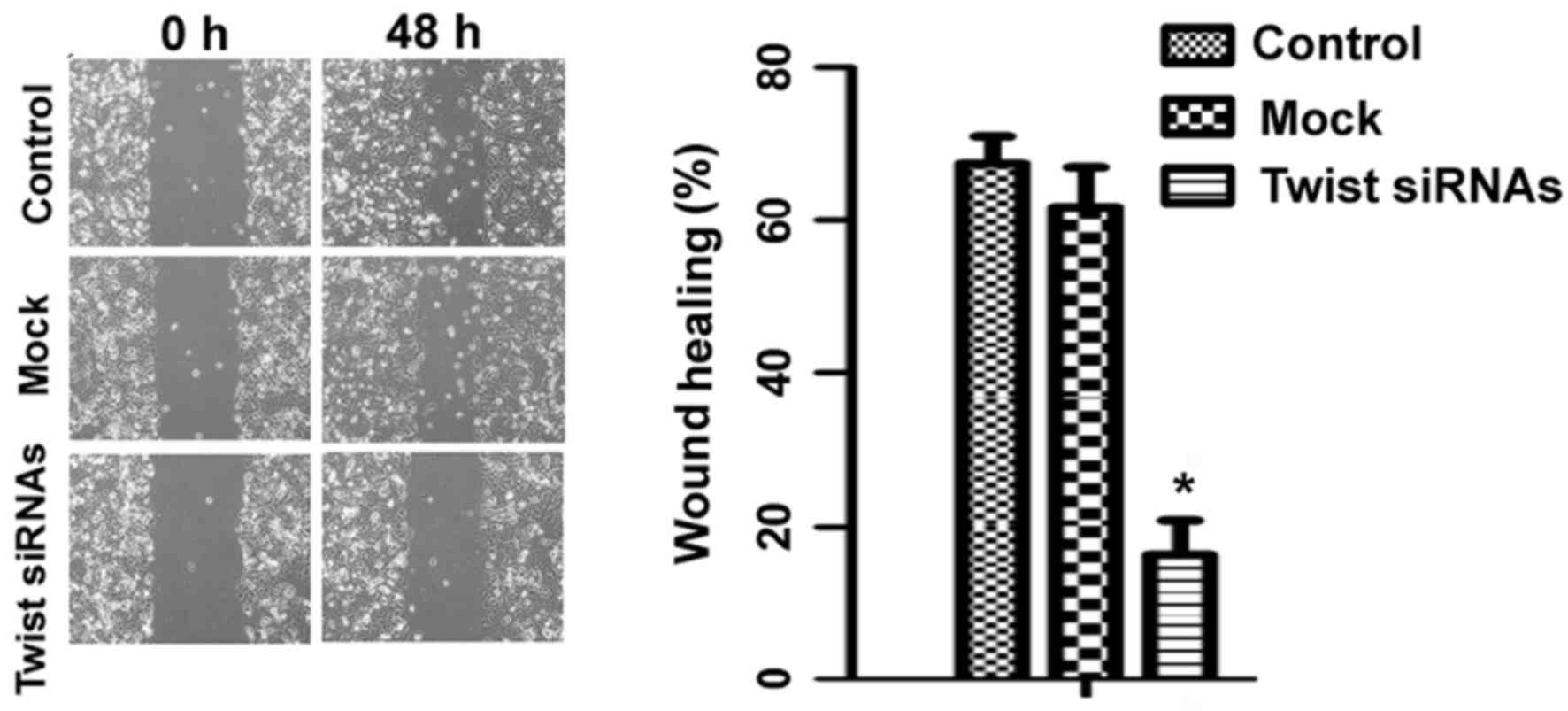
The effects of Twist knockdown on the migration of
Ishikawa cells were subsequently evaluated. In a wound-healing
assay, it was observed that knockdown of Twist significantly
attenuated the migration of Ishikawa cells, compared with control
cells (P<0.05; Fig. 4).
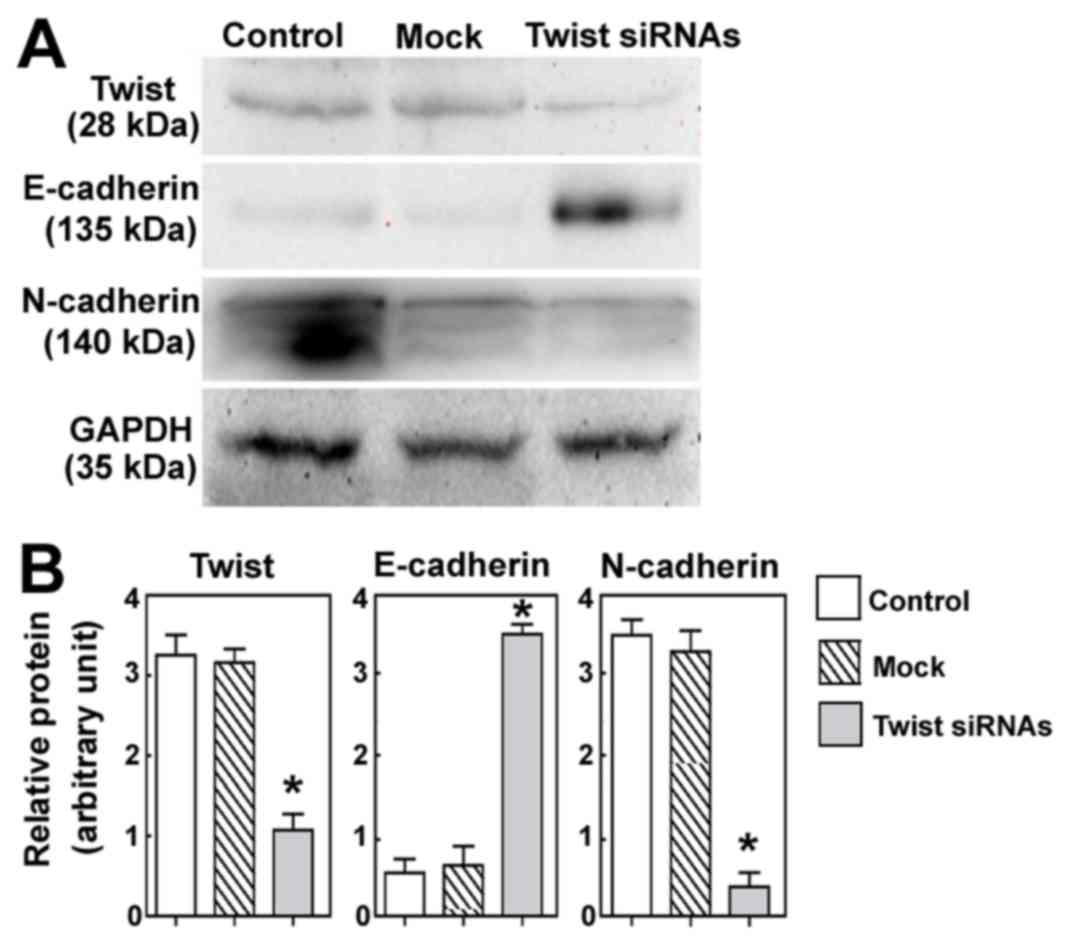
Twist downregulation affects the
expression of EMT-related proteins
The effects of Twist downregulation on the
expression of EMT-related proteins were also evaluated. As depicted
in Fig. 5, it was observed that
Twist silencing significantly induced the expression of E-cadherin
and significantly decreased the expression of N-cadherin, relative
to control cells (both P<0.05).
Discussion
Previous results have indicated that Twist
expression is correlated with the invasiveness of tumor cells and a
poor prognosis in many solid cancers, including prostate cancer and
hepatocellular carcinoma (8,14,15). In
addition, Twist has the ability to trigger EMT in a variety of
tumor cells, including prostate cancer and breast cancer (16). However, no previous results have
identified a role of Twist in the invasion and metastasis of
endometrial carcinoma. In the present study, it was determined that
knockdown of Twist led to a significant upregulation in E-cadherin
and a significant downregulation in N-cadherin. A wound scratch
assay also demonstrated that the migratory rate of Twist-knockdown
cells was significantly lower than that of control cells. These
results suggest that the decreases in cell migration mediated by
Twist silencing were associated with a reversion to an epithelial
cell phenotype, resulting in an impaired migratory capacity in
endometrial carcinoma cells.
Loss of E-cadherin expression is a critical
component of EMT. A primary mechanism by which E-cadherin
expression is inhibited is through the upregulation of its
transcriptional repressors. Yang et al (8) documented that overexpression of Twist
in human mammary epithelial cells inhibited the promoter activity
of E-cadherin. In addition, Twist overexpression in mammary and
prostate cancer cells leads to a decrease in E-cadherin expression,
thus promoting EMT and cell migration (8,14).
Consistent with these results, the current study identified a
statistically significant inverse association between Twist and
E-cadherin expression in endometrial carcinoma cells. Yang et
al (8) also demonstrated that
Twist may act as an EMT inducer, resulting in enhanced tumor
invasion and metastasis in a model of breast cancer. Collectively,
these results suggest that the delivery of Twist siRNA may be a
potential therapeutic approach for the treatment of endometrial
carcinoma.
Cell cycle control is the major regulatory mechanism
of cell growth. In particular, activation of the tumor suppressor
protein p53 serves a role in the regulation of cell cycle arrest
and apoptosis (17). Many
chemotherapeutic drugs and Chinese herbal medicines arrest the cell
cycle and subsequently induce cell death (18,19). It
has also been suggested that Twist has oncogenic properties. For
instance, it was demonstrated that overexpression of Twist in
rhabdomyosarcoma inhibits myc-induced apoptosis and interfere with
the tumor suppressive-effects of p53 (20). Furthermore, upregulation of Twist has
been associated with malignant transformation in T-cell lymphoma
(21) and forced expression of Twist
may trigger resistance in human cancer cells against drugs that
inhibit microtubule formation, including taxol and vincristine
(14). Vichalkovski et al
(22) demonstrated that
phosphorylation of the transcription factor Twist-1 by protein
kinase B at Ser42 inhibits the activity of p53 in response to DNA
damage. Indeed, it has been demonstrated that Twist participates in
development and progression of nasopharyngeal carcinoma and breast
cancers (23,24). Therefore, the present study
investigated the regulatory effect of Twist on endometrial
carcinoma cells by delivering Twist siRNAs into Ishikawa cells. The
growth of cells transfected with Twist siRNA was significantly
decreased and subsequent assays indicated that the suppressive
effects of Twist on cell proliferation occurred, at least in part,
through disruption of the S/M transition.
In conclusion, the present results indicate that
Twist affects the proliferation, migration and cell cycle
distribution of endometrial carcinoma cells, all of which may be
associated with its regulatory effects on EMT. These findings
suggest that Twist is a potential target for the treatment of
endometrial carcinoma. Further in vivo studies are now
warranted to confirm the roles of Twist in endometrial
carcinoma.
Acknowledgements
The present study was supported by the Shanghai
Medical Science Development Foundation of China (grant no.
3030503).
References
|
1
|
Jemal A, Thomas A, Murray T and Thun M:
Cancer statistics, 2002. CA Cancer J Clin. 52:23–47. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lotocki RJ, Copeland LJ, DePetrillo AD and
Muirhead W: Stage I endometrial adenocarcinoma:Treatment results in
835 patients. Am J Obstet Gynecol. 146:141–144. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wijnhoven BP, Dinjens WN and Pignatelli M:
E-cadherin-catenin cell-cell adhesion complex and human cancer. Br
J Surg. 87:992–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bremnes RM, Veve R, Gabrielson E, Hirsch
FR, Baron A, Bemis L, Gemmill RM, Drabkin HA and Franklin WA:
High-throughput tissue microarray analysis used to evaluate biology
and prognostic significance of the E-cadherin pathway in
non–small-cell lung cancer. J Clin Oncol. 20:2417–2428. 2000.
View Article : Google Scholar
|
|
6
|
Cheng L, Nagabhushan M, Pretlow TP,
Pretlow TP, Amini SB and Pretlow TG: Expression of E-cadherin in
primary and metastatic prostate cancer. Am J Pathol. 148:1375–1380.
1996.PubMed/NCBI
|
|
7
|
Sakuragi N1, Nishiya M, Ikeda K, Ohkouch
T, Furth EE, Hareyama H, Satoh C and Fujimoto S: Decreased
E-cadherin expression in endometrial carcinoma is associated with
tumor dedifferentiation and deep myometrial invasion. Gynecol
Oncol. 53:183–189. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vernon AE and LaBonne C: Tumor metastasis:
A new Twist on epithelial-mesenchymal transitions. Curr Biol.
14:R719–R721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Puisieux A, Valsesia-Wittmann S and
Ansieau S: A twist for survival and cancer progression. Br J
Cancer. 94:13–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng JJ, Zhang W, Xu XM, Zhang F, Tao WP,
Ye JJ and Ge W: Twist mediates an aggressive phenotype in human
colorectal cancer cells. Int J Oncol. 48:1117–1124. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Hou Y, Zhou M, Wen S, Zhou J, Xu
L, Tang X, Du YE, Hu P and Liu M: Twist induces
epithelial-mesenchymal transition and cell motility in breast
cancer via ITGB1-FAK/ILK signaling axis and its associated
downstream network. Int J Biochem Cell Biol. 71:62–71. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Q, Qiu MT, Zhu Z, Zhou JH, Chen L,
Zhou Y, Gu W, Wang LH, Li ZN, Xu Y, et al: Twist induces
epithelial-mesenchymal transition in cervical carcinogenesis by
regulating the TGF-β/Smad3 signaling pathway. Oncol Rep.
34:1787–1794. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C,
Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, et al: Up-regulation
of Twist in prostate cancer and its implication as a therapeutic
target. Cancer Res. 65:5153–5162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok
WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL, et al: Twist
overexpression correlates with hepatocellular carcinoma metastasis
through induction of epithelial-mesenchymal transition. Clin Cancer
Res. 12:5369–5376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang H, Massi D, Hemmings BA, Mandalà M,
Hu Z, Wicki A and Xue G: AKT-ions with a TWIST between EMT and MET.
Oncotarget. 7:62767–62777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai YJ, Lin CI, Wang CL and Chao JI:
Expression of survivin and p53 modulates honokiol-induced apoptosis
in colorectal cancer cells. J Cell Biochem. 115:1888–1899.
2014.PubMed/NCBI
|
|
18
|
Lee SM, Kwon JI, Choi YH, Eom HS and Chi
GY: Induction of G2/M arrest and apoptosis by water extract of
Strychni Semen in human gastric carcinoma AGS cells. Phytother Res.
22:752–758. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yunlan L, Juan Z and Qingshan L: Antitumor
activity of Di-n-Butyl(2,6-difluorobenzo-hydroxamato) tin (IV)
against human gastric carcinoma SGC-7901 cells via G2/M cell cycle
arrest and cell apoptosis. PLoS One. 9:e907932014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maestro R, Dei Tos AP, Hamamori Y,
Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach DH and
Hannon GJ: Twist is a potential oncogene that inhibits apoptosis.
Genes. 13:2207–2217. 1999. View Article : Google Scholar
|
|
21
|
Van Doorn R, Dijkman R, Vermeer MH,
Out-Luiting JJ, vander Raaij-Helmer EM, Willemze R and Tensen CP:
Aberrant expression of the tyrosine kinase receptor EphA4 and the
transcription factor twist in Sézary syndrome identified by gene
expression analysis. Cancer Res. 64:5578–5586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vichalkovski A, Gresko E, Hess D,
Restuccia DF and Hemmings BA: PKB/AKTphosphorylation of the
transcription factor Twist-1 at Ser42 inhibits p53 activity in
response to DNA damage. Oncogene. 29:3554–3565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Horikawa T, Yang J, Kondo S, Yoshizaki T,
Joab I, Furukawa M and Pagano JS: Twist and epithelial-mesenchymal
transition are induced by the EBV oncoprotein latent membrane
protein 1 and are associated with metastatic nasopharyngeal
carcinoma. Cancer Res. 67:1970–1978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|