Introduction
Due to rapid economic development and continuous
improvements in living standards, the prevalence of diabetes is
increasing worldwide (1). Type 2
diabetes mellitus (T2DM) is a complex metabolic disorder caused by
insulin (INS) resistance and insufficient compensatory INS
secretion (2). T2DM greatly
increases the risk of chronic kidney disease, cardiovascular
disease, myocardial infarction and stroke (3); therefore, more effective treatments and
novel prevention strategies for T2DM are required (4). The mechanisms of T2DM action, including
lipid metabolism disorders, endothelial dysfunction, chronic
inflammation and redox homeostasis imbalance, have previously been
described (5). β-cells dysfunction
contributes to inadequate INS secretion, which is the ultimate
cause of T2DM (6). It has been
demonstrated that high levels of free fatty acids or
hypertriglyceridemia and lipid ectopic deposition in non adipose
tissue are key factors contributing to the development of T2DM
(7). Total β-cells function is
determined by the number of β-cellss and their functionality.
Establishing an effective therapy to decrease β-cells lipid
toxicity may be beneficial for the treatment of T2DM.
The progression of T2DM is associated with changes
in several factors, including mitochondrial biogenesis, lipid
metabolism and β-cells development (5). A number of proteins, including
mitochondrial biogenesis-related nuclear respiratory factor (NRF),
mitochondrial transcription factor A (mtTFA), lipid
metabolism-associated carnitine palmitoyltransferase 1 (CPT-1),
acetyl-CoA carboxylase (ACC), long-chain acyl-CoA dehydrogenase
(LCAD), β-cells-associated pancreatic duodenal homeobox-1 gene
(PDX-1), mid-arm fat area (MAFA) (8)
and INS, are associated with T2DM (9). Resveratrol (RSV), a natural polyphenol
found in grapes and red wine (10),
may potentially attenuate hepatic steatosis and reduce the risk of
patients developing T2DM (11). It
has previously been demonstrated that RSV activates Sirtuin type 1
(SIRT1) to inhibit the development of T2DM (12). SIRT1 is a nuclear
NAD+-dependent class III histone deacetylase that may
regulate cell aging, life span and energy metabolism (13) by engaging in the reciprocal
co-regulation of different binding partners (14). SIRT1 is able to adjust the
deacetylase activity of various transcription factors that control
metabolic and endocrine signals and is widely involved in
regulating the mammalian cell life span, INS secretion and
glucose/lipid metabolism (15).
Genetic variation in the SIRT1 gene is associated with INS
resistance and may explain the increased risk of T2DM in the
Chinese Han population (16). SIRT1
may have a therapeutic effect on metabolic deterioration in T2DM
(17) and in the liver,
SIRT1-knockout increases plasma glucose levels, reduces INS
sensitivity and upregulates free fatty acid and cholesterol levels
(18). To explore the association
between SIRT1 and mitochondrial biogenesis, it is essential to
assess lipid metabolism and β-cells development and identify the
underlying mechanisms by which RSV alleviates T2DM.
In the present study, a T2DM rat model was
constructed following the administration of a high fat diet and
streptozotocin (STZ) injections into the abdominal cavity of rats.
It was determined that the T2DM model was successfully constructed
following the assessment of glucose and INS levels. The results
indicated that RSV treatment effectively modulated glucose and INS
levels as well as the expression of SIRT1, peroxisome
proliferator-activated receptor-γ coactivator-1α (PGC-1α), and
forkhead box protein O3 (FOXO3a). In addition, the insulinoma cell
line clone 1E (INS-1E) was treated with palmitic acid (PA). PA
suppressed the expression of SIRT1 in a dose- and time-dependent
manner. In PA-induced INS-1E cells, it was demonstrated that RSV
modulated the expression of PGC-1α and FOXO3a via SIRT1 and
regulated the expression of mitochondrial biogenesis-associated,
lipid metabolism-associated and β-cells-associated proteins. These
results provide novel evidence that RSV mitigates T2DM via SIRT1
and identified the mechanisms by which SIRT1 inhibits T2DM.
Materials and methods
Animals
A total of 30 male Sprague-Dawley (SD) rats (6–8
weeks old) weighing 200±20 g were purchased from Hunan SJA
Laboratory Animal Co., Ltd. (Changsha, China). Rats were housed at
24±1°C with 40–50% humidity in a clean environment with a 12 h
light/dark cycle. All animals had free access to food and purified
water. All procedures were approved by the Animal Ethics Committee
of the First Affiliated Hospital of Harbin Medical University
(Harbin, China). Rats were fed with a high glucose, high fat diet
(60% common chow, 10% lard, 10% egg yolk powder and 20% sucrose)
for 8 weeks. Rats then received a 40 mg/kg intraperitoneal
injection of STZ (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
once. At 72 h following STZ injection, rats with fasting blood
glucose ≥16.67 mM (based on tail vein samples) were selected as
T2DM animals for use in the following experiments (19). The normal control group (NC, n=10)
was administered the same volume of sodium citrate buffer instead
of STZ. T2DM rats were randomly assigned to either a diabetic group
(DM, n=10) or a diabetic RSV group (DR, n=10). Rats in the DR group
were administered once with 30 mg/kg RSV intragastrically
(Sigma-Aldrich; Merck KGaA). Rats in the NC and DM groups were
administered intragastrically with the same amount of 0.5%
carboxymethyl cellulose. At weeks 0, 4 and 8 following induction,
fasting and 2 h postprandial blood glucose were measured using the
glucose oxidase method on the Beckman Glucose Analyzer II (Beckman
Coulter, Inc., Brea, CA, USA) and plasma INS levels were determined
using an RIA Assay system (Linco Research, Inc., St. Charles, MO,
USA) (20) according to the
manufacturer's protocol. Following 8 weeks of different treatments,
total RNA was isolated from rat pancreas tissues using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), and proteins were extracted using radioimmunoprecipitation
assay buffer (RIPA; Beyotime Institute of Biotechnology, Haimen,
China) for western blot analysis.
Cell culture and grouping
The rat insulinoma cell line clone 1E (INS-1E;
provided by Professor Pierre Maechler; CMU; University of Geneva,
Geneva, Switzerland) was cultured at 37°C in a humidified
atmosphere containing 5% CO2 in RPMI 1640 medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA), 11 mM
glucose, 10 mM HEPES, 1 mM sodium pyruvate, 50 µM 2-mercaptoethanol
(Sigma-Aldrich; Merck KGaA), 50 µg/ml penicillin and 100 µg/ml
streptomycin. When density reached 80%, cells were pretreated with
PA (0, 0.125, 0.25, 0.5, or 1 mM) for 24 h. INS-1E cells were
treated with 0.5 mM PA for 0, 12, 24, 48 or 72 h.
Cell transfection
INS-1E cells were seeded into 6-well plates at a
density of 5×105 cells/well and divided into 5 groups: A
normal control (NC) group, a high fat (HF) group, a HF+RSV group, a
HF+RSV+SIRT small interfering (si)RNA group (HF+RSV+SIRT siRNA) and
a HF+RSV+negative control siRNA group (HF+RSV+NC siRNA). To
transfect cells with SITR siRNA or NC siRNA, cells in the
HF+RSV+SIRT siRNA and HF+RSV+NC siRNA groups were incubated in 1 ml
RPMI 1640 with SIRT1 siRNA
(5′-CACCCCAGCAACTCAGCATTCATCGAAATGAATGCTGAGTTGCTGG-3′) or NC siRNA
(5′-TTCTCCGAACGTGTCACGT-3′), respectively, and Lipofectamine 2000
(Thermo Fisher Scientific, Inc.) without FBS. The final
concentrations of siRNA and Lipofectamine 2000 used were 2 and 4
µg/ml, respectively. Following 48 h incubation at 37°C, cells in
the HF+RSV, HF+RSV+SIRT siRNA and HF+RSV+NC siRNA groups were
treated with 0.5 mM PA+10 µM RSV. The HF group received the same
amount of PA and NC cells were treated with the same amount of
vehicle. Following 24 h incubation at 37°C, total RNA or proteins
were extracted for further experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from INS-1E cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. The quality of RNA was
determined by agarose electrophoresis and the concentration was
measured using a NanoDrop ND-2000 spectrophotometer (Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA) at 260 nm and 280 nm. RT was
performed using 1 µg total RNA using a reverse transcriptase cDNA
synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocol. qPCR was performed using
SYBR-Green qPCR SuperMix (Invitrogen; Thermo Fisher Scientific,
Inc.) on a real-time RT-PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The thermocycling conditions were as follows:
94°C for 5 min followed by 35 cycles of 94°C for 30 sec, 56°C for
30 sec and 72°C for 30 sec, with a final elongation step at 72°C
for 5 min. All amplification reactions were performed in triplicate
and the relative expression of target genes were normalized against
β-actin using the 2−∆∆Cq method (21). The primers for RT-qPCR are presented
in Table I.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Genes | Forward primers
(5′-3′) | Reverse primers
(5′-3′) |
|---|
| SIRT1 |
GCTGACGACTTCGACGACG |
TCGGTCAAGAGGAGGTTGTCT |
| PGC1α |
TATGGAGTGACATAGAGTGTGCT |
GTCGCTACACCACTTCAATCC |
| NRF |
CGGAAACGGCCTCATGTGT |
CGCGTCGTGTACTCATCCAA |
| FOXO3a |
TCTTACGCCGACCTCATCAC |
ACGCTCTTGACCATCCACT |
| INS |
CCCTGTTGGTGCACTTCCT |
TCCCAGCTCCAGTTGTTCC |
| mtTFA |
TATTAGAATTTGTTAAATTTTGGGGAAT |
ACAAACAATCCTACATCCAAAACC |
| ACC |
CACATCATGAAGGAGGAGG |
GCTATCACACAGCCTGGGTC |
| CPT-1 |
ACGGGTGGATGTTCGAGATG |
GGCAGTGACGTTTGGAAGCT |
| LCAD |
TTCGTGTCCTGAGCG |
GAGGCTAATGCCATG |
| PDX-1 |
GTTCATCTCCCTTTCCCGTGG |
GGTGGTGGCTTTGGCAATG |
| MAFA |
AAAGCGGTGCTGGAGGAT |
GGTTCAGGTGGTGCTGGTA |
| β-actin |
CCTCTATGCCAACACAGTGC |
GTACTCCTGCTTGCTGATCC |
Western blotting
Proteins were extracted from rat pancreatic tissues
or INS-1E cells using RIPA buffer (Beyotime Institute of
Biotechnology, Haimen, China) and centrifuged at 15,000 × g for 30
min at 4°C. The concentration of proteins was determined using a
bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Equal amounts of protein
(25 µg/lane) were subjected to 12.5% SDS-PAGE. Proteins were
subsequently electrotransferred to a polyvinylidene fluoride
membrane and blocked with 5% non-fat milk in Tris-buffered saline
with 0.05% Tween-20 (TBST) for 1 h at room temperature. The
membrane was incubated with mouse anti-SIRT1 (sc-15404),
anti-FOXO3a (sc-20680), anti-PGC-1α (sc-13067) and anti-β-actin
(sc-1616) antibodies (all 1:1,000 dilution; all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Following
three washes with TBST, the membrane was incubated with horseradish
peroxidase-conjugated rabbit anti-mouse secondary antibody
(1:2,000; sc-203; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Membrane bands were developed using an enhanced
chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.). The
relative densities of target protein bands were normalized against
β-actin using Quantity One software v4.4 (Bio-Rad Laboratories,
Inc.).
Detection of serum superoxide
dismutase (SOD) and malondialdehyde (MDA)
Total heart blood was harvested from rats following
8 weeks of different treatments and centrifuged at 3,500 × g for 15
min at 4°C to separate the serum. Serum SOD (cat. no. SES134Hu) and
MDA (cat. no. CEA597Ge) levels were detected using a commercially
available sandwich ELISA kit (Cloud-Clone Corp., Katy, TX, USA)
according to the manufacturer's protocol. Absorbance was measured
at 450 nm on Bio-Rad 680 Microplate reader (Bio-Rad Laboratories,
Inc.). The quantity of SOD and MDA in the serum was estimated using
a constructed calibration curve.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). All results are presented as
the mean ± standard error of the mean. Significant differences in
mean values were evaluated using one-way analysis of variance with
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
RSV treatment decreases blood glucose
and INS levels in rats with STZ-induced DM
To confirm that the diabetic rat model was
successfully established, blood glucose levels were measured
following STZ injection. No significant differences in blood
glucose levels were observed in the NC group at weeks 0, 4 or 8
(Table II). Compared with the NC
group, fasting glucose levels in the DM group were significantly
higher at week 8 (P<0.05; Table
II). However, the administration of RSV significantly
attenuated the STZ-induced increase in fasting glucose (P<0.05;
Table II). The 2 h postprandial
blood glucose levels were subsequently assessed. At weeks 4 and 8
post-induction, postprandial blood glucose was significantly higher
in the DM group compared with the NC group (P<0.01; Table II); however, in the DR group, this
increase was significantly attenuated (P<0.01; Table II).
 | Table II.Fasting and 2 h postprandial blood
glucose levels at weeks 0, 4 and 8 following induction of
diabetes. |
Table II.
Fasting and 2 h postprandial blood
glucose levels at weeks 0, 4 and 8 following induction of
diabetes.
|
|
| Blood glucose
(mM) |
|---|
|
|
|
|
|---|
| Group | Time (weeks) | Fasting | 2 h
postprandial |
|---|
| NC | 0 |
5.86±0.51 |
6.14±0.62 |
|
| 4 |
5.91±0.64 |
6.24±0.64 |
|
| 8 |
5.74±0.49 |
6.05±0.59 |
| DM | 0 |
5.79±0.41 |
6.11±0.61 |
|
| 4 |
6.45±0.62 |
15.98±0.71b |
|
| 8 |
7.97±0.71a |
22.36±1.14b |
| DR | 0 |
5.84±0.47 |
6.31±0.61 |
|
| 4 |
6.24±0.59 |
12.36±0.89d |
|
| 8 |
7.01±0.68c |
13.59±0.97d |
The fasting and 2 h postprandial levels of INS were
also assessed. Compared with the NC group, fasting and 2 h
postprandial blood INS levels were significantly elevated in the DM
group at weeks 4 and 8 (P<0.05; Table III). However, INS levels in the DR
group were significantly lower at week 8 compared with the DM group
(P<0.05; Table III). Taken
together, these results suggest that STZ injection successfully
induced DM in rats and that RSV administration modulated glucose
and INS levels in rats with STZ-induced DM.
 | Table III.Fasting and 2 h postprandial blood
insulin levels at weeks 0, 4 and 8 following induction of
diabetes. |
Table III.
Fasting and 2 h postprandial blood
insulin levels at weeks 0, 4 and 8 following induction of
diabetes.
|
|
| Blood insulin
(ng/ml) |
|---|
|
|
|
|
|---|
| Group | Time (weeks) | Fasting | 2 h
postprandial |
|---|
| NC | 0 |
1.21±0.14 |
1.14±0.07 |
|
| 4 |
1.24±0.14 |
1.16±0.09 |
|
| 8 |
1.29±0.49 |
1.15±0.09 |
| DM | 0 |
1.25±0.11 |
1.11±0.06 |
|
| 4 |
1.45±0.12a |
1.37±0.11a |
|
| 8 |
1.56±0.11a |
1.47±0.14a |
| DR | 0 |
1.24±0.07 |
1.17±0.09 |
|
| 4 |
1.32±0.09 |
1.35±0.09 |
|
| 8 |
1.35±0.08b |
1.37±0.07b |
RSV modulates the expression of
PGC-1α, SIRT1 and FOXO3a in the pancreatic tissues of rats with
STZ-induced DM
To investigate the mechanism by which RSV functions
in DM, levels of PGC-1α, SIRT1 and FOXO3a mRNA and protein were
assessed using RT-qPCR and western blotting, respectively. Compared
with the NC group, levels of PGC-1α, SIRT1 and FOXO3a mRNA and
protein were significantly suppressed in the DM group (P<0.01;
Fig. 1A and B). However,
pretreatment with RSV significantly attenuated these decreases
(P<0.05; Fig. 1A and B). These
data suggest that RSV administration upregulates PGC-1α, SIRT1 and
FOXO3a expression in mice with STZ-induced DM.
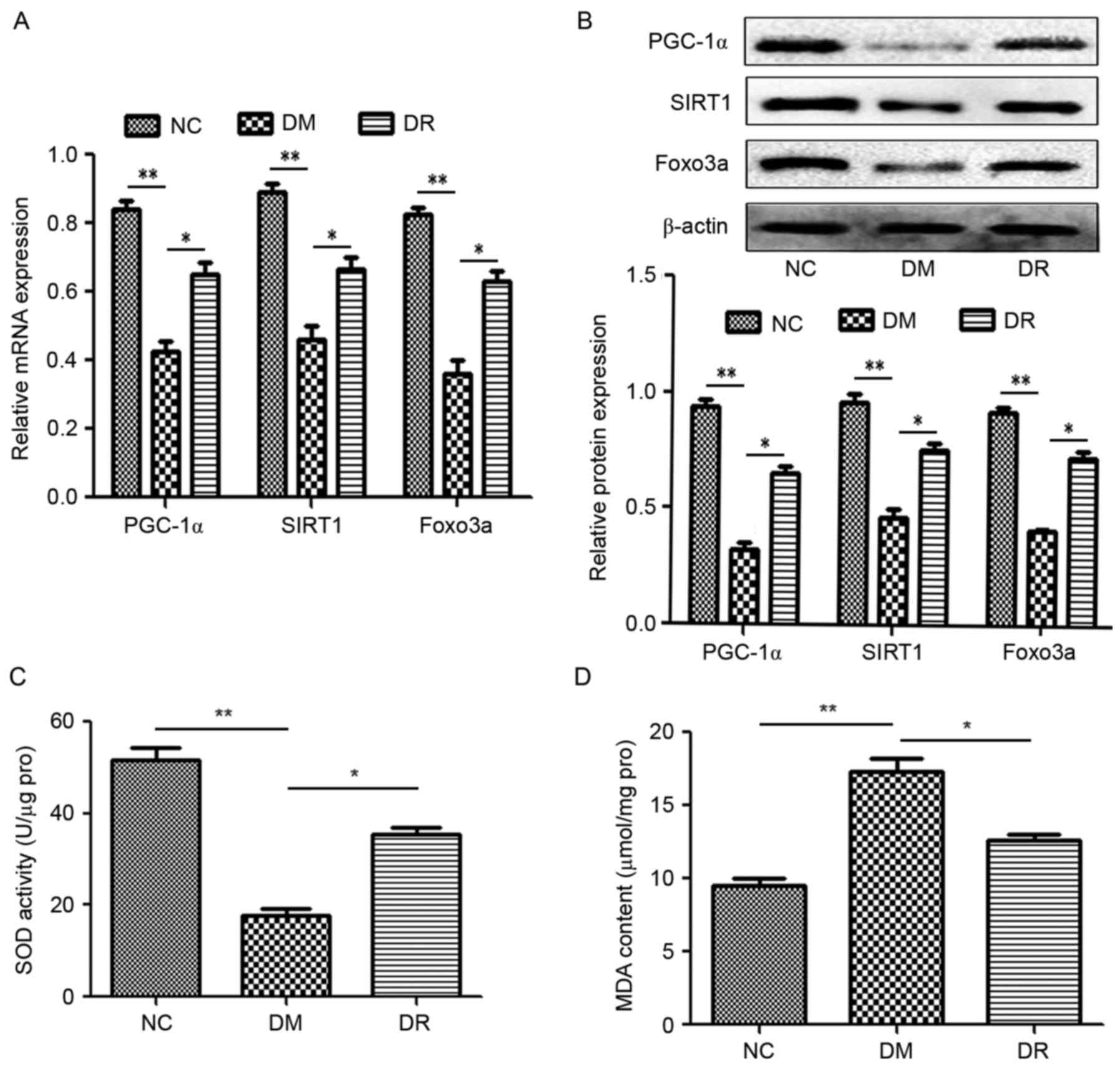 | Figure 1.(A) RSV modulates PGC-1α, SIRT1 and
FOXO3a (A) mRNA and (B) protein levels as assessed by reverse
transcription-quantitative polymerase chain reaction and western
blotting, respectively. β-actin was used as an internal control.
Pretreatment with RSV affected the activity of (C) SOD and (D) MDA
as analyzed using ELISA. *P<0.05 and **P<0.01. RSV,
resveratrol; PGC1α, peroxisome proliferator-activated receptor-γ
coactivator-1α; SIRT1, Sirtuin 1; FOXO3a, forkhead box O3a; SOD,
superoxide dismutase; MDA, malondialdehyde; NC, normal control
group; DM, streptozotocin-induced diabetic group; DR, RSV treated
streptozotocin-induced diabetic group; RSV, resveratrol. |
It has been reported that oxidaωtive stress due to
the excessive production of reactive oxygen species (ROS) and
mitochondrial dysfunction are major factors in the development of
INS resistance in T2DM (22). ELISA
assays were used to analyze the activity of SOD and MDA to evaluate
oxidative stress. SOD activity was significantly suppressed in the
DM group compared with the NC group (P<0.01; Fig. 1C). However, RSV treatment
significantly increased SOD activity in the DR group compared with
the DM group (P<0.05; Fig. 1C).
By contrast, MDA activity was significantly upregulated in the DM
group compared with the NC group (P<0.01; Fig. 1D). In the DR group, pretreatment with
RSV significantly attenuated the DM-induced increase in MDA
activity (P<0.05; Fig. 1D). These
data suggest that RSV regulates the activity of SOD and MDA in rats
with STZ-induced DM.
PA inhibits the expression of SIRT1 in
a dose- and time-dependent manner
It has been demonstrated that PA serves a role in
the development of obesity and T2DM (17). Varying concentrations of PA were used
to treat INS-1E cells and the results indicated that PA
significantly suppressed SIRT1 mRNA in a dose-dependent manner
(P<0.01; Fig. 2A). The
association between SIRT1 expression and PA induction time was
subsequently investigated and the results indicate that PA
treatment also significantly reduced SIRT1 mRNA expression in a
time-dependent manner (P<0.01; Fig.
2B).
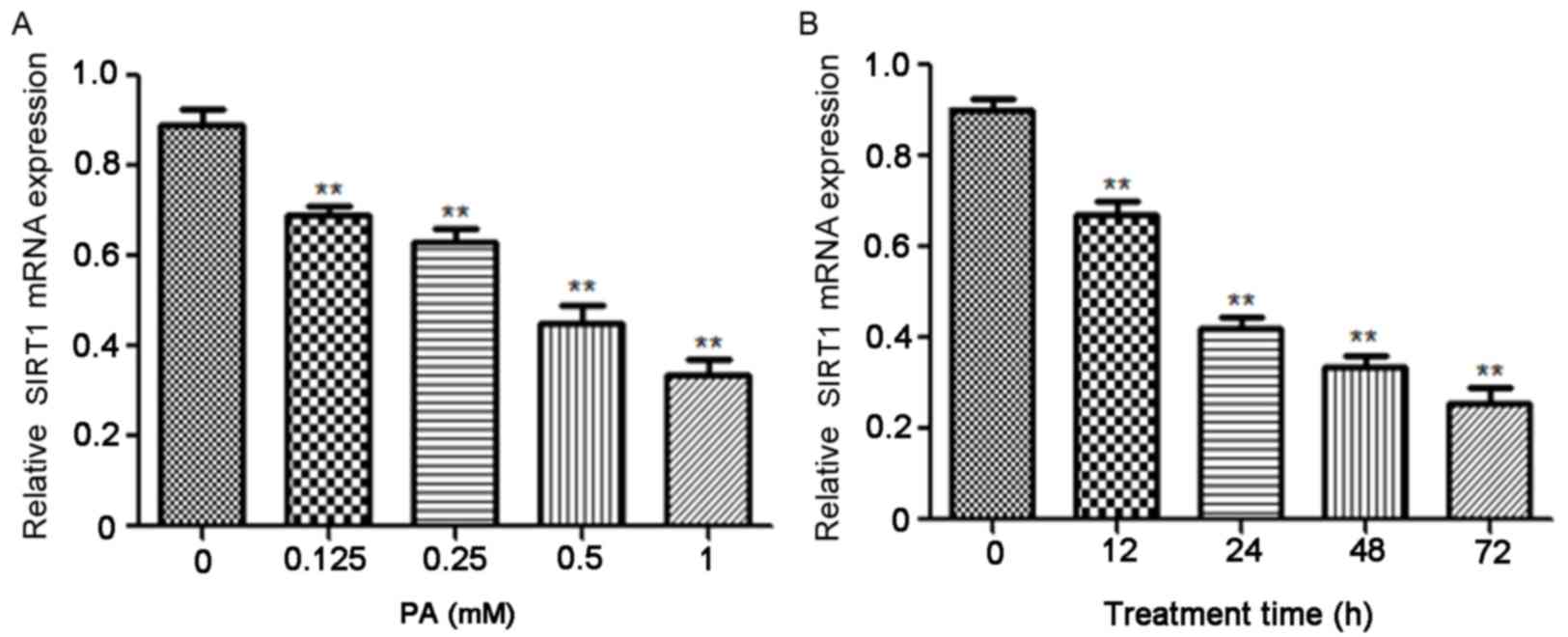 | Figure 2.PA suppressed the expression of SIRT1
in a dose- and time-dependent manner. (A) INS-1E cells were treated
with 0, 0.125, 0.25, 0.5 and 1 mM PA for 24 h and the expression of
SIRT1 mRNA was assessed using RT-qPCR. **P<0.01 vs. 0 mM PA (B)
INS-1E cells were pretreated with 0.5 mM PA for 0, 12, 24, 48 and
72 h, respectively and SIRT1 mRNA was measured using RT-qPCR.
β-actin was used as an internal control. **P<0.01 vs. 0 h. PA,
palmitic acid; SIRT1, Sirtuin 1; INS-1E, insulinoma cell line clone
1E; RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
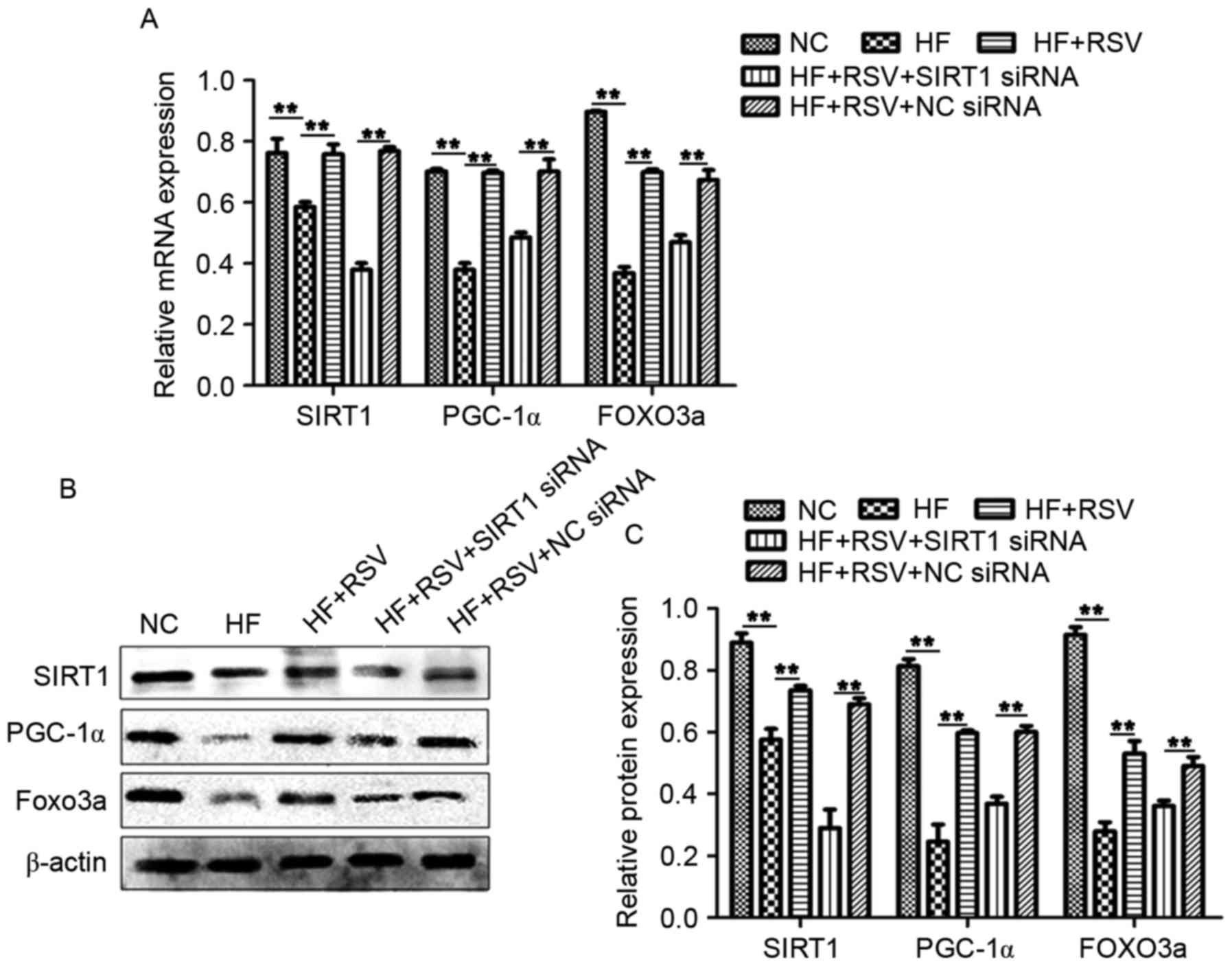
RSV affects the expression of PGC-1α
and FOXO3a via SIRT1 in PA-induced INS-1E cells
To further investigate the role of RSV in
vitro, levels of PGC-1α, SIRT1 and FOXO3a in INS-1E cells were
evaluated. PA significantly suppressed the levels of PGC-1α, SIRT1
and FOXO3a mRNA and protein compared with the NC group (P<0.01;
Fig. 3A-C); however, administration
of RSV significantly attenuated this effect (P<0.01; Fig. 3A-C). Furthermore, transfection with
SIRT1 siRNA in conjunction with PA significantly reduced the
expression of PGC-1α, SIRT1 and FOXO3a compared with the HF+RSV+NC
siRNA group (P<0.01; Fig. 3A-C).
These data suggest that RSV mitigates the HF-induced inhibition of
PGC-1α and FOXO3a expression via SIRT1.
To examine the mechanism by which SIRT1 functions,
the expression of a cascade of genes was investigated, including
mitochondrial biogenesis-associated NRF and mtTFA, lipid
metabolism-associated CPT-1, ACC and LCAD, and β-cells-associated
PDX-1, MAFA and INS. PA treatment significantly suppressed mRNA
levels of SIRT1, NRF, mtTFA, CPT-1, LCAD, PDX-1, MAFA and INS mRNA,
whereas it significantly upregulated the expression of ACC mRNA
compared with the NC group (P<0.01; Fig. 4). Treatment with RSV significantly
attenuated these PA-induced alterations in gene expression
(P<0.01; Fig. 4). However, SIRT1
knockdown significantly attenuated the PA-induced increases in
SIRT1, NRF, mtTFA, CPT-1, LCAD, PDX-1, MAFA and INS expression as
well as the PA-induced decrease in ACC expression compared with the
HF+RSV+NC siRNA group (P<0.01; Fig.
4). These data suggest that RSV modulates the expression of
NRF, mtTFA, CPT-1, LCAD, PDX-1, MAFA and INS via SIRT1 in
PA-induced INS-1E cells.
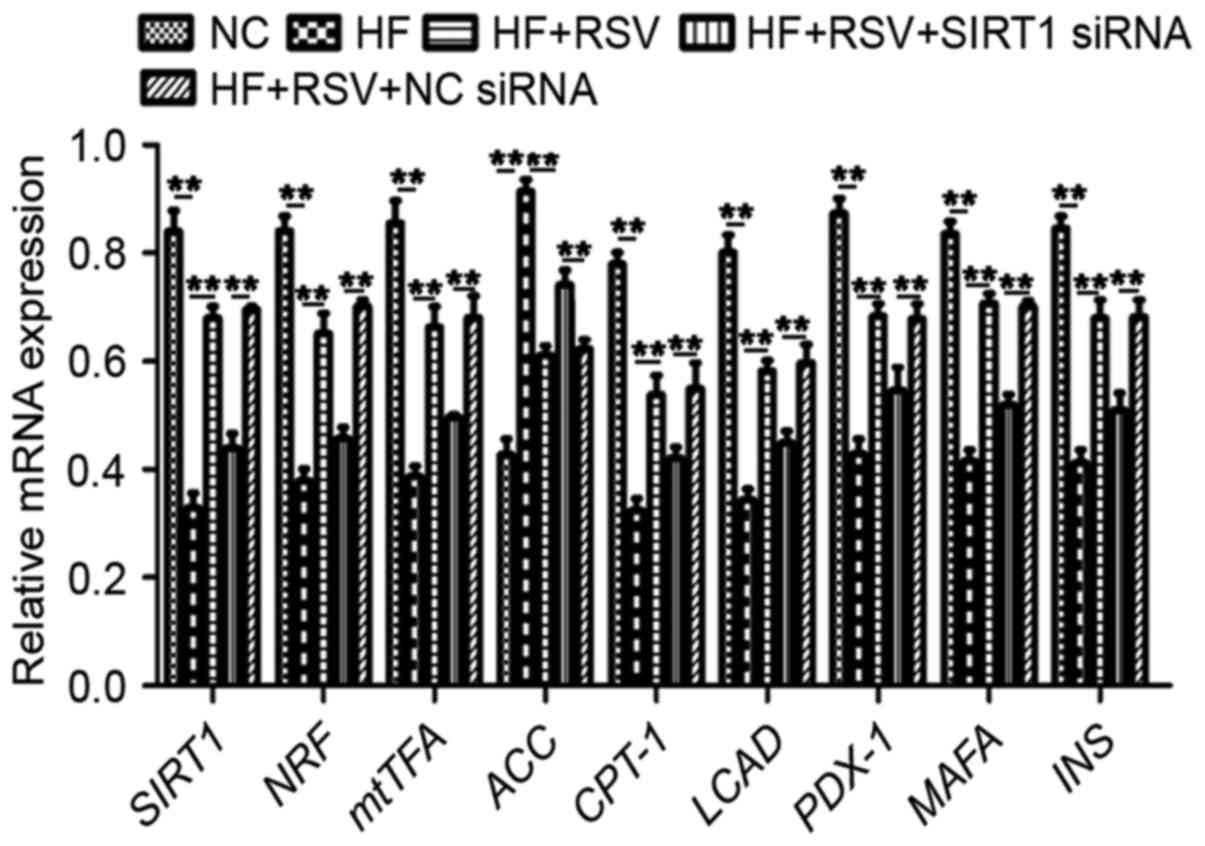 | Figure 4.RSV regulated the levels of
mitochondrial biogenesis-associated, lipid metabolism-associated
and β-cells-associated genes via SIRT1. **P<0.01. SIRT1, Sirtuin
1; INS-1E, insulinoma cell line clone 1E; PGC1α, peroxisome
proliferator-activated receptor-γ coactivator-1α; FOXO3a, forkhead
box O3a; NC, normal INS-1E cells pretreated with vehicle; HF,
INS-1E cells incubated with 30 mM PA for 24 h; HF+RSV, INS-1E cells
incubated with 30 mM PA and 10 µm RSV for 24 h; HF+RSV+SIRT siRNA,
INS-1E cells transfected with SIRT1 siRNA for 48 h and incubated
with PA 30 mM and 10 µm RSV for 24 h; HF+RSV+NC siRNA, INS-1E cells
transfected with negative control siRNA for 48 h and incubated with
PA 30 mM and 10 µm RSV for 24 h; NRF, nuclear respiratory factor;
mtTFA, mitochondrial transcription factor A; ACC, acetyl-CoA
carboxylase; CPT-1, lipid metabolism-related carnitine
palmitoyltransferase 1; LCAD, long-chain acyl-CoA dehydrogenase;
PDX-1, pancreatic duodenal homeobox-1; MAFA, mid-arm fat area; INS,
insulin; siRNA, small interfering RNA. |
RSV affects the expression of SOD and
MDA via SIRT1 in PA-induced INS-1E cells
The expression of SOD and MDA mRNA in INS-1E cells
following SIRT1 knockdown was assessed to determine the effect of
SIRT1 on oxidative stress. Compared with the NC group, PA
significantly suppressed SOD mRNA expression (P<0.01; Fig. 5A) and RSV treatment significantly
reversed this effect (P<0.01; Fig.
5A). However, SIRT1 knockdown significantly decreased SOD
expression compared with the HF+RSV+NC siRNA group (P<0.01;
Fig. 5A). The administration of PA
significantly increased MDA expression compared with the NC group
(P<0.01; Fig. 5B) and RSV
significantly attenuated this upregulation (P<0.01; Fig. 5B). SIRT1 knockdown significantly
upregulated MDA mRNA expression compared with the HF+RSV+NC siRNA
group (P<0.01; Fig. 5B). These
data indicate that RSV regulates SOD and MDA levels via SIRT1 in
PA-treated INS-1E cells.
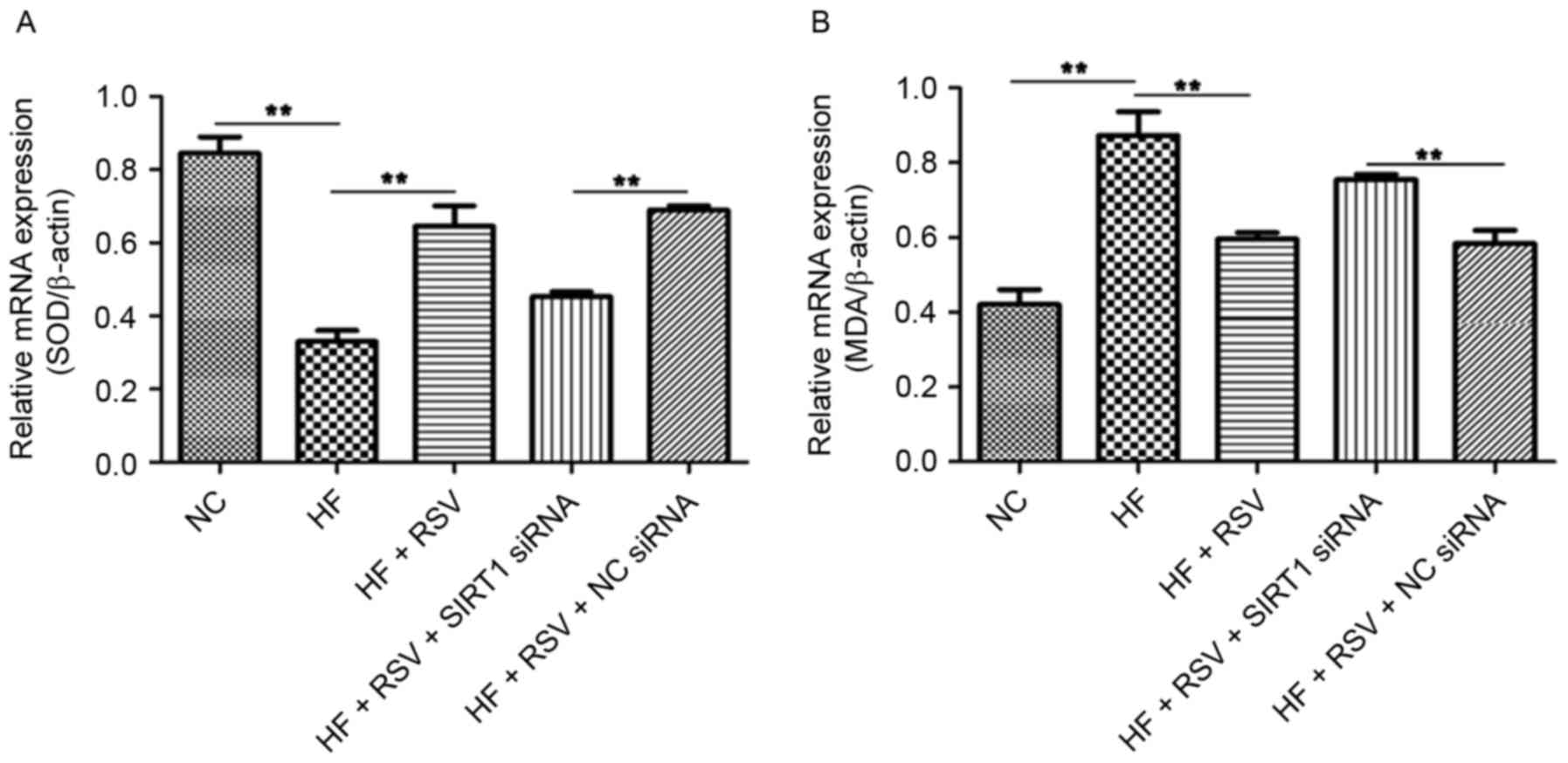 | Figure 5.RSV regulates SOD and MDA mRNA levels
via SIRT1 in INS-1E cells. The relative expression of (A) SOD and
(B) MDA was normalized to β-actin in PA-induced INS-1E cells.
**P<0.01. RSV, resveratrol; SOD, superoxide dismutase; MDA,
malondialdehyde; SIRT1, Sirtuin 1; INS-1E, insulinoma cell line
clone 1E; NC, normal INS-1E cells pretreated with vehicle; HF,
INS-1E cells incubated with 30 mM PA for 24 h; HF+RSV, INS-1E cells
incubated with 30 mM PA and 10 µm RSV for 24 h; HF+RSV+SIRT siRNA,
INS-1E cells transfected with SIRT1 siRNA for 48 h and incubated
with PA 30 mM and 10 µm RSV for 24 h; HF+RSV+NC siRNA, INS-1E cells
transfected with negative control siRNA for 48 h and incubated with
PA 30 mM and 10 µm RSV for 24 h; siRNA, small interfering RNA. |
Discussion
Over the past few decades, the incidence and
prevalence of DM have increased worldwide, primarily due to the
increased incidence of T2DM (17).
As such, there is a need to develop additional treatments and novel
prevention strategies for T2DM. In the present study, a rat model
of STZ-induced DM was constructed and the therapeutic effects of
RSV were assessed. Furthermore, PA-induced INS-1E cells were
assessed in vitro to investigate the underlying molecular
mechanisms of T2DM. The results of the present study indicate that
RSV mitigates the development of T2DM via SIRT1 by modulating
mitochondrial biogenesis, lipid metabolism and β-cells
development.
Abdominal injection of STZ is a widely used
technique for DM modeling (23). In
the present study, glucose and INS levels were assessed following
STZ administration. It was demonstrated that glucose and INS levels
were significantly upregulated, indicating that the DM model was
constructed successfully. The administration of RSV attenuated the
DM-induced alterations in glucose and INS levels, suggesting that
RSV mitigates T2DM. These data were similar to those obtained from
a previous study (11) and support
the use of RSV as a potential glucose-lowering agent in T2DM.
Furthermore, the results of an in vivo study indicated that
RSV increases SIRT1 expression and stimulates PGC-1a activity in
skeletal muscle (24). The results
of the present study demonstrate that RSV is able to mitigate the
STZ-induced inhibition of SIRT1, PGC-1α and FOXO3a, which is in
accordance with previous results (25–27). A
number of previous studies have reported that INS resistance is
associated with mitochondrial dysfunction (22) and these results may be caused by
oxidative stress (28). PGC-1α
stimulates mitochondrial biogenesis and electron transport activity
to suppress ROS production and ROS levels are also affected by MDA.
Furthermore, FOXO3a serves a central role in controlling oxidative
stress (29). SOD is an antioxidant
enzyme and to a certain extent, SOD levels represent the level of
oxidative stress (30). The results
of the present study indicated that MDA activity in rats with DM
was higher compared with the NC group; however, treatment with RSV
reversed this effect. The opposite trend was observed in SOD
activity. These data indicate that there is an association between
DM and oxidative stress and that RSV is able to successfully
attenuate these conditions.
INS secretion from pancreatic islet β-cells is
regulated by a number of factors; an increase in blood glucose is
the predominant trigger; however, fatty and amino acids may also
act as direct or indirect stimuli (31). It has been reported that PA induces
lipotoxicity in rat INS-producing cells (32). In the present study, PA was used to
induce INS secretion in INS-1E cells. The results demonstrated that
PA suppressed the expression of SIRT1 in a dose- and time-dependent
manner, suggesting that SIRT1 serves an important role in the
regulation of lipid metabolism. Furthermore, PA inhibited PGC-1α
and FOXO3a expression. SIRT1 knockdown attenuated the effects of
RSV on PA-inhibited PGC-1a and FOXO3a expression. It has previously
been reported that SIRT1 mediates the deacetylation of certain
targets, including PGC-1α, FOXO3a and p53, in mitochondrial
biogenesis, lipid metabolism and inflammation (33). The results of the present study
suggest that RSV functions via SIRT1 in PA-induced INS-1E cells.
SIRT1 regulates a range of cellular functions affecting metabolic
homeostasis and the link between metabolism and INS secretion
depends on mitochondrial function (34). PGC-1α is a mitochondrial regulator
that serves an essential role in cellular energy metabolism and the
ectopic expression of PGC-1α induces the expression of NRFs and
mtTFA prior to mitochondrial biogenesis (35). The results of the present study
demonstrate that SIRT1 knockdown modulates NRFs and mtTFA levels in
cells treated with PA and RSV. These data suggest that RSV
functions via SIRT1 to modulate mitochondrial biogenesis.
It has been suggested that INS resistance occurs
following disturbances in lipid metabolism (36). Therefore, the current study
investigated whether RSV affects lipid metabolism. It was
demonstrated that SIRT1 knockdown altered the effect of RSV on ACC,
CPT-1 and LCDA expression. A previous study reported that SIRT1
suppresses the expression of ACC to reduce lipogenesis and
upregulates CPT1 and LCAD to increase normal β-oxidation activities
(37). Furthermore, it was
demonstrated that RSV modulates ACC, CPT-1 and LCDA activity in
lipid metabolism via SIRT1, suggesting a novel link between RSV,
T2DM and lipid metabolism. PDX-1, MAFA and INS contribute to
pancreas development, β-cells differentiation and the maintenance
of mature β-cells function (38). In
the present study, SIRT1 knockdown regulated levels of PDX-1, MAFA
and INS mRNA. These results suggest that RSV may affect β-cells
functionality via SIRT1.
In conclusion, the results of the present study
suggest that RSV modulates the expression of SIRT1, PGC-1α and
FOXO3a in a rat model of STZ-injected DM. In vitro, RSV
significantly affected levels of PGC-1α and FOXO3a via SIRT1. RSV
promoted mitochondrial biogenesis to stimulate INS secretion and
enhanced fatty acid β-oxidation activity to reduce ectopic lipid
deposition and promote INS expression. The present study provides
an insight into the mechanism by which RSV prevents T2DM via SIRT1
and provides evidence that RSV may be used as a clinical treatment
to prevent the development of T2DM.
Acknowledgements
The present study was supported by the Research Fund
for Clinical Medicine, Chinese Medical Association (grant no.
13050770462), the Heilongjiang Postdoctoral Science Foundation
(grant no. LBH-Z15159), the Health Department of Heilongjiang
Province (grant no. 2016–022), the Scientific Foundation of the
First Affiliated Hospital of Harbin Medical University (grant nos.
2016B008 and 2017B001), the Technology Innovation Program of Harbin
City (grant no. 2016RAQXJ167), the National Natural Science
Foundation of China (grant no. 81370929)) and the Innovative
Science Research Project of Harbin Medical University (grant nos.
2016LCZX40 and 2016LCZX51).
Glossary
Abbreviations
Abbreviations:
|
T2DM
|
type 2 diabetes mellitus
|
|
NRF
|
nuclear respiratory factor
|
|
mtTFA
|
mitochondrial transcription factor
A
|
|
CPT-1
|
lipid metabolism-related carnitine
palmitoyltransferase 1
|
|
ACC
|
acetyl-CoA carboxylase
|
|
LCAD
|
long-chain acyl-CoA dehydrogenase
|
|
PDX-1
|
pancreatic duodenal homeobox-1
gene
|
|
MAFA
|
mid-arm fat area
|
|
INS
|
insulin
|
|
RSV
|
resveratrol
|
|
STZ
|
streptozotocin
|
|
INS-1E
|
insulinoma cell line clone 1E
|
|
SIRT1
|
Sirtuin type 1
|
|
PGC-1α
|
peroxisome proliferator-activated
receptor-γ coactivator-1α
|
|
FOXO
|
forkhead box O
|
|
PA
|
palmitic acid
|
References
|
1
|
Franz MJ, Boucher JL, Rutten-Ramos S and
VanWormer JJ: Lifestyle weight-loss intervention outcomes in
overweight and obese adults with type 2 diabetes: A systematic
review and meta-analysis of randomized clinical trials. J Acad Nutr
Diet. 115:1447–1463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galling B, Roldan A, Nielsen RE, Nielsen
J, Gerhard T, Carbon M, Stubbs B, Vancampfort D, De Hert M, Olfson
M, et al: Type 2 diabetes mellitus in youth exposed to
antipsychotics: A systematic review and meta-analysis. JAMA
Psychiatry. 73:247–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dart AB, Martens PJ, Rigatto C, Brownell
MD, Dean HJ and Sellers EA: Earlier onset of complications in youth
with type 2 diabetes. Diabetes Care. 37:436–443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liberopoulos EN, Tsouli S, Mikhailidis DP
and Elisaf MS: Preventing type 2 diabetes in high risk patients: An
overview of lifestyle and pharmacological measures. Curr Drug
Targets. 7:211–228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lotfy M, Adeghate J, Kalasz H, Singh J and
Adeghate E: Chronic complications of diabetes mellitus: A mini
review. Curr Diabetes Rev. 13:3–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saisho Y: β-cell dysfunction: Its critical
role in prevention and management of type 2 diabetes. World J
Diabetes. 6:109–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lomonaco R, Bril F, Portillo-Sanchez P,
Ortiz-Lopez C, Orsak B, Biernacki D, Lo M, Suman A, Weber MH and
Cusi K: Metabolic impact of nonalcoholic steatohepatitis in obese
patients with type 2 diabetes. Diabetes Care. 39:632–638. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin YL, Lai YH, Wang CH, Kuo CH, Liou HH
and Hsu BG: Triceps skinfold thickness is associated with lumbar
bone mineral density in peritoneal dialysis patients. Ther Apher
Dial. 21:102–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morino K, Petersen KF, Dufour S, Befroy D,
Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, et al:
Reduced mitochondrial density and increased IRS-1 serine
phosphorylation in muscle of insulin-resistant offspring of type 2
diabetic parents. J Clin Invest. 115:3587–3593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hausenblas HA, Schoulda JA and Smoliga JM:
Resveratrol treatment as an adjunct to pharmacological management
in type 2 diabetes mellitus-systematic review and meta-analysis.
Mol Nutr Food Res. 59:147–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wicklow B, Wittmeier K, T' Jong GW,
McGavock J, Robert M, Duhamel T and Dolinsky VW: Proposed trial:
Safety and efficacy of resveratrol for the treatment of
non-alcoholic fatty liver disease (NAFLD) and associated insulin
resistance in adolescents who are overweight or obese
adolescents-rationale and protocol. Biochem Cell Biol. 93:522–530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szkudelski T and Szkudelska K: Resveratrol
and diabetes: From animal to human studies. Biochim Biophys Acta.
1852:1145–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guarente L and Picard F: Calorie
restriction-the SIR2 connection. Cell. 120:473–482. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McBurney MW, Clark-Knowles KV, Caron AZ
and Gray DA: SIRT1 is a highly networked protein that mediates the
adaptation to chronic physiological Stress. Genes Cancer.
4:125–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song R, Xu W, Chen Y, Li Z, Zeng Y and Fu
Y: The expression of Sirtuins 1 and 4 in peripheral blood
leukocytes from patients with type 2 diabetes. Eur J Histochem.
55:e102011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han J, Wei M, Wang Q, Li X, Zhu C, Mao Y,
Wei L, Sun Y and Jia W: Association of genetic variants of SIRT1
with type 2 diabetes mellitus. Gene Expr. 16:177–185. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kitada M and Koya D: SIRT1 in type 2
diabetes: Mechanisms and therapeutic potential. Diabetes Metab J.
37:315–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lovis P, Gattesco S and Regazzi R:
Regulation of the expression of components of the exocytotic
machinery of insulin-secreting cells by microRNAs. Biol Chem.
389:305–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nathan DM, Buse JB, Davidson MB, Heine RJ,
Holman RR, Sherwin R and Zinman B: Professional Practice Committee,
American Diabetes Association; European Association for the Study
of Diabetes: Management of hyperglycaemia in type 2 diabetes: A
consensus algorithm for the initiation and adjustment of therapy. A
consensus statement from the American Diabetes Association and the
European Association for the Study of Diabetes. Diabetologia.
49:1711–1721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banin RM, Hirata BK, Andrade IS, Zemdegs
JC, Clemente AP, Dornellas AP, Boldarine VT, Estadella D,
Albuquerque KT, Oyama LM, et al: Beneficial effects of Ginkgo
biloba extract on insulin signaling cascade, dyslipidemia, and body
adiposity of diet-induced obese rats. Braz J Med Biol Res.
47:780–788. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tangvarasittichai S: Oxidative stress,
insulin resistance, dyslipidemia and type 2 diabetes mellitus.
World J Diabetes. 6:456–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marangoni MN, Brady ST, Chowdhury SA and
Piano MR: The co-occurrence of myocardial dysfunction and
peripheral insensate neuropathy in a streptozotocin-induced rat
model of diabetes. Cardiovasc Diabetol. 13:112014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goh KP, Lee HY, Lau DP, Supaat W, Chan YH
and Koh AF: Effects of resveratrol in patients with type 2 diabetes
mellitus on skeletal muscle SIRT1 expression and energy
expenditure. Int J Sport Nutr Exerc Metab. 24:2–13. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang XZ, Wen D, Zhang M, Xie Q, Ma L,
Guan Y, Ren Y, Chen J and Hao CM: Sirt1 activation ameliorates
renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J Cell
Biochem. 115:996–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Higashida K, Kim SH, Jung SR, Asaka M,
Holloszy JO and Han DH: Effects of resveratrol and SIRT1 on PGC-1α
activity and mitochondrial biogenesis: A reevaluation. PLoS Biol.
11:e10016032013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song X, Yang B, Qiu F, Jia M and Fu G:
High glucose and free fatty acids induce endothelial progenitor
cell senescence via PGC-1α/SIRT1 signaling pathway. Cell Biol Int.
41:1146–1159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang HH, Ma XJ, Wu LN, Zhao YY, Zhang PY,
Zhang YH, Shao MW, Liu F, Li F and Qin GJ: SIRT1 attenuates high
glucose-induced insulin resistance via reducing mitochondrial
dysfunction in skeletal muscle cells. Exp Biol Med (Maywood).
240:557–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brunet A, Sweeney LB, Sturgill JF, Chua
KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et
al: Stress-dependent regulation of FOXO transcription factors by
the SIRT1 deacetylase. Science. 303:2011–2015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Sheng M, Liu Y, Wang P, Chen Y,
Chen L, Wang W and Li B: Expression of SIRT1 and oxidative stress
in diabetic dry eye. Int J Clin Exp Pathol. 8:7644–7653.
2015.PubMed/NCBI
|
|
31
|
Tan C, Voss U, Svensson S, Erlinge D and
Olde B: High glucose and free fatty acids induce beta cell
apoptosis via autocrine effects of ADP acting on the P2Y(13)
receptor. Purinergic Signal. 9:67–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Plotz T, Hartmann M, Lenzen S and Elsner
M: The role of lipid droplet formation in the protection of
unsaturated fatty acids against palmitic acid induced lipotoxicity
to rat insulin-producing cells. Nutr Metab (Lond). 13:162016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghinis-Hozumi Y, González-Dávalos L,
Antaramian A, Villarroya F, Piña E, Shimada A, Varela-Echavarría A
and Mora O: Effect of resveratrol and lipoic acid on
sirtuin-regulated expression of metabolic genes in bovine liver and
muscle slice cultures. J Anim Sci. 93:3820–3831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wiederkehr A and Wollheim CB:
Mitochondrial signals drive insulin secretion in the pancreatic
β-cell. Mol Cell Endocrinol. 353:128–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen CH, Nagayama K, Enomoto N, Miyasaka
Y, Kurosaki M, Sakamoto N, Maekawa S, Kakinuma S, Ikeda T, Izumi N,
et al: Enhancement of mitochondrial gene expression in the liver of
primary biliary cirrhosis. Hepatol Res. 31:24–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen S, Zhao X, Ran L, Wan J, Wang X, Qin
Y, Shu F, Gao Y, Yuan L, Zhang Q and Mi M: Resveratrol improves
insulin resistance, glucose and lipid metabolism in patients with
non-alcoholic fatty liver disease: A randomized controlled trial.
Dig Liver Dis. 47:226–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen LL, Zhang HH, Zheng J, Hu X, Kong W,
Hu D, Wang SX and Zhang P: Resveratrol attenuates high-fat
diet-induced insulin resistance by influencing skeletal muscle
lipid transport and subsarcolemmal mitochondrial β-oxidation.
Metabolism. 60:1598–1609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaneto H, Miyatsuka T, Kawamori D,
Yamamoto K, Kato K, Shiraiwa T, Katakami N, Yamasaki Y, Matsuhisa M
and Matsuoka TA: PDX-1 and MafA play a crucial role in pancreatic
beta-cell differentiation and maintenance of mature beta-cell
function. Endocr J. 55:235–252. 2008. View Article : Google Scholar : PubMed/NCBI
|