Introduction
Cerebral hemorrhage is the most common type of
cerebrovascular disease in humans (accounting for 20–30%) and the
least treatable subtype of hemorrhagic stroke (mortality rate,
30–40%) (1). The most common
manifestations in the clinic are cerebral arteriosclerosis,
hypertension and intracranial vascular malformations (2). Cerebral hemorrhage is often induced by
exertion and emotions, and most patients show sudden onset during
activity. Cerebral hemorrhage usually causes severe dysfunction of
the cerebral nervous system and loss of social functioning,
self-care ability and further increases the burden on the family of
affected patients (3,4). Subarachnoid hemorrhage is one of the
most serious types of cerebral hemorrhage and usually leads to
death, as it is a devastating cerebrovascular disease with bleeding
into the subarachnoid space (5,6). While
oral administration of anti-coagulants and surgical resection are
the mainstay of cerebral hemorrhage treatment in the clinic, no
effective therapeutic schedule is currently available to improve
functional outcomes in patients with cerebral hemorrhage,
particularly subarachnoid hemorrhage (7–9).
Therefore, the development of therapeutic agents targeting cerebral
hemorrhage is urgently required and the underlying molecular
mechanisms require further elucidation in order to provide novel
targets for identifying novel treatment approaches of human
cerebrovascular diseases.
Patients with acute intracerebral hemorrhage are
frequently monitored in intensive care units (ICUs) (10). Sudden intracerebral hemorrhage is
associated with higher rates of mortality and morbidity than other
intracephalic diseases (11). The
expression levels of inflammatory factors have important roles in
the inflammatory response associated with changes in a patient's
condition and are important during monitoring and treatment at the
ICU as well as for the development of therapeutic strategies for
acute cerebral hemorrhage (12). In
the majority of patients with cerebral hemorrhage, the condition
triggers an immune activation sufficient to induce systemic
inflammatory response syndrome (13). Inflammatory response has been
reported to be associated with extra-cerebral organ dysfunction as
well as delayed cerebral ischemia and the amount of bleeding, which
is associated with poor outcome for patients with cerebral
hemorrhage at an ICU (14).
Leukocytosis has long been associated with adverse events after
acute intracerebral hemorrhage (15). Therefore, controlling and monitoring
inflammatory responses is essential for patients with acute
intracerebral hemorrhage at the ICU.
As is known, the beneficial effects of statin drugs
in reducing cardiovascular diseases have been predominantly
attributed to their lipid-lowering effects (16). Recent studies suggested that the
beneficial effects of statins are associated with their
anti-inflammatory properties (17,18). In
addition, statins have important roles in changes in endothelial
dysfunction, stabilizing the plaque and immune system regulation as
well as anti-oxidant effects (19).
Simvastatin is a statin drug that produces beneficial outcomes due
to its anti-neoplastic effects and overcomes the resistance to
serum withdrawal-induced apoptosis of lymphocytes from Alzheimer's
disease patients (20). Numerous
studies have investigated the anti-inflammatory effects of
Simvastatin in different types of human diseases, such as cancer,
chronic heart failure, diabetes and traumatic brain injury
(21–24).
Furthermore, Simvastatin inhibited the aggregation
of amyloid-β in extracellular cortical and hippocampal plaques in
cerebral hemorrhage, which is a widely accepted mechanism of action
of cerebral hemorrhage pathology inhibitors and associated with the
lowering of brain cholesterol levels in patients with cerebral
hemorrhage (25,26).
The present study investigated the anti-inflammatory
effects of Simvastatin administered to patients after cerebral
hemorrhage and hypothesized that it may protect neurons by
regulating the inflammatory response. The anti-inflammatory
properties of Simvastatin, including its capacity to inhibit
inflammatory factor expression, decrease the inflammatory cellular
plasma concentration of lymphocytes, granulocytes and mononuclear
cells, and improve brain edema as well as reduce the amount of
bleeding, were studied in patients with cerebral hemorrhage at the
ICU. The results demonstrated that Simvastatin treatment led to the
decrease of the levels of inflammatory factors, including
interleukin (IL)-4, IL-6, IL-8 and IL-10. Furthermore, inflammatory
factor levels were found to be associated with brain edema in
patients with acute cerebral hemorrhage. The amount of bleeding was
significantly depended on the inflammatory cellular plasma
concentration of lymphocytes, granulocytes and mononuclear cells.
Importantly, Simvastatin treatment produced beneficial outcomes
with regard to improving brain edema and the amount of bleeding.
However, further evaluation of additional clinical data is
essential to fully elucidate the efficacy and tolerability of
Simvastatin.
Materials and methods
Ethical approval and patient
consent
This study was approved by the Ethics Committee of
the People's Hospital of Changle County (ref no. 10/CC06/124;
Weifang, China). The phase-I study was performed from February 2006
to June 2012 according to the Guide for Chinese Clinical
Experiments of Weifang People's Hospital (Weifang, China). The
study was also performed in accordance with the European Medicines
Agency requirements. All patients provided written informed consent
prior to undergoing any procedures associated with the study.
Patients
A total of 146 patients who presented with
intracerebral hemorrhage at the ICU were randomized into two
groups, which were treated with Simvastatin or placebo,
respectively. The inclusion criteria were patients with no heart
disease or previous history of intracerebral hemorrhage. At
baseline, patient age, body mass index and time since epilepsy
diagnosis were similar between the two groups. In total, 101
patients who completed the maintenance period of the phase I study
post-surgery (minimal invasive puncture and drainage vs. endoscopic
surgery) were included in the analysis of the therapeutic effects
of Simvastatin. Patients received Simvastatin at dosages of 0.08,
0.16, 0.24, 0.30 and 0.36 mg/kg based on a previous clinical trial
(26).
18F-Fluorodeoxyglucose positron
emission tomography imaging (FDG-PET). FDG-PET was used to
analyze brain edema and the amount of bleeding by using statistical
parametric mapping (SPM) software (SPM, version 2; Wellcome
Department of Imaging Neuroscience, University College London,
London, UK). FDG-PET images were spatially normalized onto the
Montreal Neurological Institute (MNI) PET brain template (MNI,
McGill University, Montreal, QC, Canada), which defined regions of
interest. Normalized images were smoothed by convolution with a
10-mm full width at half maximum Gaussian kernel to increase the
signal-to-noise ratio. Detailed procedures for FDG-PET acquisition
and image processing were described in a previous study (27).
Vasospasm analysis
In the present clinical study, vasospasm in patients
with intracerebral hemorrhage was defined using clinical and
angiographic criteria for vasospasm. Symptomatic vasospasm was
diagnosed and recorded as abnormal neurological status as described
in a previous study (28).
ELISA
The levels of IL-4 (cat no. D4050), IL-6 (cat no.
D6050), IL-8 (cat no. D8000C) and IL-10 (cat no. DY417) (all from
R&D Systems, Inc., Minneapolis, MN, USA) in the peripheral
blood of patients with intracerebral hemorrhage was assessed by
using commercialized human interleukin ELISA kits. The ELISAs were
performed according to the manufacturer's instructions. The results
were measured at 450 nm with an ELISA reader and finally converted
to concentrations of IL-4, IL-6, IL-8 and IL-10.
Modified neurological severity score
(MNSS) analysis
The patients with intracerebral hemorrhage at the
ICU were subjected to MNSS assessment at 7, 14, 21, 28, 35 and 42
days of treatment (n=26 in Simvastatin and n=18 in placebo group).
MNSS analysis comprised sensory, motor, reflex and balance
experiments. A scale of 0–18 was used to grade neurological
function (normal score, 0; maximal deficit score, 18). The higher
the score, the higher was the severity of injury in patients with
intracerebral hemorrhage at the ICU.
Behavioral assessment
Behavioral assessment was performed on
post-operative days 7, 14, 21 and 28, 35 and 42 for intracerebral
hemorrhage patients. The assessment parameters, including left limb
movement and coordination of movement, were evaluated using the
modified Tarlov scores as follows: Severe level, possible limb
movement and partial limb paralysis (1–4 points); moderate level,
failure to jump and stand normally (4–7 points); primary level,
failure to stand while being capable of joint movement (7–9
points); normal function (9–10 points). According to the study
design, the assessment and Tarlov scoring were performed for each
patient at the ICU independently and then averaged.
Efficacy and safety assessment
Efficacy assessment included determination of the
maximum tolerated dose (MTD) in cerebral hemorrhage patients and
dose-limiting toxicity in the presence of Simvastatin. Safety
assessments included the incidence rates (≥10%) of the most
frequent treatment-associated adverse events in a 42-day treatment
period in the drug treatment groups. The efficacy and safety data
included all patients with cerebral hemorrhage receiving
Simvastatin.
Flow cytometry
Peripheral blood was drawn from patients with
cerebral hemorrhage and total leukocytes were extracted using a
Human Leukocyte Extraction kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Inflammatory cellular plasma
concentrations of lymphocytes, granulocytes and mononuclear cells
were analyzed by flow cytometry (BD FACSDiva™ v. 6.1.3 software; BD
Biosciences, San Jose, CA, USA) as previously described (29).
Statistical analysis
Statistical analysis was performed by using SPSS
19.0 software (IBM Corp., Armonk, NY, USA) and Excel (2010 version;
Microsoft Corporation, Redmond, WA, USA). Values are expressed as
the mean ± standard error of the mean. Statistical tests for data
analysis included Fisher's exact test, log-rank test, Chi-square
test, and Student's 2-tailed t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of patients with
cerebral hemorrhage
A total of 146 patients with cerebral hemorrhage who
were candidates for intravenous injection were included in the
present clinical study. The mean age of the patients was 47 years.
All patients were randomized into two groups and treated with
Simvastatin (n=88) or placebo (n=58) by intravenous injection. The
number of male patients with cerebral hemorrhage (n=94, 64.4%) was
slightly higher than that of female patients (n=52, 35.6%). The
characteristics of patients with cerebral hemorrhage are summarized
in Table I. Furthermore, 101 (69%)
patients with cerebral hemorrhage continued to complete the
maintenance period of the phase-I study.
 | Table I.Characteristics of patients with
intracerebral hemorrhage (n=146). |
Table I.
Characteristics of patients with
intracerebral hemorrhage (n=146).
| Parameter | Value |
|---|
| Gender |
|
|
Male | 94 (64.4%) |
|
Female | 52 (35.6%) |
| Mean age
(years) | 47.4 (Range:
35.4–65.8) |
| Drug therapy |
|
|
Simvastatin | 88 (60.3%) |
|
Placebo | 58 (39.7%) |
| Trial
participation |
|
|
Complete | 101 (69.2%) |
|
Incomplete | 35 (30.8%) |
| Plasma glucose
(mmol/l) | 5.4–8.7 (normal
range: 3.9–6.1) |
| Blood pressure
(mmHg) | 142±6 (normal
range: 90–140) |
Duration of treatment, dose-limiting
toxicities and MTD
The median overall duration of Simvastatin treatment
was four weeks for patients with cerebral hemorrhage at the ICU.
Within the cohort, subgroups were treated with 0.08, 0.16, 0.24,
0.30 and 0.36 mg/kg Simvastatin. As presented in Table II, 0.30 mg/kg Simvastatin once a day
was identified as the MTD and 0.24 mg/kg of Simvastatin once a day
was identified as dose-limiting toxicity. The group treated with
the lowest dose of Simvastatin presented with the lowest number of
adverse reactions. It was observed that the common
treatment-associated adverse events of Simvastatin injection were
hypertension, proteinuria, fatigue, diarrhea, vomiting, rash,
constipation and peripheral edema. For most of the patients with
cerebral hemorrhage, a reduction of the drug dose was required due
to cumulative toxicity after treatment with the MTD. Therefore,
most of the patients that were subsequently enrolled in the study
were treated with Simvastatin at a dose of 0.20 mg/kg to ensure
tolerability and therapeutic efficacy of Simvastatin. The most
common grade ≥3 adverse events in patients with cerebral hemorrhage
according to Common Toxicity Criteria were hypertension and
proteinuria (15% each; Table
III).
 | Table II.Adverse events (n) occurring during
treatment with an overall incidence of ≥10%. |
Table II.
Adverse events (n) occurring during
treatment with an overall incidence of ≥10%.
|
|
| Simvastatin
(mg/kg) |
|---|
|
|
|
|
|---|
| Adverse event | Total (%) n=40 | 0.08–0.16 (%)
n=14 | 0.24–0.30 (%)
n=16 | 0.36 (%) n=10 |
|---|
| Hypertension | 10 (25) | 2 (5) | 3 (7.5) | 5 (12.5) |
| Proteinuria | 8 (20) | 2 (5) | 2 (5) | 4 (10) |
| Fatigue | 4 (10) | 1 (2.5) | 1 (2.5) | 2 (5) |
| Diarrhea | 5 (12.5) | 1 (2.5) | 2 (5) | 2 (5) |
| Vomiting | 4 (10) | 1 (5) | 1 (2.5) | 2 (5) |
| Rash | 6 (15) | 2 (5) | 2 (5) | 2 (5) |
| Constipation | 7 (17.5) | 2 (5) | 2 (5) | 3 (7.5) |
| Peripheral
edema | 8 (20) | 2 (5) | 3 (7.5) | 3 (7.5) |
 | Table III.Treatment-associated hypertension and
proteinuria graded by Common Toxicity Criteria (≥20%). |
Table III.
Treatment-associated hypertension and
proteinuria graded by Common Toxicity Criteria (≥20%).
|
|
| Simvastatin
(mg/kg) |
|---|
|
|
|
|
|---|
| Adverse
event/grade | Total (%) n=40 | 0.08–0.16 (%)
n=14 | 0.24–0.30 (%)
n=16 | 0.36 (%) n=10 |
|---|
| Hypertension |
|
|
|
|
| 1 | 3 (7.5) | 0 (0) | 1 (2.5) | 2 (5) |
| 2 | 3 (7.5) | 1 (2.5) | 1 (2.5) | 1 (2.5) |
| 3 | 4 (10) | 1 (2.5) | 1 (2.5) | 2 (5) |
|
Total | 10 (25) | 2 (5) | 3 (7.5) | 5 (12.5) |
| Proteinuria
(%) |
|
|
|
|
| 1
(2.5) | 2 (5) | 0 (0) | 1 (2.5) | 2 (5) |
| 2
(5) | 3 (7.5) | 1 (2.5) | 0 (0) | 1 (2.5) |
| 3
(7.5) | 2 (5) | 1 (2.5) | 1 (2.5) | 1 (2.5) |
|
Total | 8 (20) | 2 (5) | 2 (5) | 4 (10) |
Efficacy of Simvastatin in patients
with cerebral hemorrhage
The efficacy of Simvastatin treatment in patients
with acute cerebral hemorrhage was assessed in this clinical study.
Clinical examination demonstrated that compared with the placebo
group, arthralgia and body pain were markedly improved in the drug
treatment groups after 4 weeks of therapy regiment. Furthermore,
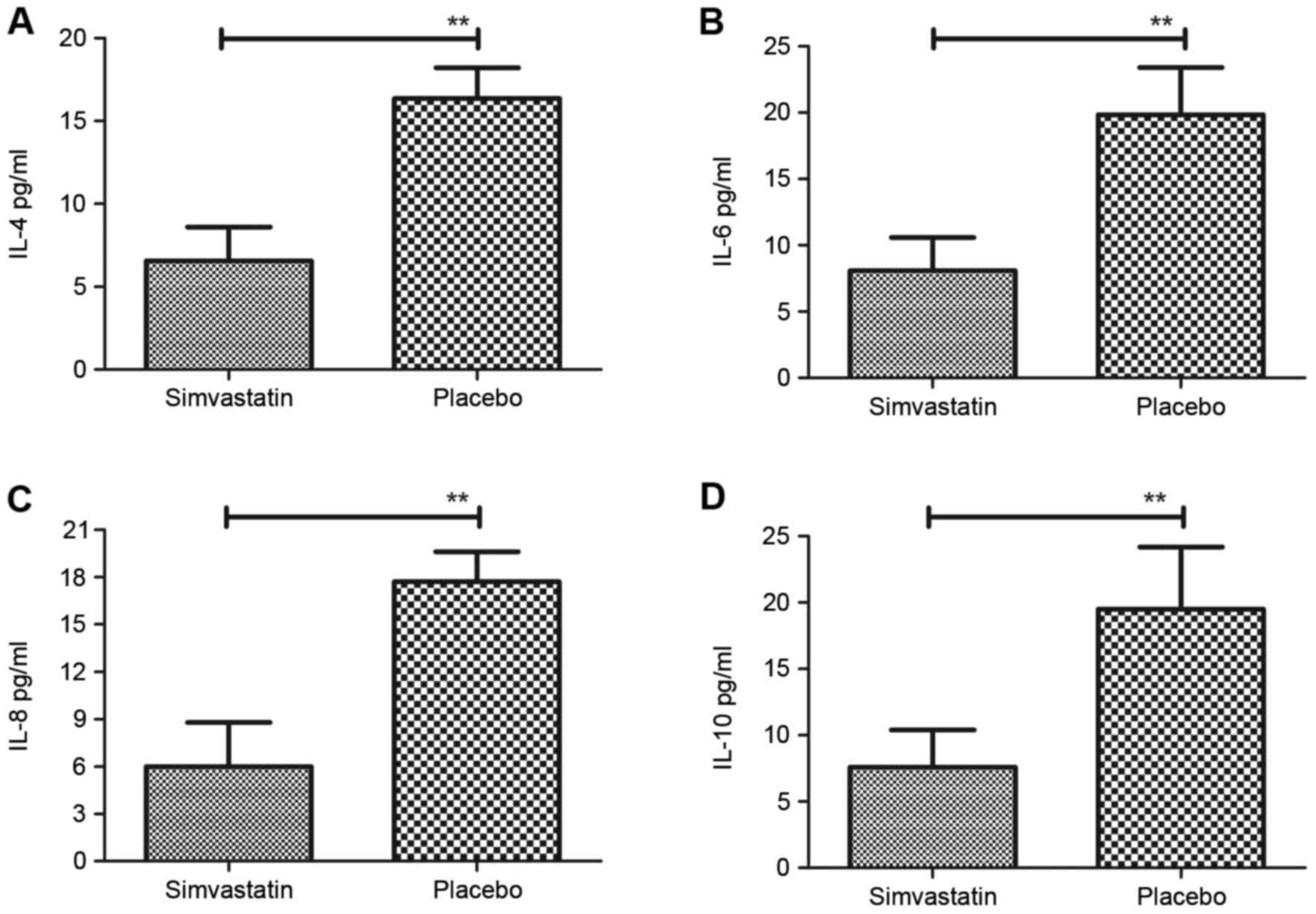
the levels of the inflammatory factors IL-4, IL-6, IL-8 and IL-10
were decreased after treatment with Simvastatin in patients with
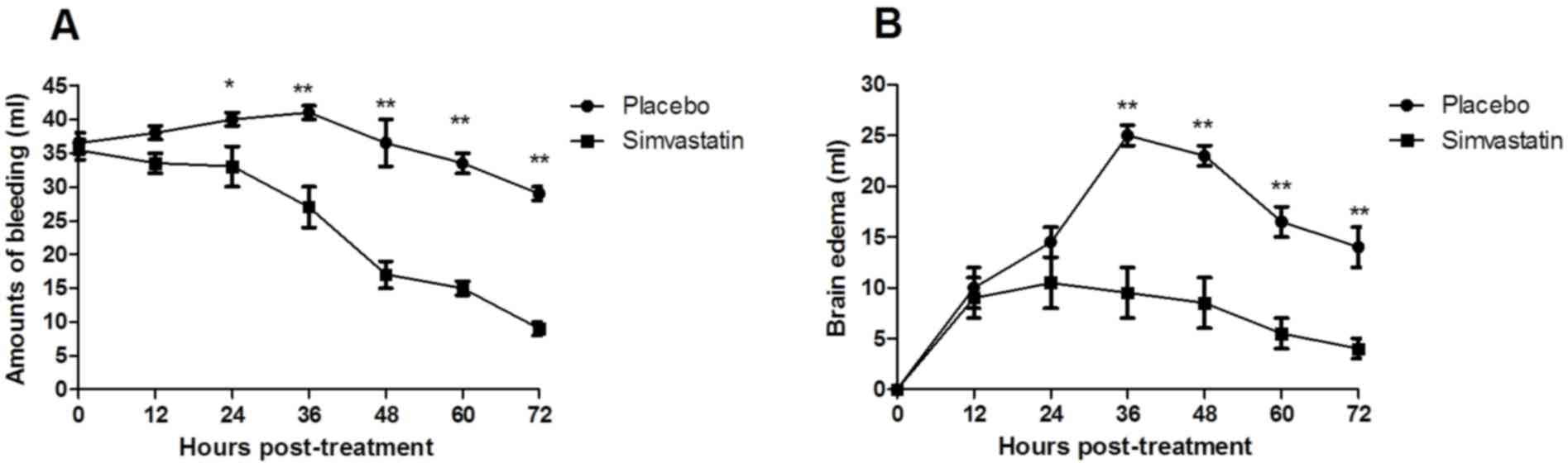
acute cerebral hemorrhage in the ICU (Fig. 1). Furthermore, systemic inflammatory
response syndrome was found to be associated with brain edema in
the patients with acute cerebral hemorrhage. Simvastatin treatment
improved the degree of brain edema and the amount of bleeding
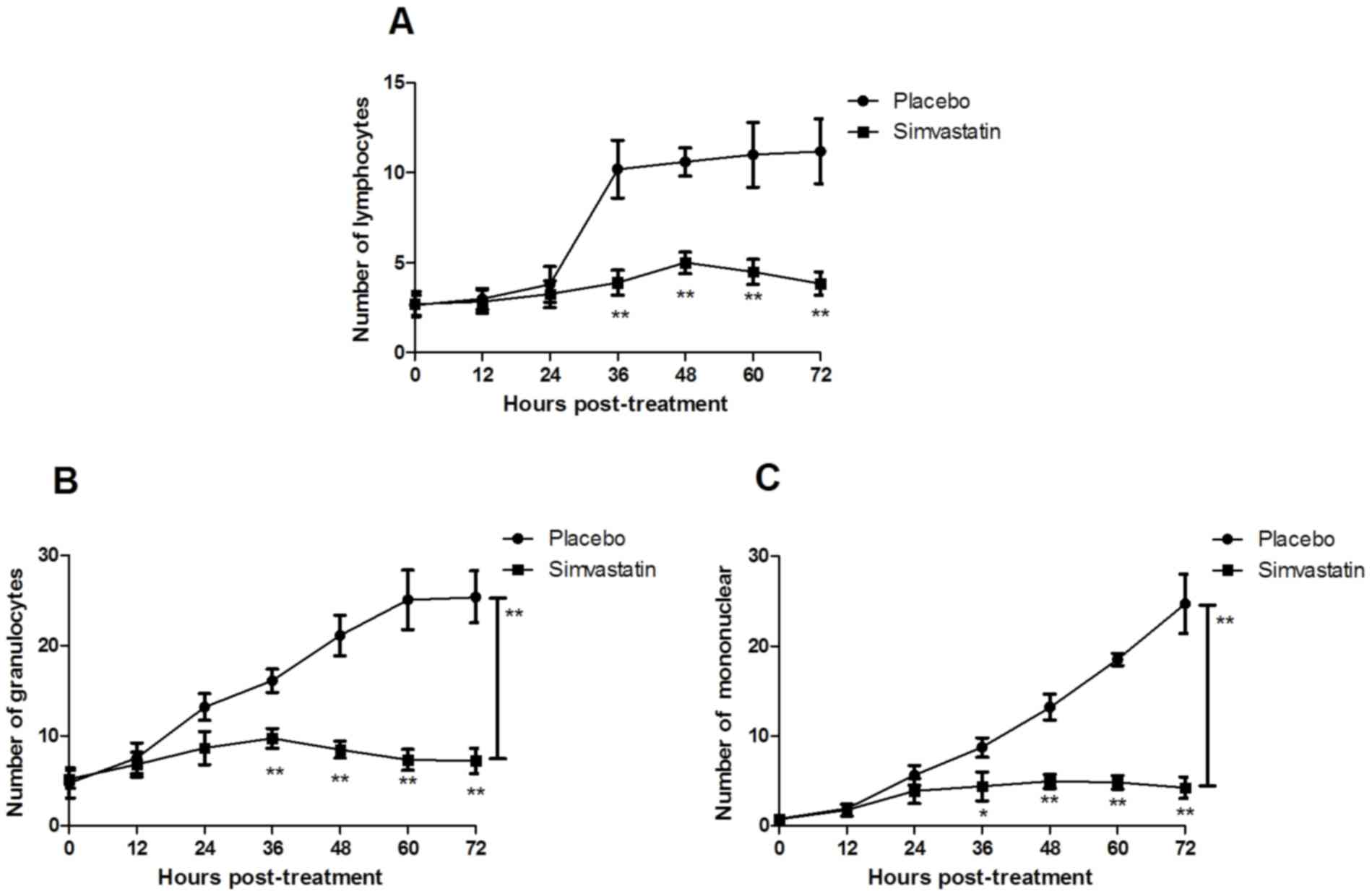
(Fig. 2). Of note, the results
indicated that the inflammatory cellular plasma concentration of
lymphocytes, granulocytes and mononuclear cells was recovered to
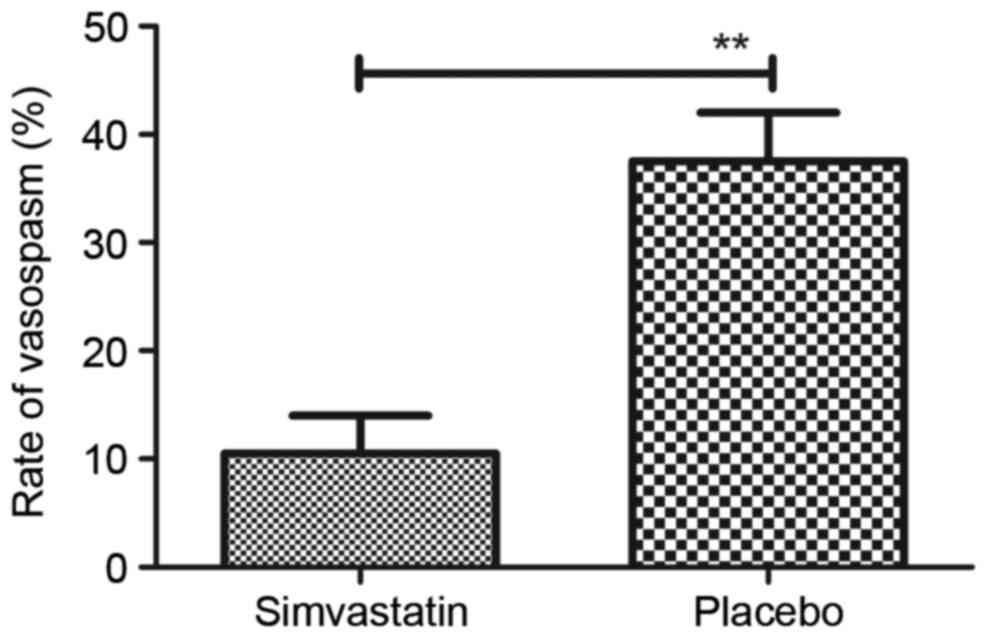
normal levels after treatment with Simvastatin (Fig. 3). Furthermore, the results revealed
that Simvastatin treatment significantly improved the frequency of
vasospasms in patients with acute cerebral hemorrhage compared with
that in the placebo group (Fig. 4).
Collectively, the results indicated that Simvastatin was efficient
in treating acute intracerebral hemorrhage and that the
inflammatory response was in parallel with the progress of patients
with acute intracerebral hemorrhage in an ICU setting, which may
provide insight for applying effective therapies for patients with
acute intracerebral hemorrhage.
Simvastatin treatment improves the
survival of patients with cerebral hemorrhage
In order to explore whether therapy with Simvastatin
was effective for patients with cerebral hemorrhage in vivo,
the recurrent activity of convulsion in cerebral hemorrhage
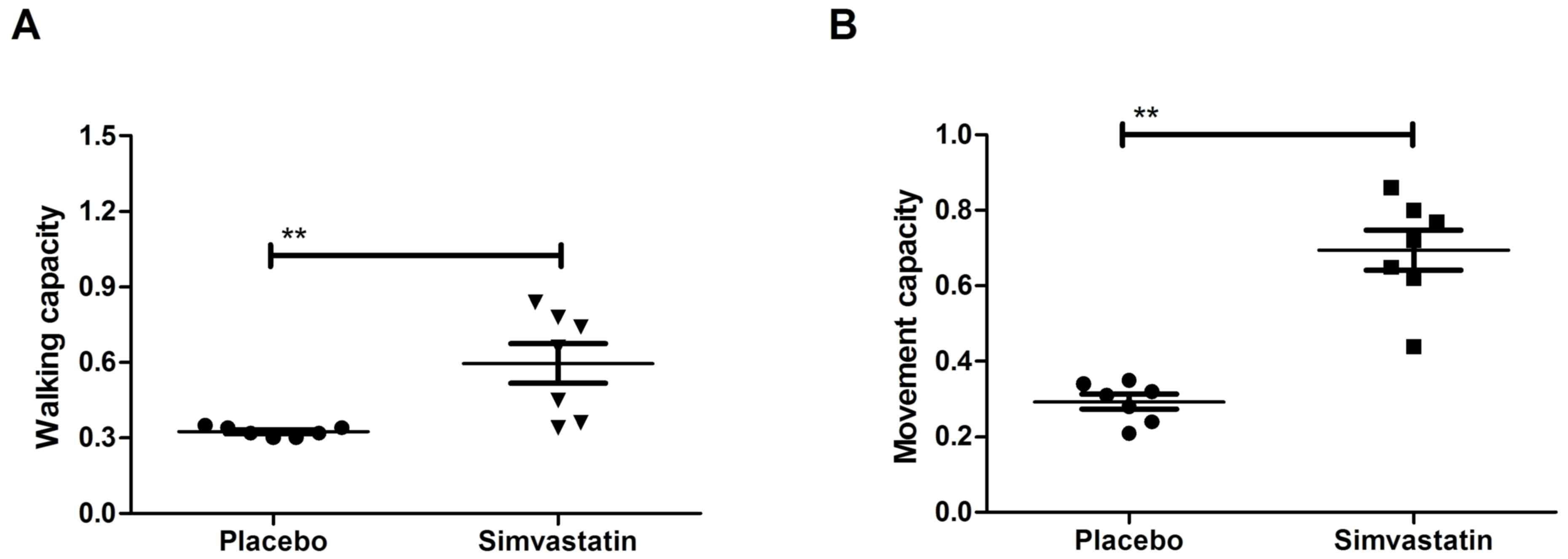
patients was assessed. The results in Fig. 5 demonstrated that movement
capacities, including limb coordination and walking, were
significantly improved in Simvastatin-treated patients with
hemorrhage lesions compared with those in patients treated with
placebo (P<0.01). However, no significant changes in arterial
blood pressure, body weight and body temperature were observed
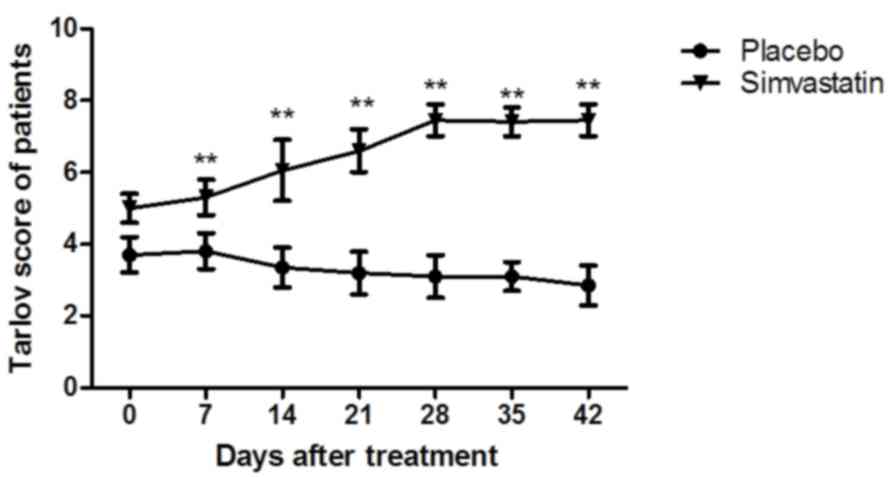
(data not shown). As presented in Fig.
6, the Tarlov scores revealed that the therapeutic effects of
Simvastatin were significant in patients with cerebral hemorrhage
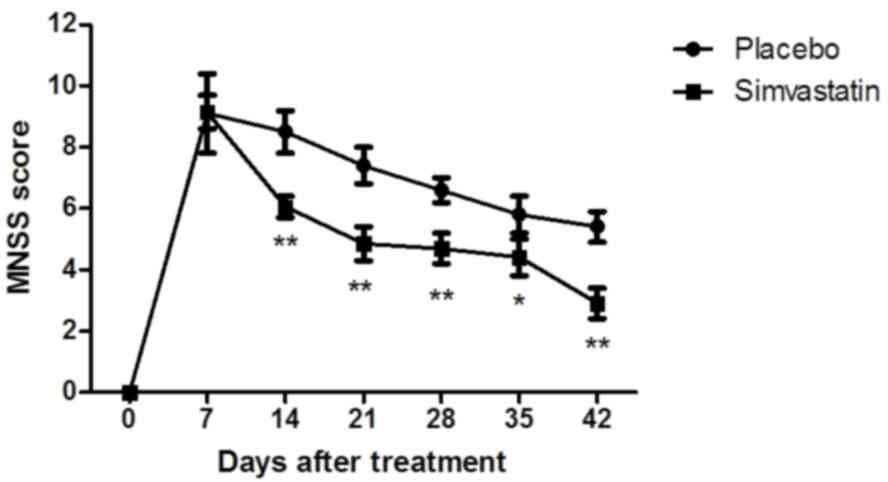
compared with those in the placebo group (P<0.01). The MNSS test
demonstrated that the Simvastatin-treated patients with cerebral
hemorrhage exhibited significant functional improvement compared
with the placebo group (Fig. 7).
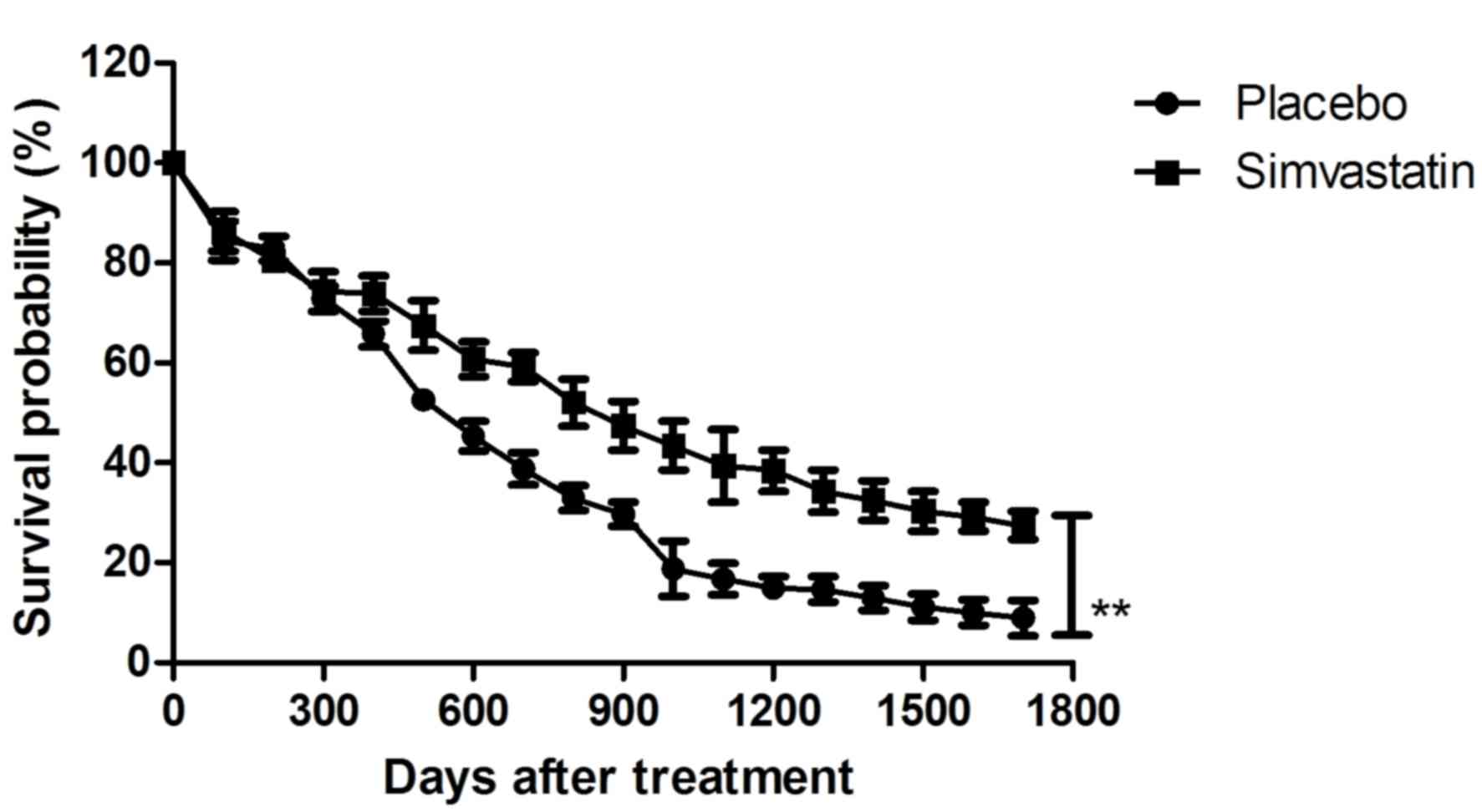
Furthermore, a long-term survival observation over a 1,664-day
period after treatment with Simvastatin in patients with cerebral
hemorrhage was performed. The results inFig. 8 revealed that the survival of
patients was prolonged after treatment with Simvastatin. Taken
together, the results suggested that Simvastatin was efficient in
patients with cerebral hemorrhage in an ICU setting.
Discussion
Intracerebral hemorrhage is the most common type of
cerebrovascular disease in humans and patients with acute
intracerebral hemorrhage are frequently monitored in ICUs (30). Spontaneous and acute cerebral
hemorrhage leads to high morbidity and mortality in ICUs worldwide
(31,32). It was previously indicated that
systemic inflammatory response syndrome is associated with
intracerebral hemorrhage (13).
Simvastatin is an efficient drug that improves the neurological
outcome after experimental intracerebral hemorrhage (33). In addition, Simvastatin was reported
to reduce the inflammatory response in the treatment of various
human diseases (34). The present
study investigated the anti-inflammatory effects of Simvastatin in
patients with acute intracerebral hemorrhage in an ICU. Recurrent
bleeding after acute intracerebral hemorrhage is a major cause of
morbidity and mortality in ICUs and a previous study suggested that
the inflammatory response is associated with spontaneous
intracerebral hemorrhage patients in an ICU setting (35). The present study revealed that
Simvastatin treatment improved the inflammatory response and had
beneficial effects in intracerebral hemorrhage patients in an ICU.
Furthermore, numerous studies have shown that cerebral hemorrhage
caused neuronal damage and further aggravated brain damage to even
lead to the development of contralateral limb dysfunction (36–38). In
cerebral hemorrhage, the blood often overflowed directly into the
brain parenchyma. The clinical data of the present study
demonstrated that contralateral limb dysfunction, frequency of
vasospasm, degree of brain edema and the amount of bleeding were
improved by treatment with Simvastatin once a day.
A previous study indicated that the possible
mechanism underlying the dysfunction of the inflammatory response
may be associated with leakage from small intracerebral arteries
(39). Dysfunction of the
inflammatory response may be an important pathophysiological factor
in intracerebral hemorrhage and other human cerebrovascular
diseases. A previous study has indicated that early inflammation
contributes to edema after intracerebral hemorrhage in an ICU
setting (40). A review on
inflammation after intracerebral hemorrhage based on available
evidences from preclinical and clinical studies suggested that
inflammatory mechanisms are involved in the progression of
intracerebral hemorrhage-induced secondary brain injury (41). In addition, the therapeutic benefit
of anti-inflammatory and angiogenesis-inducing treatments in
intracerebral hemorrhage has been investigated (42). The present study first assessed the
anti-inflammatory effects of Simvastatin in patients with acute
intracerebral hemorrhage in an ICU. The results revealed that IL-4,
IL-6, IL-8 and IL-10 levels were downregulated by Simvastatin
compared to those in the placebo group. Furthermore, in parallel
with the decrease of inflammatory factors, the degree of brain
edema and the amount of bleeding in patients with intracerebral
hemorrhage was also reduced, along with an increased survival rate.
These findings suggested that inhibition of inflammatory factors is
beneficial for reducing the degree of brain injury and promoting
functional recovery (43).
In the present study, Simvastatin was evaluated as a
therapeutic agent for treating patients with intracerebral
hemorrhage. In the majority of cases, cerebral hemorrhage is
non-traumatic and caused by rupture of vessels in the brain
parenchyma (44). The findings of
the present study were consistent with those of previous studies in
terms of Simvastatin exerting beneficial effects by inhibiting the
expression of inflammatory factors in clinical trials (45,46). The
number of detection methods used for measuring the disease
progression of intracranial hemorrhage has been expanding to
include imaging cerebral physiology, PET, computed tomography and
magnetic resonance imaging (47). In
addition, numerous molecular markers were found to be associated
with cerebral hemorrhage, which may support its diagnosis and
determination of its extent, and may further be utilized for
treating hemorrhage via target cells associated with hemostasis
(48,49). However, in terms of the overall
survival rate of patients, further development or combined
therapies are required for achieving better outcomes for patients
with intracerebral hemorrhage in an ICU setting. Of note, the
present and previous studies indicated that Simvastatin is a
prospective candidate drug for clinical therapy of patients with
cerebrovascular disease (50,51).
In conclusion, the present study proved the efficacy
of Simvastatin in treating acute intracerebral hemorrhage and
indicated that the inflammatory response was in parallel with the
progression of patients with acute intracerebral hemorrhage in an
ICU setting, which may provide insight for applying effective
therapies for patients with acute intracerebral hemorrhage. Taken
together, these findings indicated that Simvastatin exerted
beneficial effects in patients with intracerebral hemorrhage by
improving survival, vasospasm, brain edema, the amount of bleeding
as well as limb coordination and walking.
Acknowledgements
This work was supported by the National Science
foundation of China (no. 814020100354 to W.L.L.).
References
|
1
|
Sussman ES and Connolly ES Jr: Hemorrhagic
transformation: A review of the rate of hemorrhage in the major
clinical trials of acute ischemic stroke. Front Neurol. 4:692013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kobayashi T, Tadokoro H, Odai T, Hibino T
and Waki K: A delayed cerebral vasospasm with infarction is
secondary to listeria monocytogenes meningitis: MRI and MRA are
diagnostically useful. Intern Med. 54:2935–2938. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee WJ, Yeon JY, Jo KI, Kim JS and Hong
SC: Reversible cerebral vasoconstriction syndrome and posterior
reversible encephalopathy syndrome presenting with deep
intracerebral hemorrhage in young women. J Cerebrovasc Endovasc
Neurosurg. 17:239–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanamaru K, Suzuki H and Taki W: Cerebral
infarction after aneurysmal subarachnoid hemorrhage. Acta Neurochir
Suppl. 121:167–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown RJ, Epling BP, Staff I, Fortunato G,
Grady JJ and McCullough LD: Polyuria and cerebral vasospasm after
aneurysmal subarachnoid hemorrhage. BMC Neurol. 15:2012015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jenson AV, Rodriguez GJ, Alvarado LA,
Cruz-Flores S and Maud A: Higher rate of intracerebral hemorrhage
in hispanic patients with cerebral Cavernous Malformation. J Vasc
Interv Neurol. 8:1–4. 2015.PubMed/NCBI
|
|
7
|
Dabus G and Nogueira RG: Current options
for the management of aneurysmal subarachnoid hemorrhage-induced
cerebral vasospasm: A comprehensive review of the literature.
Interv Neurol. 2:30–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sehba FA, Pluta RM and Macdonald RL: Brain
injury after transient global cerebral ischemia and subarachnoid
hemorrhage. Stroke Res Treat. 2013:8271542013.PubMed/NCBI
|
|
9
|
Matsumoto H and Yoshida Y: Rapid
progression of cerebral infarction after intraventricular
hemorrhage in adult moyamoya disease. J Korean Neurosurg Soc.
54:411–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Medeiros de Bustos E and Moulin T: French
Society of Intensive Care: Specific treatment for intracerebral
hemorrhage. Experts' recommendations: Stroke management in the
intensive care unit. Rev Neurol (Paris). 168:522–526. 2012.(In
French). PubMed/NCBI
|
|
11
|
Ohwaki K, Yano E, Nagashima H, Nakagomi T
and Tamura A: Impact of infection on length of intensive care unit
stay after intracerebral hemorrhage. Neurocrit Care. 8:271–275.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma L, Shen X, Gao Y, Wu Q, Ji M, Luo C,
Zhang M, Wang T, Chen X and Tao L: Blocking B7-1/CD28 pathway
diminished long-range brain damage by regulating the immune and
inflammatory responses in a mouse model of intracerebral
hemorrhage. Neurochem Res. 41:1673–1683. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boehme AK, Hays AN, Kicielinski KP, Arora
K, Kapoor N, Lyerly MJ, Gadpaille A, Shiue H, Albright K, Miller D,
et al: Systemic inflammatory response syndrome and outcomes in
intracerebral hemorrhage. Neurocrit Care. 25:133–140. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schuiling WJ, Dennesen PJ and Rinkel GJ:
Extracerebral organ dysfunction in the acute stage after aneurysmal
subarachnoid hemorrhage. Neurocrit care. 3:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McGirt MJ, Mavropoulos JC, McGirt LY,
Alexander MJ, Friedman AH, Laskowitz DT and Lynch JR: Leukocytosis
as an independent risk factor for cerebral vasospasm following
aneurysmal subarachnoid hemorrhage. J Neurosurg. 98:1222–1226.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Panichi V, Paoletti S, Mantuano E,
Manca-Rizza G, Filippi C, Santi S, Taccola D, Donadio C, Tramonti
G, Innocenti M, et al: In vivo and in vitro effects of simvastatin
on inflammatory markers in pre-dialysis patients. Nephrol Dial
Transplant. 21:337–344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Montazerolghaem M, Ning Y, Engqvist H,
Karlsson Ott M, Tenje M and Mestres G: Simvastatin and zinc
synergistically enhance osteoblasts activity and decrease the acute
response of inflammatory cells. J Mater Sci Mater Med. 27:232016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Encarnação IC, Xavier CC, Bobinski F, dos
Santos AR, Corrêa M, de Freitas SF, Aragonez A, Goldfeder EM and
Cordeiro MM: Analysis of bone repair and inflammatory process
caused by simvastatin combined with PLGA+HA+βTCP Scaffold. Implant
Dent. 25:140–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shahbazian H, Atrian A, Yazdanpanah L,
Lashkarara GR and Mohtashami Zafar A: Anti-inflammatory effect of
simvastatin in hemodialysis patients. Jundishapur J Nat Pharm Prod.
10:e179622015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartolomé F, Muñoz U, Esteras N, Alquezar
C, Collado A, Bermejo-Pareja F and Martín-Requero A: Simvastatin
overcomes the resistance to serum withdrawal-induced apoptosis of
lymphocytes from Alzheimer's disease patients. Cell Mol Life Sci.
67:4257–4268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pinchuk TV, Fedulaev YN, Khairetdinova GA,
Denisova NN, Chura OV and Logunova IY: Anti-inflammatory effects of
simvastatin in patients with chronic heart failure. Bull Exp Biol
Med. 157:552–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lacerda L, Reddy JP, Liu D, Larson R, Li
L, Masuda H, Brewer T, Debeb BG, Xu W, Hortobágyi GN, et al:
Simvastatin radiosensitizes differentiated and stem-like breast
cancer cell lines and is associated with improved local control in
inflammatory breast cancer patients treated with postmastectomy
radiation. Stem Cells Transl Med. 3:849–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan C, Liu W, Yue Y, Jin H, Wang X and
Wang XJ: Inhibitory effect of β-elemene on human breast cancer
cells. Int J Clin Exp Pathol. 7:3948–3956. 2014.PubMed/NCBI
|
|
24
|
Kirmizis D, Papagianni A, Dogrammatzi F,
Efstratiadis G and Memmos D: Anti-inflammatory effects of
simvastatin in diabetic compared to non-diabetic patients on
chronic hemodialysis. J Diabetes. 5:492–494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugawara T, Ayer R, Jadhav V, Chen W,
Tsubokawa T and Zhang JH: Simvastatin attenuation of cerebral
vasospasm after subarachnoid hemorrhage in rats via increased
phosphorylation of Akt and endothelial nitric oxide synthase. J
Neurosci Res. 86:3635–3643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McGirt MJ, Lynch JR, Parra A, Sheng H,
Pearlstein RD, Laskowitz DT, Pelligrino DA and Warner DS:
Simvastatin increases endothelial nitric oxide synthase and
ameliorates cerebral vasospasm resulting from subarachnoid
hemorrhage. Stroke. 33:2950–2956. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teoh EJ, McGowan DR, Bradley KM, Belcher
E, Black E, Moore A, Sykes A and Gleeson FV: 18F-FDG PET/CT
assessment of histopathologically confirmed mediastinal lymph nodes
in non-small cell lung cancer using a penalised likelihood
reconstruction. Eur Radiol. 26:4098–4106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsukawa H, Tanikawa R, Kamiyama H,
Tsuboi T, Noda K, Ota N, Miyata S, Suzuki G, Takeda R and Tokuda S:
Effects of Clot removal by meticulous irrigation and continuous
low-dose intravenous nicardipine on symptomatic cerebral vasospasm
in patients with aneurysmal subarachnoid hemorrhage treated by
clipping. World Neurosurg. 84:1798–1803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Filipova J, Rihova L, Vsianska P, Kufova
Z, Kryukova E, Kryukov F and Hajek R: Flow cytometry in
immunoglobulin light chain amyloidosis: Short review. Leuk Res. Jul
13–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sreekrishnan A, Dearborn JL, Greer DM, Shi
FD, Hwang DY, Leasure AC, Zhou SE, Gilmore EJ, Matouk CC, Petersen
NH, et al: Intracerebral hemorrhage location and functional
outcomes of patients: A systematic literature review and
meta-analysis. Neurocrit Care. 25:384–391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rannikmäe K, Woodfield R, Anderson CS,
Charidimou A, Chiewvit P, Greenberg SM, Jeng JS, Meretoja A, Palm
F, Putaala J, et al: Reliability of intracerebral hemorrhage
classification systems: A systematic review. Int J Stroke.
11:626–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pittella JE and da Silva Gusmão SN:
Intracerebral hemorrhage due to cerebral amyloid angiopathy after
head injury: Report of a case and review of the literature.
Neuropathology. 36:566–572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karki K, Knight RA, Han Y, Yang D, Zhang
J, Ledbetter KA, Chopp M and Seyfried DM: Simvastatin and
atorvastatin improve neurological outcome after experimental
intracerebral hemorrhage. Stroke. 40:3384–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shao H, Shen Y, Liu H, Dong G, Qiang J and
Jing H: Simvastatin suppresses lung inflammatory response in a rat
cardiopulmonary bypass model. Ann Thorac Surg. 84:2011–2018. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang KW, Cho CL, Chen HJ, Liang CL,
Liliang PC, Tsai YD, Wang HK and Lu K: Molecular biomarker of
inflammatory response is associated with rebleeding in spontaneous
intracerebral hemorrhage. Eur Neurol. 66:322–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chamnanvanakij S, Margraf LR, Burns D and
Perlman JM: Apoptosis and white matter injury in preterm infants.
Pediatr Dev Pathol. 5:184–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Riggs AJ and Riggs JE: Epilepsy's role in
the historical differentiation of religion, magic, and science.
Epilepsia. 46:452–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao F, Guo Y, Zhang H, Wang S, Wang J, Wu
JM, Chen Z and Ding MP: Anterior thalamic nucleus stimulation
modulates regional cerebral metabolism: An FDG-MicroPET study in
rats. Neurobiol Dis. 34:477–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo FQ, Li XJ, Chen LY, Yang H, Dai HY,
Wei YS, Huang YL, Yang YS, Sun HB, Xu YC and Yang ZL: Study of
relationship between inflammatory response and apoptosis in
perihematoma region in patients with intracerebral hemorrhage.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 18:290–293. 2006.(In
Chinese). PubMed/NCBI
|
|
40
|
Loftspring MC, Johnson HL, Feng R, Johnson
AJ and Clark JF: Unconjugated bilirubin contributes to early
inflammation and edema after intracerebral hemorrhage. J Cereb
Blood Flow Metab. 31:1133–1142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J: Preclinical and clinical research
on inflammation after intracerebral hemorrhage. Prog Neurobiol.
92:463–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liao W, Zhong J, Yu J, Xie J, Liu Y, Du L,
Yang S, Liu P, Xu J, Wang J, et al: Therapeutic benefit of human
umbilical cord derived mesenchymal stromal cells in intracerebral
hemorrhage rat: Implications of anti-inflammation and angiogenesis.
Cell Physiol Biochem. 24:307–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee ST, Chu K, Sinn DI, Jung KH, Kim EH,
Kim SJ, Kim JM, Ko SY, Kim M and Roh JK: Erythropoietin reduces
perihematomal inflammation and cell death with eNOS and STAT3
activations in experimental intracerebral hemorrhage. J Neurochem.
96:1728–1739. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu L and Chen G: Signaling pathway in
cerebral vasospasm after subarachnoid hemorrhage: News update. Acta
Neurochir Suppl. 121:161–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Diringer MN, Dhar R, Scalfani M, Zazulia
AR, Chicoine M, Powers WJ and Derdeyn CP: Effect of high-dose
simvastatin on cerebral blood flow and static autoregulation in
subarachnoid hemorrhage. Neurocrit Care. 25:56–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McGirt MJ, Ambrossi Garces GL, Huang J and
Tamargo RJ: Simvastatin for the prevention of symptomatic cerebral
vasospasm following aneurysmal subarachnoid hemorrhage: A
single-institution prospective cohort study. J Neurosurg.
110:968–974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kidwell CS, Chalela JA, Saver JL, Starkman
S, Hill MD, Demchuk AM, Butman JA, Patronas N, Alger JR, Latour LL,
et al: Comparison of MRI and CT for detection of acute
intracerebral hemorrhage. JAMA. 292:1823–1830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roever L and Levine SR: Cerebral
hemorrhage following thrombolytic therapy for stroke: Are
neutrophils really neutral? Neurology. 85:1360–1361. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang DN, Hou XW, Yang BW, Lin Y, Shi JP
and Wang N: Quantity of cerebral microbleeds, antiplatelet therapy,
and intracerebral hemorrhage outcomes: A systematic review and
meta-analysis. J Stroke Cerebrovasc Dis. 24:2728–2737. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou HX, Gao LH, Meng LL, Zhang YX, Wei ZF
and Si DW: Preventive and therapeutic effect of simvastatin on
secondary inflammatory damage of rats with cerebral hemorrhage.
Asian Pac J Trop Med. 10:152–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin C, Zhao Y, Wan G, Zhu A and Wang H:
Effects of simvastatin and taurine on delayed cerebral vasospasm
following subarachnoid hemorrhage in rabbits. Exp Ther Med.
11:1355–1360. 2016. View Article : Google Scholar : PubMed/NCBI
|