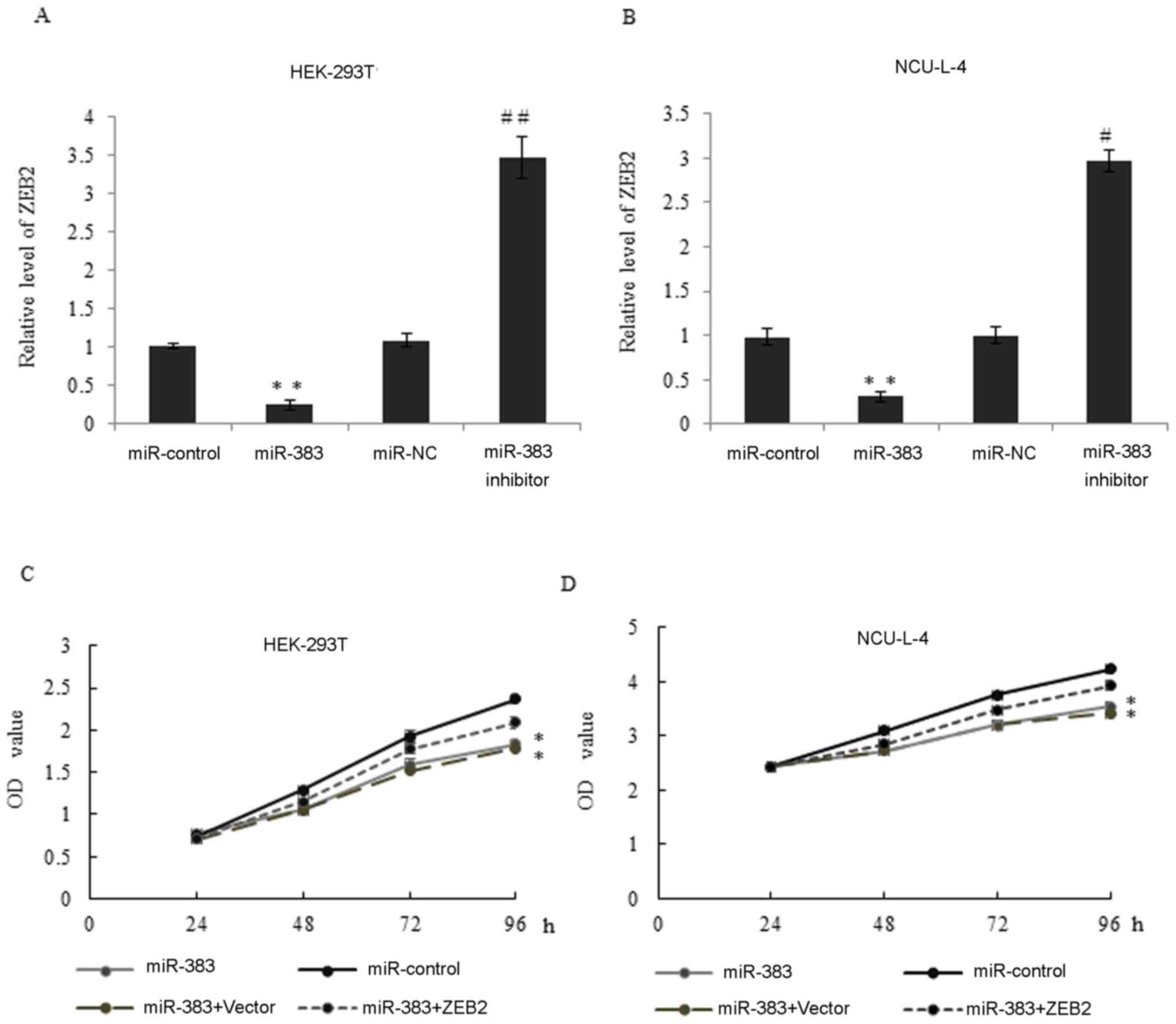
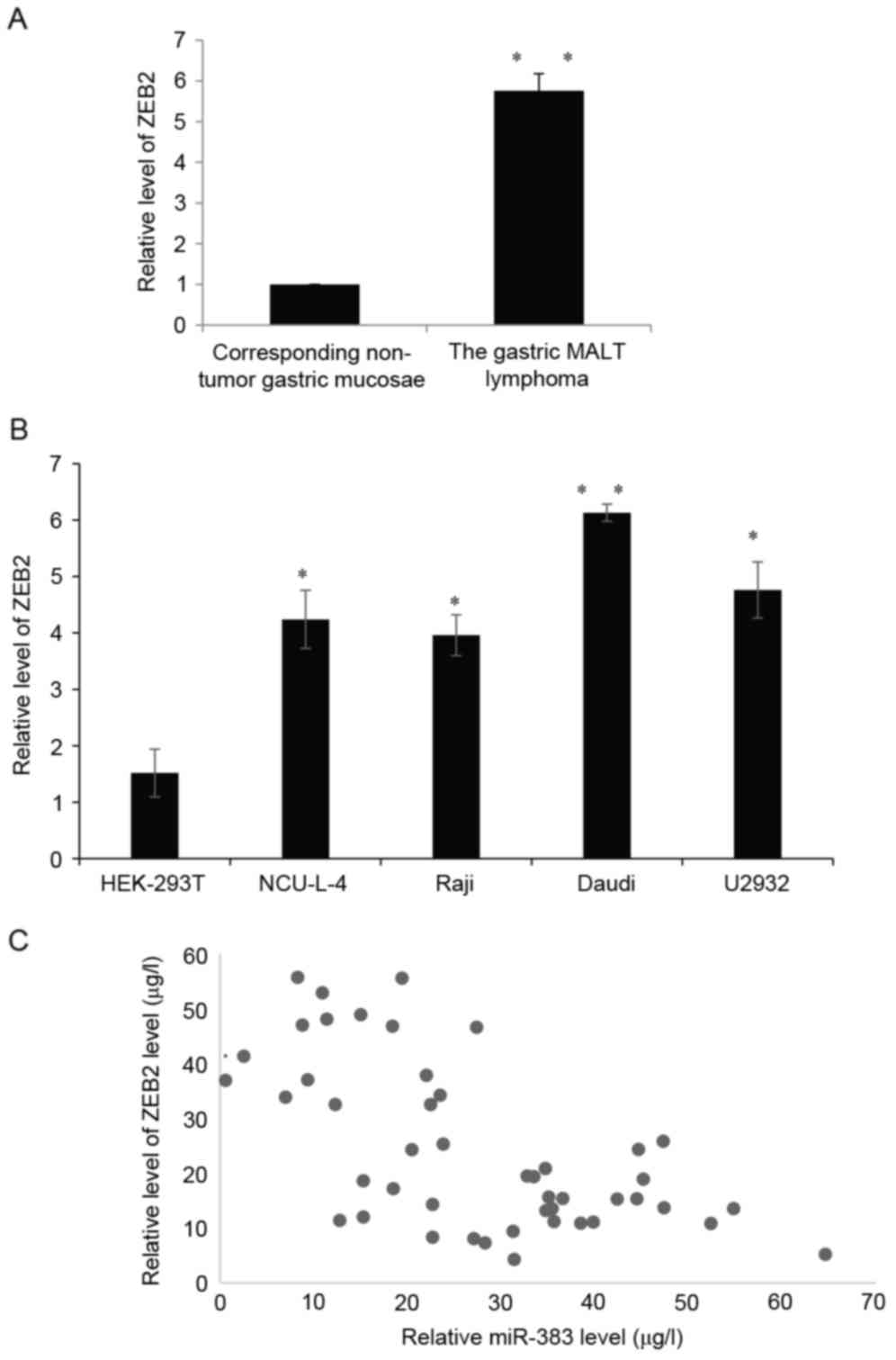
|
1
|
Nakamura S, Matsumoto T, Iida M, Yao T and
Tsuneyoshi M: Primary gastrointestinal lymphoma in Japan: A
clinicopathologic analysis of 455 patients with special reference
to its time trends. Cancer. 97:2462–2473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sabattini E, Bacci F, Sagramoso C and
Pileri SA: WHO classification of tumours of haematopoietic and
lymphoid tissues in 2008: An overview. Pathologica. 102:83–87.
2010.PubMed/NCBI
|
|
3
|
Nakamura S, Sugiyama T, Matsumoto T,
Iijima K, Ono S, Tajika M, Tari A, Kitadai Y, Matsumoto H, Nagaya
T, et al: Long-term clinical outcome of gastric MALT lymphoma after
eradication of Helicobacter pylori: A multicentre cohort follow-up
study of 420 patients in Japan. Gut. 61:507–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruskoné-Fourmestraux A, Fischbach W,
Aleman BM, Boot H, Du MQ, Megraud F, Montalban C, Raderer M, Savio
A and Wotherspoon A; EGILS group, : EGILS consensus report. Gastric
extranodal marginal zone B-cell lymphoma of MALT. Gut. 60:747–758.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng KS, Chan YK and Yeung YW: Treatment
outcome of gastric extra-nodal marginal zone B-cell lymphoma of
mucosa-associated lymphoid tissue type in the Hong Kong Chinese
population: A single centre experience. J Gastrointest Oncol.
4:198–202. 2013.PubMed/NCBI
|
|
6
|
Andriani A, Miedico A, Tedeschi L, Patti
C, Di Raimondo F, Leone M, Schinocca L, Romanelli A, Bonanno G,
Linea C, et al: Management and long-term follow-up of early stage
H. pylori-associated gastric MALT-lymphoma in clinical practice: An
Italian, multicentre study. Dig Liver Dis. 41:467–473. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fischbach W, Goebeler-Kolve ME, Dragosics
B, Greiner A and Stolte M: Long term outcome of patients with
gastric marginal zone B cell lymphoma of mucosa associated lymphoid
tissue (MALT) following exclusive Helicobacter pylori eradication
therapy: Experience from a large prospective series. Gut. 53:34–37.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wündisch T, Thiede C, Morgner A, Dempfle
A, Günther A, Liu H, Ye H, Du MQ, Kim TD, Bayerdörffer E, et al:
Long-term follow-up of gastric MALT lymphoma after Helicobacter
pylori eradication. J Clin Oncol. 23:8018–8024. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ke XS, Liu CM, Liu DP and Liang CC:
MicroRNAs: Key participants in gene regulatory networks. Curr Opin
Chem Biol. 7:516–523. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herranz H and Cohen SM: MicroRNAs and gene
regulatory networks: Managing the impact of noise in biological
systems. Genes Dev. 24:1339–1344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lytle JR, Yario TA and Steitz JA: Target
mRNAs are repressed as efficiently by microRNA-binding sites in the
5′ UTR as in the 3′ UTR. Proc Natl Acad Sci USA. 104:pp. 9667–9672.
2007; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Standart N and Jackson RJ: MicroRNAs
repress translation of m7Gppp-capped target mRNAs in vitro by
inhibiting initiation and promoting deadenylation. Genes Dev.
21:1975–1982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsushima K, Isomoto H, Inoue N, Nakayama
T, Hayashi T, Nakayama M, Nakao K, Hirayama T and Kohno S: MicroRNA
signatures in Helicobacter pylori-infected gastric mucosa. Int J
Cancer. 128:361–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gebauer N, Kuba J, Senft A, Schillert A,
Bernard V and Thorns C: MicroRNA-150 is up-regulated in extranodal
marginal zone lymphoma of MALT type. Cancer Genomics Proteomics.
11:51–56. 2014.PubMed/NCBI
|
|
15
|
Brabletz S and Brabletz T: The ZEB/miR-200
feedback loop - a motor of cellular plasticity in development and
cancer? EMBO Rep. 11:670–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Si W, Huang W, Zheng Y, Yang Y, Liu X,
Shan L, Zhou X, Wang Y, Su D, Gao J, et al: Dysfunction of the
reciprocal feedback loop between GATA3- and ZEB2-nucleated
repression programs contributes to breast cancer metastasis. Cancer
Cell. 27:822–836. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang WT, Kuo SH, Cheng AL and Lin CW:
Inhibition of ZEB1 by miR-200 characterizes Helicobacter
pylori-positive gastric diffuse large B-cell lymphoma with a less
aggressive behavior. Mod Pathol. 27:1116–1125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartl R, Frisch B, Burkhardt R, Kettner G,
Mahl G, Fateh-Moghadam A and Sund M: Assessment of bone marrow
histology in the malignant lymphomas (non-Hodgkin's): Correlation
with clinical factors for diagnosis, prognosis, classification and
staging. Br J Haematol. 51:511–530. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Musshoff K: Clinical staging
classification of non-Hodgkin's lymphomas. Strahlentherapie.
153:218–221. 1977.(In German). PubMed/NCBI
|
|
20
|
Roseau G: Role of echoendoscopy in the
study of gastric diseases with fold thickening and in gastric
lymphomas. Gastroenterol Hepatol. 25:19–25. 2002.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheson BD, Pfistner B, Juweid ME, Gascoyne
RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca
E, et al: Revised response criteria for malignant lymphoma. J Clin
Oncol. 25:579–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asenjo LM and Gisbert JP: Prevalence of
Helicobacter pylori infection in gastric MALT lymphoma: A
systematic review. Rev Esp Enferm Dig. 99:398–404. 2007.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin WC, Tsai HF, Kuo SH, Wu MS, Lin CW,
Hsu PI, Cheng AL and Hsu PN: Translocation of Helicobacter pylori
CagA into Human B lymphocytes, the origin of mucosa-associated
lymphoid tissue lymphoma. Cancer Res. 70:5740–5748. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu YY, Tsai HF, Lin WC, Hsu PI, Shun CT,
Wu MS and Hsu PN: Upregulation of CCL20 and recruitment of CCR6+
gastric infiltrating lymphocytes in Helicobacter pylori gastritis.
Infect Immun. 75:4357–4363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tasaki K, Shichishima A, Furuta M, Yoshida
S, Nakamura N and Abe M: CD5-positive mucosa-associated lymphoid
tissue (MALT) lymphoma of ocular adnexal origin: Usefulness of
fluorescence in situ hybridization for distinction between mantle
cell lymphoma and MALT lymphoma. Pathol Int. 57:101–107. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stathis A, Chini C, Bertoni F, Proserpio
I, Capella C, Mazzucchelli L, Pedrinis E, Cavalli F, Pinotti G and
Zucca E: Long-term outcome following Helicobacter pylori
eradication in a retrospective study of 105 patients with localized
gastric marginal zone B-cell lymphoma of MALT type. Ann Oncol.
20:1086–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zucca E and Dreyling M; ESMO Guidelines
Working Group, : Gastric marginal zone lymphoma of MALT type: ESMO
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 5 Suppl 21:v175–v176. 2010. View Article : Google Scholar
|
|
29
|
Liu TY, Dei PH, Kuo SH and Lin CW: Early
low-grade gastric MALToma rarely transforms into diffuse large cell
lymphoma or progresses beyond the stomach and regional lymph nodes.
J Formos Med Assoc. 109:463–471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong EJ, Ahn JY, Jung HY, Park H, Ko YB,
Na HK, Jung KW, Kim do H, Lee JH, Choi KD, et al: Helicobacter
pylori eradication therapy is effective as the initial treatment
for patients with H. pylori-negative and disseminated gastric
Mucosa-Ass. Gut Liver. 10:706–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vasilatou D, Sioulas AD, Pappa V,
Papanikolaou IS, Triantafyllou K, Dimitriadis GD and Papageorgiou
SG: The role of miRNAs and epigenetic mechanisms in primary gastric
mucosa-associated lymphoid tissue lymphoma. Future Oncol.
12:1587–1593. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thorns C, Kuba J, Bernard V, Senft A,
Szymczak S, Feller AC and Bernd HW: Deregulation of a distinct set
of microRNAs is associated with transformation of gastritis into
MALT lymphoma. Virchows Arch. 460:371–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shang Y, Zang A, Li J, Jia Y, Li X, Zhang
L, Huo R, Yang J, Feng J, Ge K, et al: MicroRNA-383 is a tumor
suppressor and potential prognostic biomarker in human non-small
cell lung caner. Biomed Pharmacother. 83:1175–1181. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Z, Zeng X, Tian D, Xu H, Cai Q, Wang J
and Chen Q: MicroRNA-383 inhibits anchorage-independent growth and
induces cell cycle arrest of glioma cells by targeting CCND1.
Biochem Biophys Res Commun. 453:833–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lian J, Tian H, Liu L, Zhang XS, Li WQ,
Deng YM, Yao GD, Yin MM and Sun F: Downregulation of microRNA-383
is associated with male infertility and promotes testicular
embryonal carcinoma cell proliferation by targeting IRF1. Cell
Death Dis. 1:e942010. View Article : Google Scholar : PubMed/NCBI
|