Introduction
Methamphetamine hydrochloride (MA) is a widely
abused psychostimulant with an estimated 35 million users
worldwide; thus, it has become a public health problem (1). A number of animal and clinical studies
have demonstrated that MA abuse induces immunosuppressive effects,
thereby increasing susceptibility to infectious diseases (2). Lipopolysaccharide (LPS), an
immunostimulant, may mediate the immune response associated with
gram-negative bacterial infection. Although previous studies have
demonstrated the detrimental effect of MA on host immunity
(3,4), the effect of MA following stimulation
with LPS on the immune response has not yet been described.
It has been demonstrated that mast cells (MCs) serve
an important role in innate and acquired immune responses (5,6), such
that certain diseases are associated with changes in the number of
MCs at affected sites (7,8). MCs are abundant at the borders of the
external environment, including the intestinal mucosa where MCs
function as sentinel cells during immune defense (9,10).
Cluster of differentiation 117 (CD117, also known as c-kit) is a
primary receptor and MCs marker and it has been demonstrated that a
loss-of-function mutation in c-kit causes MCs deficiency in mice
(11). The type I high affinity
immunoglobulin E receptor (FcεRI) is an another receptor and marker
of MCs that excites FcεRI and activates MCs (12). It has been demonstrated that LPS
induces rodent MCs to secrete a variety of cytokines, including
tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-4, IL-6,
IL-10, IL-13 and chemokine ligand-5 (CCL-5) (13,14).
MC-mediated cytokine production is often greater than that from
other immunocytes, including macrophages and T cells (15,16). In
addition, MCs serve an important role in the lung (17); indeed, thymic MCs have been
implicated in infection-induced thymus involution (18). However, it is remains unknown whether
MA modulates MCs activation and the subsequent production of
cytokines stimulated by LPS.
The present study assessed the effect of MA on MC
activation and release of cytokines stimulated by LPS in C57BL/6J
mice. The expression of CD117 and FcεRI was measured in mouse
intestines and it was demonstrated that MCs released cytokines in
the lung and thymus tissues following treatment with MA and LPS
stimulation. To further verify the effect of MA on the response of
MCs mediated by LPS, LPS-stimulated cytokine production following
MA treatment in mouse bone marrow-derived mast cells (BMMCs) was
examined. The results of the present study demonstrate that MA may
regulate MC activation and LPS-stimulated cytokine production.
Materials and methods
Animals
A total of 48 C57BL/6J mice aged 6-week-old
(weighing ~20 g; 24 males: 24 females) were purchased from the
Laboratory Animal Department of Xi'an Jiaotong University Medical
School (Xi'an, China). C57BL/6J mice were housed in a specific
pathogen-free facility (temperature, 22±3°C; relative humidity,
60±5%) maintained on a 12-h light/dark cycle. All the mice had free
access to food and water. Experiments were approved by the Animal
Ethics Committee of Xi'an Jiaotong University and all treatment
procedures were performed in accordance with the guidelines of the
Institutional Animal Care and Use Committee of Xi'an Jiaotong
University.
Reagents
MA was purchased from the National Institute for the
Control of Pharmaceutical and Biological Products (Beijing, China).
MA was dissolved in sterile 0.9% physiological saline and injected
intramuscularly (i.m.) at a dose of 5 mg MA/kg. LPS (derived from
Escherichia coli; serotype O55:B5; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was dissolved in sterile saline and
injected i.m. at a dose of 150 µg/kg.
Animal treatments
The 6-week-old sex-matched C57BL/6J mice were
randomly divided into 4 groups (n=12), including a normal saline
(NS) NS+NS group (control), a MA+NS group, an NS+LPS group and a
MA+LPS group. Mice received four i.m. injections of either 5 mg/kg
MA or NS at 2 h intervals. Mice then received one i.m. injection of
either LPS (150 µg/kg) or NS 24 h following the first MA injection.
The 5 mg/kg dose of MA was selected based on the results of a
previous study (19) and a
preliminary experiment in the present study indicated that 150
µg/kg LPS was the most appropriate dose. Mice were sacrificed by
CO2 asphyxiation and tissues, including lung and thymus
tissues, were obtained for histological analysis and cytokine
measurement.
Immunohistochemistry
Intestine tissues were fixed in 10% formalin
solution at room temperature for 24 h, and then samples were
embedded in paraffin and 5-µm thick paraffin sections were
prepared. The paraffin sections were deparaffinized and rehydrated
in a descending alcohol series. Antigen retrieval was performed
using a highly compressed heating method in a citrate buffer
solution (95°C, 5–10 min). Endogenous peroxidase activity was
blocked using a solution of methanol-0.3%
H2O2 incubated for 30 min at room
temperature. Slides were incubated with the rabbit anti-mouse
polyclonal antibodies for CD117 and FcεRI (Beijing Bo Orson
Biological Technology Co., Ltd., Beijing, China; dilutions, 1:100
and 1:200, respectively; cat. nos. bs-0672 and bs-13167R,
respectively) overnight at 4°C. Slides were then washed three times
with PBS (pH 7.4) and incubated with a secondary antibody
(anti-rabbit immunoglobulin G; dilution, 1:500; cat. no. bs-0295M;
Beijing Bo Orson Biological Technology Co., Ltd.) for 2–3 h at room
temperature. Finally, 3,3′-diaminobenzidine (Dr. Wuhan's Biological
Engineering Co., Ltd., Wuhan, China) was used for coloration at
room temperature for 5 min. The chromogenic reaction was monitored
every 3 min using an optical microscope. Following washing,
sections were air-dried, dehydrated in ascending concentrations of
ethanol, cleared with xylene and mounted under a cover slip with
Permount. A total of 10 random fields per slide were examined and
analyzed. The images were captured using a microscope (Leica
microsystem GmbH, Wetzlar, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the lungs and thymus of
mice and subsequently purified using a TRIzol kit (Invitrogen,
Thermo Fisher Scientific Inc., Waltham, MA, USA). Nucleic acid
concentration and purity (A260/A280) was measured using a
microplate instrument. Residual genomic DNA was removed by
incubation with RNase-free deoxyribonuclease (Takara Bio, Inc.,
Otsu, Japan). Reverse transcription was performed using a
PrimeScript™ RT reagent kit (Takara Bio, Inc.) following
the manufacturer's protocol. The resulting cDNA was subjected to
qPCR using a Stratagene Mx 3005p real-time PCR Detection system
(Agilent Technologies Inc., Santa Clara, CA, USA) using SYBR Green
II (Takara Bio, Inc.) as a double-strand DNA-specific dye to
quantify the expression of TNF-α, IL-1β, IL-6, IL-10, IL-4, IL-13
and CCL-5 in the lung and thymus of mice. The primer sequences were
as follows: IL-1β forward, (F) 5′-GTCACAAGAAACCATGGCACAT-3′ and
reverse, (R) 5′-GCCCATCAGAGGCAAGGA −3′; IL-4 F,
5′-ACGGAGATGGATGTGCCAAAC-3′ and R, 5′-AGCACCTTGGAAGCCCTACAGA-3′;
IL-6 F, 5′-CTGCAAGAGACTTCCATCCAGTT-3′ and R,
5′-AGGGAAGGCCGTGGTTGT-3′; IL-10 F, 5′-GCCAGAGCCACATGCTCCTA-3′ and
R, 5′-GATAAGGCTTGGCAACCCAAGTAA-3′; IL-13 F,
5′-CGGCAGCATGGTATGGAGTG-3′ and R, 5′-ATTGCAATTGGAGATGTTGGTCAG-3′;
TNF-α F, 5′-GGCTGCCCCGACTACGT-3′ and R,
5′-ACTTTCTCCTGGTATGAGATAGCAAAT-3′; CCL-5 F,
5′-GGAGTATTTCTACACCAGCAGCAAG-3′ and R,
5′-GGCTAGGACTAGAGCAAGCAATGAC-3′; and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) F, 5′-GCACCGTCAAGGCTGAGAAC-3′ and R:
5′-TGGTGAAGACGCCAGTGGA-3′. All primers were synthesized by Bao
Bioengineering Co., Ltd. (Dalian, China). The thermo cycling
conditions of qPCR were as follows: Initial denaturation at 95°C
for 30 sec; followed by 40 cycles at 95°C for 5 sec and 60°C for 30
sec; 1 cycle at 95°C for 60 sec, 55°C for 30 sec, and 95°C for 30
sec. Following the completion of qPCR, specificity was assessed
using a melting curve analysis. The results were quantified using
the 2−ΔΔCq method (20).
GAPDH was utilized as a reference gene.
Cytokine analysis of lung and
thymus
The lungs and thymus of mice were homogenized at 4°C
using tissue protein extraction reagent (Xi'an FengZu Biotechnology
Co., Ltd., Xi'an, China) with a complete mini protease inhibitor
cocktail and complete mini phosphatase inhibitor cocktail tablets
(Roche Applied Science, Pleasanton, CA, USA), using 1 inhibitor
tablet per 10 ml tissue protein extraction reagent. Tissue
homogenates were centrifuged at 12,000 × g for 15 min at 4°C. The
total protein concentration in the supernatants of lung and thymus
homogenates was determined using a BCA kit (Zhuhai Jian Kangyuan
Biopharmaceutical Co., Jian Kangyuan Group Corporation, Guangdong,
China). Supernatants were then diluted using a tissue protein
extraction reagent to a final protein concentration of 500 µg/ml
and stored at 80°C until further use. Cytokines TNF-α, IL-1β, IL-6,
IL-10, IL-4, IL-13 and CCL-5 in the supernatants were measured
using ELISA (eBioscience; Thermo Fisher Scientific Inc., Waltham,
MA, USA; cat. nos. BMS607-3, BMS6002, BMS603-2, BMS614-2, BMS613,
BMS6015 and BMS6009INST, respectively) following the manufacturer's
protocol.
BMMC preparation and cytokine
measurements
BMMCs were obtained from the femurs of 6-week-old
C57BL/6J mice, following a previously described protocol (21). Cells were cultured at 37°C in RPMI
1640 medium (Gibco; Thermo Fisher Scientific) supplemented with 10%
fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.),
10 ng/ml IL-3, 10 ng/ml stem cell factor (SCF), 2 mM L-glutamine, 1
mM sodium pyruvate, 1 mM HEPES, 50 µM 2-mercaptoethanol, 100 U/ml
penicillin and 100 µg/ml streptomycin. IL-3 and SCF were purchased
from PeproTech Inc. (Rocky Hill, NJ, USA). After 4 weeks, flow
cytometry was used to identify whether BMMCs were composed of
>95% MCs. BMMCs were then incubated with fluorescence-labeled
antibodies, including anti-CD117-flourescein isothocyanate (FITC;
dilution, 1:100; cat. no. 553354; BD Biosciences Franklin Lakes,
NJ, USA) and anti-FcεR1-APC (dilution, 1:200, cat. no. 17-5898-82;
eBioscience, Thermo Fisher Scientific Inc.) for 1 h at 4°C.
BMMCs from C57BL/6J mice were treated with 100 µM/l
MA and 1 µg/ml LPS for 24 h at 37°C. The concentration of TNF-α,
IL-6, IL-4, IL-13 and CCL-5 cytokines present in the supernatants
was then quantified using ELISA kits (eBioscience, Thermo Fisher
Scientific Inc.; cat. nos. BMS607-3, BMS603-2, BMS613, BMS6015 and
BMS6009INST) according to the manufacturer's protocol.
Statistical analysis
All the analysis was performed using SPSS software
version 15.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance was used to determine the difference among groups.
Comparisons of all pairs were completed using the Turkey-Kramer
test. Data were expressed as the mean ± standard error of the mean
and P<0.05 was considered to indicate a statistically
significant difference.
Results
MA suppresses LPS-stimulated MC
activation in the intestines of C57BL/6J mice
C57BL/6J mice received four i.m. injections of MA (5
mg/kg) or saline and were then injected with LPS or saline 24 h
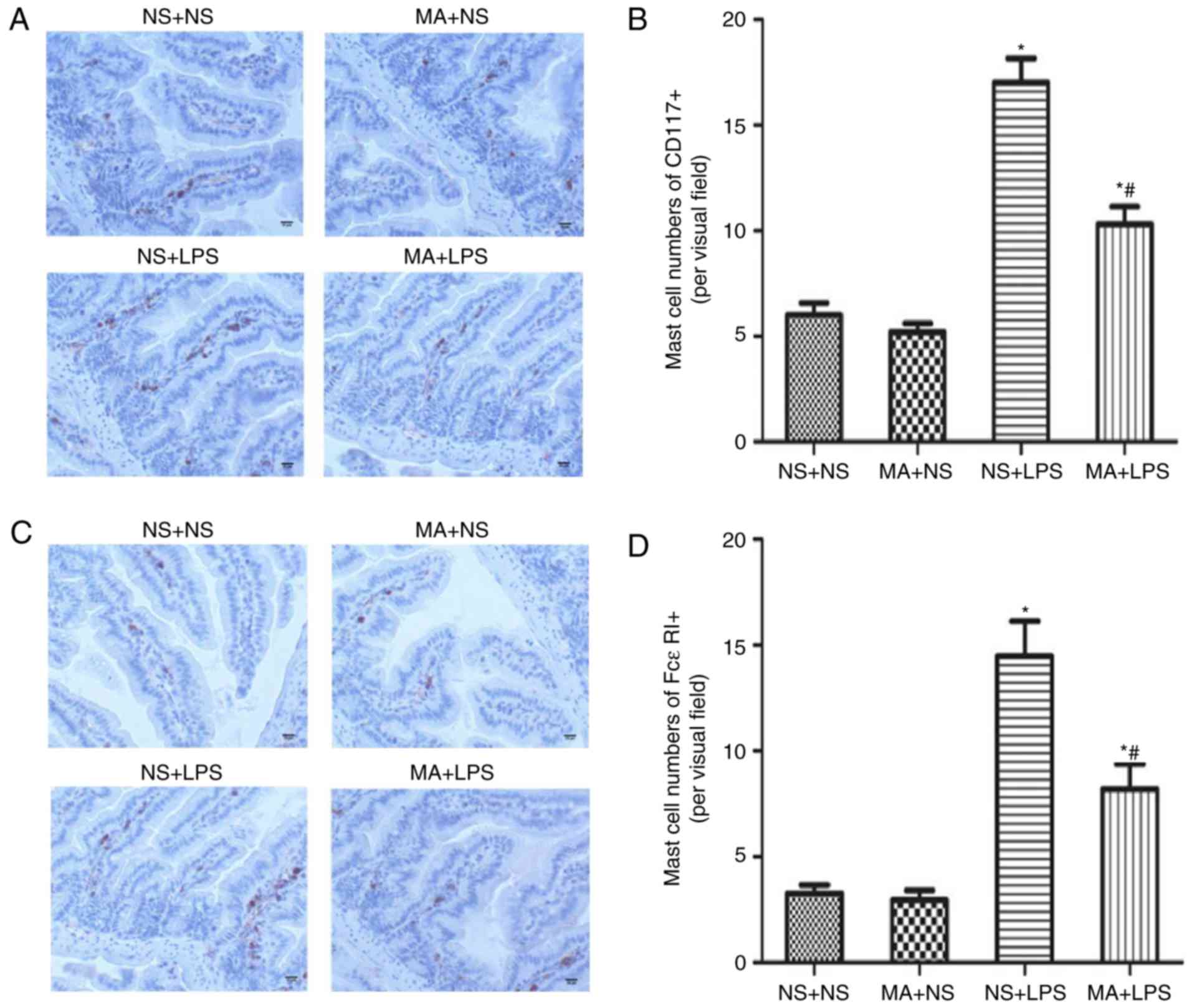
following the first MA injection. Fig.
1 presents the results of immunohistochemical staining for
CD117+ and FcεRI+ in C57BL/6J mice. NS+LPS
mice exhibited a significant increase in intestinal
CD117+ and FcεRI+ compared with the NS+NS
group (P<0.05; Fig. 1B and D). No
effect on CD117+ or FcεRI+ cells was
identified in MA+NS treated mice. However, it was demonstrated that
significantly fewer intestinal CD117+ and
FcεRI+ cells were present in MA+LPS treated mice,
compared with mice that received NS+LPS (P<0.05; Fig. 1B and D).
MA suppresses LPS-stimulated
production of inflammatory cytokines in the lungs of C57BL/6J
mice
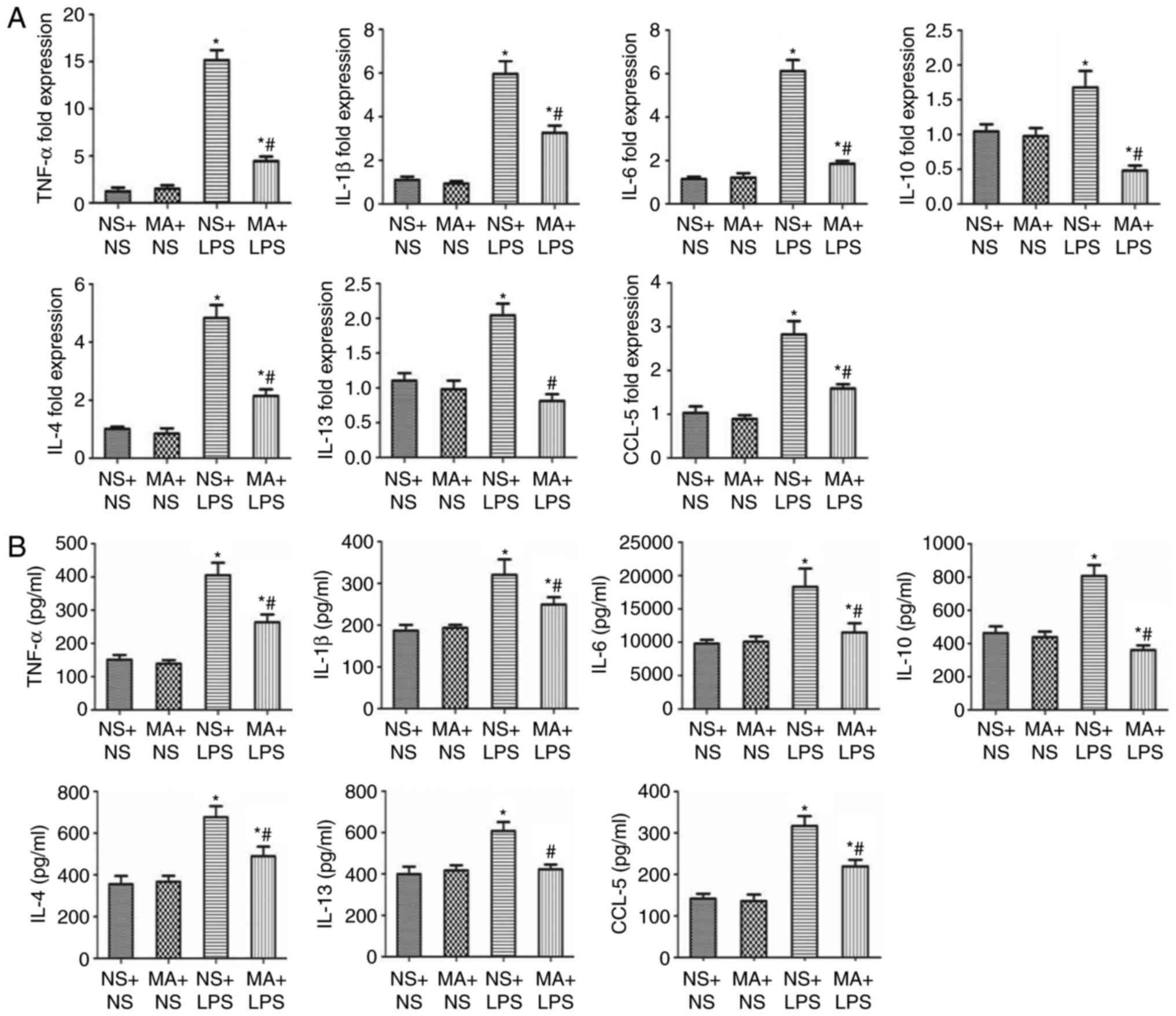
Mice treated with NS+LPS exhibited a significant
increase in the mRNA and protein levels of the pro-inflammatory
cytokines TNF-α, IL-1β and IL-6 in the lungs, compared with NS+NS
mice (P<0.05; Fig. 2). MA+NS
treatment had no effect on cytokine production. However, the mRNA
and protein levels of all the pro-inflammatory cytokines were
significantly decreased in the MA+LPS group, compared with mice in
treated with NS+LPS (P<0.05; Fig.
2). NS+LPS treatment induced a significant increase in IL-10
mRNA and protein expression (P<0.05; Fig. 2). No significant difference in IL-10
production was identified between the MA+NS and the NS+NS groups;
however, MA treatment significantly suppressed the LPS-mediated
increase in IL-10 mRNA and protein expression (P<0.05; Fig. 2).
MCs increase in number following the T helper 2
(Th2) response. Therefore, the effect of MA on LPS-stimulated Th2
cytokine/chemokine production in the lung was assessed. NS+LPS
treatment significantly increased the mRNA and protein expression
of the Th2 cytokines/chemokines IL-4, IL-13 and chemokine ligand-5
(CCL-5) in the lungs of mice (P<0.05; Fig. 2). MA+NS treatment had no significant
effect on the production of Th2 cytokines/chemokines at either
level in the lungs of mice. However, the mRNA and protein levels of
Th2 cytokines/chemokines were significantly decreased in MA+LPS
mice, compared with mice that received NS+LPS (P<0.05; Fig. 2).
MA suppresses the LPS-stimulated
production of inflammatory cytokines in the thymus of C57BL/6J
mice
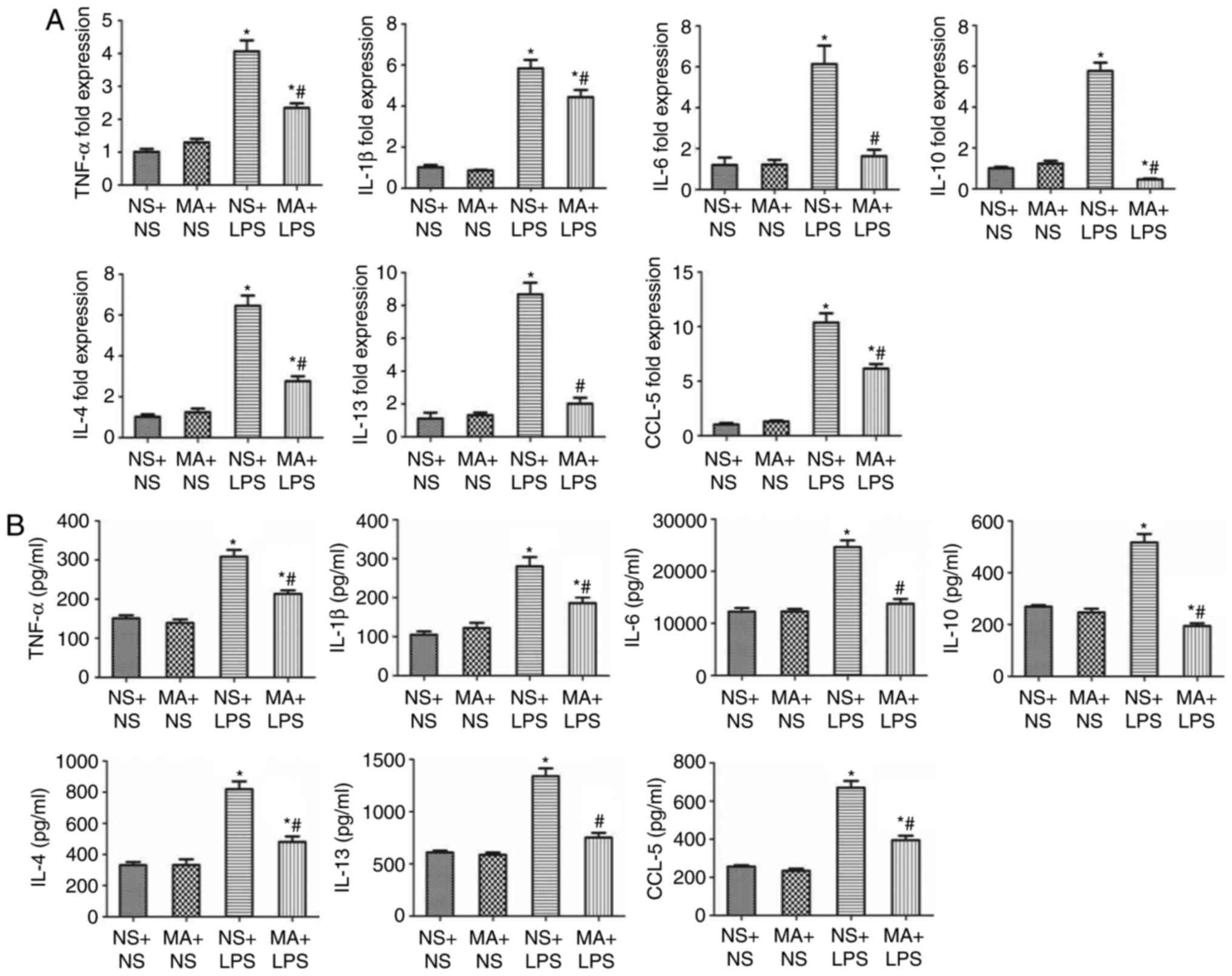
NS+LPS treatment significantly increased the
production of the pro-inflammatory cytokines TNF-α, IL-1β and IL-6
at the mRNA and protein level in the thymus of mice compared with
the NS+NS group (P<0.05; Fig. 3A and
B). No significant differences were identified in the mRNA or
protein expression of thymic pro-inflammatory cytokines between
mice treated with MA+NS and those treated with NS+NS. However, the
mRNA and protein levels of the pro-inflammatory cytokines were
significantly reduced in the thymus of the mice treated with MA+LPS
compared with the NS+LPS group (P<0.05; Fig. 3).
NS+LPS treatment significantly increased the mRNA
and protein levels of the anti-inflammatory cytokine IL-10 in the
thymus of mice (P<0.05; Fig. 3).
MA+NS treatment alone had no effect on thymic IL-10 mRNA or protein
expression. However, MA treatment significantly suppressed the
LPS-stimulated increase in IL-10 mRNA and protein expression in the
thymus of mice compared with the NS+NS group (P<0.05; Fig. 3).
NS+LPS treated mice exhibited a significant increase
in the production of the Th2 cytokine/chemokines IL-4, IL-13 and
CCL-5 at the mRNA and protein levels in the thymus of mice
(P<0.05; Fig. 3). No difference
in the expression of Th2 cytokines/chemokine was observed between
MA+NS treated mice and the NS+NS group. However, the expression of
Th2 cytokine/chemokine mRNA and protein was significantly reduced
in the group treated with MA+LPS, compared with mice that received
NS+LPS treatment (P<0.05; Fig.
3).
MA suppresses the LPS-stimulated
inflammatory cytokine production in the BMMCs of C57BL/6J mice
To verify the suppressive effects of MA on
LPS-stimulated cytokine production, BMMCs were cultured and
supernatant cytokine levels were measured using ELISA. BMMCs
produced significantly higher levels of TNF-α, IL-6, IL-4, IL-13
and CCL-5 in LPS-treated mice compared with NS+NS mice (P<0.05;
Fig. 4). However, a significant
decrease in cytokine/chemokine production was identified in the
BMMCs of mice in the MA+LPS group compared with the LPS group
(P<0.05; Fig. 4). IL-1β and IL-10
were not measured in BMMCs, as the concentrations were too low.
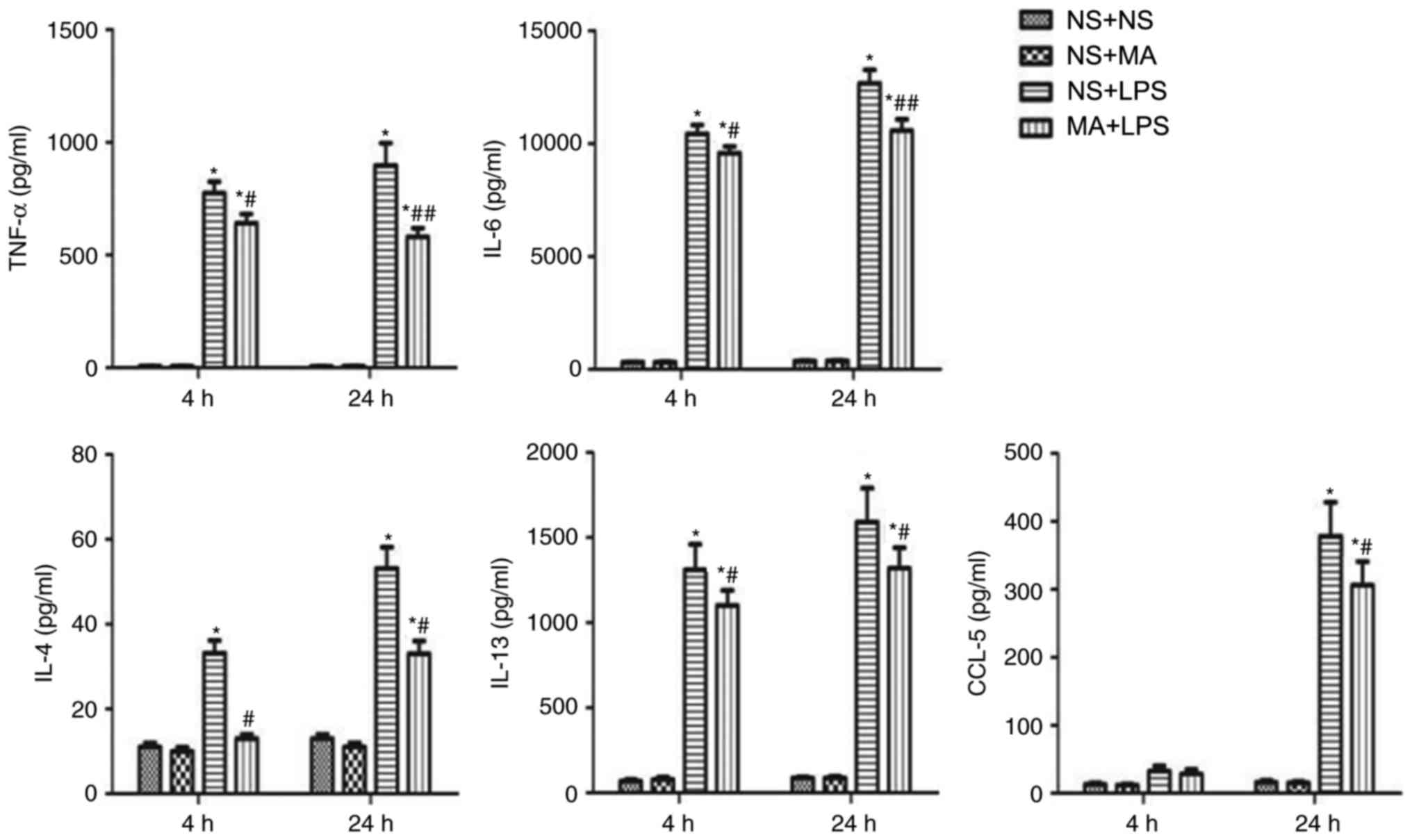 | Figure 4.Effect of MA on LPS-stimulated
cytokine production in the BMMCs of C57BL/6J mice. BMMCs were
treated with NP, MA and/or LPS at the indicated concentrations (MA,
100 µM; LPS, 1 µg/ml; MA+LPS, 100 µM MA+1 µg/ml LPS) for 4 h or 24
h at 37°C. Following treatment, cytokine concentrations in cell
supernatants were evaluated using ELISA. Data are expressed as the
mean ± standard error of the mean of four samples. *P<0.05 vs.
NS+NS group at the same time-point; #P<0.05 vs. LPS
group at the same time-point; ##P<0.01 vs. NS+LPS
group at the same time-point. MA, methamphetamine; LPS,
Lipopolysaccharide; BMMCs, bone marrow-derived mast cells; TNF-α,
tumor necrosis factor α; IL, interleukin; CCL-5, chemokine
ligand-5. |
Discussion
MA is a potent stimulant of the central nervous
system and its abuse causes severe psychological and physical
effects. Previous studies have revealed that MA negatively impacts
immune responses, which may contribute to the higher rate and rapid
progression of certain infections found in drug abusers (22,23). The
present study therefore assessed the effects of MA on MC activation
and cytokine/chemokine production in C57BL/6J mice that received
LPS stimulation.
The results demonstrated that MA treatment
suppressed LPS-mediated MC activation in the intestines of C57BL/6J
mice. MCs are concentrated at interfaces between the host and
environment, including the intestinal tract, where they limit the
spread of invading pathogens (24).
MCs are considered to function as effecter cells during the innate
and adaptive immune responses (25–27).
CD117 and FcεRI are primary MC surface receptors associated with MC
activation (7,12,28–30). The
present study demonstrated that MA+LPS treatment significantly
decreased the expression of intestinal CD117 and FcεRI. However,
the effect of MA and LPS on MCs themselves was not assessed in the
present study. Therefore, further experiments should be conducted
to investigate the expression profiles of MC cytokines stimulated
with varying doses of LPS and MA.
Previous studies have demonstrated that LPS induces
MCs to secrete cytokines, thus promoting the innate and adaptive
immune responses (31,32). The most studied MC derived cytokine
in the innate immune response is TNF-α, which induces the early
influx of neutrophils and clearance of pathogens (33–35). It
has been demonstrated that IL-6 produced by MCs increases the
survival rates of patients with Klebsiella pneumoniae and
sepsis by stimulating neutrophil activity (36). IL-10 is an anti-inflammatory cytokine
which suppresses the synthesis of inflammatory cytokines, including
TNF-α and IL-6 (37). It has been
demonstrated that MCs can mediate negative immunomodulatory
functions by producing IL-10 in response to chronic irradiation
with UVB light (38). In addition,
certain MC derived Th2-type cytokines, including IL-4 and IL-13,
influence B-cell development and function (11). MCs are also an important source of
chemokines, including CCL5, which is involved in Th2-type responses
(10). The results of the present
study demonstrate that MA treatment suppresses MC derived,
LPS-stimulated, inflammatory cytokine production in the lungs and
thymus of C57BL/6J mice. It was also revealed that MA has a
suppressive effect on the production of LPS-stimulated inflammatory
cytokines in the BMMCs of mice. The results indicated that MA abuse
leads to immunosuppressive effects, which may increase the risk of
infection. However, the present study did not assess the effect of
MA on other immune cells. Further studies are required to improve
understanding regarding the effects of MA on immune function.
In conclusion, the present study demonstrated that
MA may be involved in the regulation of LPS-stimulated MCs
activation and cytokine production. This may be responsible for the
immune dysfunction and increased susceptibility to infectious
diseases associated with MA abuse. Further studies are required to
explore the mechanism underlying the immunomodulatory effects of
MA.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81273196 and
81430048) and the Fundamental Research Funds for the Central
Universities of China (grant no. xjj2016109).
Glossary
Abbreviations
Abbreviations:
|
MA
|
methamphetamine
|
|
LPS
|
lipopolysaccharide
|
|
MCs
|
mast cells
|
|
NS
|
normal saline
|
|
BMMCs
|
bone marrow-derived mast cells
|
|
SCF
|
stem cell factor
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL
|
interleukin
|
|
CCL-5
|
chemokine ligand-5
|
References
|
1
|
Chomchai C and Chomchai S: Global patterns
of methamphetamine use. Curr Opin Psychiatr. 28:269–274. 2015.
View Article : Google Scholar
|
|
2
|
Mravčík V, Florián Z, Nečas V and Štolfa
J: Infectious and other somatic comorbidity in problem drug
users-results of a cross-sectional study with medical examination.
Epidemiol Mikrobiol Imunol. 65:56–62. 2016.(In Czech). PubMed/NCBI
|
|
3
|
Patel D, Desai GM, Frases S, Cordero RJ,
DeLeon-Rodriguez CM, Eugenin EA, Nosanchuk JD and Martinez LR:
Methamphetamine enhances Cryptococcus neoformans pulmonary
infection and dissemination to the brain. MBio. 4(pii): e00400–13.
2013.PubMed/NCBI
|
|
4
|
Peerzada H, Gandhi JA, Guimaraes AJ,
Nosanchuk JD and Martinez LR: Methamphetamine administration
modifies leukocyte proliferation and cytokine production in murine
tissues. Immunobiology. 218:1063–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arthur G and Bradding P: New developments
in mast cell biology: Clinical implications. Chest. 150:680–693.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamanishi Y and Karasuyama H: Basophils
and mast cells in immunity and inflammation. Semin Immunopathol.
38:535–537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galli SJ, Kalesnikoff J, Grimbaldeston MA,
Piliponsky AM, Williams CM and Tsai M: Mast cells as ‘tunable’
effector and immunoregulatory cells: Recent advances. Annu Rev
Immunol. 23:749–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ryan JJ, Kashyap M, Bailey D, Kennedy S,
Speiran K, Brenzovich J, Barnstein B, Oskeritzian C and Gomez G:
Mast cell homeostasis: A fundamental aspect of allergic disease.
Crit Rev Immunol. 27:15–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galli SJ, Maurer M and Lantz CS: Mast
cells as sentinels of innate immunity. Curr Opin Immunol. 11:53–59.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marshall JS: Mast-cell responses to
pathogens. Nat Rev Immunol. 4:787–799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galli SJ, Nakae S and Tsai M: Mast cells
in the development of adaptive immune responses. Nat Immunol.
6:135–142. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abramson J and Pecht I: Regulation of the
mast cell response to the type 1 Fc epsilon receptor. Immunol Rev.
217:231–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Supajatura V, Ushio H, Nakao A, Akira S,
Okumura K, Ra C and Ogawa H: Differential responses of mast cell
Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin
Invest. 109:1351–1359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sandig H and Bulfone-Paus S: TLR signaling
in mast cells: Common and unique features. Front Immunol.
3:1852012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leal-Berumen I, Conlon P and Marshall JS:
IL-6 production by rat peritoneal mast cells is not necessarily
preceded by histamine release and can be induced by bacterial
lipopolysaccharide. J Immunol. 152:5468–5476. 1994.PubMed/NCBI
|
|
16
|
Gupta AA, Leal-Berumen I, Croitoru K and
Marshall JS: Rat peritoneal mast cells produce IFN-gamma following
IL-12 treatment but not in response to IgE-mediated activation. J
Immunol. 157:2123–2128. 1996.PubMed/NCBI
|
|
17
|
Moiseeva EP and Bradding P: Mast cells in
lung inflammation. Adv Exp Med Biol. 716:235–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marinova T, Philipov S and Aloe L: Nerve
growth factor immunoreactivity of mast cells in acute involuted
human thymus. Inflammation. 30:38–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buchanan JB, Sparkman NL and Johnson RW: A
neurotoxic regimen of methamphetamine exacerbates the febrile and
neuroinflammatory response to a subsequent peripheral immune
stimulus. J Neuroinflammation. 7:822010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue L, Li X, Ren HX, Wu F, Li M, Wang B,
Chen FY, Cheng WY, Li JP, Chen YJ and Chen T: The dopamine D3
receptor regulates the effects of methamphetamine on LPS-induced
cytokine production in murine mast cells. Immunobiology.
220:744–752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Najera JA, Bustamante EA, Bortell N,
Morsey B, Fox HS, Ravasi T and Marcondes MC: Methamphetamine abuse
affects gene expression in brain-derived microglia of SIV-infected
macaques to enhance inflammation and promote virus targets. BMC
Immunol. 17:72016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loftis JM: Commentary: Methamphetamine
mediates immune dysregulation in a murine model of chronic viral
infection. Front Microbiol. 6:14732015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abraham SN and St John AL: Mast
cell-orchestrated immunity to pathogens. Nat Rev Immunol.
10:440–452. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galli SJ, Grimbaldeston M and Tsai M:
Immunomodulatory mast cells: Negative, as well as positive,
regulators of immunity. Nat Rev Immunol. 8:478–486. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stelekati E, Orinska Z and Bulfone-Paus S:
Mast cells in allergy: Innate instructors of adaptive responses.
Immunobiology. 212:505–519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rao KN and Brown MA: Mast cells:
Multifaceted immune cells with diverse roles in health and disease.
Ann N Y Acad Sci. 1143:83–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gilfillan AM and Tkaczyk C: Integrated
signalling pathways for mast-cell activation. Nat Rev Immunol.
6:218–230. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kinet JP: The high-affinity IgE receptor
(Fc epsilon RI): From physiology to pathology. Annu Rev Immunol.
17:931–972. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rivera J and Gilfillan AM: Molecular
regulation of mast cell activation. J Allergy Clin Immunol.
117:1214–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dietrich N, Rohde M, Geffers R, Kröger A,
Hauser H, Weiss S and Gekara NO: Mast cells elicit proinflammatory
but not type I interferon responses upon activation of TLRs by
bacteria. Proc Natl Acad Sci USA. 107:pp. 8748–8753. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takenaka H, Ushio H, Niyonsaba F,
Jayawardana ST, Hajime S, Ikeda S, Ogawa H and Okumura K:
Synergistic augmentation of inflammatory cytokine productions from
murine mast cells by monomeric IgE and toll-like receptor ligands.
Biochem Biophys Res Commun. 391:471–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Henz BM, Maurer M, Lippert U, Worm M and
Babina M: Mast cells as initiators of immunity and host defense.
Exp Dermatol. 10:1–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maurer M, Theoharides T, Granstein RD,
Bischoff SC, Bienenstock J, Henz B, Kovanen P, Piliponsky AM, Kambe
N, Vliagoftis H, et al: What is the physiological function of mast
cells? Exp Dermatol. 12:1–910. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maurer M and Metz M: The status quo and
quo vadis of mast cells. Exp Dermatol. 14:923–929. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sutherland RE, Olsen JS, McKinstry A,
Villalta SA and Wolters PJ: Mast cell IL-6 improves survival from
Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J
Immunol. 181:5598–5605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Glocker EO, Kotlarz D, Klein C, Shah N and
Grimbacher B: IL-10 and IL-10 receptor defects in humans. Ann N Y
Acad Sci. 1246:102–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grimbaldeston MA, Nakae S, Kalesnikoff J,
Tsai M and Galli SJ: Mast cell-derived interleukin 10 limits skin
pathology in contact dermatitis and chronic irradiation with
ultraviolet B. Nat Immunol. 8:1095–1104. 2007. View Article : Google Scholar : PubMed/NCBI
|