Introduction
As a novel microtubule-binding protein, JWA is
essential in regulating cancer cell migration through
mitogen-activated protein kinase cascades and cytoskeletal F-actin
pathways (1). It has been reported
that JWA is essential for promoting cell survival and protection
from DNA damage induced by oxidative stress, which may result in
cancer cell apoptosis by chemical methods (2,3).
Previous studies have associated the JWA gene with reduced cancer
risk in various types of cancer, including gastric cancer (4), bladder cancer (5) and hepatocellular carcinoma (6). In addition, previous results have
demonstrated the important role of JWA downregulation in promoting
cell invasion using Matrigel-coated chambers and accelerating
melanoma cell migration and adhesion (7). However, the potential role of JWA in
the malignant transformation of cells has not been fully
elucidated.
Epithelial-mesenchymal transition (EMT) is a
biological process accompanied by mesenchymal gene activation and
epithelial gene repression. EMT has been implicated in the loss of
function of multiple adhesion proteins, including E-cadherin during
tumor progression, and increased cell proliferation, invasion and
migration (8–10). Based on tumor xenograft models and
cell culture studies, previous results have indicated that the
activation of EMT may promote tumor cell dissociation and
metastasis to distant organs (9,11).
The present study aimed to explore the role of JWA
knockdown (JWA−/−) on the malignant transformation of
mouse embryonic fibroblast (MEF) cells. In the present study, the
effects of JWA−/− on MEF cell proliferation, migration,
invasion and colony formation were investigated. The effects of
JWA−/− on the regulation of EMT-related proteins and the
tumorigenicity of MEF cells were explored. The findings of the
present study may provide insightful information into the potential
mechanisms of JWA in carcinogenesis.
Materials and methods
Preparation of MEF cells
All experiments were conducted in accordance with
the Animal Care and Use Committee of the Model Animal Research
Centre and approved by the Nanjing Medical University and the
Animal Care Ethics Committee of Nanjing Medical University
(Nanjing, China). The conditional JWA−/− murine model
used in the present study was constructed by the Model Animal
Research Center of Nanjing University (Nanjing, China) and
generated according to a previous study (12). Embryos from JWA+/− ×
JWA+/− crossed female mice were obtained on day 13.5 of
gestation (12). Once the heads and
all visible organs of the embryos, such as the heart and spleen
were removed, the embryos were placed in a 50-ml centrifuge tube
and minced with scissors. A total of 5 ml 0.25% trypsin, which was
inactivated using 5 ml of Dulbecco's modified Eagle medium (DMEM)
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) was added to the tube and incubated at 37°C for
20 min. Subsequently, the cells of embryo were centrifuged at 1,500
× g for 5 min at room temperature and resuspended in 15 ml fresh
medium. After standing for 10 min, the top layer of cell suspension
(10 ml) was collected and plated in a 100-mm dish. The cells were
cultured at 37°C in humidified atmosphere containing 5%
CO2. All experiments were conducted in accordance with
the Animal Care and Use Committee of the Model Animal Research
Centre and approved by Nanjing Medical University and the Animal
Care Ethics Committee of Nanjing Medical University (Nanjing,
China).
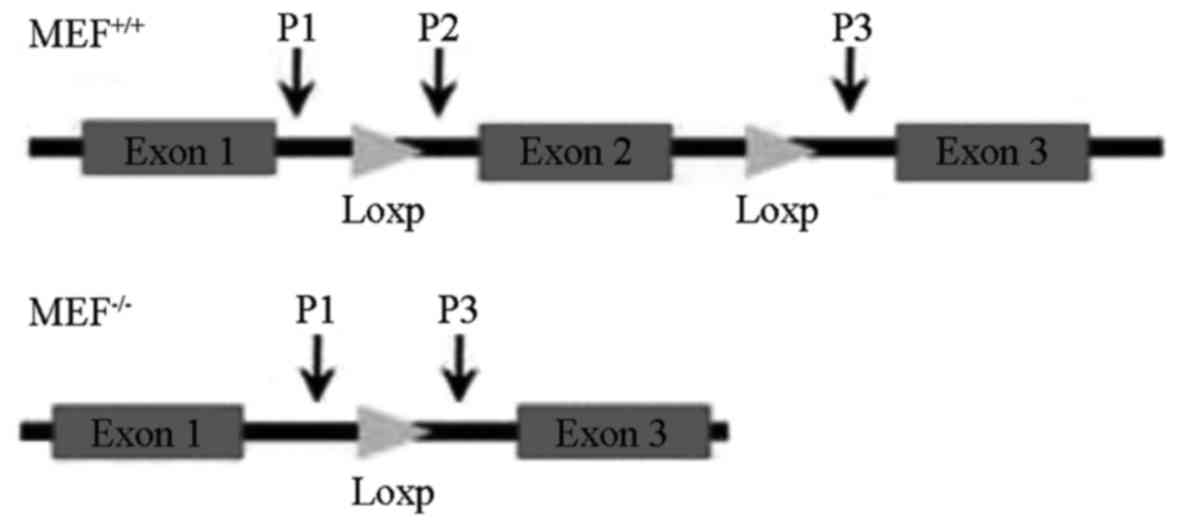
Identification of the JWA gene
Genomic DNA from MEF cells was extracted using
standard protocols to detect the JWA gene. The sequences of primers
for detecting wild type and null JWA alleles were as follows:
Wild-type and null JWA forward primer P1: 5′-CCACTGTTTCCTCTGTTG-3′;
wild-type reverse primer P2: 5′-GTGAAAACCACTGAGAACC-3′; and null
JWA reverse primer P3, 5′-CAGATGTTCCTCGTGTATC-3′. The JWA gene
structure is presented in Fig. 1.
The extracted genomic DNA was amplified by polymerase chain
reaction. Taq DNA polymerase and PCR kit were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). The PCR procedures were as
follows: Initial denaturation step at 94°C for 10 min, followed by
40 cycles of denaturation at 95°C for 15 sec, annealing at 60°C for
1 min and elongation at 72°C for 45 sec, and a final extension at
72°C for 10 min. The products were analyzed by 1.5% agarose
electrophoresis.
MEF cell proliferation
MEF cell proliferation was analyzed using the Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan)
according to the manufacturer's instructions. A total of 1,000
cells for each of MEF with wild-type JWA and JWA−/− MEF
cells were collected and inoculated into 96-well plates, which were
routinely cultured. A total of 10 µl CCK-8 reagent was added into
the wells and the absorbance was measured at 450 nm using an ELISA
spectrophotometer (Hyphen Biomed, Neuville-sur-oise, France),
following incubation for 1 h.
MEF cell migration and invasion
assays
Cell migration and invasion assays were performed
using the Transwell invasion assay. In the upper chamber of the
Transwell unit (Corning Incorporated, Corning, NY, USA), 6.5-mm
diameter polycarbonate filters with 8-µm pore size (EMD Millipore,
Billerica, MA, USA) were inserted. For the migration assay, 200 µl
cell suspension with a density of 2×105 cells/ml were
seeded in serum-free DMEM in the upper chamber and incubated for 8
h at 37°C. MEF cells were fixed in methanol, stained with 1%
crystal violet solution for 15 min at room temperature and counted.
A total of 9 random fields were counted using a light microscope at
×200 magnification (Olympus BX41; Olympus Corporation, Tokyo,
Japan). For invasion assay, MEF cells were suspended in serum-free
DMEM at a density of 5×105 cells/ml. Subsequently, 200
µl of cell suspension were added into the upper chamber and 500 µl
of culture medium supernatant were added into the lower chamber.
Following incubation at 37°C in a humidified atmosphere containing
5% CO2 for 24 h, the cells on the surface layer of cells
in the upper chamber were swabbed with cotton-swappers. Cells in
the lower chamber were fixed with 4% paraformaldehyde, stained with
crystal violet, washed with distilled water, dried at room
temperature and counted following the same steps as those in the
migration assay.
Colony formation assay
The MEF cells in logarithmic phase were harvested
and resuspended in DMEM with 10% heat-inactivated FBS, penicillin
(100 IU/ml) and streptomycin (100 µg/ml). The molten 0.3% agarose
was placed in a 60 mm culture dish. The cell suspension was applied
onto the base layer and cultured in the incubator at 37°C in a
humidified environment containing 5% CO2 for 2 weeks.
The cells were counted using a hemocytometer under a light
microscope. Clusters containing >50 cells were identified as a
colony. The colony forming rate was calculated as: Colony forming
rate = number of colonies/number of total cells seeded × 100.
Expression of EMT-related proteins and
western blotting
The effects of JWA knockout on PARP-1 and
EMT-related proteins were observed. MEF cells (1×106)
were seeded in a 100 mm culture dish. NU1025 (Sigma-Aldrich; Merck
KGaA Darmstadt, Germany), a specific inhibitor of PARP-1, was used
to inhibit the function of PARP-1 in MEF cells. NU1025 was
dissolved in dimethylsulfoxide (DMSO) and added into the wells to
give a final concentration of 50 µmol/l. DMSO of same volume was
used as a blank control. The small interfering (si)RNA of PARP-1
(PARP-1 siRNA: sc-29438; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) was also used to inhibit the function of PARP-1
simultaneously. The MEF cells were transfected with 60 µl of 10
µmol/l PARP-1 siRNA according to the manufacturer's protocol
(Lipofectamine® 3000, Invitrogen; Thermo Fisher
Scientific, Inc.). A mimical nonsense siRNA was used as blank
control. A 24 h interval was left before subsequent
experimentation.
The EMT-related proteins in MEF cells, including
poly (ADP-ribose) polymerase-1 (PARP-1), vimentin, β-catenin and
E-cadherin, were extracted for western blotting as described
previously (12). In brief, the
cells were lysed in radioimmunoprecipitation buffer (50 mmol/l
Tris-HCl pH 7.2, 150 mmol/l NaCl, 1% NP40, 0.1% SDS, 0.5%
deoxycholic acid sodium, 1 mmol/l PMSF, 25 mmol/l MgCl2,
and supplemented with phosphatase inhibitor cocktail). The protein
concentrations were determined using the bicinchoninic acid assay
method (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins
(20 µg per lane) were separated by 12.5% SDS-PAGE and
electroblotted onto polyvinyl difluoride membranes. Membranes were
blocked with 5% milk at 37°C for 1 h and subsequently incubated
with specific primary antibodies for 1 h at room temperature.
Monoclonal rabbit anti-PARP-1 (1:1,000; EMD Millipore; MABE365),
mouse anti-vimentin (1:500; EMD Millipore; MABT121), mouse
anti-β-catenin monoclonal (1:200; BD Biosciences, San Jose, CA,
USA; 610153), mouse anti-E-cadherin (1:200; BD Biosciences; 610404)
and mouse anti-β-actin (1:2,000; Beyotime Institute of
Biotechnology, Nantong, China; AA128) were used as the primary
antibodies as described above. The relevant proteins were stained
with the secondary antibody (goat anti-mouse IgG-HRP, 1:2,000;
Santa Cruz Biotechnology, Inc.; sc-2005,) for 1 h at room
temperature. Immunoreactive bands were detected using Beyond
electrochemiluminescence (BeyoECL) Plus kit (P0018; Beyotime
Institute of Biotechnology, Haimen, China). Protein bands were
visualized and measured using ImageJ software (version 1.44;
National Institutes of Health, Bethesda, MA, USA; data not shown),
following normalization to the corresponding β-actin level.
Nude mouse xenograft assay
To determine the effects of JWA−/− on the
tumorigenicity of MEF cells in vivo, nude murine xenograft
assays were performed. A total of 10 female nude mice (8–9 weeks
old, weight 16–20 g) were obtained from the Experimental Animal
Center of the Chinese Academy of Medical Sciences (Beijing, China).
Mice were maintained in a temperature-controlled room (23°C),
relative humidity of 50% with a 12-h light/dark cycle and free
access to food and water. A xenograft assay was performed. The mice
were randomly divided into two groups (n=5 per group): MEF cells
with wild type JWA (JWA+/+) and JWA−/− MEF
cell groups. MEF cells were suspended in DMEM and adjusted to a
density of 2×106 cells/ml. Subsequently, 200 µl cell
suspension of JWA+/+ or JWA−/− MEF cells was
injected subcutaneously into nude mice backs. The volume and weight
of the formed tumors were measured in 4 weeks after
inoculation.
Statistical analysis
Statistical analyses were performed using SPSS 16.0
(SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± or +
standard deviation. Student's t-test was used to determine the
differences between the two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Knockout of JWA in MEF cells
A MEF cell line with JWA−/− was
constructed and the wild-type MEF cells (JWA+/+) were
used as the negative control. Genotyping of JWA−/− mice
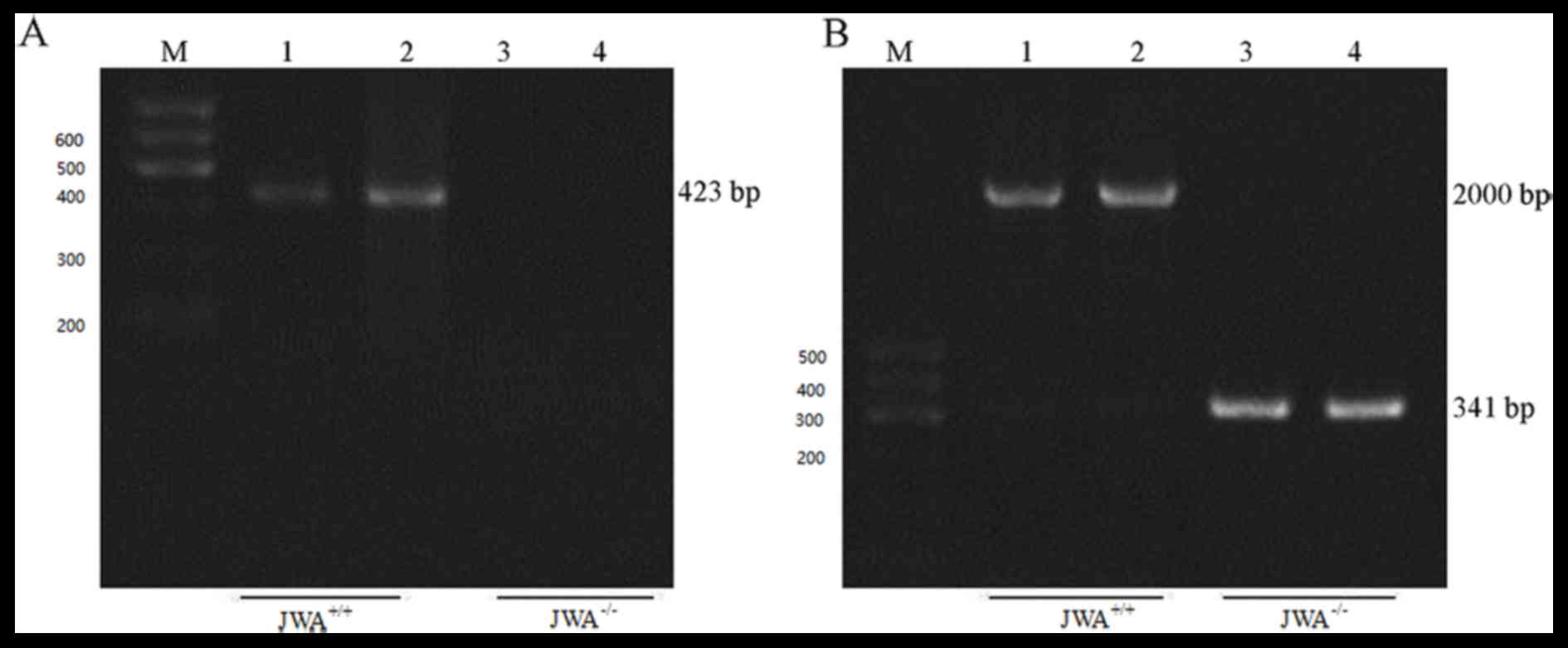
was performed at the genomic DNA level (Fig. 2). Using P1 and P2 primers, a 423-bp
band was observed in wild-type MEF cells, which indicated that
primer 1 and 2 amplified a 423-bp fragment between exon 1 and 2. No
bands were present in the JWA−/− MEF cells, which
suggested that the deletion of exon 2 lead to JWA−/−
(Fig. 2A). In addition, P1 and 3
primers amplified a fragment of ~2,000 bp between exons 1 and 3 in
the wild-type MEF cells, whereas a 341 bp band was observed in
JWA−/− MEF cells, which suggested that P1 and 3 primers
amplified the remaining 341-bp fragment between exons 1 and 3
(Fig. 2B).
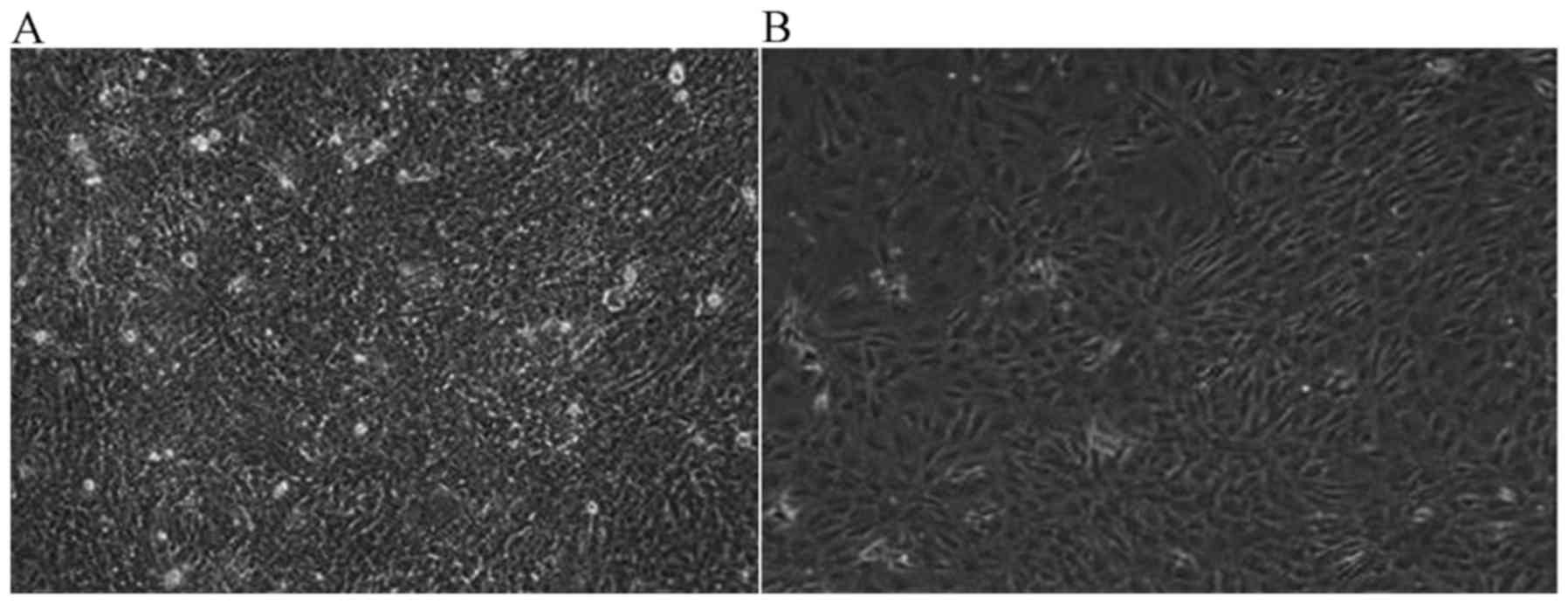
JWA-/- induces malignant
transformation in MEF cells
Once JWA−/− MEF cells were cultured for 6
months, malignant transformation was observed in the
JWA−/− MEF cells. Under the light microscope (1:40), the
proliferation activity of JWA−/− MEF cells was notably
increased compared with wild-type MEF cells. The number of cells
was markedly increased and once the saturation density was reached,
cells began to grow overlapping one another. Compared with
wild-type MEF cells, the difference was marked. In Fig. 3A, JWA−/− MEF cells covered
the bottom of the culture dish. The cell nuclei of
JWA−/− MEF with malignant transformation became large
and the contact inhibition of growing cells disappeared. However,
the MEF cells with wild-type JWA began to die and only a few
scattered cell colonies were observed (Fig. 3B).
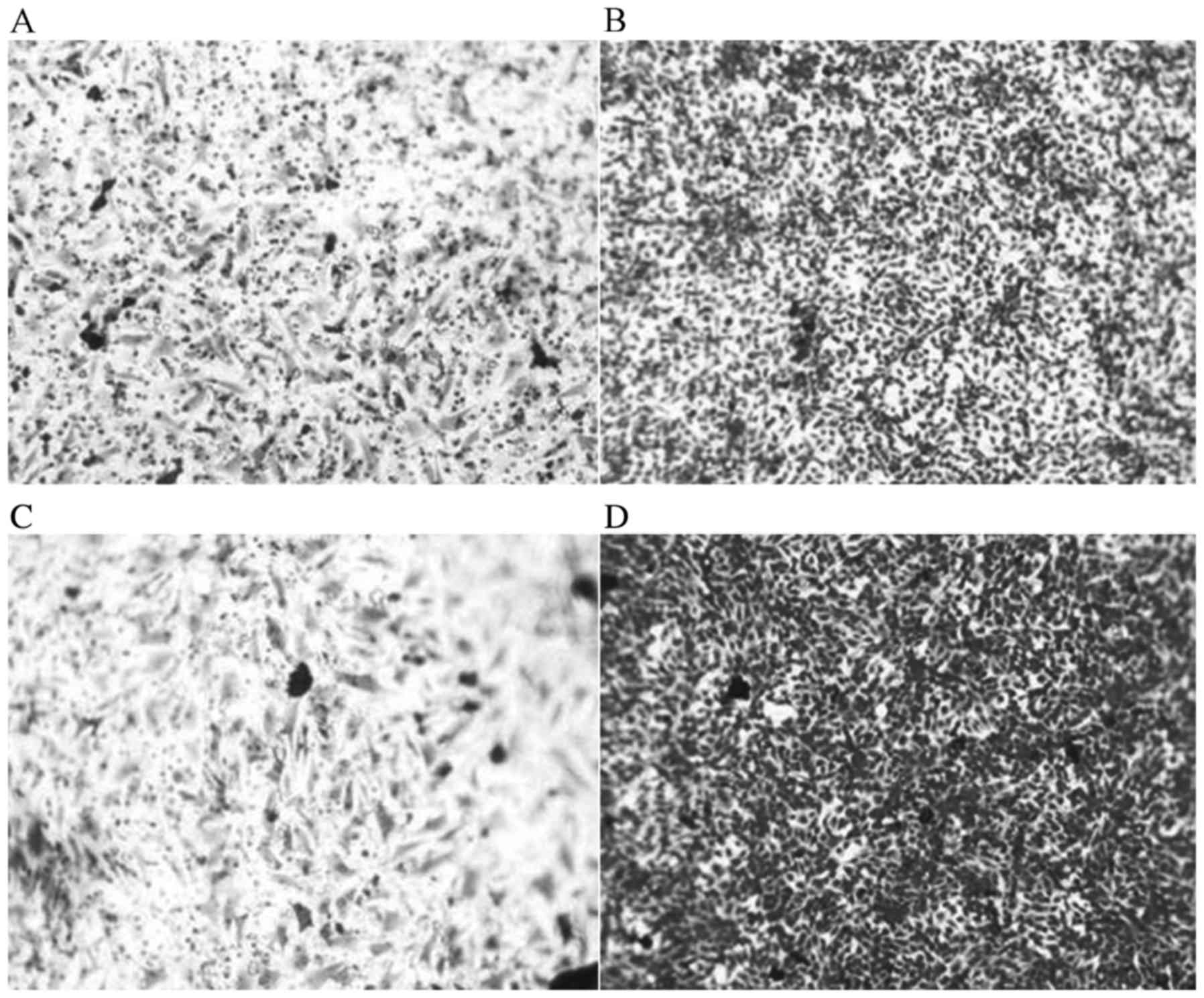
Effects of JWA-/- on cell
proliferation, migration, invasion and colony formation
The migration and invasion abilities of MEF cells
with JWA−/− were markedly increased compared with those
of the MEF cells with wild type JWA (JWA+/+). The
results of migration ability of MEF cells indicated that
JWA−/− promoted cell migration and invasion of MEF cells
with wild type JWA. The number of MEF cells with JWA−/−
was markedly greater than MEF cells with wild type JWA (Fig. 4). The results of the invasion assay
indicated that the number of MEF cells with JWA−/− was
increased (Fig. 4). As presented in
Fig. 4D, a large number of
JWA−/− MEF cells penetrated into the lower chamber.
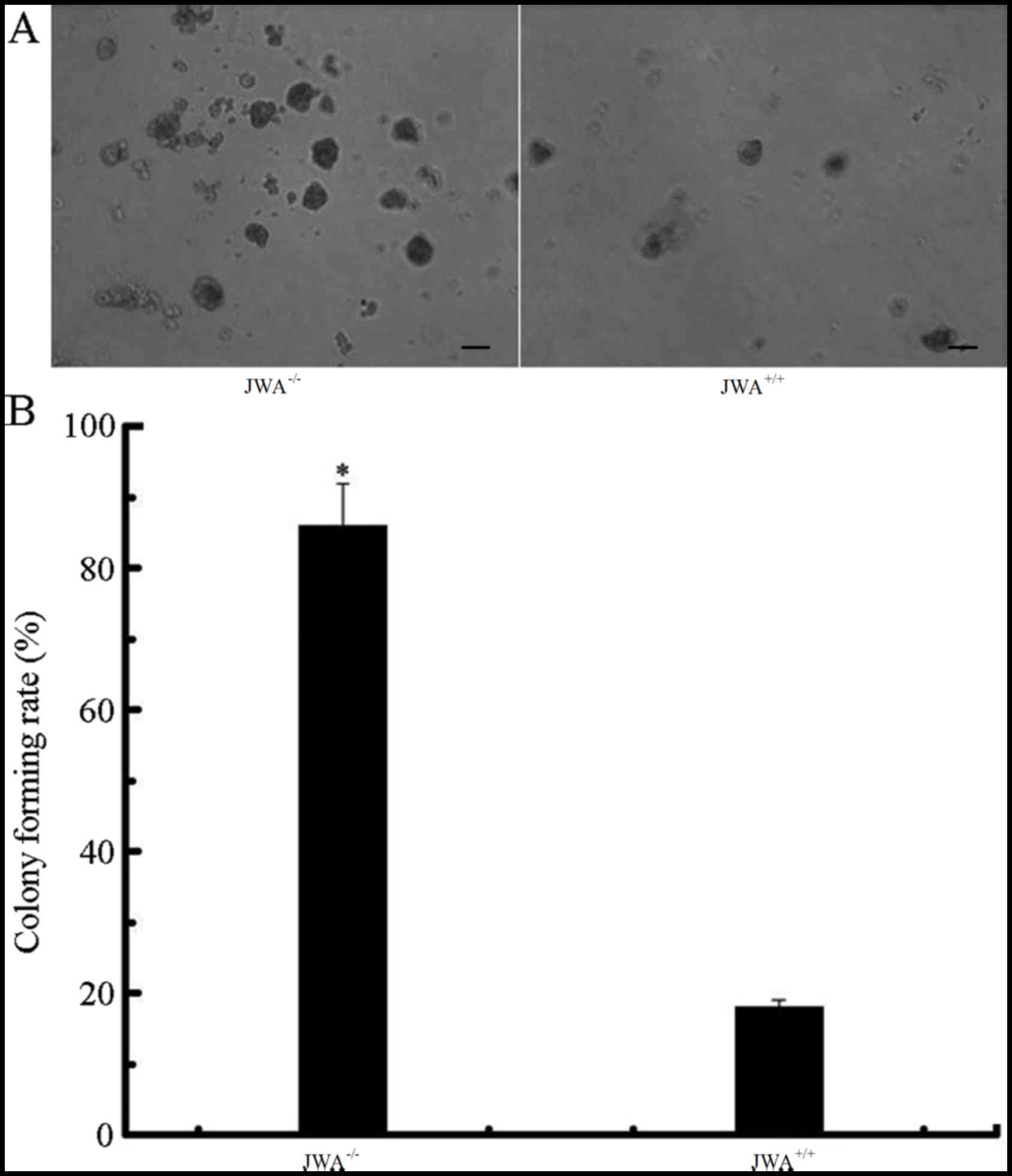
Colony formation assay results suggested that the number of
colonies of JWA−/− MEF cells was notably increased when
compared with the wild-type MEF cells (Fig. 5A). Quantitative analysis demonstrated
that the rate of colony formation of JWA−/− MEF cells
was 83.4±5.2%, and the JWA+/+ MEF cells was 19.6±1.3%.
The colony-forming rate in JWA−/− MEF cells was
significantly greater than that of the MEF cells with wild type JWA
(P<0.05, Fig. 5B).
Effects of JWA-/- on the regulation of
EMT-related proteins
JWA−/− markedly altered the expression
levels of EMT-related proteins. Western blot analysis revealed that
JWA−/− upregulated the protein expression levels of
PARP-1, vimentin and β-catenin, and downregulated the protein
expression levels of E-cadherin (Fig.
6A). Following the application of PARP-1 inhibitor NU1025 or
relevant siRNA, the protein expression levels of PARP-1, vimentin
and β-catenin were reduced compared with blank DMSO group or mock
group as mimical control (Fig.
6B).
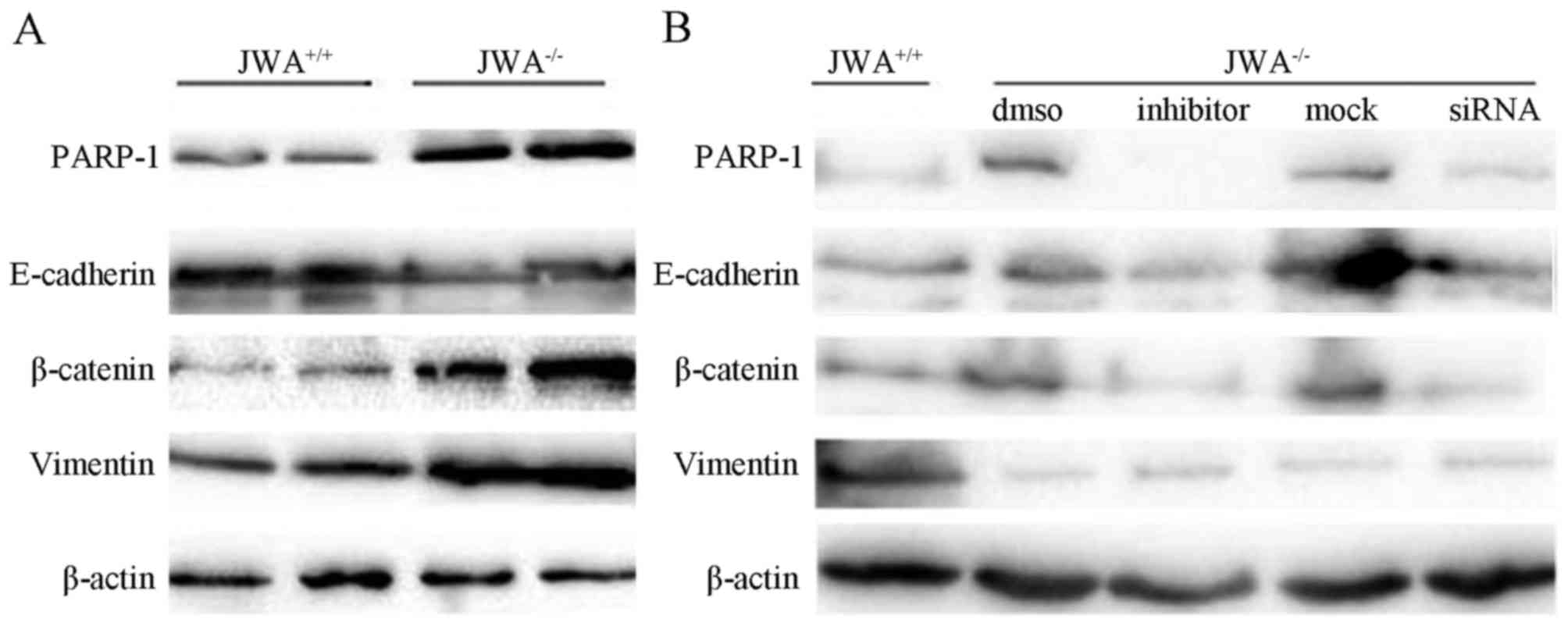 | Figure 6.Western blottinng results of JWA
knockout on PARP-1 inhibition and the regulation of EMT-related
protein expression. (A) The effects of JWA knockout.
JWA−/− upregulated the expression levels of PARP-1,
vimentin and β-catenin, and downregulated the expression of
E-cadherin. (B) The effects of inhibitor NU1025 and siRNA. The
application of PARP-1 inhibitor NU1025 reduced the expression of
PARP-1, vimentin and β-catenin compared with the DMSO group. DMSO
was blank organic solvent without NU1025. The application of
relevant siRNA reduced the expression of PARP-1, vimentin and
β-catenin compared with the mock group in a similar manner.
JWA+/+, wild-type JWA; JWA−/−, JWA knockout;
PARP-1, poly(ADP-ribose) polymerase-1; NU1025, PARP-1 inhibitor;
DMSO, pure organic solvent without NU1025; siRNA, small interfering
RNA for PARP-1, mock: Nonsense RNA. |
Effects of JWA-/- on the
tumorigenicity of MEF cells
In 8 weeks following subcutaneous injections of MEF
cells with wild type JWA, no control mice exhibited tumor
formation. However, the group injected JWA−/− MEF
exhibited obvious tumor formation (Fig.
7). The tumor volume was 2.56±0.36 cm3 and the tumor
weight was 1.74±0.43 g in the JWA−/− MEF group.
Discussion
JWA has been previously reported as a novel
regulator in inhibiting melanoma cell adhesion, invasion and
metastasis (7) and has been
demonstrated to have prognostic and predictive roles in gastric
cancer (13). In the present study,
JWA−/− was able to increase MEF cell proliferation, and
stimulate cell migration, cell invasion and colony formation,
ultimately promoting malignant transformation in MEF cells.
Additionally, JWA−/− was able to upregulate the
expression levels of EMT-related proteins (PARP-1, vimentin and
β-catenin) and downregulate the expression levels of
E-cadherin.
JWA has been recognized as a typical tumor
suppressor and stress response gene (3,7). It has
been demonstrated that JWA is an essential signaling gene in the
regulation of tumor cell migration and differentiation (14). Additionally, JWA protein expression
levels are closely correlated with the occurrence, invasion and
metastasis of malignant tumors (15). Using liver cells with different
metastatic potential, a previous study revealed that reduced
expression levels of JWA protein resulted in increased metastasis
potential (6). Previous results have
indicated that downregulation of JWA expression may affect cell
function, such as proliferation, apoptosis, migration and invasion
via the mitogen-activated protein kinase/extracellular
signal-regulated kinase pathway of mitogen-activated protein kinase
signaling cascades (16).
In the present study, the proliferation of MEF cells
was increased in JWA−/− MEF cells, and cell migration
and invasion were promoted. Previous studies have indicated that
loss of JWA was able to increase cell migration and metastasis
(1,7,17). The
loss of JWA combined with p53 mutation has been demonstrated to
promote aggressiveness and metastasis of gastric cancer cells,
which may be attributed to the fact that JWA is a member of the DNA
repair pathway and may have inhibitory roles in gastric
carcinogenesis. The expression of JWA gene may contribute to better
chemotherapy outcome in gastric carcinogenesis (4). As a multi-functional
microtubule-associated protein, JWA is associated with DNA damage
repair and apoptosis in various physiological contexts and inhibits
multiple steps of metastasis, including cell invasion, cell
adhesion and angiogenesis in diverse carcinoma (18).
Overall, the results of the present study suggest
that JWA−/− may induce MEF cell malignant transformation
by having important roles in promotion of cell proliferation,
migration, invasion and colony formation. These results were
consistent with the findings of previous numerous researches on JWA
gene (19).
The present results indicated that the protein
expression levels of PARP-1, vimentin and β-catenin were
upregulated and E-cadherin was downregulated. PARP-1 is considered
an important molecule in regulating lung cell proliferation, as a
previous study revealed that mice lacking PARP-1 exhibited
excessive lung cell proliferation and hyperplasia (20). Furthermore, microglial activation is
associated with cell proliferation and the increased release of
pro-inflammatory cytokines (21). A
previous study reported that PARP-1 promoted microglial activation
and proliferation (22).
Additionally, vimentin has been indicated to have a crucial role in
cell division and proliferation (23). Previous results have suggested that
the association of vimentin and epigallocatechin gallate regulates
cell proliferation (24). β-catenin
expression and vascular endothelial cadherin binding requires the
inhibition of vascular endothelial growth factor-induced cell
proliferation (25).
E-cadherin-associated β-catenin is implicated in inhibiting cell
proliferation through regulating the Hippo signaling pathway
(26). The findings of the present
study suggest that JWA may mediate cell proliferation by regulating
the expression of EMT-related proteins. However, further studies
are required to investigate this hypothesis.
In conclusion, the present study demonstrated that
JWA−/− was able to induce malignant transformation in
MEF cells by altering cell proliferation, migration, invasion and
colony formation. The role of JWA in mediating cell proliferation
may involve regulating the expression of EMT-related proteins. The
present findings suggest that JWA may function as an anti-oncogene
in cancer.
Acknowledgements
The authors would like to thank Professor Jianwei
Zhou from the School of Public Health, Nanjing Medical University
for his guidance and help in the design of this study. This study
was supported by Top-notch Academic Programs Project of Jiangsu
Higher Education Institutions (TAPP).
References
|
1
|
Chen H, Bai J, Ye J, Liu Z, Chen R, Mao W,
Li A and Zhou J: JWA as a functional molecule to regulate cancer
cells migration via MAPK cascades and F-actin cytoskeleton. Cell
Signal. 19:1315–1327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen R, Qiu W, Liu Z, Cao X, Zhu T, Li A,
Wei Q and Zhou J: Identification of JWA as a novel functional gene
responsive to environmental oxidative stress induced by
benzo[a]pyrene and hydrogen peroxide. Free Radic Biol Med.
42:1704–1714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang S, Gong Z, Chen R, Liu Y, Li A, Li G
and Zhou J: JWA regulates XRCC1 and functions as a novel base
excision repair protein in oxidative-stress-induced DNA
single-strand breaks. Nucleic Acids Res. 37:1936–1950. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Wang S, Xia X, Chen Y, Zhou Y, Wu
X, Zhang J, He S, Tan Y, Qiang F, et al: Synergistic role between
p53 and JWA: prognostic and predictive biomarkers in gastric
cancer. PLoS One. 7:e523482012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li CP, Zhu YJ, Chen R, Wu W, Li AP, Liu J,
Liu QZ, Wei QY, Zhang ZD and Zhou JW: Functional polymorphisms of
JWA gene are associated with risk of bladder cancer. J Toxicol
Environ Health A. 70:876–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu X, Chen H, Gao Q, Bai J, Wang X, Zhou
J, Qiu S, Xu Y, Shi Y, Wang X, et al: Downregulation of JWA
promotes tumor invasion and predicts poor prognosis in human
hepatocellular carcinoma. Mol Carcinog. 53:325–336. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bai J, Zhang J, Wu J, Shen L, Zeng J, Ding
J, Wu Y, Gong Z, Li A, Xu S, et al: JWA regulates melanoma
metastasis by integrin αVβ3 signaling. Oncogene. 29:1227–1237.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong Z, Shi Y, Zhu Z, Li X, Ye Y, Zhang J,
Li A, Li G and Zhou J: JWA deficiency suppresses
dimethylbenz[a]anthracene-phorbol ester induced skin papillomas via
inactivation of MAPK pathway in mice. PLoS One. 7:e341542012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang S, Wu X, Chen Y, Zhang J, Ding J,
Zhou Y, He S, Tan Y, Qiang F, Bai J, et al: Prognostic and
predictive role of JWA and XRCC1 expressions in gastric cancer.
Clin Cancer Res. 18:2987–2996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi GZ, Yuan Y, Jiang GJ, Ge ZJ, Zhou J,
Gong DJ, Tao J, Tan YF and Huang SD: PRAF3 induces apoptosis and
inhibits migration and invasion in human esophageal squamous cell
carcinoma. BMC Cancer. 12:972012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Ge Z, Tan Y, Jiang G, Feng J, Wang
H and Shi G: Downregulation of JWA expression in human esophageal
squamous cell carcinoma and its clinical significance. Oncol Res.
20:157–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu YY, Ma TL, Ge ZJ, Lin J, Ding WL, Feng
JK, Zhou SJ, Chen GC, Tan YF and Cui GX: JWA gene regulates PANC-1
pancreatic cancer cell behaviors through MEK-ERK1/2 of the MAPK
signaling pathway. Oncol Lett. 8:1859–1863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang S, Shen Q, Mao WG, Li AP, Ye J, Liu
QZ, Zou CP and Zhou JW: JWA, a novel signaling molecule, involved
in the induction of differentiation of human myeloid leukemia
cells. Biochem Biophys Res Commun. 341:440–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S, Gong Z, Chen R, Liu Y, Li A, Li G
and Zhou J: JWA regulates XRCC1 and functions as a novel base
excision repair protein in oxidative-stress-induced DNA
single-strand breaks. Nucleic Acids Res,. 37:1936–1950. 2009.
View Article : Google Scholar
|
|
19
|
Chen Y, Huang Y, Huang Y, Xia X, Zhang J,
Zhou Y, Tan Y, He S, Qiang F, Li A, et al: JWA suppresses tumor
angiogenesis via Sp1-activated matrix metalloproteinase-2 and its
prognostic significance in human gastric cancer. Carcinogenesis.
35:1–451. 2014. View Article : Google Scholar
|
|
20
|
Pagano A, Métrailler-Ruchonnet I,
Aurrand-Lions M, Lucattelli M, Donati Y and Argiroffo CB: Poly
(ADP-ribose) polymerase-1 (PARP-1) controls lung cell proliferation
and repair after hyperoxia-induced lung damage. Am J Physiol Lung
Cell Mol Physiol. 293:L619–L629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kreutzberg GW: Microglia: A sensor for
pathological events in the CNS. Trends Neurosci. 19:312–318. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kauppinen TM and Swanson RA: Poly
(ADP-ribose) polymerase-1 promotes microglial activation,
proliferation, and matrix metalloproteinase-9-mediated neuron
death. J mmunol. 174:2288–2296. 2005. View Article : Google Scholar
|
|
23
|
Chou YH and Goldman RD: Goldman,
Intermediate filaments on the move. J Cell Biol. 150:F101–F106.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ermakova S, Choi BY, Choi HS, Kang BS,
Bode AM and Dong Z: The intermediate filament protein vimentin is a
new target for epigallocatechin gallate. J Biol Chem.
280:16882–16890. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grazia Lampugnani M, Zanetti A, Corada M,
Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A,
Kemler R, Daniel TO and Dejana E: Contact inhibition of
VEGF-induced proliferation requires vascular endothelial cadherin,
β-catenin, and the phosphatase DEP-1/CD148. J Cell Biol.
161:793–804. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim NG, Koh E, Chen X and Gumbiner BM:
E-cadherin mediates contact inhibition of proliferation through
Hippo signaling-pathway components. Proc Natl Acad Sci USA. 108:pp.
11930–11935. 2011; View Article : Google Scholar : PubMed/NCBI
|