Introduction
Staphylococcus aureus (S. aureus) is
an important Gram-positive human pathogen that causes various
diseases, including typical skin and soft-tissue infections, as
well as life-threatening invasive diseases (1). S. aureus pneumonia,
characterized by localized necrosis and inflammation, is one of the
most prevalent S. aureus-mediated diseases and occurs in
~13.3% of all invasive staphylococcal infections (2,3).
However, due to the ability of S. aureus to develop
resistance to a broad range of antibiotics, a decline in the
efficiency of current treatments has been observed, and there is an
obvious requirement for the development of novel antibiotics or
therapeutic strategies against staphylococcal infections.
Recently, the innate immune pathway involving the
inflammasome was demonstrated to have a key role in several
diseases, including atherosclerosis (4), Alzheimer's disease (5) and type 2 diabetes (6). The nucleotide-binding domain and
leucine-rich repeat containing gene (NLR) family, pyrin domain
containing 3 protein (NLRP3) inflammasome consists of the NLR
family member NLRP3, the adaptor protein apoptosis-associated
speck-like protein containing a C-terminal caspase recruitment
domain (ASC) and the effector protein caspase-1 (7). The NLRP3 inflammasome has been
demonstrated to be activated in response to a broad range of
exogenous and endogenous stimuli, including infecting
microorganisms such as Sendai virus (8), adenovirus (9) and Candida albicans (10), as well as S. aureus (11), and Shigella flexneri (12). Furthermore, S. aureus
α-hemolysin was demonstrated to induce interleukin (IL)-1β
secretion and caspase-1 activation in human and mouse monocytic
cells through the NLRP3 inflammasome (13). Furthermore, the activation of NLRP3
was found to have a key role in a murine model of severe pneumonia
induced by S. aureus α-hemolysin (14), suggesting the NLRP3 inflammasome as a
potential target for pharmacologic interventions in severe S.
aureus pneumonia.
Resveratrol (3,5,4′-trihydroxystilbene or
3,5,4′-stilbenetriol) is a natural polyphenolic compound contained
in several plant species, such as grapes, berries and peanuts
(15). Resveratrol has been shown to
have various activities, including an-inflammatory (16), anti-bacterial (17) and anti-oxidant effects (18). Furthermore, resveratrol has also been
demonstrated to exert an anti-inflammatory effect on S.
aureus-induced keratitis by decreasing cell surface Toll-like
receptor 2 and downregulating the expression of IL-8 (19). A previous study found that
resveratrol protects S. aureus-induced lung inflammation in
human lung epithelial cells (20).
These findings suggested that resveratrol may be effective in
ameliorating S. aureus pneumonia. In addition, numerous
studies have indicated that resveratrol inhibits the activation of
NLRP3-mediated inflammation in serious diseases, such as myocardial
ischemia/reperfusion injury (21),
metaflammation (22) and chronic
kidney disease (23). Thus, in the
present study, it was hypothesized that resveratrol ameliorates
S. aureus-induced pneumonia. Furthermore, it was
investigated whether resveratrol exerts this effect via inhibition
of the activation of the NLRP3 inflammasome pathway.
Materials and methods
Reagents and bacteria
S. aureus 8325-4 and DU 1090 were purchased
from Wenzhou Kont Biology & Technology Co. Ltd (Jiangsu,
China). S. aureus strains were cultured in tryptone soya
broth (Oxoid, Basingstoke, UK). TNF-α (438208), IL-1β (437008) and
IL-18 (437008) ELISA kits were purchased from BioLegend Inc. (San
Diego, CA, USA). TRIzol solution, a
reverse-transcription-polymerase chain reaction (RT-PCR) kit and a
fluorescence quantitative PCR kit were purchased from Takara Bio
Inc. (Otsu, Japan). Antibodies against NLRP3 (13158) and ASC
(67824) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Antibodies against caspase-1 (sc-514) and GAPDH
(sc-25778) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Horseradish peroxidase-conjugated anti-rabbit
immunoglobulin G antibody (074–1506) was purchased from KPL
(Gaithersburg, MD, USA). Resveratrol, purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany), was suspended in PBS for the
animal studies.
Mouse model of S. aureus
pneumonia
A total of 93 female C57BL/6J mice (age, 8 weeks;
weight, 18–22 g) were purchased from the Experimental Animal Center
of Soochow University (Suzhou, China). Mice were divided into three
groups: S. aureus + PBS group, S. aureus +
resveratrol group and DU 1090 + PBS group. The DU 1090 group was
designed as the comparative group for S. aureus infected
group treated with PBS. After anesthesia with 80 mg/kg ketamine and
15 mg/kg xylazine (Hubei Xinkang Pharmaceutical Chemical Co., Ltd;
intraperitoneally) (24), S.
aureus pneumonia was induced by addition of 30 µl of S.
aureus suspension (4×108 CFUs, S. aureus
8325-4, Wenzhou Kont Biology & Technology Co., Ltd., Jiangsu,
China) into the left nostril. Mice in the DU 1090 + PBS group were
intranasally infected with 30 µl DU 1090 (4×108 CFUs;
Wenzhou Kont Biology & Technology Co., Ltd.). The infected mice
were subcutaneously administered PBS or 30 mg/kg resveratrol 2 h
after infection and then at 12 h intervals. The mice were housed at
30°C with 45–75% humidity. Mice had free access to food and water
with a 12 h light/dark cycle. The mortality was monitored over a
72-h time course.
All animal studies were performed according to the
experimental practices and standards developed by the Animal
Welfare and Research Ethics Committee of Zhangjiagang City Jingfeng
People's Hospital (Suzhou). The experimental protocols were
approved by the animal care committee of Zhangjiagang City Jingfeng
People's Hospital (Suzhou).
Hematoxylin and eosin (H&E)
staining analysis
Lungs were removed and fixed at room temperature in
10% formalin for one day. Formalin-fixed tissues were processed,
paraffin-embedded, thin sectioned (5 µm), stained with H&E
(hematoxylin for 5 min and eosin for 20 sec at room temperature)
and visualized by light microscopy (Olympus, Tokyo, Japan). A total
of 9 mice were used for H&E analysis.
ELISA
Bronchoalveolar lavage fluid (BALF) collection was
performed twice by intratracheal instillation of 500 µl PBS. The
lung tissues were then harvested and homogenized with PBS. After
centrifugation (1,500 × g, 10 min, 4°C), the supernatants of BALF
and lung tissue lysates were individually used for cytokine
measurements. Cytokine levels were measured using ELISA kits for
interleukin (IL)-1β, IL-18 and tumor necrosis factor (TNF)-α.
RT quantitative (q)PCR
Total RNA of lung tissues was isolated using TRIzol
(Takara Bio, Inc.) and DNA was removed from each RNA preparation
using RNase-free DNase I (Takara Bio Inc.) according to the
manufacturer's instructions. For RT, total RNA (1 µg) was reverse
transcribed to first-strand cDNA in 20 µl of mixture containing 25
mM MgCl2 (4 µl), reverse transcription 10X buffer (2
µl), 10 mM dNTP mixture (2 µl), recombinant RNase inhibitor (0.5
µl), AMV reverse transcriptase (15 µl and Oligo (dT) primers (0.5
µg; RNA PCR kit; cat. no. RR019B; Takara Bio, Inc.). The reaction
conditions were as follows: 42°C for 60 min followed by 95°C for 5
min. qPCR was performed using a 7000 Sequence Detection System
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) using SYBR Premix Ex Taq (Takara Bio, Inc.) according to the
manufacturer's protocol. GAPDH is used as an internal control. The
PCR reaction system contained 2 µg template cDNA, ligase, dNTP,
buffer 10× buffer, 200 µmol/l of each dNTP, 20 pmol of each primer,
1.5 mmol/l Mg2+ and nuclease-free water. PCR was performed using
the following program: 94°C for 5 min, followed by 30 cycles of
94°C for 30 sec, 58°C for 60 sec and 72°C for 45 sec. Primers used
in this study are listed in Table I.
The relative expressions were calculated using the
2−ΔΔCq method (25).
 | Table I.Primer sequences used for polymerase
chain reaction in the present study. |
Table I.
Primer sequences used for polymerase
chain reaction in the present study.
| Gene | Forward | Reverse |
|---|
| NLRP3 |
5′-GGTCCTCTTTACCATGTGTGCTTC-3′ |
5′-AAGTCATGTGGCTGAAGCTGTA-3′ |
| ASC |
5′-CTTGTCAGGGGATGAACTCAA-3′ |
5′-CTGGTCCACAAAGTGTCCTGT-3′ |
| Caspase-1 |
5′-CGTGGAGAGAAACAAGGAGTG-3′ |
5′-AATGAAAAGTGAGCCCCTGAC-3′ |
| GAPDH |
5′-AGGTCGGTGTGAACGGATTTG-3′ |
5′-GGGGTCGTTGATGGCAACA-3′ |
Western blot analysis
Total protein extraction of mouse lung tissues was
performed for western blot analysis using lysis buffer (10 mM
Tris-HCl, 1 mM EDTA, and 250 mM sucrose, pH 7.4, containing 15
µg/ml aprotinin, 5 µg/ml leupeptin, 0.1 mM phenylmethanesulfonyl
fluoride, 1 mM NaF and 1 mM Na3VO4). A total
of 20 mg protein was separated 12% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA).
Membranes were incubated with primary antibodies, including
antibodies against NLRP3 (1:1,000 dilution), ASC (1:1,000
dilution), Caspase-1 (1:300 dilution), pro-Caspase-1 (1:300
dilution, ERP16883; Abcam, Cambridge, MA, USA) and GAPDH (1:500
dilution) overnight at 4°C. Subsequent to secondary antibody
incubation with horseradish peroxidase-conjugated goat anti-rabbit
IgG H&L (1:10,000; ab97051; Abcam) for 2 h at room temperature,
immunoreactive bands were visualized by incubation with lumiGLO
reagent (Cell Signaling Technologies, Inc.) and exposed to X-ray
film according to the manufacturer's instructions. Relative
quantitation of western blots was performed with normalization to
the amount of GAPDH protein levels.
Statistical analysis
The experimental data were assessed using an
independent Student's t-test with SPSS 13.0 statistical software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Resveratrol protects mice from S.
aureus infection
To evaluate the effectiveness of resveratrol on
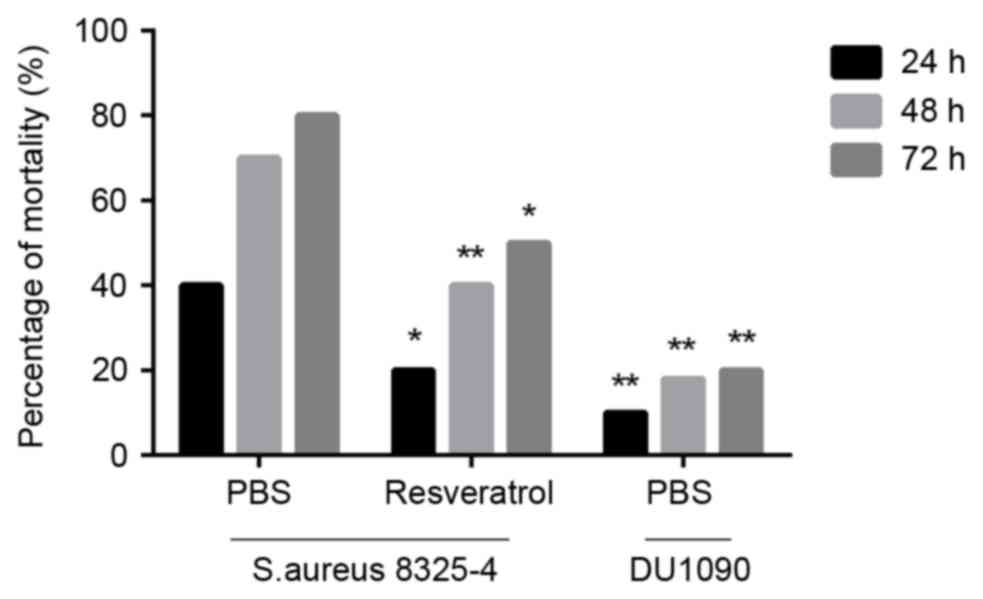
S. aureus pneumonia, mice were intranasally administered
S. aureus at a lethal dose. As displayed in Fig. 1, the mortality rate of S.
aureus-infected mice treated with resveratrol was significantly
lower than that of those treated with PBS (P<0.05 at 24 and 72 h
and P<0.01 at 48 h). These results suggested that resveratrol
protects against S. aureus infection at a lethal dose.
Resveratrol alleviates S. aureus
pneumonia in mice
To investigate the effect of resveratrol treatment
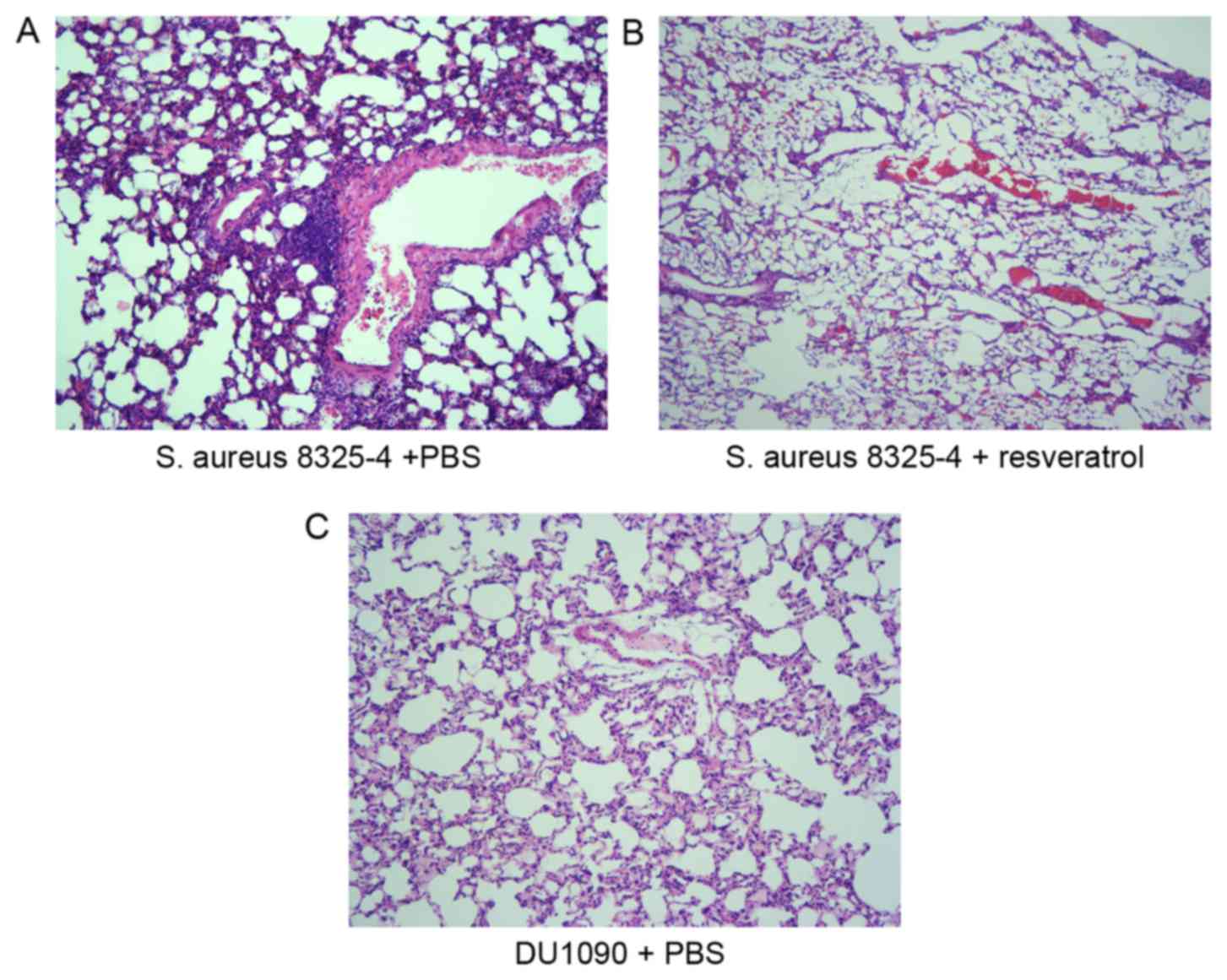
on pneumonia-associated lung injury and pathological changes,
histopathological analysis of the lungs was performed at 24 h after
infection. H&E staining demonstrated that S. aureus
infection induced severe alveolar destruction and massive
inflammatory cell aggregation in the lung tissue (Fig. 2A), while treatment with resveratrol
obviously reduced these changes in the lung tissue (Fig. 2B). These results suggested that
resveratrol alleviated S. aureus pneumonia in mice.
Resveratrol decreases inflammatory
cytokine production in S. aureus pneumonia
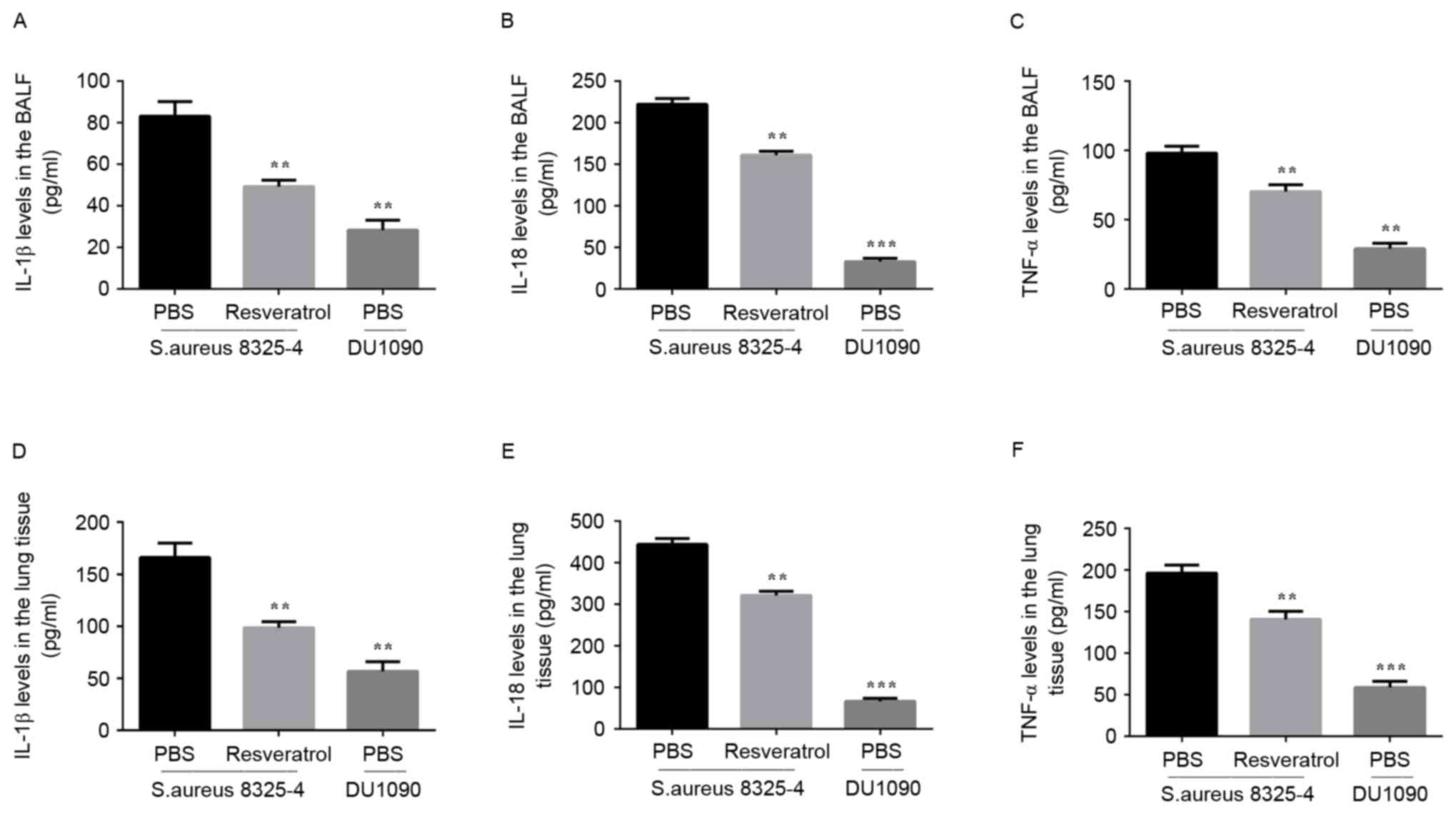
The present study then investigated the secretion of
inflammatory cytokines in the BALF and lung tissues of mice
infected with S. aureus. The mean value of IL-1β, IL-18 and
TNF-α expression in BALF and lung tissues in two S. aureus
and DU 1090 groups are as follows: IL-1β (BALF: 83.062, 49.332,
28.404, lung: 166.123, 98.664, 56.808, IL-18 (BALF: 222.02,
160.694, 33.09, lung: 444.042, 321.386, 66.183) and TNF-α (BALF:
98.083, 70.325, 29.223, lung: 196.165, 140.651, 58.447). As
illustrated in Fig. 3, S.
aureus significantly increased the levels of IL-1β, IL-18 and
TNF-α in the BALF. Compared to the model group, mice treated with
resveratrol showed a lower level of IL-1β (P<0.01), IL-18
(P<0.01) and TNF-α (P<0.01) in the BALF. Similar results were
also observed in the lung tissues (Fig.
3D-F). These results indicated that resveratrol treatment
decreases the inflammatory response by reducing the production of
inflammatory cytokines in mice with S. aureus pneumonia.
Resveratrol inhibits the activation of
the NLRP3 inflammasome in S. aureus-infected mice
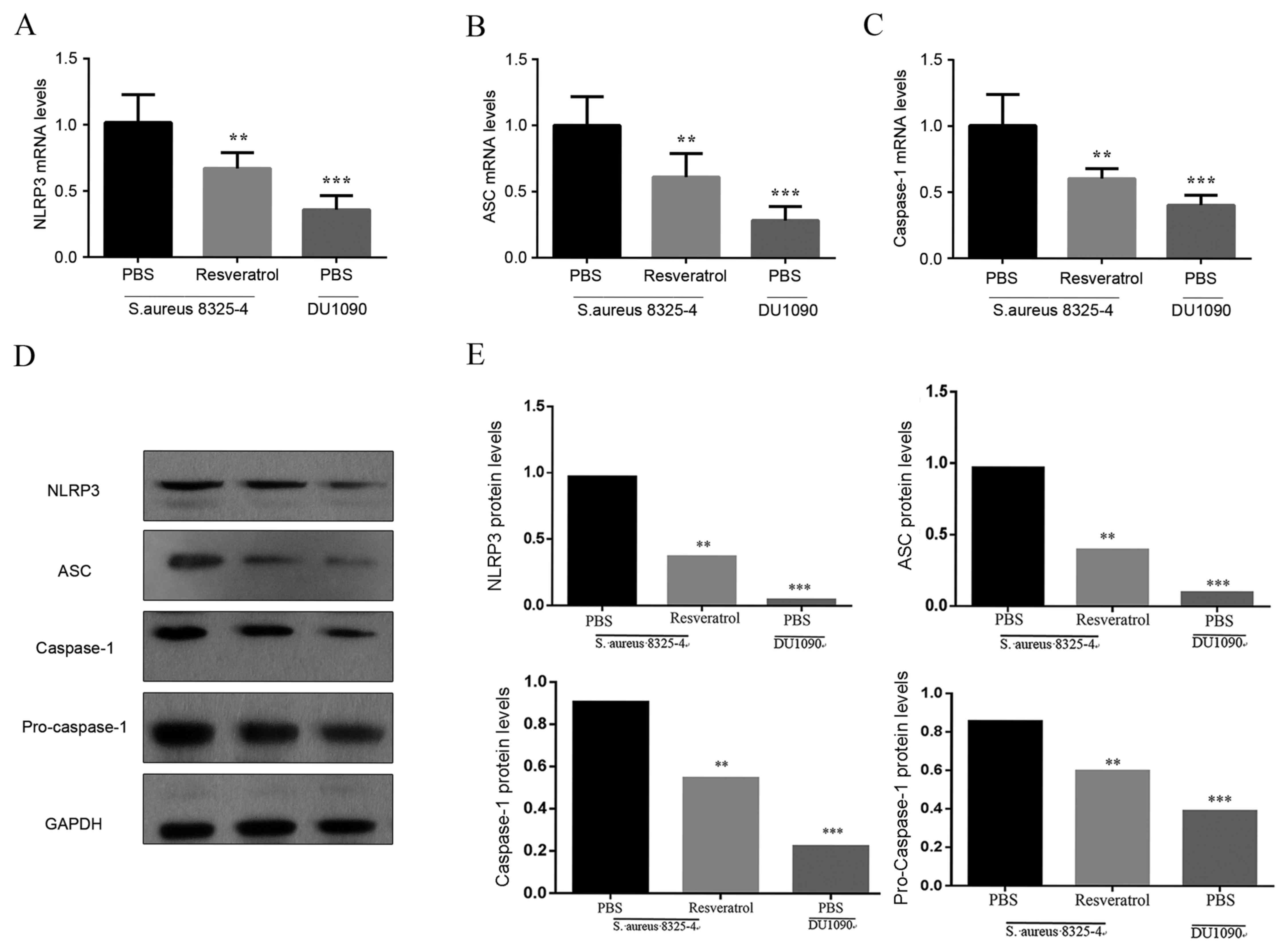
To analyze the impact of resveratrol on the NLRP3
inflammasome in the lungs of infected mice, RT-qPCR and western
blot analyses were used to estimate their expression in lungs of
pneumonia model mice at 24 h after S. aureus infection. As
illustrated in Fig. 4A-C, the mRNA
expression levels of NLRP3, ASC and caspase-1 in the lungs were
obviously increased in S. aureus-infected mice in comparison
with mice in the DU 1090 group indicating the activation of the
NLRP3 inflammasome. Furthermore treatment with resveratrol
significantly decreased the mRNA levels of NLRP3, ASC and caspase-1
in this animal model. In addition, western blot analysis revealed
that S. aureus significantly increased the protein levels of
NLRP3 and ASC compared with the PBS treated DU 1090 infected group,
and stimulated the activation of caspase-1 (Fig. 4D), which was attenuated by the
treatment with resveratrol. These results demonstrated that
resveratrol inhibited the activation of the NLRP3 inflammasome in
the lungs of S. aureus-infected mice.
Discussion
To the best of our knowledge, the present study was
the first to demonstrate that resveratrol exerts a protective
effect against lung injury in a murine model of S. aureus
pneumonia, and that the effect is probably associated with the
reduction of the inflammatory response and inhibition of the NLRP3
inflammasome.
Increasing evidence indicated that certain natural
products protect mice from S. aureus pneumonia (26–28). A
previous study demonstrated that resveratrol had obvious
anti-bacterial activities against clinical isolates of
methicillin-resistant S. aureus (29). Similarly, the present results showed
that treatment with resveratrol significantly reduced the mortality
in a murine model of S. aureus pneumonia. Consistent with a
previous study (26), the
histopathology results showed that S. aureus induced severe
alveolar destruction and massive inflammatory cell aggregation in
the lung. Of note, resveratrol significantly ameliorated S.
aureus-induced lung injury in mice, suggesting a protective
effect of resveratrol against S. aureus pneumonia in
mice.
S. aureus pneumonia induces a strong host
inflammatory reaction, which causes rapid and excessive recruitment
of inflammatory cells (30). The
present study found that pro-inflammatory cytokines, including
IL-1β, IL-18 and TNF-α, were increased in the BALF and lungs.
However, resveratrol obviously reduced the elevated levels of
IL-1β, IL-18 and TNF-α in the BALF and lungs induced by S.
aureus infection in mice. These results suggested that
resveratrol may have therapeutic potential by reducing the
inflammatory response in mice with S. aureus-infected lung
tissues.
Several studies reported that the NLRP3 inflammasome
has a crucial role in the host immune response to S. aureus
and may be an important target pathway for the treatment of S.
aureus pneumonia (14,31). In accordance with this, the present
study found that resveratrol treatment decreased the mRNA and
protein expression of NLRP3, ASC and caspase-1 in the lung tissue
of mice with S. aureus pneumonia. As is known, activation of
the NLRP3 inflammasome promotes the cleavage and activation of
caspase-1, which in turn is a central stimulator of the
pro-inflammatory cytokines IL-1β and IL-18, resulting in
inflammation (32,33); this is consistent with the results of
the present study regarding pro-inflammatory cytokine secretion
observed in BALF and lung tissues in mice infected with S.
aureus. Collectively, these results suggested that resveratrol
exerts a protective effect against S. aureus pneumonia,
probably via suppression of the NLRP3 inflammasome. However,
further study required to clarify the precise mechanism by which
resveratrol inhibits the activation of the NLRP3 inflammasome.
In conclusion, the present study demonstrated that
resveratrol alleviated S. aureus pneumonia in mice by
inhibiting NLRP3-mediated inflammation. The results suggested that
resveratrol is a potentially useful agent for the treatment of
S. aureus pneumonia and associated infectious diseases.
References
|
1
|
Lowy FD: Staphylococcus aureus
infections. N Engl J Med. 339:520–532. 1998. View Article : Google Scholar
|
|
2
|
Klevens RM, Morrison MA, Nadle J, Petit S,
Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM,
et al: Invasive methicillin-resistant Staphylococcus aureus
infections in the United States. JAMA. 298:1763–1771. 2007.
View Article : Google Scholar
|
|
3
|
Kitur K and Prince A: Staphylococcus
aureus activation of NLRP3 inflammasome and necroptosis through
MLKL exacerbates pneumonia. FASEB J. 29 1 Suppl:S148.52015.
|
|
4
|
Duewell P, Kono H, Rayner KJ, Sirois CM,
Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr
M, et al: NLRP3 inflammasomes are required for atherogenesis and
activated by cholesterol crystals. Nature. 464:1357–1361. 2010.
View Article : Google Scholar
|
|
5
|
Heneka MT, Kummer MP, Stutz A, Delekate A,
Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, et
al: NLRP3 is activated in Alzheimer/'s disease and contributes to
pathology in APP/PS1 mice. Nature. 493:674–678. 2013. View Article : Google Scholar
|
|
6
|
Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ and
Jo EK: Upregulated NLRP3 inflammasome activation in patients with
type 2 diabetes. Diabetes. 62:194–204. 2013. View Article : Google Scholar
|
|
7
|
Jin C and Flavell RA: Molecular mechanism
of NLRP3 inflammasome activation. J Clin Immunol. 30:628–631. 2010.
View Article : Google Scholar
|
|
8
|
Kanneganti TD, Body-Malapel M, Amer A,
Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton
JT, Inohara N and Núñez G: Critical role for Cryopyrin/Nalp3 in
activation of caspase-1 in response to viral infection and
double-stranded RNA. J Biol Chem. 281:36560–36568. 2006. View Article : Google Scholar
|
|
9
|
Muruve DA, Périlli V, Zaiss AK, White LR,
Clark SA, Ross PJ, Parks RJ and Tschopp J: The inflammasome
recognizes cytosolic microbial and host DNA and triggers an innate
immune response. Nature. 452:103–107. 2008. View Article : Google Scholar
|
|
10
|
Wilson D, Thewes S, Zakikhany K, Fradin C,
Albrecht A, Almeida R, Brunke S, Grosse K, Martin R, Mayer F, et
al: Identifying infection-associated genes of Candida
albicans in the postgenomic era. FEMS Yeast Res. 9:688–700.
2009. View Article : Google Scholar
|
|
11
|
Mariathasan S, Weiss DS, Newton K, McBride
J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM and
Dixit VM: Cryopyrin activates the inflammasome in response to
toxins and ATP. Nature. 440:228–232. 2006. View Article : Google Scholar
|
|
12
|
Willingham SB, Bergstralh DT, O'Connor W,
Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, Flavell
RA, Deshmukh M, et al: Microbial pathogen-induced necrotic cell
death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3
and ASC. Cell Host Microbe. 2:147–159. 2007. View Article : Google Scholar
|
|
13
|
Craven RR, Gao X, Allen IC, Gris D,
Wardenburg Bubeck J, McElvania-TeKippe E, Ting JP and Duncan JA:
Staphylococcus aureus alpha-hemolysin activates the
NLRP3-inflammasome in human and mouse monocytic cells. PLoS One.
4:e74462009. View Article : Google Scholar
|
|
14
|
Kebaier C, Chamberland RR, Allen IC, Gao
X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL and Duncan
JA: Staphylococcus aureus α-hemolysin mediates virulence in
a murine model of severe pneumonia through activation of the NLRP3
inflammasome. J Infect Dis. 205:807–817. 2012. View Article : Google Scholar
|
|
15
|
Chun-Fu W, Jing-Yu Y, Fang W and Xiao-Xiao
W: Resveratrol: Botanical origin, pharmacological activity and
applications. Chin J Nat Med. 11:1–15. 2013.
|
|
16
|
Bollmann F, Art J, Henke J, Schrick K,
Besche V, Bros M, Li H, Siuda D, Handler N, Bauer F, et al:
Resveratrol post-transcriptionally regulates pro-inflammatory gene
expression via regulation of KSRP RNA binding activity. Nucleic
Acids Res. 42:12555–12569. 2014. View Article : Google Scholar
|
|
17
|
Hwang D and Lim YH: Resveratrol
antibacterial activity against Escherichia coli is mediated by
Z-ring formation inhibition via suppression of FtsZ expression. Sci
Rep. 5:100292015. View Article : Google Scholar
|
|
18
|
Gülçin İ: Antioxidant properties of
resveratrol: A structure-activity insight. Innov Food Sci Emerg
Technol. 11:210–218. 2010. View Article : Google Scholar
|
|
19
|
Marino A, Santoro G, Spataro F, Lauriano
ER, Pergolizzi S, Cimino F, Speciale A, Nostro A, Bisignano G and
Dugo G: Resveratrol role in Staphylococcus aureus-induced
corneal inflammation. Pathog Dis. 68:61–64. 2013. View Article : Google Scholar
|
|
20
|
Lee IT, Lin CC, Hsu CK, Wu MY, Cho RL and
Yang CM: Resveratrol inhibits Staphylococcus aureus-induced
TLR2/MyD88/NF-κB-dependent VCAM-1 expression in human lung
epithelial cells. Clin Sci (Lond). 127:375–390. 2014. View Article : Google Scholar
|
|
21
|
Dong W, Yang R, Yang J, Yang J, Ding J, Wu
H and Zhang J: Resveratrol pretreatment protects rat hearts from
ischemia/reperfusion injury partly via a NALP3 inflammasome
pathway. Int J Clin Exp Patho. 8:8731–8741. 2015.
|
|
22
|
Yang SJ and Lim Y: Resveratrol ameliorates
hepatic metaflammation and inhibits NLRP3 inflammasome activation.
Metabolism. 63:693–701. 2014. View Article : Google Scholar
|
|
23
|
Chang YP, Ka SM, Hsu WH, Chen A, Chao LK,
Lin CC, Hsieh CC, Chen MC, Chiu HW, Ho CL, et al: Resveratrol
inhibits NLRP3 inflammasome activation by preserving mitochondrial
integrity and augmenting autophagy. J Cell Physiol. 230:1567–1579.
2015. View Article : Google Scholar
|
|
24
|
Veterinary Anesthetic and Analgesic
Formulary. 3rd edition. Version G. University of Colorado Denver,
Anschutz Medical Campus; 2012
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Zhang Y, Wang JF, Dong J, Wei JY, Wang YN,
Dai XH, Wang X, Luo MJ, Tan W, Deng XM and Niu XD: Inhibition of
α-toxin production by subinhibitory concentrations of naringenin
controls Staphylococcus aureus pneumonia. Fitoterapia.
86:92–99. 2013. View Article : Google Scholar
|
|
27
|
Qiu J, Niu X, Dong J, Wang D, Wang J, Li
H, Luo M, Li S, Feng H and Deng X: Baicalin protects mice from
Staphylococcus aureus pneumonia via inhibition of the
cytolytic activity of α-hemolysin. J Infect Dis. 206:292–301. 2012.
View Article : Google Scholar
|
|
28
|
Dong J, Qiu J, Wang J, Li H, Dai X, Zhang
Y, Wang X, Tan W, Niu X, Deng X and Zhao S: Apigenin alleviates the
symptoms of Staphylococcus aureus pneumonia by inhibiting
the production of alpha-hemolysin. FEMS Microbiol Lett.
338:124–131. 2013. View Article : Google Scholar
|
|
29
|
Su Y, Ma L, Wen Y, Wang H and Zhang S:
Studies of the in vitro antibacterial activities of several
polyphenols against clinical isolates of methicillin-resistant
Staphylococcus aureus. Molecules. 19:12630–12639. 2014.
View Article : Google Scholar
|
|
30
|
Zhao G, Wu H, Jiang K, Rui G, Zhu Z, Qiu
C, Guo M and Deng G: IFN-τ inhibits S. aureus-induced inflammation
by suppressing the activation of NF-κB and MAPKs in RAW 264.7 cells
and mice with pneumonia. Int Immunopharmacol. 35:332–340. 2016.
View Article : Google Scholar
|
|
31
|
Muñoz-Planillo R, Franchi L, Miller LS and
Núñez G: A critical role for hemolysins and bacterial lipoproteins
in Staphylococcus aureus-induced activation of the Nlrp3
inflammasome. J Immunol. 183:3942–3948. 2009. View Article : Google Scholar
|
|
32
|
Netea MG, Nold-Petry CA, Nold MF, Joosten
LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G,
Heinhuis B, Devesa I, et al: Differential requirement for the
activation of the inflammasome for processing and release of
IL-1beta in monocytes and macrophages. Blood. 113:2324–2335. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Strowig T, Henao-Mejia J, Elinav E and
Flavell R: Inflammasomes in health and disease. Nature.
481:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|