Introduction
Orthopedic infections, which are a major
complication of medical implant surgery, can have serious
implications on patient health. Orthopedic infections can cause
delayed recovery, chronic osteomyelitis complications or even
failure of surgery (1). Orthopedic
infections are primarily caused by Staphylococcus aureus and
Streptococcus pyogenes (2). The
severity of S. aureus orthopedic infection is directly associated
with the toxins that it generates. S. aureus bacteria can produce a
variety of toxins, including bowel poison element, toxic shock
toxin, coagulase proteases and haemolysin (3), and has toxin a specific pathogenicity.
S. aureus can also express adhesive molecules, which allow
bacterial adhesion to host cells or material surfaces. This
facilitates the formation of biofilms that makes it increasingly
difficult to treat infections in medical implants (4).
According to the World Health Organization, the
prevalence of joint disease in people >55 years old is 80%, and
joint disease, including arthritis and femoral head necrosis, are
an important public health issue (5). Artificial joint replacement, which
began in the 20th century, is one of the most successful orthopedic
surgeries for the treatment of severe joint diseases. Joint
replacement can help relieve joint pain and restore joint function.
However, the most serious complication following artificial joint
replacement is infection around the implant (6). As cases of artificial joint replacement
increase each year, so does the incidence of infections around the
implant. The use of antibiotics to control surgical infections has
significant clinical efficacy. However, bacterial drug resistance
is becoming increasingly common, making it more difficult to treat
infections. Therefore, there is an urgent requirement to identify
novel methods to treat such infections. Bacteriocins, which are
secreted by lactic acid bacteria, are small molecule peptides that
exhibit a unique antibacterial mechanism that bacteria rarely
develop resistance to (7).
The present study aimed to isolate broad-range
bacteriocins from Lactobacillus rhamnosus (ATCC 53103) and
investigate their antibacterial effect on S. aureus in a
rabbit model of knee implant infection. The serum concentrations of
C-reactive protein (CRP) and interleukin (IL)-6 were measured in
order to analyze the antibacterial effect of bacteriocins. The
results of the present study highlight a potential novel method for
the treatment of knee implant infections in vivo.
Materials and methods
Strains and culture conditions
L. rhamnosus (ATCC 53103) was purchased from the
American Type Culture Collection (Manassas, VA, USA). The bacteria
were maintained on de Man, Rogosa and Sharpe (MRS) agar plates
(Difco; BD Biosciences, Franklin Lakes, NJ, USA) and cultured
overnight in MRS broth (Difco; BD Biosciences) at 20°C with gentle
agitation. S. aureus (ATCC 29213; American Type Culture
Collection) was used in the present study to screen for the
antibacterial activity of bacteriocins from L. rhamnosus.
Cultures of S. aureus were grown overnight in brain-heart
infusion broth (Difco; BD Biosciences) at 37°C, centrifuged (3,000
× g at 4°C for 5 min), and the final concentrations of the cultures
were adjusted to match the turbidity of a McFarland 0.5 standard
(0.5×105 CFU/ml) using a spectrophotometer (Densimat;
BioMérieux UK Ltd., Basingstoke, Hampshire, UK).
Production of crude bacteriocins
Crude bacteriocin preparations were prepared as
previously described (8). Briefly,
L. rhamnosus cultures were initially grown in MRS broth at
37°C for 24 h. The cultures were then centrifuged (7,000 × g at 4°C
for 10 min) and cell-free supernatants were then aspirated. The pH
of the supernatants was adjusted to 6.5, treated with catalase (5
mg/ml) and filtered through 0.22 µm pore size filters (EMD
Millipore, Billerica, MA, USA).
Purification of bacteriocins
Crude bacteriocin preparations were saturated with
70% ammonium sulphate at 4°C to precipitate the proteins. The
pellets were recovered by centrifugation at 10,000 × g at 4°C for
30 min, vacuum-dried and dissolved in 1 ml deionized water. The 10
ml suspensions were then applied to Sephadex G-100 columns (1.6×36
cm) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) that were
pre-equilibrated with a phosphate buffer (pH 7.0), as previously
described (9). The flow rate was
adjusted to 24 ml/h, and the proteins were collected and adjusted
to a total protein concentration of 2.5 mg/ml. The protein
suspension was stored at −70°C until required. A bacteriocins
suspension (10 µl; 1 mg/ml) was loaded into a 12.5%
Tricine-SDS-PAGE and the molecular weight of the bacteriocins was
determined according to the methods of Biosa G (10). A total of 10 µl of the low molecular
weight (10–120 kDa) BenchMark marker (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used as a standard.
Animals
A total of 12 New Zealand White female rabbits
provided by the Zhejiang Province Academy of Medical Sciences
(Hangzhou, China) weighing 2.5–3.0 kg and aged 70–100 days old were
used in the present study. Animals were housed in individual cages
in a temperature-controlled room (23°C) with a 12 h light/dark
cycle. A total of 6 rabbits were used for validation of the
infection model, while the other 6 were assessed as part of the
control group. All animals were handled in strict accordance with
good animal practice as defined by the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (11) and all animal work was approved by the
College of Medicine of Ningbo University Chancellor's Animal
Research Committee (Yuyao, China).
Model validation
The right knee of each rabbit was replaced with a
tibial component using a Silastic implant (Dow Corning, Midland,
MI, USA). The surgery was performed as described previously
(12). Briefly, the rabbits were
anesthetized via inhalation of 2% isoflurane, which was maintained
by the intramuscular injection of ketamine (25 mg/kg) followed by
continuous inhalation of 1% isoflurane. The right leg of each
rabbit was shaved 24 h prior to the surgery and the skin was
cleaned with an iodine solution just before surgery. A longitudinal
skin incision was made to expose the knee. The epiphyseal plates
were removed following dislocation of the tibia. The metaphysis was
exposed and the cancellous bone of the medullary cavity of the
proximal metaphysis was reamed. The stem of the nail-shaped
silicone implant (14 mm long) was inserted into the intramedullary
canal of the tibia, such that the implant head (15×5 mm) replaced
the tibial plateau. The deep fascia and skin were finally closed to
complete the procedure.
Immediately after the surgical wound was closed, the
12 rabbits were infected by the intra-articular injection of 0 5 ml
inoculum containing 3×105 CFU/ml S. aureus. Then,
6 rabbits were injected with 1 ml of the bacteriocin suspension 4 h
after the injection of S. aureus, and the other 6 rabbits
were injected with 1 ml sterile saline solution.
Detection of biofilm formation on the
implants
To test for biofilm formation in the rabbit model
used in the present study, the implants were harvested from
euthanized rabbits 5 days after surgery. The specimens were fixed
with 2.5% glutaraldehyde at 4°C for 1 h. Following fixation, the
tissues were stained with 0.01% acridine orange solution at 4°C for
2 h (Sigma-Aldrich; Merck KGaA), as described previously (13), and washed three times with PBS. The
50-µm-thick sections were then observed under an Eclipse 80i
microscope (Nikon Corporation, Tokyo, Japan) equipped with an argon
laser at an excitation wavelength of 488 nm. All images were
quantified using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc., Rockville, MD, USA). The fluorescence
intensities of the biofilms were expressed as integrated optical
density (IOD) values, which were calculated by the multiplications
of the area and the density of the biofilm (14).
Determination of the serum levels of
inflammatory cytokines
Blood samples were collected at 1, 2, 3, 4 and 5
days after the injection of bacteriocin or saline. The samples were
centrifuged at 7,000 × g at 4°C for 5 min and then stored at −70°C
until required. CRP and IL-6 levels were measured using commercial
ELISA kits for CRP (cat. no. E-EL-RB0005) and IL-6 (cat. no.
E-EL-RB0014; both Elabscience Biotechnology Co., Ltd., Wuhan,
China), respectively, according to the manufacturer's protocol All
measurements were performed in triplicate. The levels of CRP and
IL-6 were expressed as mg/ml protein.
Statistical analysis
All tests were repeated three times. The results are
presented as the mean ± standard deviation. Data analysis was
performed using SPSS software (version 14.0; SPSS, Inc., Chicago,
IL, USA). The results between two groups were compared using a
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Biofilm formation is decreased on
implants after bacteriocin treatment
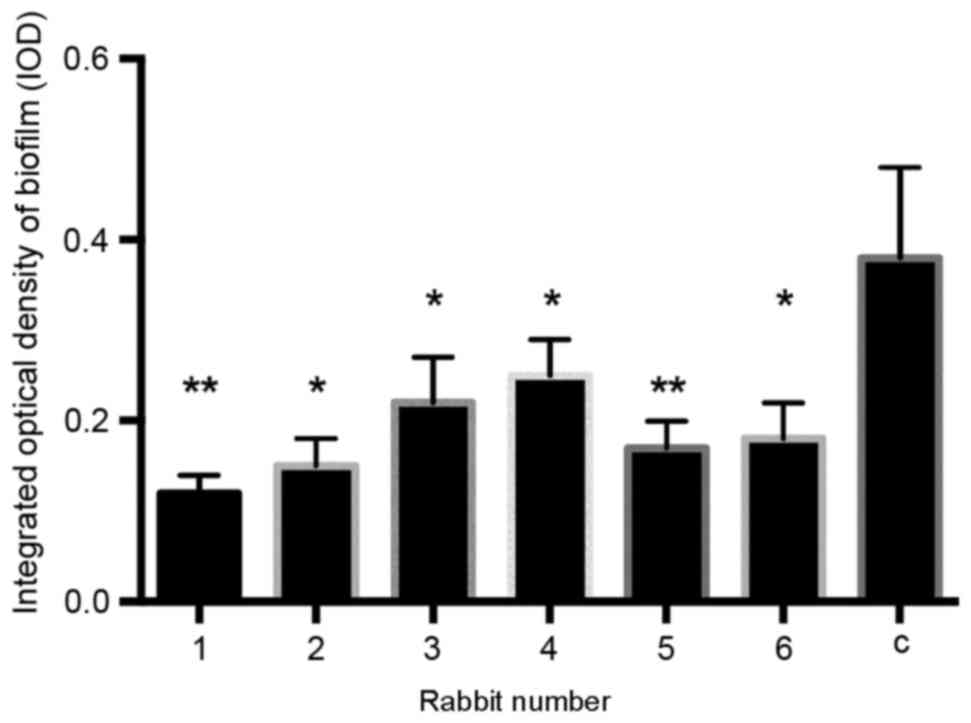
The IOD values of the biofilms the 6 infected
rabbits revealed that there was a significant decrease in biofilm
formation compared with the control group 5 days after surgery and
infection (P<0.01; Fig. 1).
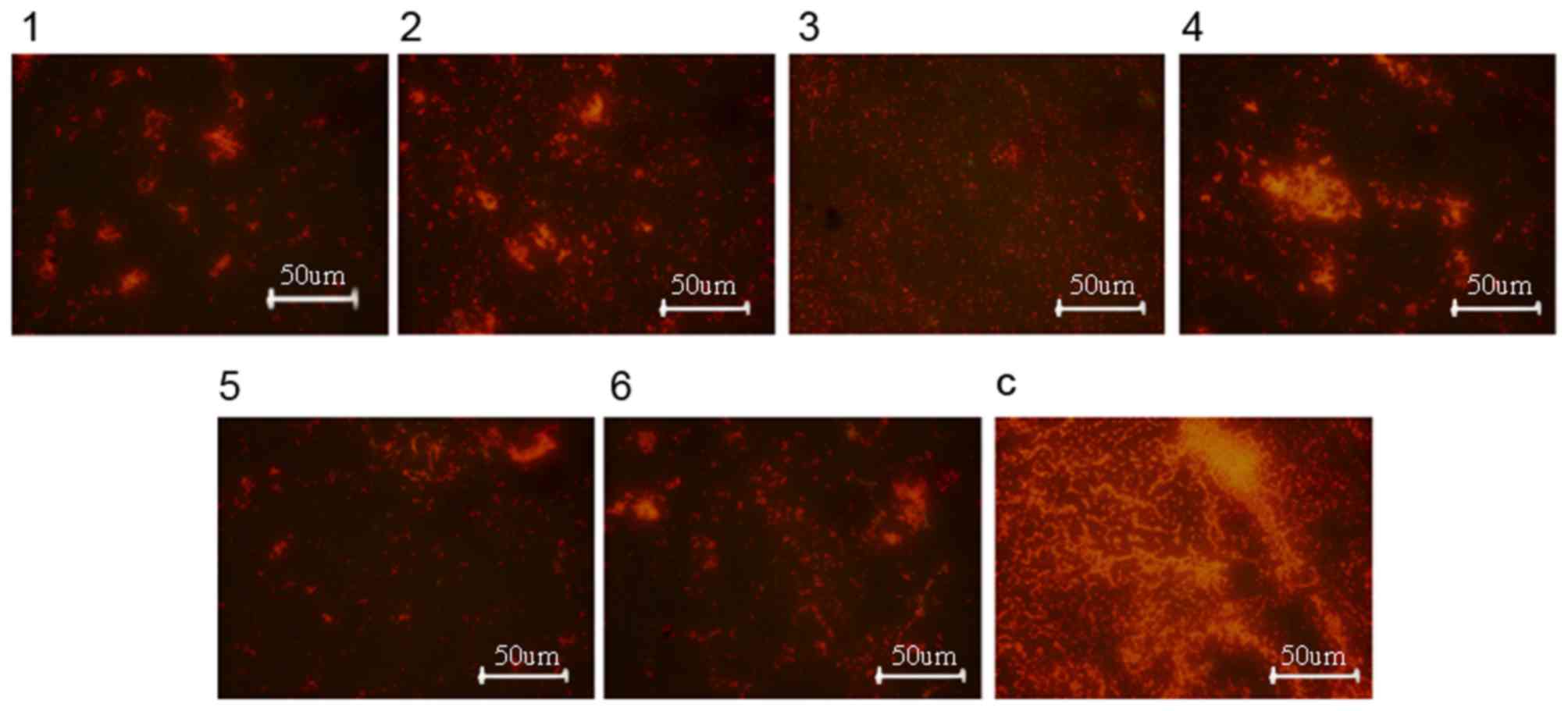
Fluorescence microscopy of the biofilms revealed that the density
of bacteria in the rabbits injected with the bacteriocin suspension
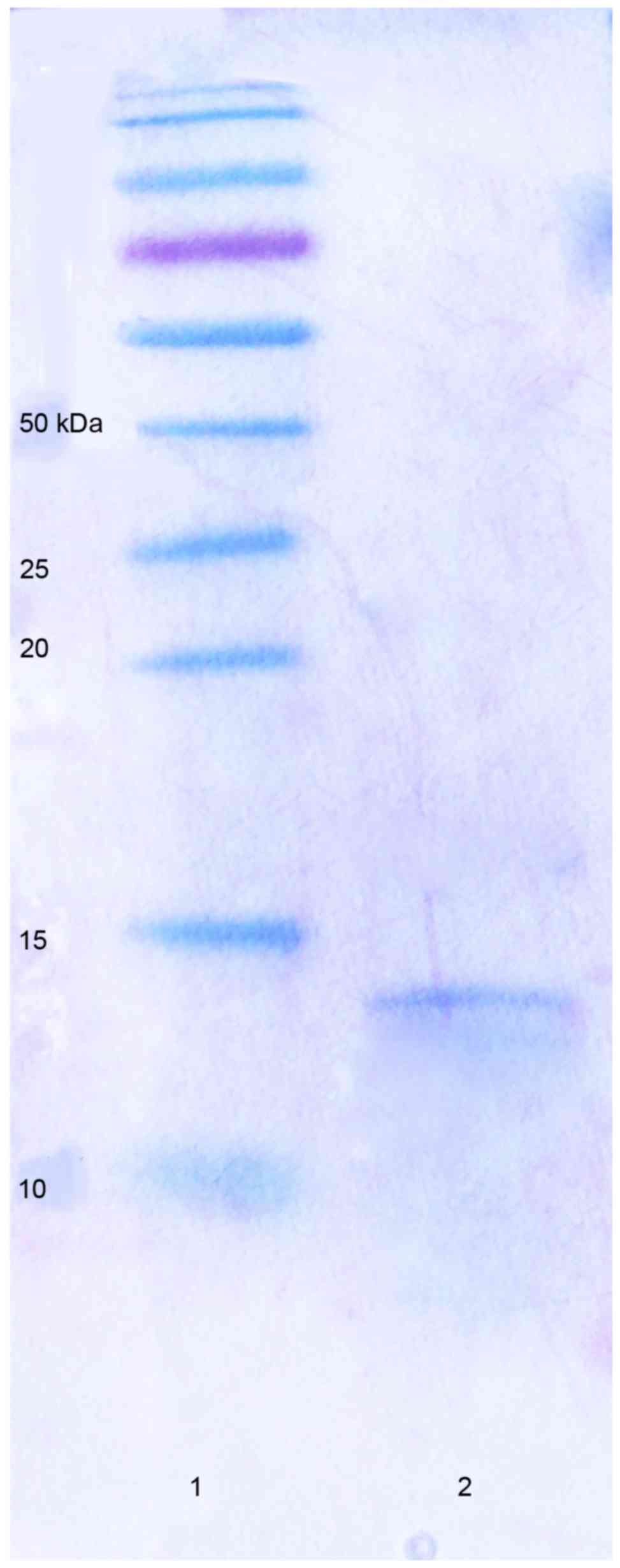
was markedly lower than that of the control group (Fig. 2). The Tricine-SDS-PAGE of the
bacteriocin suspension is presented in Fig. 3. The molecular weight of the
bacteriocin was found to be 10–15 kDa.
Bacteriocin treatment attenuates S.
aureus infection-induced inflammation
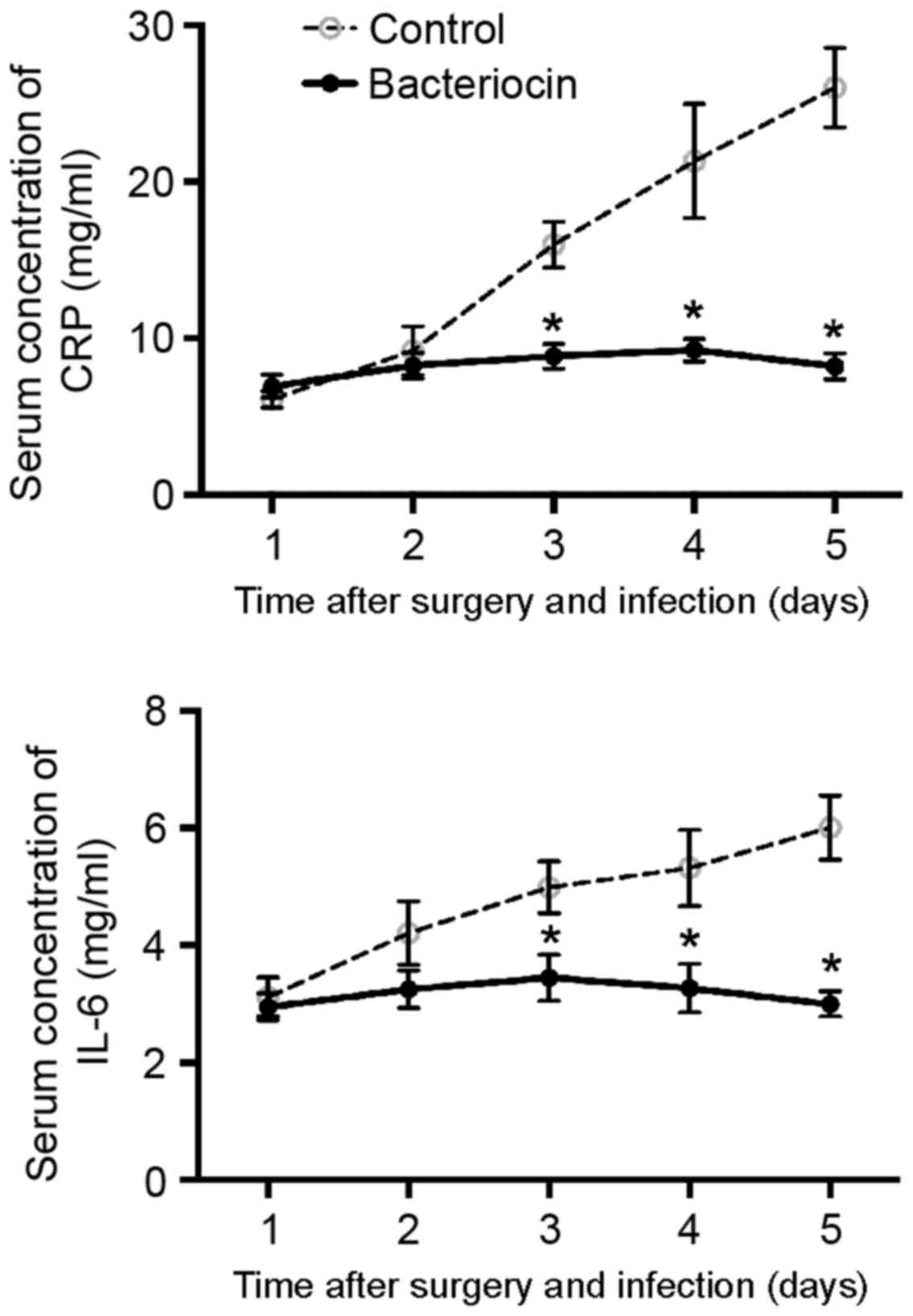
Due to the infection with S. aureus following
the surgery, the levels of CRP and IL-6 in the control group
increased, reaching maximum levels at 5 days post-surgery (Fig. 4). However, the levels of CRP and IL-6
in the rabbits treated with bacteriocin remained at a steady low
level, and were significantly lower than the levels in the control
group at days 3, 4 and 4 post-surgery (all P<0.05; Fig. 4).
Discussion
S. aureus is a pathogenic bacterium that is
the cause of numerous infections (15). As one of the most important pathogens
in orthopaedic infections (16), the
treatment of S. aureus is of the utmost importance. However,
the treatment of S. aureus infections may be complicated due
to antibiotic resistance and/or biofilm formation (17). Traditional antimicrobial agents
frequently lead to drug resistance and are not effective in
removing biofilms (18). Therefore,
there is an urgent requirement to identfy for novel antibacterial
agents.
The ability of lactic acid bacteria to adjust the
balance of intestinal microflora, enhance immunity and resistance,
and promote the growth and development of the gut has been widely
applied in clinical settings (19).
Bacteriocins are a class of antibacterial peptides secreted by
lactic acid bacteria (20), the
majority of which are water-soluble. Bacteriocins exhibit marked
antibacterial effects against numerous gram-positive bacteria
(21). In the present study,
bacteriocins were identified to possess significant inhibitory
effects on S. aureus biofilm formation, which is consistent
with previous results from similar studies (22).
CRP is an acute phase reaction protein that is
indicative of septicemia (23). CRP
levels are raised during acute infection, tissue damage, the
presence or a malignant tumor and myocardial infarction (23). CRP levels can quickly drop when the
patient recovers. CRP is thus a sensitive index in the acute phase
of disease and facilitates the early diagnosis of infection. The
liver synthesizes increased levels of CRP upon inflammation and
tissue damage, which are alleviated by the inflammatory response
(24). Therefore, the detection of
serum CRP is clinically important in order to understand and treat
inflammation. IL-6 is a proinflammatory factor that is produced by
several types of cells in the body (25). IL-6 has a variety of biological
activities, including regulation of the immune response under
normal conditions and causing immune damage in pathological
conditions (26). A previous in
vivo study indicated that CRP levels are positively correlated
with IL-6 levels, and that CRP can increase IL-6 production
(27). The present study identified
that bacteriocins attenuated the typical increase in CRP and IL-6
levels following infection, indicating that bacteriocins are a
potential agent for the control of infection following orthopedic
surgery.
In the present study, the standard laboratory strain
of S. aureus was used. However, in clinical settings
infections following orthopedic surgery tend to be caused by
drug-resistant S. aureus (28). The infection produced in the rabbit
model may be different to human infections. The results of the
present study may therefore have a number of limitations and
further research is required in order to investigate the effects of
bacteriocins on drug-resistant S. aureus.
In conclusion, the present study demonstrated the
antibacterial effect of bacteriocins in the rabbit model of S.
aureus infection following knee replacement. This results
indicates that bacteriocins may be a potentially agent to control
infection following orthopedic surgery.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Berthelot P, Grattard F, Cazorla C, Passot
JP, Fayard JP, Meley R, Bejuy J, Farizon F, Pozzetto B and Lucht F:
Is nasal carriage of Staphylococcus aureus the main acquisition
pathway for surgical-site infection in orthopaedic surgery? Europ J
Clin Microbiol. 4:373–382. 2001.
|
|
2
|
Malawski SK and Lukawski S: Pyogenic
infection of the spine. Clin Orthop Relat Res. 1–66. 1991.
|
|
3
|
Hennekinne JA, De Buyser ML and Dragacci
S: Staphylococcus aureus and its food poisoning toxins:
Characterization and outbreak investigation. FEMS Microbiol Rev.
36:815–36. 2012. View Article : Google Scholar
|
|
4
|
Kusunoki T, Hailman E, Juan TS,
Lichenstein HS and Wright SD: Molecules from Staphylococcus aureus
that bind CD14 and stimulate innate immune responses. J Exp Med.
182:1673–1682. 1995. View Article : Google Scholar
|
|
5
|
Tennant A, Fear J, Pickering A, Hillman M,
Cutts A and Chamberlain MA: Prevalence of knee problems in the
population aged 55 years and over: Identifying the need for knee
arthroplasty. BMJ Clin Res. 310:1291–1293. 1995. View Article : Google Scholar
|
|
6
|
Zimmerli W and Ochsner PE: Management of
infection associated with prosthetic joints. Infection. 31:99–108.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nes IF, Diep DB, Håvarstein LS, Brurberg
MB, Eijsink V and Holo H: Biosynthesis of bacteriocins in lactic
acid bacteria. Antonie Van Leeuwenhoek. 70:113–128. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Janes ME, Nannapaneni R and Johnson MG:
Identification and characterization of two bacteriocin-producing
bacteria isolated from garlic and ginger root. J Food Prot.
62:899–904. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Ji J, Chen X, Jiang M, Rui X and
Dong M: Structural elucidation and antioxidant activities of
exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohyd
Polym. 102:351–359. 2014. View Article : Google Scholar
|
|
10
|
Biosa G, Addis MF, Tanca A, Pisanu S,
Roggio T, Uzzau S and Pagnozzi D: Comparison of blood serum peptide
enrichment methods by Tricine SDS-PAGE and mass spectrometry. J
Proteom. 75:93–99. 2011. View Article : Google Scholar
|
|
11
|
Ortiz-Butron R, Pacheco-Rosado J,
Hernández-Garcia A, Briones-Velasco M and Rocha L: Mild thyroid
hormones deficiency modifies benzodiazepine and mu-opioid receptor
binding in rats. Neuropharmacology. 44:111–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Research C, . Guide for the care
and use of laboratory animals. National Academies Press; 2010,
PubMed/NCBI
|
|
13
|
Slauterbeck J, Clevenger C, Lundberg W and
Burchfield DM: Estrogen level alters the failure load of the rabbit
anterior cruciate ligament. J Ortho Res. 17:405–408. 1999.
View Article : Google Scholar
|
|
14
|
Jackson S, Coulthwaite L, Loewy Z, Scallan
A and Verran J: Biofilm development by blastospores and hyphae of
Candida albicans on abraded denture acrylic resin surfaces. J
Prosth Dent. 112:988–993. 2014. View Article : Google Scholar
|
|
15
|
Wang YC, Yo YT, Lee HY, Liao YP, Chao TK,
Su PH and Lai HC: ALDH1-bright epithelial ovarian cancer cells are
associated with CD44 expression, drug resistance, and poor clinical
outcome. Am J Patho. 180:1159–1169. 2012. View Article : Google Scholar
|
|
16
|
Costerton JW, Cheng KJ, Geesey GG, Ladd
TI, Nickel JC, Dasgupta M and Marrie TJ: Bacterial biofilms in
nature and disease. Ann Rev Microbiol. 41:435–464. 1987. View Article : Google Scholar
|
|
17
|
Uçkay I, Hoffmeyer P, Lew D and Pittet D:
Prevention of surgical site infections in orthopaedic surgery and
bone trauma: State-of-the-art update. J Hosp Infect. 84:5–12. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davies D: Understanding biofilm resistance
to antibacterial agents. Nat Rev Drug Discov. 2:114–123. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong K, Wang BJ, Liu M, Jiang KY, Qiu CW,
Luo ZY, Fan RY and Wang L: The influence of lactic acid bacteria
and metabolites on intestinal microflora and nonspecific immunity
of juvenile sea cucumber (Apostichopus japonicus). Mar Sci.
37:7–12. 2013.
|
|
20
|
Klaenhammer TR: Genetics of bacteriocins
produced by lactic acid bacteria. FEMS Microbiol Rev. 12:39–85.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reid G, Jass J, Sebulsky MT and Mccormick
JK: Potential uses of probiotics in clinical practice. Clin
Microbiol Rev. 16:658–672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zaghian S, Emtiazi G and Shokri D: A
bacteriocin with broad antimicrobial activity produced by newly
isolated nitrogen-fixing bacillus strains. J Isfahan Med Sch.
30:2260–2269. 2013.
|
|
23
|
Macintyre SS, Schultz D and Kushner I:
Biosynthesis of C-reactive protein. Ann NY Acad Sci. 389:76–87.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilson AM, Ryan MC and Boyle AJ: The novel
role of C-reactive protein in cardiovascular disease: Risk marker
or pathogen. Int J Cardiol. 106:291–297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li YP and Stashenko P: Proinflammatory
cytokines tumor necrosis factor-alpha and IL-6, but not IL-1,
down-regulate the osteocalcin gene promoter. J Immun. 148:788–794.
1992.PubMed/NCBI
|
|
26
|
Moore TC, Cody L, Kumm PM, Brown DM and
Petro TM: IRF3 helps control acute TMEV infection through IL-6
expression but contributes to acute hippocampus damage following
TMEV infection. Virus Res. 178:226–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saiki R, Hayashi D, Ikuo Y, Nishimura K,
Ishii I, Kobayashi K, Kan C, Toida T, Kashiwagi K and Igarashi K:
Acrolein stimulates the synthesis of IL-6 and C-reactive protein
(CRP) in thrombosis model mice and cultured cells. J Neurochem.
127:652–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Campoccia D, Montanaro L and Arciola CR:
The significance of infection related to orthopedic devices and
issues of antibiotic resistance. Biomater. 27:2331–2339. 2006.
View Article : Google Scholar
|