Introduction
It is predicted that chronic obstructive pulmonary
disease (COPD), one of the major causes of morbidity and mortality
worldwide, will be the third leading cause of death by 2030
(1). COPD is characterized by
progressive airflow obstruction and pulmonary inflammation,
typically the result of repeated exposure to cigarette smoke (CS)
and lipopolysaccharide (LPS), an important endotoxin of
gram-negative bacteria (2).
The pathophysiology of COPD involves the
infiltration of multiple inflammatory cells, including macrophages,
neutrophils and T lymphocytes (3).
Cytokines are the key orchestrators of chronic inflammatory
diseases and at least 50 cytokines have thus far been reported to
be associated with COPD (4).
Interleukin-1β (IL-1β) is a proinflammatory cytokine that is able
to activate macrophages in patients with COPD to release
inflammatory cytokines, chemokines and matrix metalloproteinase 9
(5). Tumor necrosis factor (TNF)-α
secreted by macrophages is an important cytokine in the innate
immune response, which protects against invading organisms prior to
activation of the adaptive immune system (6). Dysregulation of the TNF-α response is
associated with several inflammatory diseases (7). Transforming growth factor (TGF)-β is a
multifunctional growth factor that is generated from a latent
precursor via stress-activated responses (8). It facilitates the proliferation of
fibroblasts and airway smooth muscle cells, deposition of
extracellular matrix and epithelial repair (8). In addition, it has immune regulatory
actions that are mainly mediated by regulatory T (Treg) cells
(9). Treg cells suppress the immune
system partially via the secretion of IL-10 (9). A previous study indicated that an
increase in the IL-17A/IL-10 ratio in mice exposed to chronic CS
may be associated with immune self-tolerance in COPD (10).
Nuclear factor-κB (NF-κB) is an indispensable
protein transcription factor for the many important proinflammatory
molecules, including critical enzymes (cyclooxygenase-2 and
inducible nitric oxide synthase) and most cytokines (IL-1β, IL-6
and TNF-α) (6,11). NF-κB is activated when it is
phosphorylated by inhibitor of NF-κB (IκB) kinase and dissociates
from IκBα (6). NF-κB then
translocates to the nucleus for further gene transcription via
binding to the promoter of NF-κB-responsive genes.
Hedyotis diffusa Willd (HDW), a traditional
Chinese herb, contains many chemicals, including triterpenoids,
ferulic acid, sterols, iridoid glycosides, polypeptides,
flavonoids, ursolic acids, oleanolic acids and polysaccharides,
some of which have anti-inflammatory or antioxidative effects
(12). Due to its antibacterial
activity, it is extensively employed to treat inflammatory
diseases, including appendicitis and bronchitis (12). However, thus far there have been few
studies clearly demonstrating the anti-inflammatory mechanisms of
HDW in COPD. In the present study, it was demonstrated that HDW
exhibits a powerful anti-inflammatory ability by inhibiting the
NF-κB signaling pathway, indicating that HDW has the potential to
treat airway inflammation diseases.
Materials and methods
Plant material, extraction and
isolation, high performance liquid chromatography-mass spectrometry
(HPLC-MS) analysis
HDW was collected in Zhangshu (China) in September
2015 and identified by Professor Lai from the Medical College of
Nanchang University (Nanchang, China). A voucher specimen (no.
20150910A) was deposited at the Natural Product Laboratory of the
Medical Experiment Education Department, Medical College of
Nanchang University. Fresh HDW leaves and stems (2.5 kg) were cut
into very small pieces (~6 mm) and extracted with 25 l of 85%
ethanol using a refluxing and filtering method (13). The ethanol solvent of the filtrate
was evaporated using a rotary evaporator (Shanghai Yarong
Biochemistry Instrument Factory, Shanghai, China). The resultant
solution was concentrated to a relative density of 1.05 and a dry
powder was obtained using a spray drying method at 40°C for 2 h
(14).
The HDW powder was dissolved in dimethyl sulfoxide
(DMSO) and the final concentration of DMSO was adjusted to 0.1%
(v/v) in Roswell Park Memorial Institute 1640 culture medium
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). DMSO alone served as a control in all cases.
A Shimadzu (Kyoto, Japan) 30A series HPLC-MS
instrument was used for HPLC. Chromatographic separation was
performed on a Shim-pack Gist C18x 2 µm column (75×2.1
mm; Shimadzu) at 35°C using a column oven. Methanol and water with
0.05% acetic acid were used as the mobile phases. The injection
volume was 5 µl and the flow rate was 0.2 ml/min.
For HPLC-MS, the detector of an AB SCIEX
(Framingham, MA, USA) Triple TOF5600 instrument was set to an
electrospray ion source with negative polarity ionization. The
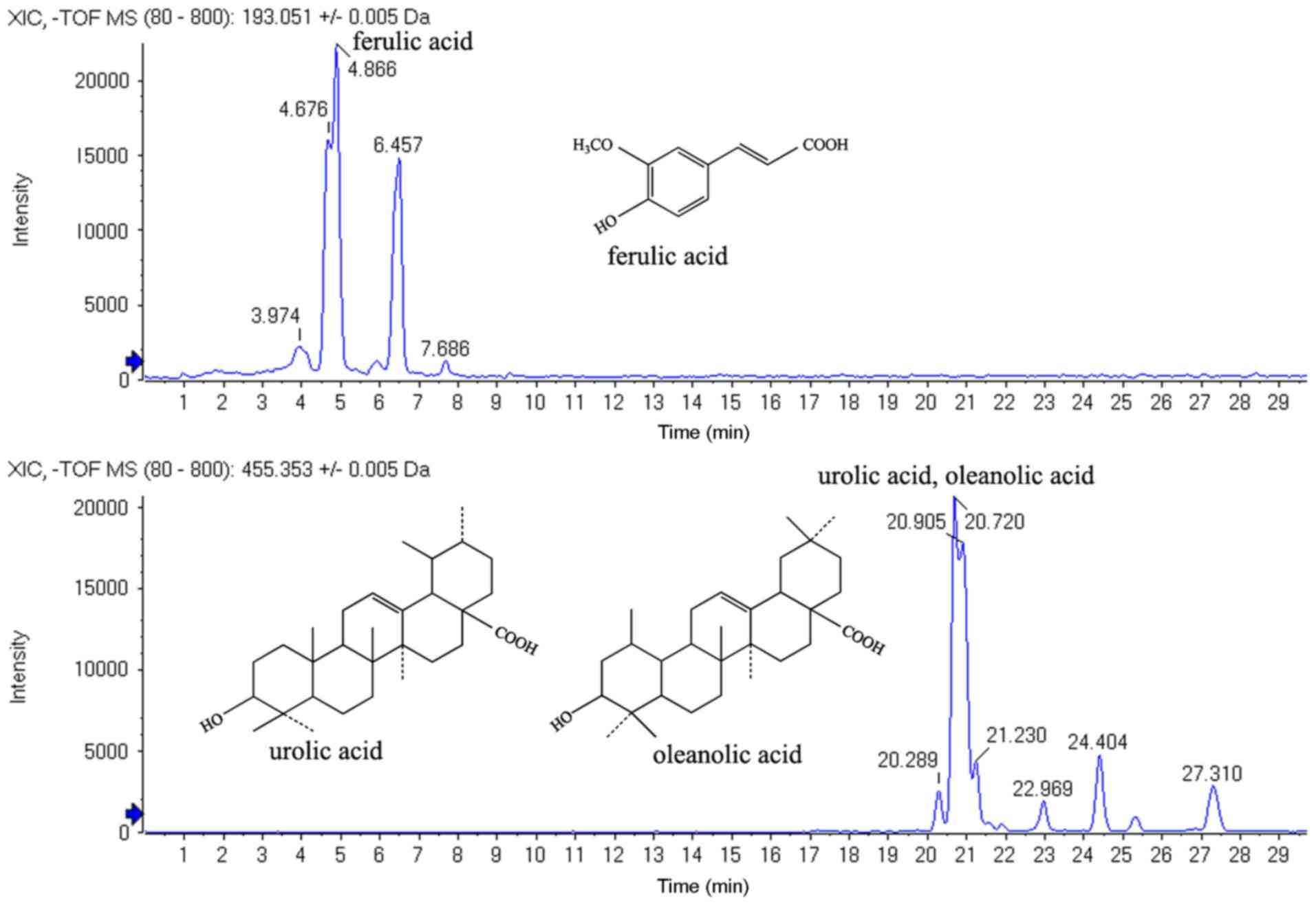
scanning range was m/z 80–800. The HPLC-MS results revealed
that the components of the ethanol fraction of HDW contained
ferulic, oleanolic and ursolic acids. The observed relative
retention time for oleanolic and ursolic acids was 20.77 min and
was 4.83 min for ferulic acids (Fig.
1).
Reagents
ELISA kits for IL-1β (cat. no. 432604), TNF-α (cat.
no. 430904), TGF-β (cat. no. 433007) and IL-10 (cat. no. 431414)
were obtained from Biolegend Systems (San Diego, CA, USA). The
ELISA kits were used according to the manufacturers' protocol.
Rabbit anti-mouse IgG primary antibodies against phosphorylated
(p)-IκBα at Ser 32 (cat. no. 2859), IκBα (cat. no. 4812), p65 (cat.
no. 8242), Lamin B (cat. no. 13435) and β-actin (cat. no. 4967)
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Secondary antibody (horseradish peroxidase-conjugated goat
anti-rabbit IgG; cat. no. sc-2004) was obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Dexamethasone was obtained
from Shanghai Xinyi Pharmaceutical Co., Ltd. (Shanghai, China) and
Lushan cigarettes were obtained from Jiangxi Tobacco (Nanchang,
China).
Culture and induction of BEAS-2B
cells
BEAS-2B cells obtained from the Center of Cells,
Suzhou University (Suzhou, China) were grown in Eagle's minimum
essential medium (Beijing Solarbio Science & Technology Co.,
Ltd.) supplemented with 10% fetal bovine serum (Beijing Transgen
Biotech Co., Ltd., Beijing, China), 2 mmol/l L-glutamine, 100
units/ml penicillin and 100 µg/ml streptomycin in a humidified
atmosphere of 5% CO2 at 37°C for 4 days.
The BEAS-2B cells were plated on a 35-mm dish
(5×103 cells/µl) and incubated overnight in complete
growth medium as aforementioned. The cells were incubated with 20,
10, 1 or 0.1 µmol/l HDW or DMSO for 1 h, followed by treatment with
LPS (10 µg/ml) or D-Hanks for 30 min (for the phosphorylation and
degradation of IκBα) or 24 h (for the level of nuclear NF-κB/p65),
respectively.
Western blotting
Nuclear and total proteins were collected from the
cultured cells using the Nuclear and Cytoplasmic Proteins
Extraction kit (Applygen Technologies Inc., Beijing, China)
according to the manufacturer's protocol. Supernatants were assayed
for protein content using a BCA Protein Assay kit (Beijing Solarbio
Science & Technology Co., Ltd.). Cell lysates (40 mg) were
separated by 10% SDS-PAGE and transferred onto nitrocellulose
acetate membranes. Following blocking with 5% nonfat dry milk
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) in TBS-T buffer (20
mM Tris base, 150 mM NaCl and 0.1% Tween-20) for 2 h at room
temperature. Membranes were blotted with primary antibodies in
TBS-T buffer (1:1,000; phospho-IκBα, IκBα, p65, Lamin B and
β-actin) overnight at 4°C and were subsequently incubated with
horseradish peroxidase-conjugated secondary goat IgG (1:2,500
dilution) for 1 h at room temperature. Bands were visualized using
an ECL Advance Western Blotting Detection kit (Tiangen Biotech Co.,
Ltd., Beijing, China) using a chemiluminescence detector (LAS 4000
mini; GE Healthcare Life Sciences, Little Chalfont, UK).
Cell proliferation assay
BEAS-2B cells were seeded in 96-well plates (100 µl;
5×103 cells/µl) and allowed to attach overnight prior to
treatment with HDW (0, 0.1, 1, 10 µmol/l) at 37°C for 24 h. Cell
Counting Kit-8 (CCK8; Beyotime Institute of Biotechnology, Haimen,
China) reagent was added into each well and incubated at 37°C for 2
h. The Optical density (OD) at 490 nm was recorded using a
microculture plate reader. The cell growth inhibition rate was
calculated as follows: (ODcontrol -
ODHDW)/ODcontrol ×100.
Animals and cigarette smoke-induced
pulmonary inflammation
A total of 75 adult male Kunming mice (age, 6–8
weeks; 18–20 g) were obtained from the Animal Center of Nanchang
University and housed at 24±1°C with 50% relative humidity under a
12 h light/dark cycle and were allowed free access to standard
mouse food and water in environmentally controlled pathogen-free
conditions during the experiments. All protocols were approved by
the Institutional Animal Experimental Ethics Committee of Nanchang
University.
The method used to establish the cigarette
smoke-induced COPD animal model was adapted from Chen et al
(2). Mice were randomly divided into
5 groups (each n=15): A saline-treated group, a cigarette
smoke+lipopolysaccharide (SL)-challenged group, an SL+HDW (50
mg/kg)-treated group, an SL+HDW (100 mg/kg)-treated group and a
dexamethasone-treated group. Except for the saline-treated group,
mice in other groups were challenged with CS in a 4 l homemade
Plexiglas container for 5 min/day for 30 days and were infused with
20 µg of LPS in 30 µl of normal saline on days 1 and 14. For the
SL+HDW (50 mg/kg)-treated, SL+HDW (100 mg/kg)-treated and
dexamethasone-treated groups, mice were respectively gavaged with
HDW (100 mg/kg body weight) and dexamethasone (1 mg/kg body weight)
1 h prior to CS challenges for 1 week. At 24 h following the final
administration of HDW, mice were weighed and sacrificed. The weight
of thymus and spleen was then assessed in each group.
Bronchoalveolar lavage fluid (BALF)
analysis
Mouse tracheas were surgically exposed and
cannulated with a blunt 20-gauge needle. BALF was obtained by
injecting 3 sequential 1-ml aliquots of PBS and withdrawing as much
fluid as possible, following which the BALF was pooled. Cell
suspensions in BALF were centrifuged at a speed of 700 × g for 10
min at 4°C and the supernatants were collected and stored at-80°C
for cytokine assays. Cytokine levels (IL-1β, TNF-α, TGF-β and
IL-10) in the BALF were measured using ELISA kits according to the
manufacturer's protocol. The detection limit for each assay was 5
pg/ml. Cells were resuspended in 300 µl of 0.1% BSA/PBS (Beijing
Solarbio Science & Technology Co., Ltd.). Absolute cell counts
were determined using a MEK-7222k automatic hematology analyzer
(Nihon Kohden, Tokyo, Japan).
Lung histology
The lung histology was analyzed as previously
described (2). The right lungs of
mice from each group were fixed in a solution of 4% buffered
formalin for 1 week at room temperature. Sections 3 µm thick were
deparaffinized by immersing in xylene followed by dehydratation in
ethanol. Following a 5 min wash with PBS three times, sections were
stained with a hematoxylin solution at room temperature for 5 min.
After a 5 min wash with PBS three times, sections were stained with
eosin solution at room temperature for 5 min. A morphometric
quantification of the stained lung sections was performed using a
customized digital image processing system. Infiltrated airway
inflammatory cells contain abundant cytoplasmic particles and based
on images of the H&E-stained lungs, the severity of
peri-bronchial inflammation was recorded semiquantitatively as
previously described: 0, no inflammatory cells; 1, a few
inflammatory cells; 2, a ring of inflammatory cells 1 cell-layer
deep; 3, a ring of inflammatory cells 2 cells deep; 4, a ring of
inflammatory cells 3–4 cells deep; and 5, a ring of inflammatory
cells >4 cells deep.
The slides were examined and scored by a pathologist
who was blinded to the treatment. The stained cell images were
assessed at ×10 and ×20 magnification with a light Olympus
VS120-S5-W whole-slide imaging system (Olympus Corporation, Tokyo,
Japan). A total of six bronchioles were randomly evaluated in each
slide and the average inflammation scores were calculated.
Statistical analysis
All experiments were performed at least three times.
All values are expressed as the mean ± standard error of the mean.
One-way analysis of variance with Tukey's post hoc test was used to
compare results. All data were analyzed using SPSS 17.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of HDW on airway inflammation
in mice
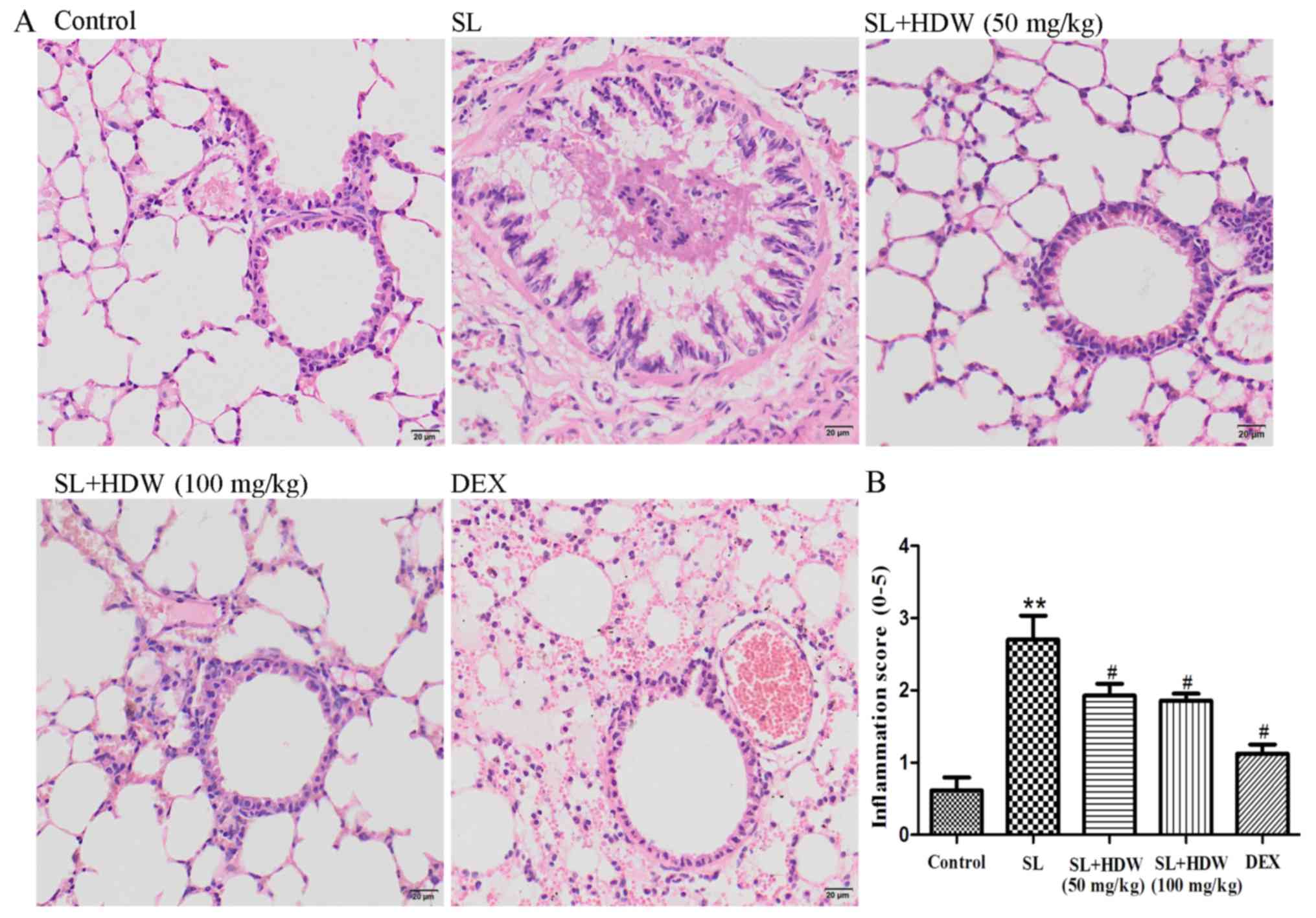
To clarify the possible anti-inflammatory effects of
HDW, lung tissues were isolated from mice 24 h following the final
CS challenge. Lung tissue sections were stained with H&E.
Compared with the control group the mice challenged with SL for 30
days had significantly increased pulmonary inflammation (P<0.01;
Fig. 2). In the SL+HDW (50 mg/kg and
100 mg/kg) mice, pulmonary inflammation was significantly lower
compared with in the SL-treated mice (P<0.05; Fig. 2). The treatment of SL-challenged mice
with dexamethasone inhibited pulmonary inflammation (P<0.05;
Fig. 2). However, the potent side
effects of dexamethasone resulted in a decrease in murine spleen
weight and size and body weight and the thymus markedly decreased
in size in certain mice (data not shown). In the SL+dexamethasone
group, 5/15 mice succumbed during the whole experimental
period.
Effects of HDW on inflammatory cells
in BALF
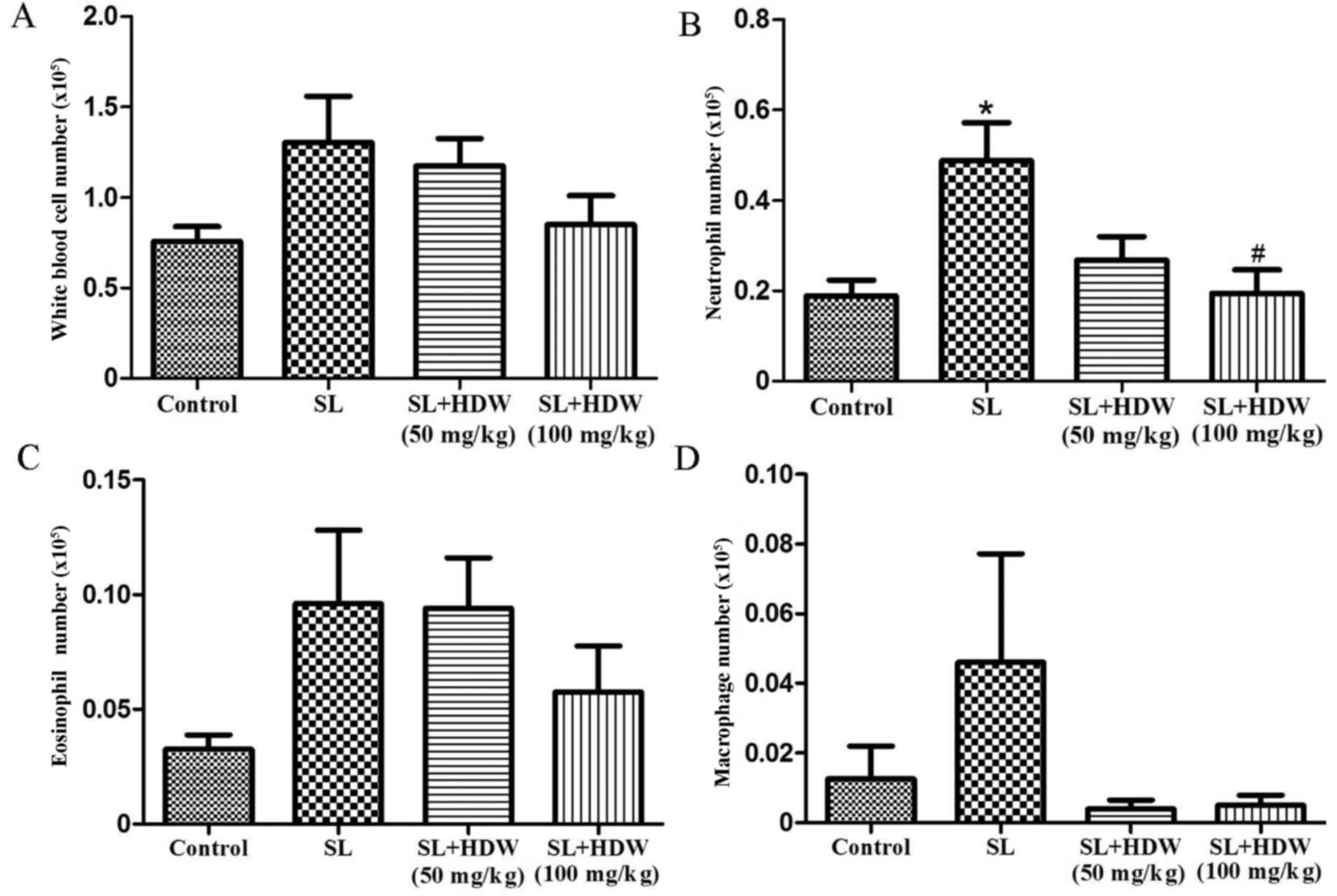
To determine the effect of HDW on inflammatory cells
in SL-induced mice, BALF was harvested 24 h following the last CS
challenge. BALF from mice in the CS group had a higher number of
total white blood cells (Fig. 3A),
neutrophils (P<0.05; Fig. 3B),
eosinophils (Fig. 3C) and
macrophages (Fig. 3D). In the SL+HDW
(50 mg/kg) group, the number of inflammatory cells was markedly
decreased compared with the CS group (Fig. 3). Treatment with 100 mg/kg HDW
significantly ameliorated the CS-induced increase in neutrophils in
the BALF (P<0.05; Fig. 3B).
Effect of HDW on cytokine levels in
BALF
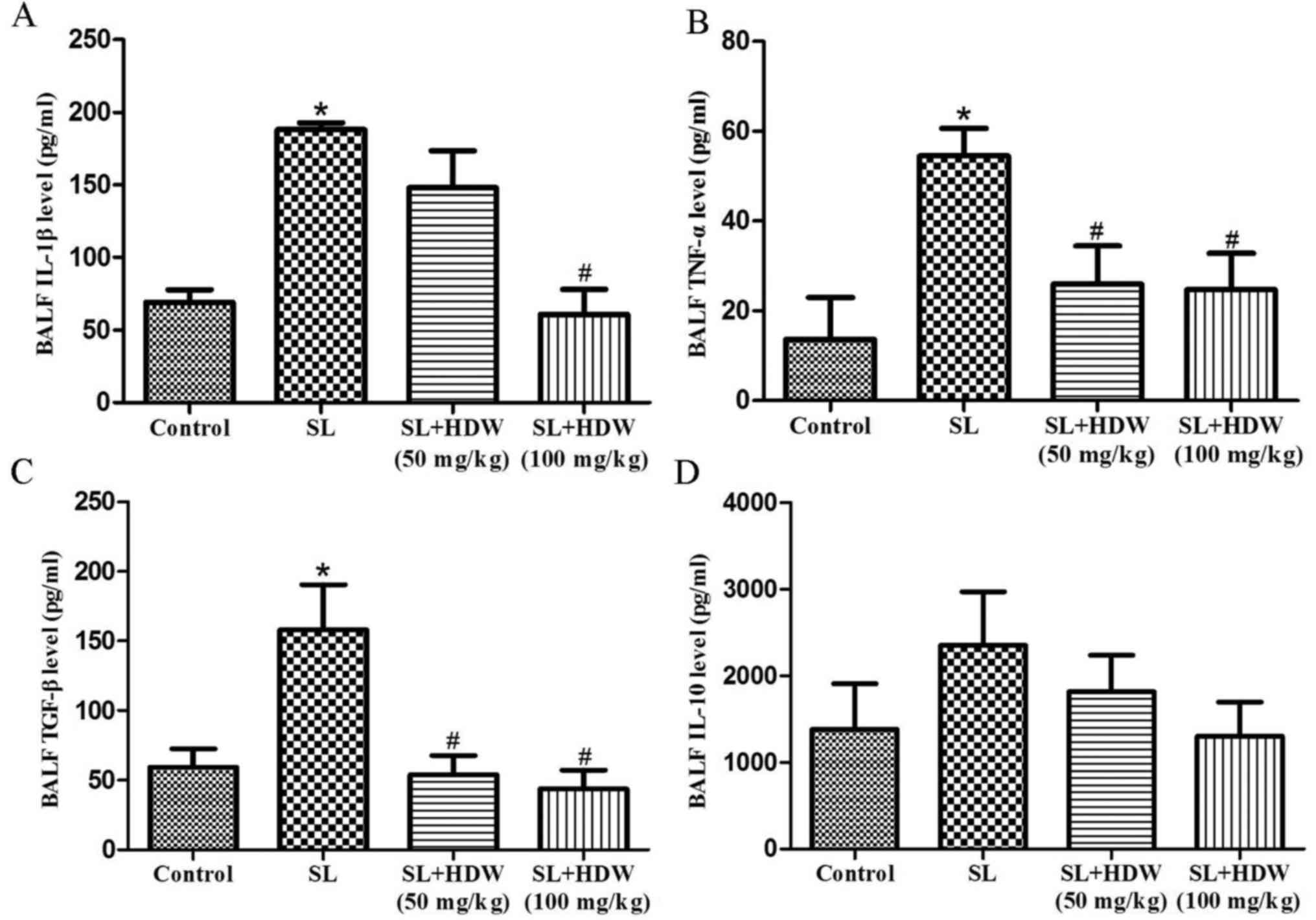
To evaluate the effects of HDW on cytokine levels in
the a COPD mouse model, ELISA kits were used to measure IL-1β,
TNF-α, TGF-β and IL-10 expression in the BALF. Levels of IL-1β,
TNF-α and TGF-β were significantly elevated in the CS group
compared with the control (P<0.05; Fig. 4A-C) and induced a marked increase in
IL-10 expression (Fig. 4D).
Treatment with 50 mg/kg HDW significantly inhibited the CS-induced
increase in TNF-α and TGF-β (P<0.05; Fig. 4B and C), whereas 100 mg/kg also
caused a significant decrease in IL-1β (Fig. 4A).
Effect of HDW on BEAS-2B cell
proliferation and LPS-induced NF-κB pathway activity
BEAS-2B cells were treated with HDW (20, 10, 1 or
0.1 µmol/l) for 1 h and challenged with LPS for 12 h. IκBα, p-IκBα
and NF-κB/p65 were measured using western blotting. LPS treatment
markedly upregulated the expression of p-IκBα and downregulated the
expression of IκBα and NF-κB/p65, respectively (Fig. 5A and B). These results suggest that
the NF-κB signaling pathway may serve a role in the
anti-inflammatory effect of HDW on airway-derived epithelial
inflammation.
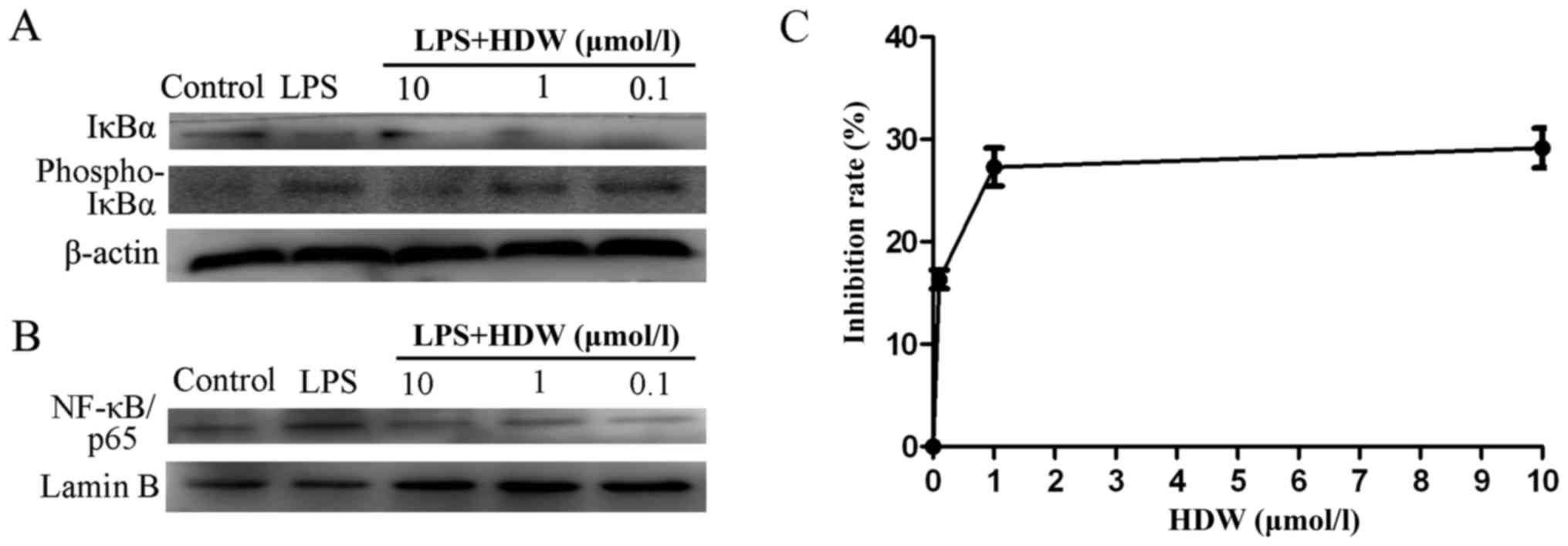 | Figure 5.Effect of HDW on NF-κB activity and
cellular proliferation in BEAS-2B cells. (A) Cells were pretreated
with HDW (20, 10, 1 or 0.1 µmol/l) for 1 h and then treated with
LPS (10 µg/ml) or D-Hanks for 30 min. Western blotting was
performed to assess the phosphorylation of IκBα. (B) Cells were
pretreated with HDW (20, 10, 1 or 0.1 µmol/l) for 1 h and then
treated with LPS (10 µg/ml) or D-Hanks for 24 h. Western blotting
was performed to assess nuclear NF-κB/p65 expression. (C) The
inhibition rates of BEAS-2B cells treated with HDW (20, 10, 1 or
0.1 µmol/l) were detected using Cell Counting Kit-8 assays. HDW,
Hedyotis diffusa Willd; NF-κB, nuclear factor-κB; LPS,
lipopolysaccharide; IκBα, inhibitor of NF-κB; phospho,
phosphorylated. |
BEAS-2B cells were treated with HDW in vitro.
Cell proliferation inhibition was measured using a CCK8 assay.
BEAS-2B cells were pretreated with 0.1, 1 or 10 µmol/l HDW for 24 h
and the resulting IC50 value was 9.05 µmol/l. As shown
in Fig. 5C, when the HDW
concentration was raised to 1 or 10 µmol/l, the inhibition rate was
increased to 25 and 31%, respectively. HDW inhibited the growth of
BEAS-2B cells.
Discussion
COPD is a chronic inflammatory disease characterized
by progressive and irreversible airflow obstruction, chronic
bronchitis, small airway remodeling and mucous overproduction
(2). Cigarette smoking is considered
to be a major cause of this disease. CS and LPS stimuli are used as
a preclinical strategy for evaluating the main pathological
characteristics of COPD (14).
Consequently, a strategy combining CS and LPS stimuli was used in
the present study to replicate the chronic inflammation associated
with COPD. The COPD inflammatory pathway is characterized by
elevated numbers of macrophages, neutrophils and lymphocytes
(2). Cigarette smoking activates
epithelial cells and macrophages to secrete inflammatory factors,
which results in inflammatory cell recruitment and tissue damage
(15). In the present study, the
results demonstrate that HDW regulates cytokine (IL-1β, TNF-α,
TGF-β and IL-10) release in BALF. Histopathological examinations
revealed that HDW suppresses inflammatory cell infiltration and
airway remodeling compared with SL-induced mice. Furthermore, HDW
decreased activation of the NF-κB signaling pathway. However,
dexamethasone treatment in the present study had potent side
effects, resulting in a decrease in spleen size and weight and body
weight (in some mice, the thymus almost completely disappeared) in
the dexamethasone treated group. Several mice succumbed during the
experimental period.
The focus of the present study was the effect of HDW
on chronic lung inflammation induced by SL. The effect of HDW on
IL-1β, TNF-α, TGF-β and IL-10 was assessed. These inflammatory
cytokines are responsible for the inflammation observed in
SL-challenged mice (16).
Proinflammatory cytokines (TNF-α and IL-1β) were upregulated in the
BALF of mice with COPD increased the degree of airway inflammation,
partly via NF-κB activation. In the present study, treating
SL-challenged mice with HDW led to a decrease in lung IL-1β levels
compared with SL-challenged mice. It has been reported that TNF-α
induces and influx of inflammatory cells into the lungs, pulmonary
fibrosis and emphysema in animal COPD models (17). TNF-α accelerates neutrophil migration
by boosting the expression of IL-8 and endothelial cell adhesion
molecules (18). In vivo,
increased levels of TNF-α in the BALF of patients are associated
with stable COPD (18). In the
present study, levels of TNF-α were increased in SL-induced mice
compared with the untreated controls. However, treatment with HDW
reduced the level of TNF-α in BALF. TGF-β is a profibrotic cytokine
that can be released by macrophages, epithelial cells, fibroblasts
and eosinophils (2,3,10). In
the present study, treatment with HDW reduced TGF-β expression in
BALF and suppressed lung IL-10 levels compared with SL-challenged
mice. NF-κB serves a significant role in lung pathology by
regulating the expression of vital proinflammatory cytokines and
chemokines (6). Upregulation of
proinflammatory cytokines promotes cellular activation and enhances
the infiltration of immune cells into airway tissues. The
activation of NF-κB in COPD arises largely in response to the
concerted action of inflammatory cytokines, including IL-1β and
TNF-α (19).
Previous studies have been performed to evaluate
whether crude extracts of HDW have potent antitumor activity
(13,20). Three major classes of HDW
constituents, the ferulic, oleanolic and ursolic acids, have been
identified as bioactive components of the herb. In the present
study, HDW reduced the levels of IL-1β, TNF-α, TGF-β and IL-10 in
BALF and suppressed activation of the NF-κB pathway in an
SL-challenged COPD mouse model. These findings provide further
evidence that HDW may be a suitable treatment for COPD.
Acknowledgements
The authors thank Dr Jinlong Chen for the extraction
and isolation of plant material. The authors also thank Mr. Fan Liu
for performing HPLC-MS analysis.
Funding
The present study was supported by the Department of
Science and Technology Program Funds of Jiangxi Province, China
(grant nos. 20142BAB205001 and 20151BAB205085).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RL contributed in the conception and design and
revision of the manuscript, the acquisition, analysis and
interpretation of data, and helped perform the animal and cell
biology experiments. PW prepared the initial draft of the
manuscript, contributed in the acquisition, analysis and
interpretation of data, and helped perform the animal and cell
biology experiments. CW, JC, CL, YX and QW contributed in the
acquisition, analysis and interpretation of data, and helped
perform the animal and cell biology experiments. JL, HH and JZ
helped revise the manuscript and performed the histology and
immunohistochemistry experiments.
Ethics approval and consent to
participate
All protocols involving mice were approved by the
Institutional Animal Experimental Ethics Committee of Nanchang
University (Nanchang, China).
Consent for publication
Approved.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DEX
|
dexamethasone
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
CS
|
cigarette smoke
|
|
HDW
|
Hedyotis diffusa Willd
|
|
SL
|
cigarette smoke+lipopolysaccharide
|
References
|
1
|
Mathers C and Loncar D: Projections of
global mortality and burden of disease from 2002 to 2030. PLoS Med.
3:e4422006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Yang X, Zhang W, Peng D, Xia Y, Lu
Y, Han X, Song G, Zhu J and Liu R: Therapeutic effects of
resveratrol in a mouse model of LPS and cigarette smoke-induced
COPD. Inflammation. 39:1949–1959. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen J, Zhou H, Wang J, Zhang B, Liu F,
Huang J, Li J, Lin J, Bai J and Liu R: Therapeutic effects of
resveratrol in a mouse model of HDM-induced allergic asthma. Int
Immunopharmacol. 25:43–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
MacNee W: Pathogenesis of chronic
obstructive pulmonary disease. Proc Am Thorac Soc. 2:pp. 258–266;
discussion 290–291. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Culpitt SV, Rogers DF, Shah P, De Matos C,
Russell RE, Donnelly LE and Barnes PJ: Impaired inhibition by
dexamethasone of cytokine release by alveolar macrophages from
patients with chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 167:24–31. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu R, Bai J, Xu G, Xuan L, Zhang T, Meng
A and Hou Q: Multi-allergen challenge stimulates steriod-resistant
airway inflammation via NF-κB-mediated IL-8 expression.
Inflammation. 36:845–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi IW, Sun K, Kim YS, Ko HM, Im SY, Kim
JH, You HJ, Lee YC, Lee JH, Park YM and Lee HK: TNF-alpha induces
the late-phase airway hyperresponsiveness and airway inflammation
through cytosolic phospholipase A(2) activation. J Allergy Clin
Immunol. 116:537–543. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan YY and Flavell RA: Regulatory T cells,
transforming growth factor-beta, and immune suppression. Proc Am
Thorac Soc. 4:pp. 271–276. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Peng W, Weng Y, Ying H, Li H, Xia
D and Yu W: Imbalance of Th17/Treg cells in mice with chronic
cigarette smoke exposure. Int Immunopharmacol. 14:504–512. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sutliff RL, Kang BY and Hart CM: PPARgamma
as a potential therapeutic target in pulmonary hypertension. Ther
Adv Respir Dis. 4:143–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu H, Liang QH, Xiong XG, Chen J, Wu D,
Wang Y, Yang B, Zhang Y, Zhang Y and Huang X: Anti-inflammatory
effects of the bioactive compound ferulic acid contained in
oldenlandia diffusa on collagen-induced arthritis in rats. Evid
Based Complement Alternat Med. 2014:5738012014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai Q, Lin J, Wei L, Zhang L, Wang L, Zhan
Y, Zeng J, Xu W, Shen A, Hong Z and Peng J: Hedyotis diffusa Willd
inhibits colorectal cancer growth in vivo via inhibition of STAT3
signaling pathway. Int J Mol Sci. 13:6117–6128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cipollina C, Di Vincenzo S, Gerbino S,
Siena L, Gjomarkaj M and Pace E: Dual anti-oxidant and
anti-inflammatory actions of the electrophilic
cyclooxygenase-2-derived 17-oxo-DHA in lipopolysaccharide- and
cigarette smoke-induced inflammation. Biochim Biophys Acta.
1840:2299–2309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herr C, Han G, Li D, Tschernig T, Dinh QT,
Beißwenger C and Bals R: Combined exposure to bacteria and
cigarette smoke resembles characteristic phenotypes of human COPD
in a murine disease model. Exp Toxicol Pathol. 67:261–269. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Özdemir ÖM, Gözkeser E, Bir F and Yenisey
C: The effects of resveratrol on hyperoxia-induced lung injury in
neonatal rats. Pediatr Neonatol. 55:352–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Churg A, Cosio M and Wright JL: Mechanisms
of cigarette smoke-induced COPD: Insights from animal models. Am J
Physiol Lung Cell Mol Physiol. 294:L612–L631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lundblad LK, Thompson-Figueroa J, Leclair
T, Sullivan MJ, Poynter ME, Irvin CG and Bates JH: Tumor necrosis
factor-alpha overexpression in lung disease: A single cause behind
a complex phenotype. Am J Respir Crit Care Med. 171:1363–1370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
King PT: Inflammation in chronic
obstructive pulmonary disease and its role in cardiovascular
disease and lung cancer. Clin Transl Med. 4:682015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edwards MR, Bartlett NW, Clarke D, Birrell
M, Belvisi M and Johnston SL: Targeting the NF-kappaB pathway in
asthma and chronic obstructive pulmonary disease. Pharmacol Ther.
121:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Z, Liu M, Liu M and Li J:
Methylanthraquinone from Hedyotis diffusa WILLD induces
Ca(2+)-mediated apoptosis in human breast cancer cells. Toxicol In
Vitro. 24:142–147. 2010. View Article : Google Scholar : PubMed/NCBI
|