Introduction
Cerebral ischemia, also known as cerebral apoplexy,
is a cerebrovascular disease characterized by reduction in cerebral
blood flow. Cerebral ischemia accounts for 85% of all cases of
stroke, and is the second major cause of disability and death
(1). At present, the most effective
way to treat cerebral ischemia is intravenous injection of
plasminogen activator for thrombolysis. However, this treatment
strategy is often accompanied by cerebral ischemia-reperfusion
injury (CIRI). CIRI refers to aggravated nerve injury and
dysfunction after reperfusion in patients with cerebral ischemia.
The mechanism of CIRI is related to oxidative stress, inflammation,
calcium overload, and other factors (2,3), among
which oxidative stress is caused by excessive production of
reactive oxygen species (ROS), which plays an important role in the
pathogenesis of ischemic stroke by exacerbating brain damage
(4). Antioxidative therapy against
ROS, which can prevent neuronal oxidative damage, may represent a
promising method in the radical treatment of ischemic stroke.
Clinical trials showed that application of antioxidants that can
clear ROS failed to achieve satisfactory results (5). Therefore, novel drugs that can
effectively treat CIRI are needed.
Panax notoginoside (PNS), a valuable traditional
Chinese herbal medicine, is a phytoestrogen isolated from the dried
roots of the perennial herb, Panax notoginseng. The main active
ingredients of PNS include ginsenoside Rb1, Rg1, and
notoginsenoside R1 (NGR1) (6).
Numerous studies have shown that PNS has therapeutic effects on
various neurodegenerative disorders, and the use of PNS during
global cerebral ischemia-reperfusion and focal ischemia-reperfusion
can significantly reduce cerebral edema. PNS also has a
satisfactory effect on acute cerebral infarction (6). Neuronal apoptosis plays an important
role in the pathogenesis of CIRI. Studies have shown that PNS can
inhibit the expression and activation of caspase-3 by regulating
the expression of Bcl-2 family genes to reduce neuronal apoptosis
caused by CIRI. This results in increased survival of neurons after
cerebral ischemia (7). In addition,
ginsenoside Rb1 and Rg1 have neuroprotective effects on mice with
CIRI (8). In recent years, studies
have reported that NGR1 can regulate various biological processes,
such as antioxidation, anti-inflammation, and anti-apoptosis, and
has neuroprotective activities (9).
Brain-derived neurotrophic factor (BDNF) is a nerve
growth factor that is widely distributed in brain tissue. BDNF can
affect neurons by significantly increasing the expression of its
receptor, TrkB, which is expressed on the surface of neurons. BDNF
can also inactivate injury factors in cells by activating TrkB
(10). Studies have shown that
cerebral ischemia and CIRI can increase the expression of BDNF,
thereby enhancing the ability of local neurons to resist injury, to
protect the patients from CIRI (11). The aim of this study was to
investigate the protective effect of NGR1 on rats with CIRI, and
its molecular mechanism, to provide a theoretical basis and
potential molecular target for the treatment of CIRI.
Materials and methods
Experimental animals
Sixty specific pathogen-free grade adult male
Sprague-Dawley rats (250–300 g) were purchased from the
Experimental Animal Center of Chinese Academy of Sciences
(Shanghai, China). Animals were kept at 23–25°C with relative
humidity of 45–55% and light cycle of 12 h. Rats were allowed free
access to food and water. They were randomly divided into four
groups including the sham-operation group (Sham), cerebral
ischemia-reperfusion model group (CIR), NGR1 treatment group
(NGR1), and nimodipine positive control group (NDC), with 15 rats
in each group. All animal experiments were carried out strictly in
accordance with the Guidelines of Proper Care and Use of Laboratory
Animals in Research established by the National Institute. The
study was approved by the Ethics Committee of the Second Affiliated
Hospital of Kunming Medical University (Yunnan, China).
Reagents
NGR1 (purity >98%; Shanghai Ronghe Pharmaceutical
Technology Development Co., Ltd., Shanghai, China); Nimodipine
(Shandong Xinhua Pharmaceutical Co., Ltd., Shandong, China);
chloral hydrate and triphenyl tetrazolium chloride (TTC)
(Sigma-Aldrich, St. Louis, MO, USA); Annexin V/propidium iodide
(PI) cell apoptosis detection kit (Invitrogen, Carslbad, CA, USA);
rabbit anti-rat Bcl-2, Bax, and β-actin primary monoclonal
antibodies (cat. nos. 3498, 2772 and 8457), and HRS goat
anti-rabbit secondary polyclonal antibody (cat. no. 7074; Cell
Signaling Technology, Inc., Danvers, MA, USA); Modified BCA kit
(Sangon, Shanghai, China); TRIzol reagent, Prime Script®
RT reagent kit with gDNA Eraser and SYBR® Premix Ex Taq™
II (Takara, Liaoning, China). All primers were synthesized by
Sangon.
Establishment of the rat model of
CIRI
The rat model of CIRI was established using the
bilateral common carotid artery occlusion (BCCAO) method described
by Schmidt-Kastner et al (12). All surgical tools were autoclaved in
advance, and the entire procedure was carried out in a sterile
environment. Rats were fasted at 6 h before surgery. After
anesthesia by intraperitoneal injection of chloral hydrate at a
dose of 350 mg/kg, a surgical blade was used to make an incision in
the middle of the neck. At the bilateral common carotid artery, the
surrounding tissue and vagus nerves were quickly and carefully
separated and exposed. Next, the bilateral common carotid artery
was ligated with two 5-0 threads, and the threads were removed 20
min later to restore cerebral blood perfusion. Surgical incisions
were sutured, and rats were placed in a 37°C incubator to allow
their body temperature to return to normal. Rats were transferred
to cages when they were awakened and righting reflex was
restored.
Animal grouping and drug
treatment
Sham: The bilateral common carotid artery was
exposed but not ligated, and the incision was sutured using a
conventional method; CIR: Rats were treated with BCCAO surgery, the
bilateral common carotid artery was ligated and reperfusion was
performed for 3 h, 20 min later, followed by intragastric
administration of 0.5 ml saline; NGR1: Rats were treated with BCCAO
surgery, the bilateral common carotid artery was ligated, and
reperfusion was performed for 3 h, 20 min later, followed by
intragastric administration of NGR1 at a dose of 100 mg/kg; NDC:
Rats were treated with BCCAO surgery, the bilateral common carotid
artery was ligated, and reperfusion was performed for 3 h, 20 min
later, followed by intragastric administration of nimodipine at a
dose of 1 mg/kg.
Determination of cerebral infarction
area
Three rats from each group that recovered (24 h
after surgery) were treated with excessive urethane for euthanasia.
The neck of rats was cut, and the brain was harvested after
craniotomy, and stored at −80°C for 15 min. After, brain tissue was
sectioned with a thickness of roughly 2 mm followed by staining in
2% TTC solution at 37°C for 30 min, and soaking in 10%
paraformaldehyde solution overnight. Living brain tissue was
stained brick red in color, while the areas of infarction were
stained gray. The percentage of cerebral infarction area to total
brain tissue area was calculated using Image-Pro Plus software
(Media Cybernetics, Rockville, MD, USA).
Analysis of hippocampal neuron
apoptosis
Analysis of hippocampal neuron apoptosis was
performed according to the instructions of the Annexin V/PI cell
apoptosis assay kit. Three rats returned to normal state in each
group (24 after surgery) were used to isolate hippocampal neurons.
After washing twice with precooled PBS, hippocampal neurons were
incubated with 100 µl 1X binding buffer containing 5 µl Annexin V
and 1 µl PI for 15 min. After, 400 µl 1X binding buffer was added
and apoptosis was analyzed immediately using FACSCalibur flow
cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Real-Time qPCR analysis
Three rats returned to normal state in each group
(24 after surgery) were used to isolate total RNA according to the
instructions of the kit. After cDNA synthesis by reverse
transcription, the reaction system for real-time PCR was prepared,
followed by PCR on a CFX-96 Real-Time PCR Detection system (Bio-Rad
Laboratories, New York, NY, USA) to measure the expression of BDNF
mRNA. All data were processed using the 2−ΔΔCq method
with the sham group as the control group, and β-actin as the
endogenous control. The primer sequences are shown in Table I.
 | Table I.Sequences of primers used in real-time
qPCR. |
Table I.
Sequences of primers used in real-time
qPCR.
| Gene | Primer sequences
(5′-3′) |
|---|
| BDNF-F |
CTGGAGAAAGTCCCGGTAT |
| BDNF-R |
GGTAGTCGGCATFGCGAGT |
| ACTB-F |
CAGGGCGTGATGGTGGGCA |
| ACTB-R |
CAAACATCATCTGGGTCATCTTCTC |
Western blot analysis
Three rats returned to normal state of each group
(24 after surgery) were used to isolate hippocampal tissue. The
tissue was stored in liquid nitrogen before use. Hippocampal tissue
was homogenized in cell lysis buffer, followed by centrifugation at
4°C (2,500 × g for 10 min) to collect the supernatant. Protein
concentration was determined by a modified BCA kit. A total of 50
µg of protein from each sample was subjected to 10% SDS-PAGE,
followed by electrotransfer to PVDF membranes. After blocking with
TBST buffer containing 5% skim milk at room temperature for 2 h,
membranes were incubated with rabbit anti-rat Bcl-2, Bax, or
β-actin primary monocolonal antibodies (1:1,000) overnight at 4°C.
After washing three times with 0.05% TBST, the membranes were
incubated with HRL goat anti-rabbit secondary polyclonal antibody
(1:2,000) at room temperature for 1 h under vibration. An ECL
detection system (Thermo Fisher Scientific, Waltham, MA, USA) was
used to detect the signals, and ImageJ software (National
Institutes of Health, Bethesda, MD, USA) was used to calculate the
gray value of each band. Relative protein levels are presented as
the percentage of the value of the target protein to that of
β-actin.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). All experiments were
repeated three times, and data are presented as mean ± standard
deviation. Single factor analysis of variance and two-tailed t-test
were performed for comparisons between groups. p<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of NGR1 treatment on cerebral
infarction area
The area of cerebral infarction at 24 h after
surgery was measured by TTC staining. As shown in Fig. 1, compared with the Sham group, the
area of cerebral infarction in the CIR group was significantly
enlarged, while the cerebral infarction area was significantly
smaller in the NGR1 and NDC groups compared with the CIR group.
Compared with the NDC group, the cerebral infarction area was
significantly smaller in the NGR1 group. These data suggest that
NGR1 can significantly reduce the area of cerebral infarction after
CIRI in rats, and this effect is stronger than that of the positive
control.
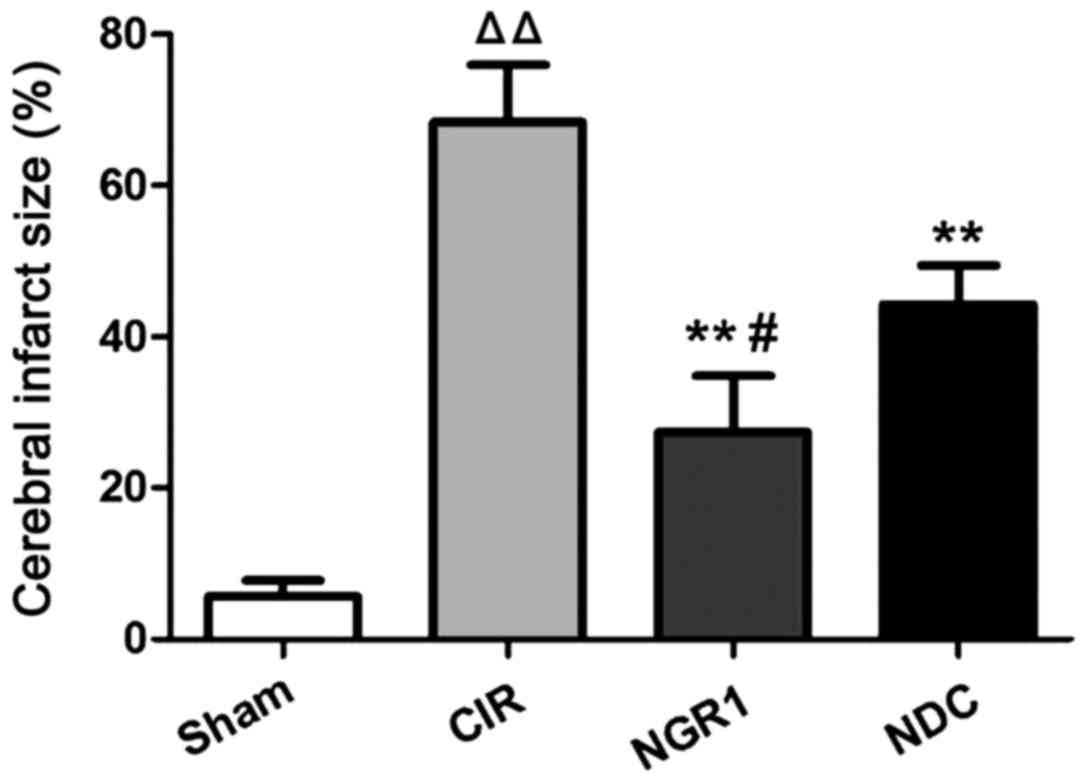 | Figure 1.Area of cerebral infarction in each
group at 24 h after surgery. TTC staining showed that compared with
the Sham group, the area of cerebral infarction in the CIR group
was significantly enlarged, while the cerebral infarction area was
significantly smaller in the NGR1 and NDC groups compared with the
CIR group. Compared with the NDC group, the cerebral infarction
area was significantly smaller in the NGR1 group.
△△Compared with the Sham group, p<0.01; **compared
with the CIR group, p<0.01; #compared with the NDC
group, p<0.05. TTC, triphenyl tetrazolium chloride; CIR,
cerebral ischemia-reperfusion model group; NGR1, notoginsenoside R1
treatment group; NDC, nimodipine positive control group. |
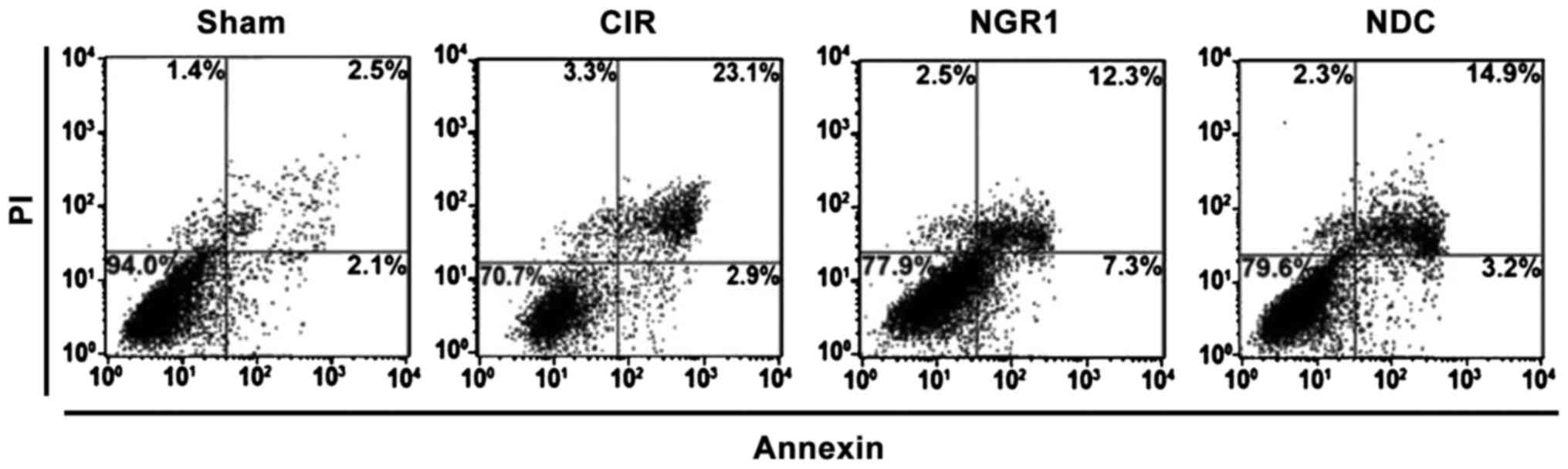
Effects of NGR1 treatment on apoptosis
of hippocampal neurons
Apoptosis of hippocampal neurons was detected by the
Annexin V/PI double staining method. As shown in Fig. 2, the upper right quadrant scatterplot
represents apoptotic cells. Compared with the Sham group, the ratio
of apoptotic hippocampal neurons was significantly increased, while
the ratio of apoptotic hippocampal neurons was significantly lower
in the NGR1 and NDC groups compared with the CIR group. Compared
with the NDC group, the ratio of apoptotic hippocampal neurons was
significantly decreased in the NGR1 group, indicating that NGR1 can
significantly decrease the ratio of apoptotic hippocampal neurons
in rats with CIRI, and this effect is stronger than that of the
positive control.
Effect of NGR1 treatment on
hippocampal expression of BDNF mRNA
The relative levels of BDNF mRNA in the hippocampus
of each group were measured by qRT-PCR. As shown in Fig. 3, compared with the Sham group, the
expression of BDNF mRNA was significantly increased in the CIR
group, while the expression of BDNF mRNA was significantly higher
in the NGR1 and NDC groups compared with the CIR group. In
addition, the expression of BDNF mRNA was significantly higher in
the NGR1 group than in the NDC group. These data suggest that CIRI
in rats can significantly increase the expression of BDNF mRNA,
while NGR1 treatment can further increase the expression of BDNF
mRNA in rats with CIRI, and this effect is stronger than that of
the positive control.
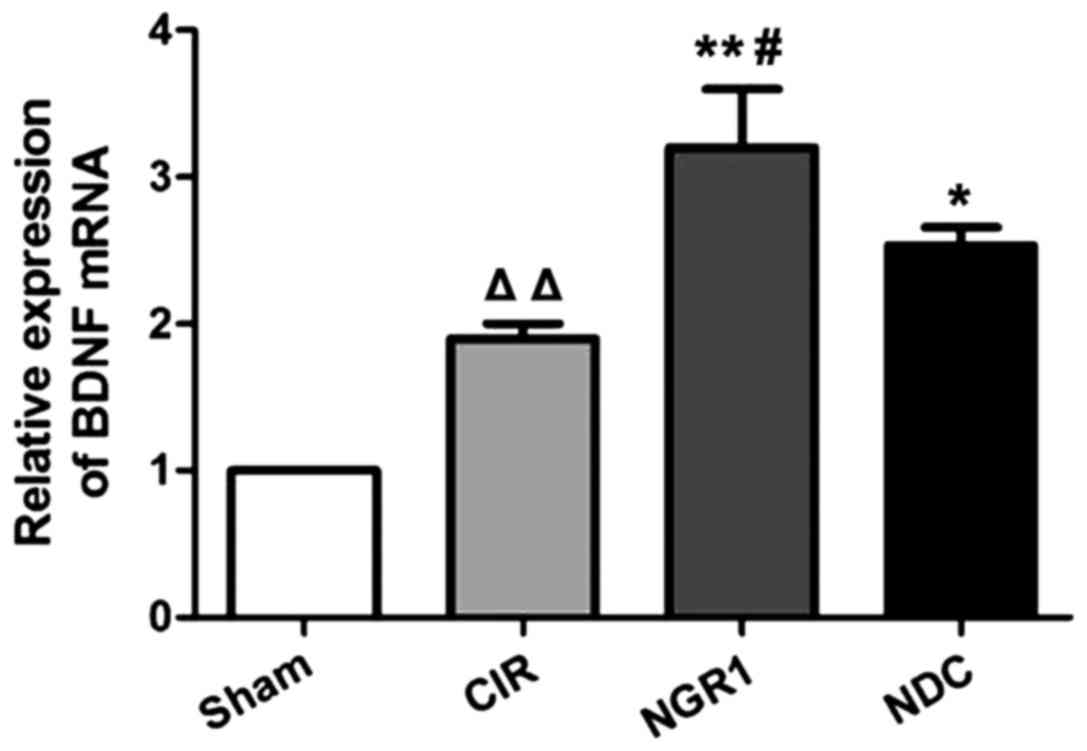 | Figure 3.Relative hippocampal expression of
BDNF mRNA in each group at 2 h after surgery. qRT-PCR showed that
compared with the Sham group, the expression of BDNF mRNA was
significantly increased in the CIR group, while the expression of
BDNF mRNA was significantly higher in the NGR1 and NDC groups
compared with the CIR group. In addition, the expression of BDNF
mRNA was significantly higher in the NGR1 group compared with the
NDC group. △△Compared with the Sham group, p<0.01;
**compared with the CIR group, p<0.01; #compared with
the NDC group, p<0.05. BDNF, brain-derived neurotrophic factor;
CIR, cerebral ischemia-reperfusion model group; NGR1,
notoginsenoside R1 treatment group; NDC, nimodipine positive
control group. |
Effects of NGR1 treatment on
hippocampal Bcl-2 and Bax protein expression
Western blot analysis was used to measure the
expression of Bcl-2 and Bax protein in the hippocampus in each
group. As shown in Fig. 4, compared
with the Sham group, the levels of the anti-apoptotic factor,
Bcl-2, were significantly decreased, and the levels of the
pro-apoptotic factor, Bax, were significantly increased in the CIR
group, while the levels of Bcl-2 were significantly higher and
those of Bax were significantly lower in the NGR1 and NDC groups
compared with the CIR group. In addition, compared with the NDC
group, the levels of Bcl-2 were significantly increased and those
of Bax were significantly decreased in the NGR1 group compared with
the NDC group. These data indicate that NGR1 treatment can
significantly increase the expression of Bcl-2 protein and
significantly decreased the expression of Bax protein in the
hippocampus of rats with CIRI.
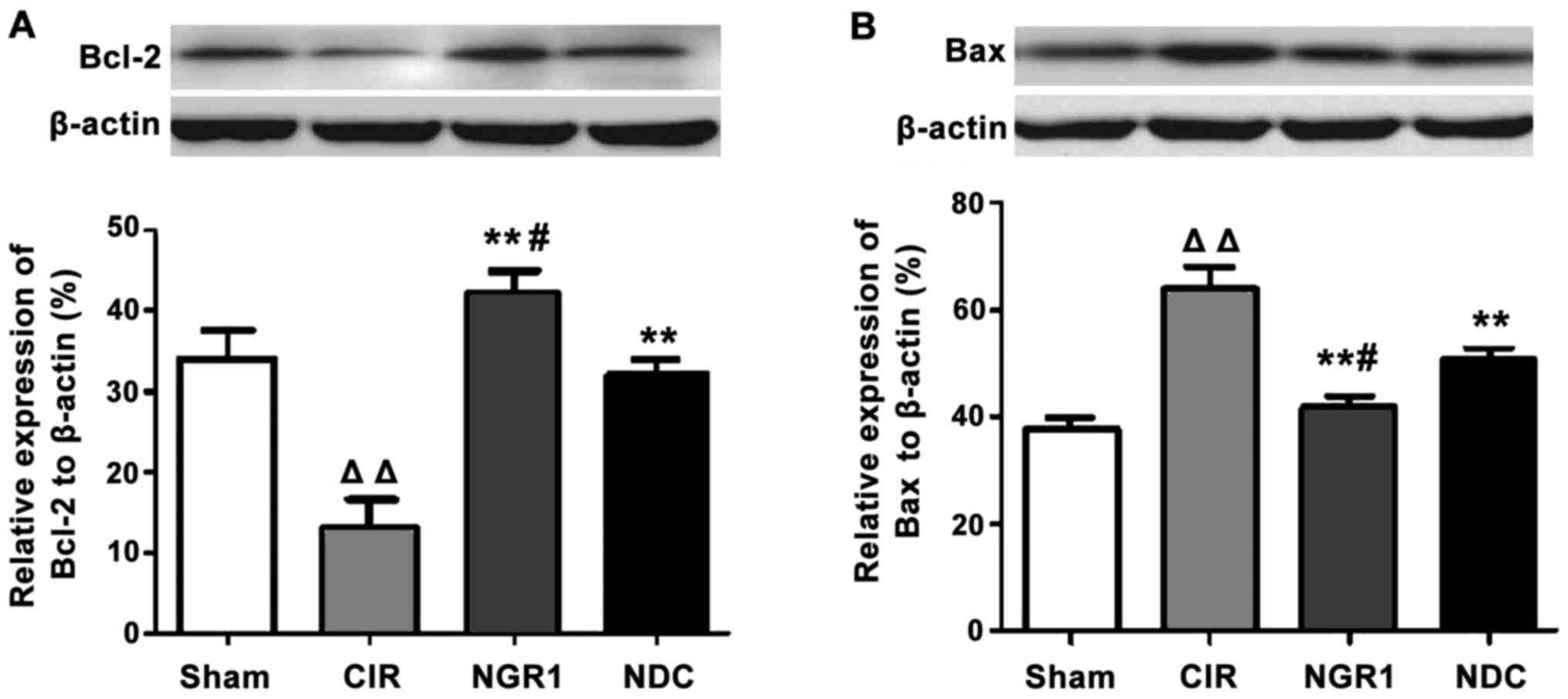 | Figure 4.Hippocampal expression of Bcl-2 and
Bax protein in each group at 24 h after surgery. Western blot
analysis showed that the levels of the anti-apoptotic factor,
Bcl-2, were significantly decreased and the levels of the
pro-apoptotic factor, Bax, were significantly increased in the CIR
group compared with the Sham group. The levels of Bcl-2 protein
were significantly higher and the levels of Bax protein were
significantly lower in the NGR1 and NDC groups compared with the
CIR group. Compared with the NDC group, the levels of Bcl-2 protein
were significantly increased and the levels of Bax protein were
significantly decreased in the NGR1 group compared with the NDC
group. △△Compared with the Sham group, p<0.01;
**compared with the CIR group, p<0.01; #compared with
the NDC group, p<0.05. CIR, cerebral ischemia-reperfusion model
group; NGR1, notoginsenoside R1 treatment group; NDC, nimodipine
positive control group. |
Discussion
Numerous studies have shown that oxidative stress
plays an important role in the pathogenesis of acute ischemic
stroke, and is an important cause of the ischemic injury cascade.
In addition, oxidative stress can induce cellular necrosis and
apoptosis (13). Studies have shown
that cellular necrosis in the core part of the ischemic area can be
induced in the transient state or within a few hours under
oxidative stress, while cellular necrosis is slow in the semi-dark
area and is mainly caused by Bcl-2 family-mediated apoptosis
(14).
BDNF is a neurotrophic factor that is widely
distributed in the cerebral cortex, hippocampus, and striatum. It
has been shown that BDNF plays an important role in protecting the
patients from CIRI, and its high expression is beneficial for the
improvement of pathologic conditions of the brain, and for the
repair of neuronal damage in CIRI (15). The mechanism of this protection is
complex, and is mainly achieved by stabilizing intracellular
Ca2+ concentration balance by antagonizing the toxicity
of excitatory amino acids, antagonizing cytotoxicity caused by
nitric oxide, enhancing the activity of antioxidant enzymes,
inhibiting the activity of caspase-3, and regulating the expression
of Bcl-2 and Bax, which in turn reduces the occurrence of apoptosis
and necrosis, and promotes the regeneration of damaged neurons
(16).
Neurosurgical treatment targeting all aspects of
cerebral ischemia or ischemia-reperfusion injury has received
increasing attention. At present, neuroprotective drugs mainly
include active oxygen free radical scavengers, anti-apoptotic
agents, and anti-inflammatory agents, among which nimodipine is a
commonly used drug in the treatment of cerebral ischemia.
Nimodipine can improve blood circulation during the recovery of
acute cerebrovascular disease and ischemic neurological disorders
(such as hypertension and migraine) and cerebral vasospasm after
subarachnoid hemorrhage caused by various factors. In addition,
nimodipine can be used in the treatment of ischemic neuronal injury
and vascular dementia. However, these chemicals can easily cause
side effects, and drug tolerance can develop after long-term use,
which in turn leads to unsatisfactory treatment outcomes. Numerous
studies have shown that PNS is more efficient than neuroprotection
in improving hypoxia tolerance, building immunity, anti-aging
function, and other aspects (17).
Monomeric saponins of PNS, such as NGR1, have shown satisfactory
efficacy in the treatment of cerebrovascular, hematological, and
neurological diseases, and for inflammation (18). Moreover, studies have shown that PNC
can protect the activity of endogenous superoxide dismutase to
reduce CIRI by removing free radicals produced by xanthine oxidase
(19), and reduce the occurrence of
neuronal apoptosis during ischemia-reperfusion by inhibiting the
expression and activation of the key apoptotic protease, caspase-3.
Other studies found that PNC can protect the brain by inhibiting
the expression of Bax and reducing the rate of apoptosis of nerve
cells after the occurrence of cerebral ischemia and hypoxia caused
by asphyxia and reperfusion injury (20).
Our study found that NGR1 treatment in rats with
CIRI significantly reduced the area of cerebral infarction, and
significantly increased the expression of the BDNF gene in the
hippocampus, which in turn protected hippocampal neurons from CIRI.
This protective effect is achieved possibly by increasing the
expression of the anti-apoptotic factor, Bcl-2, and synergistically
reducing the expression of the Bcl-2 antagonist factor (Bax), which
in turn regulates the expression of Bcl-2 family genes and inhibits
neuronal cell apoptosis. This is consistent with the aforementioned
findings. In addition, we found that the protective effects of NGR1
on rats with CIRI were stronger than those of the positive control,
nimodipine. Therefore, NGR1 may represent a novel drug with
promising prospects for the treatment of CIRI and other
cerebrovascular diseases or acute ischemic stroke.
In conclusion, our study provided new insights into
the treatment of CIRI, and a theoretical basis for the screening of
drugs for the treatment of CIRI.
References
|
1
|
Nagy Z and Nardai S: Cerebral
ischemia/repefusion injury: From bench space to bedside. Brain Res
Bull. 134:30–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iadecola C and Alexander M: Cerebral
ischemia and inflammation. Curr Opin Neurol. 14:89–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allen CL and Bayraktutan U: Oxidative
stress and its role in the pathogenesis of ischaemic stroke. Int J
Stroke. 4:461–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Collins VE, Macleod MR, Donnan GA, Horky
LL, van der Worp BH and Howells DW: 1,026 experimental treatments
in acute stroke. Ann Neurol. 59:467–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhan HQ, Zhang WX, Yan FL, Liu FQ and
Zhang XY: Effects of notoginsenoside-Rg1 on the expression of
apoptosis factors in brain tissue after ischemia injury. Guangdong
Yixue. 2014.(In Chinese).
|
|
7
|
Li J, Zhu P, Si Y, Xu Hong and Wu H:
Research on the effects of the total Saponin from Sanqi (the dried
root of Panax Notoginseng) on proapoptotic caspase-3 in the
forebrain in the rats with intracerebral hemorrhage. J Beijing Univ
Tradit Chin Med. 26:22–25. 2003.
|
|
8
|
Lu T, Jiang Y, Zhou Z, Yue X, Wei N, Chen
Z, Ma M, Xu G and Liu X: Intranasal ginsenoside Rb1 targets the
brain and ameliorates cerebral ischemia/reperfusion injury in rats.
Biol Pharm Bull. 34:1319–1324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang HS and Wang SQ: Notoginsenoside R1
inhibits TNF-alpha-induced fibronectin production in smooth muscle
cells via the ROS/ERK pathway. Free Radic Biol Med. 40:1664–1674.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrer I, Ballabriga J, Martí E, Pérez E,
Alberch J and Arenas E: BDNF up-regulates TrkB protein and prevents
the death of CA1 neurons following transient forebrain ischemia.
Brain Pathol. 8:253–261. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang KH, Ge SX, Xu BY, Yan JL and Wu LO:
Variation of BDNF mRNA on focalcerebral ischemia reperfusion injury
in rats with notogisenoside-Rg1. Zhong Yao Cai. 30:313–316.
2007.(In Chinese). PubMed/NCBI
|
|
12
|
Schmidt-Kastner R, Aguirre-Chen C, Saul I,
Yick L, Hamasaki D, Busto R and Ginsberg MD: Astrocytes react to
oligemia in the forebrain induced by chronic bilateral common
carotid artery occlusion in rats. Brain Res. 1052:28–39. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan PH: Reactive oxygen radicals in
signaling and damage in the ischemic brain. J Cereb Blood Flow
Metab. 21:2–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Erfani S, Khaksari M, Oryan S, Shamsaei N,
Aboutaleb N, Nikbakht F, Jamali-Raeufy N and Gorjipour F: Visfatin
reduces hippocampal CA1 cells death and improves learning and
memory deficits after transient global ischemia/reperfusion.
Neuropeptides. 49:63–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Endres M, Fan G, Hirt L, Fujii M,
Matsushita K, Liu X, Jaenisch R and Moskowitz MA: Ischemic brain
damage in mice after selectively modifying BDNF or NT4 gene
expression. J Cereb Blood Flow Metab. 20:139–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kiprianova I, Sandkühler J, Schwab S,
Hoyer S and Spranger M: Brain-derived neurotrophic factor improves
long-term potentiation and cognitive functions after transient
forebrain ischemia in the rat. Exp Neurol. 159:511–519. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang XP, Ding H, Lu JD, Tang YH, Deng BX
and Deng CQ: Study on protective effect of Panax notoginseng
saponins on brain ischemic reperfusion damage. Chin J Clin
Neurosci. 10:902002.
|
|
18
|
Gu B, Nakamichi N, Zhang WS, Nakamura Y,
Kambe Y, Fukumori R, Takuma K, Yamada K, Takarada T, Taniura H, et
al: Possible protection by notoginsenoside R1 against glutamate
neurotoxicity mediated by N-methyl-D-aspartate receptors composed
of an NR1/NR2B subunit assembly. J Neurosci Res. 87:2145–2156.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou X, Cui G, Tseng HH, Lee SM, Leung GP,
Chan SW, Kwan YW and Hoi MP: Vascular contributions to cognitive
impairment and treatments with traditional Chinese medicine. Evid
Based Complement Alternat Med. 2016:96272582016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han JY, Li Q, Ma ZZ and Fan JY: Effects
and mechanisms ofcompound Chinese medicine and major ingredients
onmicrocirculatory dysfunction and organ injury induced
byischemia/reperfusion. Pharmacol Ther. 177:146–173. 2017.
View Article : Google Scholar : PubMed/NCBI
|