Introduction
Gastric cancer is the second leading cause of
cancer-associated mortality worldwide due to its high incidence.
Various inherited and environmental factors, including genetic
characteristics, infectious agents and dietary habits serve an
essential role in the development of gastric cancer (1). Furthermore, signaling pathways,
including the epidermal growth receptor, vascular endothelial
growth factor, phosphoinositide 3-kinase/protein kinase
b/mechanistic target of rampamycin (PI3K/AKT/mTOR) and hepatocyte
growth factor/hepatocyte growth factor receptor signaling pathways
have been demonstrated as being associated with gastric cancer in a
molecular classification study (2).
Surgical resection and chemo-radiation are the most efficient
strategies for treating gastric cancer (3). However, due to the lack of convenient,
noninvasive biomarkers for routine population screening, diagnosis
of early stage gastric cancer is challenging in the majority of
patients (3). In addition,
peritoneal dissemination and local metastases occur in the late
stages of gastric cancer (3). A
previous study suggested that microRNAs (miRNAs or miRs) are
important regulators of the oncogenesis pathway and may be used as
clinical biomarkers (4). The
emergence of miRNAs serving as biomarkers has provided a novel
method of diagnosing cancer and determining the prognosis for
various types of cancer, including gastric cancer (3).
The mechanism of epigenetic regulation and control
does not include sequence alteration of the coding gene and is
important in post-translational regulation as an independent
process (5). It is important in
tumor formation and development, especially with the abnormal
expression and dysfunction of miRNA, which is one of the components
of the p53 tumor suppressor network (6,7). miR-34a
is a member of the miRNA family, which has tumor suppressor
properties, promotes apoptosis and cell arrest, and has an aging
function (8). miR-34a has gradually
drawn considerable attention due to its tumor suppressor effect. It
serves an essential role as a tumor suppressor gene in many types
of tumors through impacting various potential tumor associated
genes, including sirtuin 1, SNAIL and zinc finger E-box-binding
homeobox 1 (9,10). The present study aimed to compare the
difference in expression of miR-34a between gastric adenocarcinoma
and paired paricarcinomatous, various gastric cancer cells and
gastric epithelial cells in order to determine the expression of
miR-34a in human gastric cancer and whether miR-34a targets its
downstream gene to affect the biological function of gastric
cancer. A previous study demonstrated that miR-34a targets receptor
tyrosine kinases and platelet-derived growth factor receptor to
inhibit gastric cancer via the PI3K/Akt pathway (11). Therefore, in the present study
sirtuin 1 (SIRT1) was predicted as the potential tumor associated
target gene of miR-34a, as bioinformatics technology indicated that
it was associated with cell proliferation and apoptosis. SIRT1 is a
nicotinamide adenine dinucleotide (NAD)-dependent deacetylase that
regulates cellular processes, including energy metabolism,
cell-cycle progression, apoptosis, aging and migration (7). Therefore, SIRT1-3′-untranslated region
(UTR) recombinant plasmid and luciferase reporter vector were
constructed in the present study to verify the prediction and the
impact of miR-34a on cell proliferation and apoptosis of gastric
cancer cells by targeting SIRT1 was subsequently investigated.
Materials and methods
Gastric cancer and pericarcinomatous
tissue specimens
Gastric cancer and pericarcinomatous tissue
specimens were obtained from the First Affiliated Hospital of
Bengbu Medical College (Bengbu, China). Gastric cancer samples were
obtained from surgical cases immediately following diagnosis by
gastroscope and pathology biopsy between January 2015 and December
2015. None of the patients were treated via radiotherapy and
chemotherapy prior to surgery. Cancer tissues were derived from
tumor lesions and pericarcinomatous tissue specimens were taken
from normal stomach tissue >5 cm away from the tumor edge. All
specimens were stored in liquid nitrogen in eppendorf tubes filled
with RNA preserving fluid (RNAStore; cat no. DP408-02; Tiangen
Biotech., Co., Ltd., Beijing, China) following sampling. Clinical
data were recorded for pathology research and subsequent
statistical analysis. A total of 38 patients with confirmed gastric
adenocarcinoma following surgical diagnosis were selected for the
present study. There were 27 male patients and 11 female patients,
and the age range was 32–79 years. Gastric cancer stages were
identified according to the 7th edition of the Tumor Node
Metastasis Staging System issued by International Union Against
Cancer in 2010 (12), which was used
for subsequent analysis between clinical pathology parameters and
miR-34a expression. The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Bengbu Medical
College and written informed consent was obtained from all
participants.
Cell strains, vectors and
reagents
Human normal gastric epithelial cell line GES-1,
human gastric cancer strain AGS, SGC-7901, MKN-45, BGC-823 and 293
cells were purchased from Shanghai Institute of Biochemistry and
Cell Biology (Shanghai, China). Escherichia coli strain DH5α
(BeNa Culture Collection) were cultured in LB medium at 37°C for 24
h (BeNa Culture Collection) and stored at −20°C in the laboratory.
The TRIzoI RNA extraction kit was purchased from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and the reverse
transcription kit M-MLV was obtained from Promega Corporation
(Madison, WI, USA). A SYBR premix ex taq kit was purchased from
Axygen Scientific, Inc. (Union City, CA, USA), and the upstream and
downstream primers of the targeting gene and internal reference
genes were purchased from Guangzhou Ribo Bio Co., Ltd. (Guangzhou,
China). Over-expression hsa-miR-34a vector, GV272 luciferase report
empty vector and GV259, pHelper 1.0, pHelper 2.0 lentiviral empty
vector were purchased from Shanghai Genechem Co., Ltd. (Shanghai,
China). The dual-luciferase reporter assay system and plasmid
extraction kit were purchased from Promega Corporation and the
X-tremegene HP transfection reagents were purchased from Roche
Applied Science (Penzberg, Germany). Lipofectamine 2000 was
purchased from Invitrogen; Thermo Fisher Scientific, Inc.
XbaI, XhoI and BamHI restriction enzymes and
T4DNA ligase were purchased from New England Biolabs, Inc.
(Ipswich, MA, USA). The In-Fusion™ PCR Cloning kit was
purchased from Clontech Laboratories, Inc. (Mountainview, CA, USA).
The agarose gel recovery kit was purchased from Takara Bio, Inc.
(Otsu, Japan). Rabbit anti-human SIRT1 antibody was obtained from
Cell Signaling Technology, Inc. (Danvers, MA, USA), GAPDH antibody
(cat no. AF0006), SIRT1 antibody (cat no. AF1267), horseradish
peroxide conjugated goat-anti-rabbit Immunoglobulin g (H+L)
secondary antibody (cat no. A0208) and the ECL kit were purchased
from Beyotime Institute of Biotechnology (Haimen, China). The MTT
kit was purchased from Beijing Dingguo Changsheng Biotechnology
Co., Ltd. (Beijing, China) and the cell apoptosis kit was purchased
from eBioscience (Thermo Fisher Scientific, Inc.).
miR-34a expression in gastric cancer
and normal tissue detected by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzoI from an RNA extraction kit was used to
isolate RNA from the gastric cancer and normal tissues according to
the manufacturer's protocol. A Nanodrop 2000 spectrophotometer
(Nanodrop; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA) was
used to identify the total RNA concentration. The M-MLV kit was
used to synthesize cDNA through RT and U6 was adopted as the
internal reference according to the manufacturer's protocol.
Upstream and downstream primer sequences were as follows: miR-34a,
forward 5′-TGGCAGTGTCTTAGCTGGTTGT-3′ and reverse
5′-CATTGGTGTCGTTGTGCTCT-3′; and U6, forward
5′-CTCGCTTCGGCAGCACATATA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′,
and 2X SYBR premix ex taq was used for qPCR. qPCR thermocycling
conditions were set as 98°C for 5 min, 96°C for 30 sec, 62°C for 30
sec and 73°C for 1.5 min for 35 cycles and 4°C for storage. The
experiment was performed in triplicate and PCR quantification was
performed using the 2−ΔΔCq method (13).
miR-34a target gene prediction
miR-34a target gene predication was performed using
the miRWalk database (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/index.html)
and the overlap of the miRanda (http://34.236.212.39/microrna/home.do), TargetScan
(http://www.targetscan.org/vert_71/)
and PICTAR (http://pictar.mdc-berlin.de/) online analysis results
was selected for further analysis.
SIRT1 gene and 3′-UTR luciferase
reporter recombinant plasmid construction
The wild type 3′-UTR gene sequence and mutation type
SIRT1 gene sequence was downloaded from Genbank (https://www.ncbi.nlm.nih.gov/nuccore/215982795/?Report=genbank)
by inputting accession number NM_012238. Double enzyme digestion of
the target gene SIRT1 and GV272 luciferease reporter vector was
performed using XbaI/XbaI. The exchanging reaction
was performed in ddH2O and the In-Fusion exalter enzyme
system at 25°C for 30 min and 42°C for 15 min. The GV272
self-ligation empty vector was used as a negative control and the
GV272 vector binding to GAPDH was used as a positive control.
Following transfection of the vectors into the competent DH5α
monoclonal colony, the cells were enzyme digested and purified
followed by PCR amplification and 10% agarose gel electrophoresis.
The Taq DNA polymerase (cat no. D7205) was purchased from Beyotime
Institute of Biotechnology (Haimen, China). Additionally, the
primers used in PCR were as follows: miR-34a, forward,
5′CTTGAACTCCTGGGGCCTGAAG3′ and reverse, 5′GCCAAAGAAACACTCACAGCT3′;
and SIRT1, forward, 5′TAGCCTTGTCAGATAAGGAAGGA3′ and reverse,
5′ACAGCTTCACAGTCAACTTTGT3′. The amplification conditions consisted
of 2 min at 95°C, followed by 40 cycles of 95°C for 15 sec and 60°C
for 30 sec and storage at 4°C. The agarose gel electrophoresis was
stained using ethidium bromide and visualized under UV light at 254
nm. Fragment sequencing was conducted by Beijing SBS Genetech Co.,
Ltd (Beijing, China).
Cell co-transfection and luciferase
activity verification
Using trypsin, 293 cells growing in log-phase were
harvested and 1×105 cells were transfected. Transfection
was performed in triplicate in 96-well plates. Plasmids were
transfected using the X-tremeGene HP DNA transfection reagent and
100 µl opti-MEM (cat no. 31985-070, Shanghai Bai Gen Biological
Technology Co., Ltd., Shanghai, China) at a ratio of 1 µg plasmid
to 2 µl X-tremeGene HP reagent. Following incubation at 37°C for 6
h, the medium was replaced with fresh culture medium containing 10%
bovine serum. Following incubation for 24 h, the
Dual-Luciferase® Reporter assay system (cat no. E1910;
‘Promega Corporation) was used to verify the transfection
efficiency according to the manufacturer's protocols. Experimental
groups are presented in Table I.
 | Table I.Cell groups co-transfected with
recombinant vectors. |
Table I.
Cell groups co-transfected with
recombinant vectors.
| Group | Transfection
vectors |
|---|
| Group 1 | 3′-UTR empty
plasmid + miRNA empty plasmid |
| Group 2 | 3′-UTR empty
plasmid + miR-34a over-expression plasmid |
| Group 3 | SIRT1-3′-UTR
recombinant plasmid + miRNA empty plasmid |
| Group 4 | SIRT1-3′-UTR
recombinant plasmid + miR-34a over-expression plasmid |
| Group 5 | SIRT1-3′-UTR mutant
plasmid + miRNA empty plasmid |
| Group 6 | SIRT1-3′-UTR mutant
plasmid + miR-34a over-expression plasmid |
hsa-miR-34a over-expressing lentiviral
vector and cell transfection construction
The hsa-miR-34a over-expression lentiviral vector
system was constructed via combination ligation (hsa-miR-34a,
5′-UGGCAGUGUCUUAGCUGGUUGU-3′; negative control,
5′-TTCTCCGAACGTGTCACGTCTC-3′). The pHelper 1.0, pHelper 2.0
lentiviral vectors and has-miR-34a plasmids were purchased from
Shanghai Genechem Co., Ltd. (Shanghai, China). SGC-7901 cells
(5×106) were transfected with lentivirus using
X-tremegene HP (Roche Diagnostic, Basel, Switzerland) and incubated
in opti-MEM medium at 37°C with 5% CO2 for 6 h. The
cells were grouped into three groups: Group A transfected with 10
µl lentiviral stock solution; Group B transfected with 1 µl
lentivirus and Group C transfected with 0.1 µl lentivirus. The
three concentration of lentivirus were set to avoid the 100%
positive and 100% negative of lentiviral transfection. Following
transfection and incubation for 3 days, the cells were employed for
the subsequent experiment. The CON group was defined as the
non-transfected group; the NC group was defined as the negative
control group transfected with empty lentiviruses; and the miR-34a
group was the group transfected with miR-34a overexpression
vectors. TCID50 (Tissue Culture Infective Dose) was used to
calculate the lentiviral titer (14).
Determination of the expression of
SIRT1 mRNA by RT-qPCR
Total RNA from the cells transfected with miR-34a
plasmids was extracted using a TRIzoI RNA extraction kit, and cDNA
was synthesized by reverse transcription with GoTaq® DNA
Polymerase (cat no. M3005, Promega Corporation, Madison, WI, USA)
and amplified by qPCR using the M-MLV RT kit (cat no. 28025-013;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The temperature protocol was 93°C for 12
min for reverse transcription. A probe with FAM fluorophore at the
5′ end and TAMARA fluorophore at the 3′ end from Shanghai Shinegene
Molecular Biotechnology (Shanghai, China) was employed for
detection. The primer sequences used were as follows: SIRT1,
forward 5′-GACTTCAGGTCAAGGGAT-3′ and reverse
5′-CGTGTCTATGTTCTGGGTA-3′; and GAPDH, forward
5′-TGACTTCAACAGCGACACCCA-3′ and reverse
5′-CACCCTGTTGCTGTAGCCAAA-3′. The PCR reaction was set as 96°C for 5
min, 95°C for 30 sec, 66°C for 30 sec and 76°C for 1 min for 32
cycles and 4°C for storage. The expression of SIRT1 mRNA was
relatively quantified using the 2−ΔΔCq method (13). This experiment was performed in
triplicate and there were three wells per row for each
experiment.
SIRT1 protein expression determined by
western blot analysis
Cells were lysed using radioimmunoprecipitation
buffer (cat no. 211-40; AmyJet Scientific Co., Ltd., Wuhan, China)
and total protein concentration was verified using the BCA method.
A total of 2 µg per lane protein was used for the SDS-PAGE assay
with 10% gel electrophoresis. Proteins were then transferred to
PVDF membranes and blocked with 5% milk at room temperature for 1
h. The PVDF membrane was incubated with rabbit anti-human SIRT1
primary antibody (cat no. AF0282; 1:10,000; Beyotime Institute of
Biotechnology) for 24 h at 4°C. GAPDH was used as the reference
protein stated above. The secondary antibody (1:10,000) was added
to the membrane and incubated for 1 h with agitation at room
temperature. An ECL kit was used to visualize the blots and the gel
image was recorded by a Chemiluminescent imager (Thermo Fisher
Scientific, Inc.). Version 1.5.2 ImageJ analytical software
(National Institutes of Health, Bethesda, MD, USA) was used for
quantification. Each sample was tested three times
independently.
Cell proliferation determined using an
MTT assay
The inoculated SGC-7901 cells (5×105)
were plated on 96-well plates following trypsin digestion and cell
counting, and 5 wells in a row were used for each sample. Cells
were incubated at 37°C with 5% CO2 for 5 days. MTT stain
was performed according to the manufacturer's protocol. Formazan
was dissolved by DMSO. Then, a microplate reader was used to
measure the OD490 value of solution.
Cell apoptosis profile detection by
flow cytometry
SGC-7901 cells (5×105) were digested with
trypsin and samples were plated in 96-well plates in triplicate. 5
µl Annexin V-APC (Wuhan Bot Biological Technology Co., Ltd., Wuhan,
China) was added to 100 µl cell suspension and incubated at room
temperature in the dark for 15 min. The cell apoptosis profile of
SGC-7901 was detected by flow cytometry using a flow cytometer
(FACScan; BD Biosciences). Version 2.5 WinMDI from Purdue
University Cytometry Laboratories (West Lafayette, USA) was
employed for data analysis.
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. Data are expressed as the mean ±
standard deviation and non-normal distribution data was shown by
boxplot. A Student t-test was adopted for paired comparison and
one-way ANOVA analysis of variance followed by Tukey's test were
performed for comparisons of multiple groups. According to the
analysis of variance homogeneity by Bartlett's test, the
corresponding t- and P-values were selected on the basis of
P<0.05 indicating a statistically significant difference.
Results
Expression of miR-34a in gastric
cancer tissues
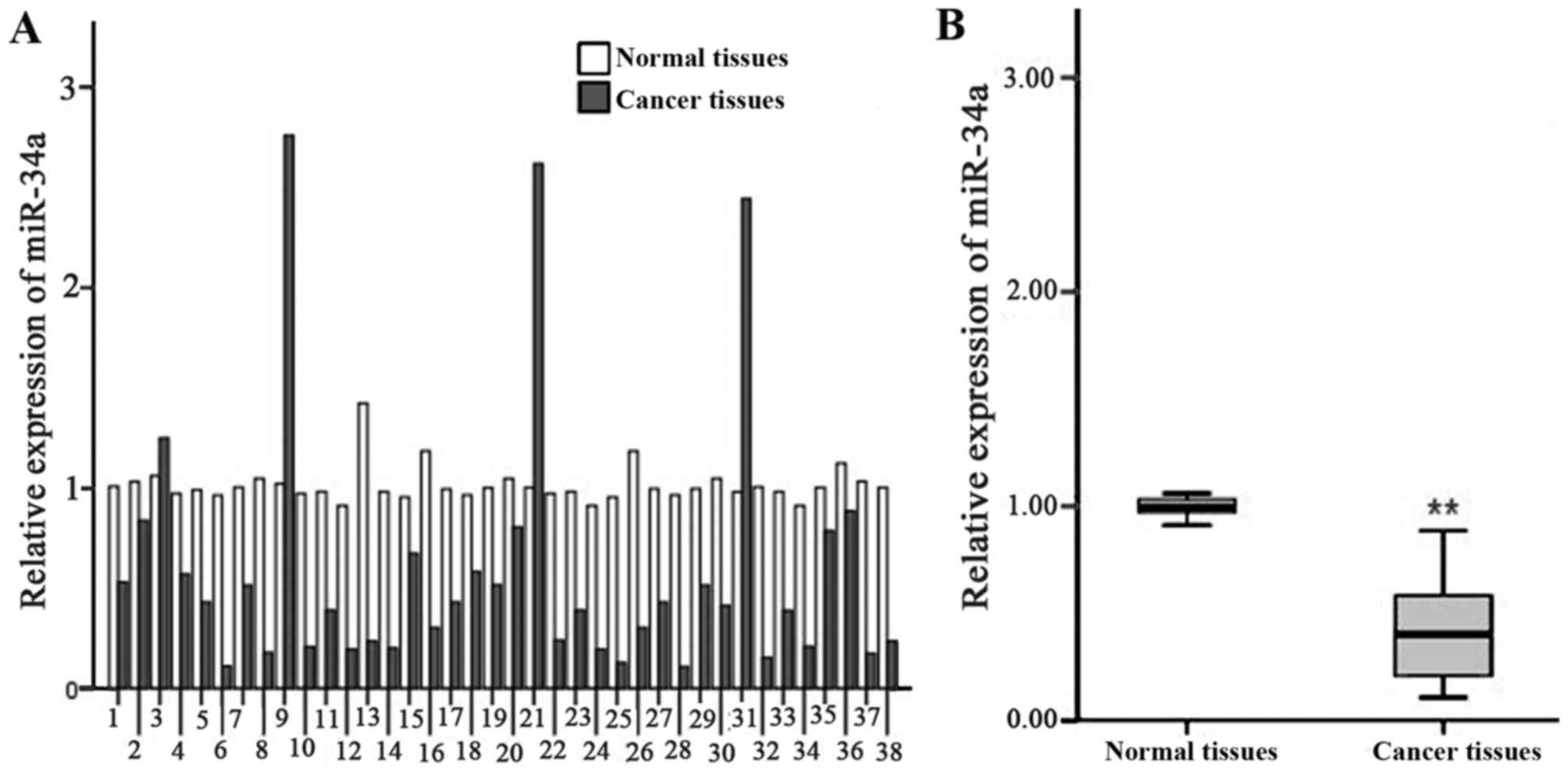
The results of RT-qPCR analysis indicated that there
was a significant decrease in the relative expression of miR-34a in
gastric cancer tissue compared with that in paired
pericarcinomatous tissue (0.59±0.65 vs. 1.01±0.09; t=4.007;
P<0.01; Fig. 1). T-test analysis
on the pathology parameters and relative expression of miR-34a
suggested that the expression of miR-34a was significantly
decreased in low differentiated gastric cancer cells N2, N3
compared with that of high differentiated and middle differentiated
gastric cells N0, N1 (P<0.01). There was no distinct association
between tumor size and infiltration degree with the expression of
miR-34a (Table II).
 | Table II.Association between pathological
parameters and miR-34a expression level. |
Table II.
Association between pathological
parameters and miR-34a expression level.
| Pathology
parameters | Cases (n) | Relative expression
of miR-34a in gastric cancer cells | P-value |
|---|
| Tumor size |
|
| 0.643 |
| ≥5
cm | 11 | 0.67±0.77 |
|
| <5
cm | 27 | 0.56±0.61 |
|
| Degree of
differentiation |
|
| 0.004a |
| High,
middle differentiation | 15 | 1.05±0.84 |
|
| Low
differentiation | 23 | 0.29±0.14 |
|
| Infiltration
degree |
|
| 0.804 |
| T1,
T2 | 13 | 0.55±0.58 |
|
| T3,
T4 | 25 | 0.61±0.69 |
|
| Lymphatic
metastasis |
|
| 0.007a |
| N0,
N1 | 16 | 0.98±0.85 |
|
| N2,
N3 | 22 | 0.31±0.18 |
|
Relative expression of miR-34a in
human gastric cancer cells and GES
The results of RT-qPCR indicated that the relative
expression of miR-34a in human gastric cell strains AGS (0.24±0.01;
P<0.05), SGC-7901 (0.03±0.00; P<0.01), MKN-45 (0.31±0.04;
P<0.05) and BGC-823 (0.25±0.05; P<0.05) was significantly
decreased compared with GES cells (0.98±0.07; Fig. 2). These differences were significant
with respective t-values of t=17.522, 22.879, 13.836 and 14.393.
The biggest decrease in the relative expression of miR-34a compared
with GES cells was in the gastric cancer cell line SGC-7901
(P<0.01). Therefore, this cell line was selected for the
subsequent lentivirus infection and cell viability tests.
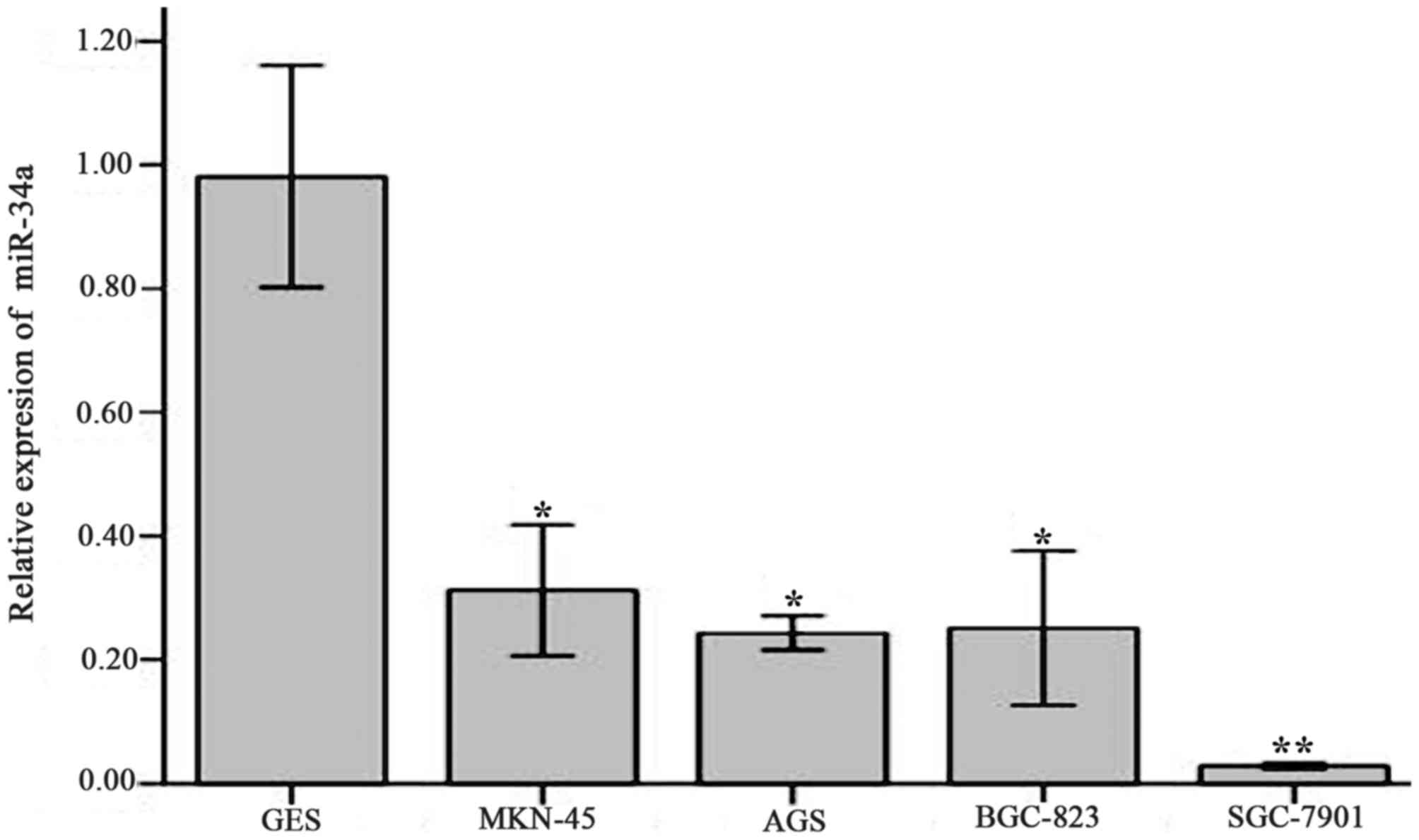 | Figure 2.Relative expression of miR-34a in
various cell strains compared with GES. The relative expression was
determined using reverse transcription-quantitative polymerase
chain reaction. miR-34a relative expression in cell strains GES,
MNK-45, AGS, BGC-823, SGC-7901 was 0.98±0.07, 0.31±0.04, 0.24±0.01,
0.25±0.05 and 0.03±0.00, respectively. Values are expressed as the
mean ± standard deviation and experiments were performed in
triplicate. *P<0.05, **P<0.01 vs. GES. miR-34a, microRNA-34a;
GES, human normal gastric mucosa epithelial cells. |
miR-34a target gene prediction
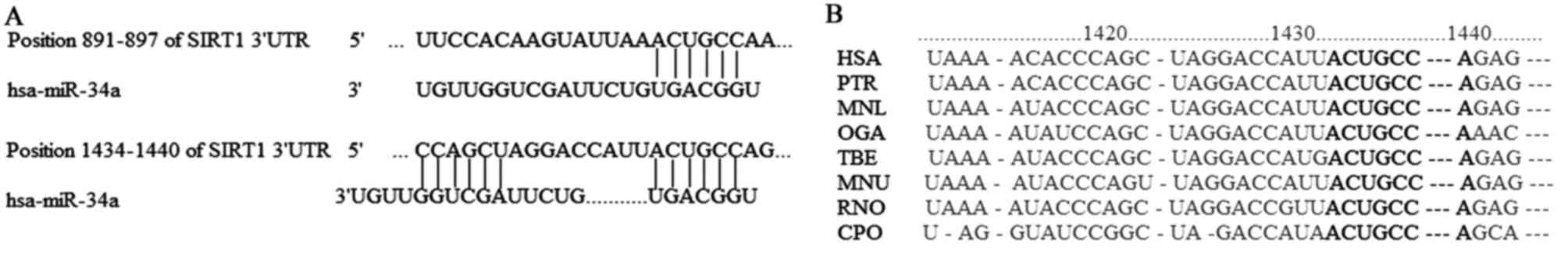
The results of the bioinformatic analysis indicated
that there were two miR-34a binding sites in the SIRT1-3′-UTR
region from 891–897 bp and 1,434-1,440 bp, which are associated
with tumor proliferation and apoptosis. The free energy of binding
was −20.1 and −37.5 kcal/mol respectively (data not shown).
Analysis of species homology implied that the base sequence of
SIRT1-3′-UTR region from 1,434-1,440 bp was highly conserved among
different species (Fig. 3).
Therefore, SIRT1 was chosen for subsequent experiments in the
present study.
Construction of SIRT-1 3′-UTR region
luciferase reporter vector
The agarose gel electrophoresis indicated that the
base pairs of the recombinant SIRT1-3′-UTR luciferase reporter
vector plasmid were 752 bp and the base pairs of converter of
negative control were 453 bp following double restriction enzyme
digestion (Fig. 4).
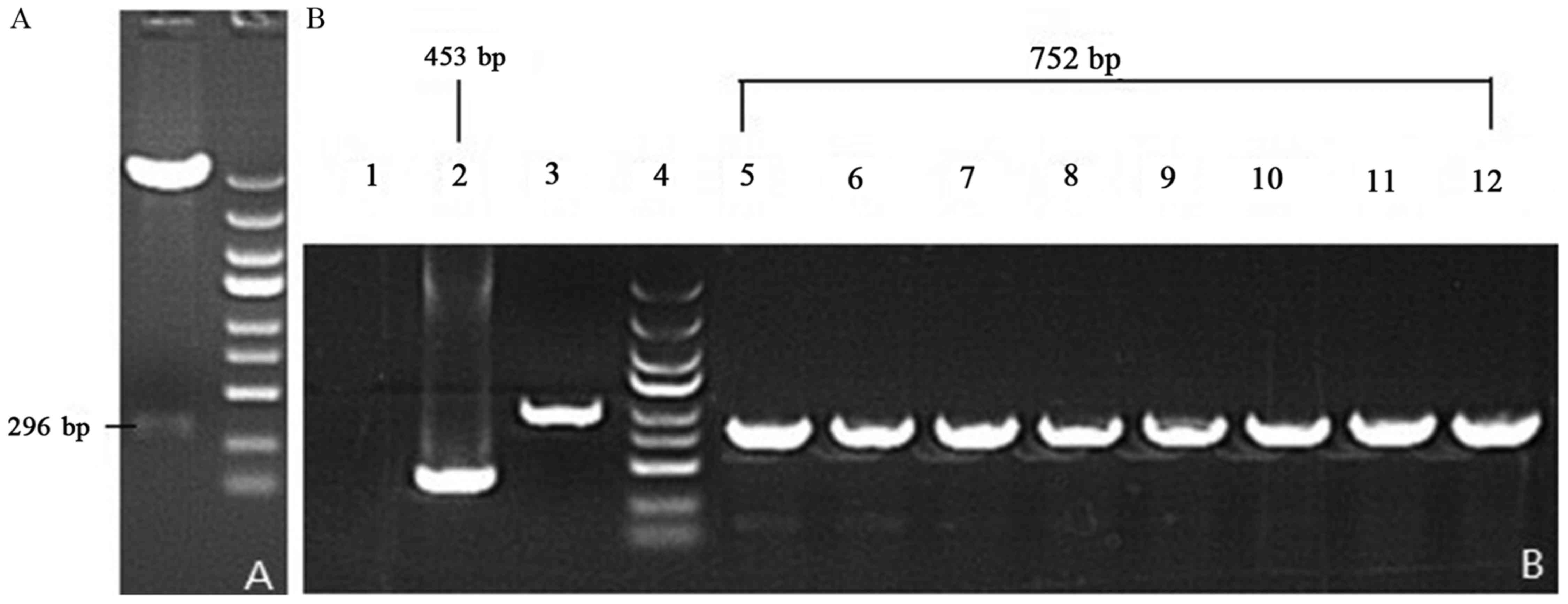 | Figure 4.Gel images of PCR products of
recombinant vectors. Following PCR, the double enzyme restriction
digestion and agarose gel electrophoresis was performed to
determine the fragment size. (A) Enzyme-digested SIRT1-3′-UTR
fragments. (B) Lane 1, ddH2O; lane 2, negative control
(empty vector self-ligation); lane 3, positive control (GAPDH);
lane 4, MW scale (molecular weight of marker protein); lanes 5–12,
recombinant SIRT1-3′-UTR luciferase reporter vector plasmid. PCR,
polymerase chain reaction; miR-34a, microRNA-34a; SIRT1, sirtuin 1;
UTR, untranslated region. |
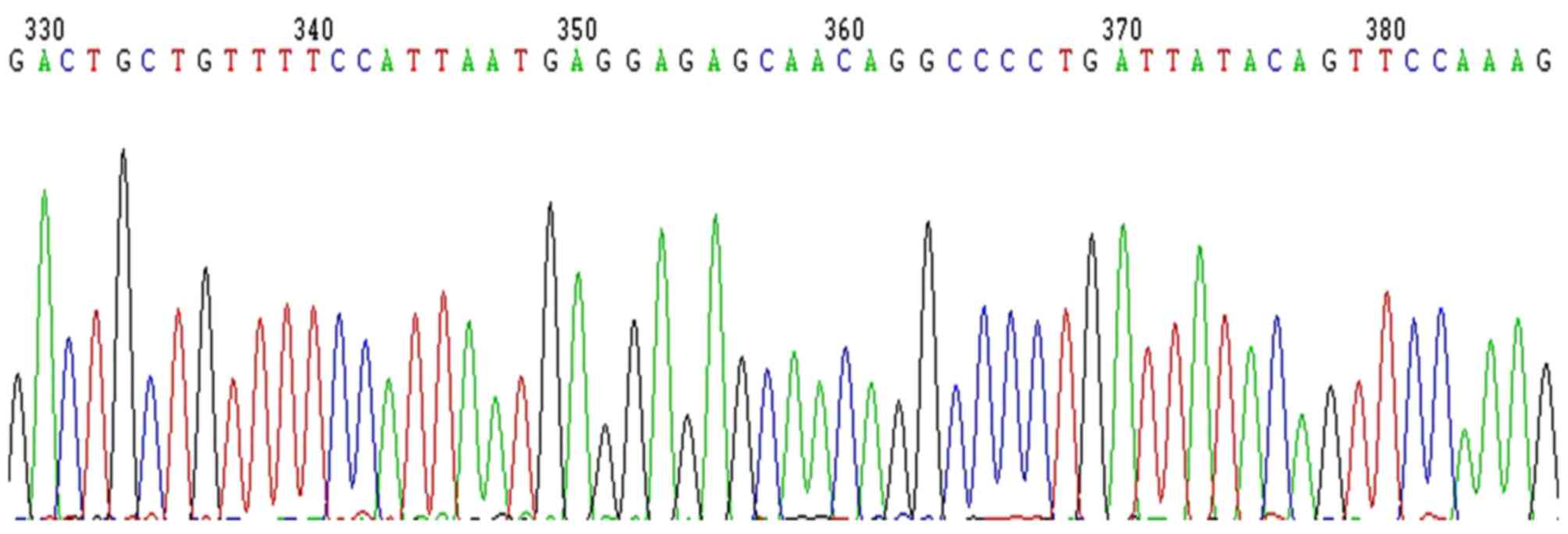
Therefore, 752 bp-453 bp=299 bp and the segment size
of SIRT1-3′-UTR is 293 bp following deduction of the 6 bp enzyme
digestion sites (Fig. 4). This
result was consistent with the GenBank search result without site
mutation (Fig. 5).
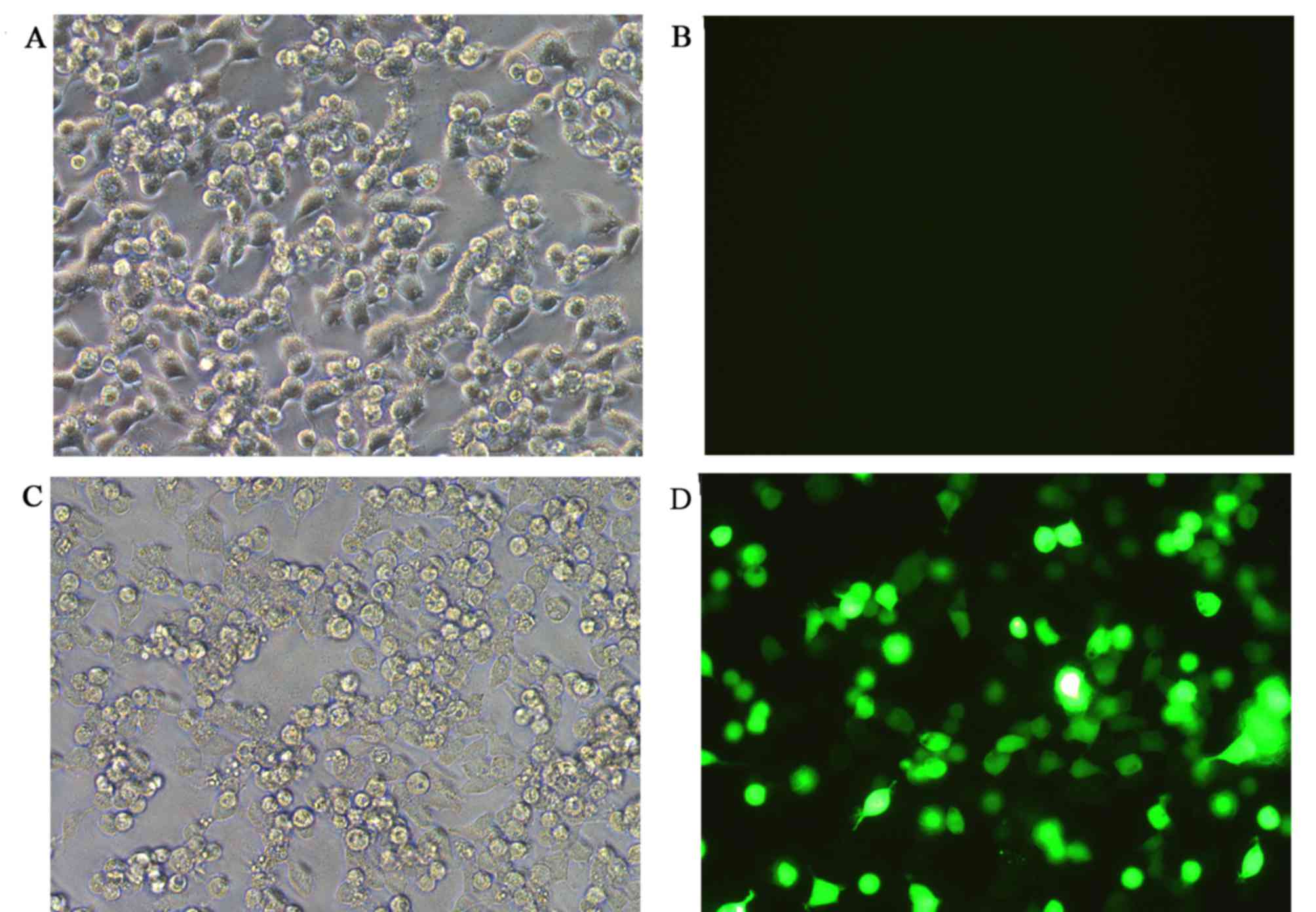
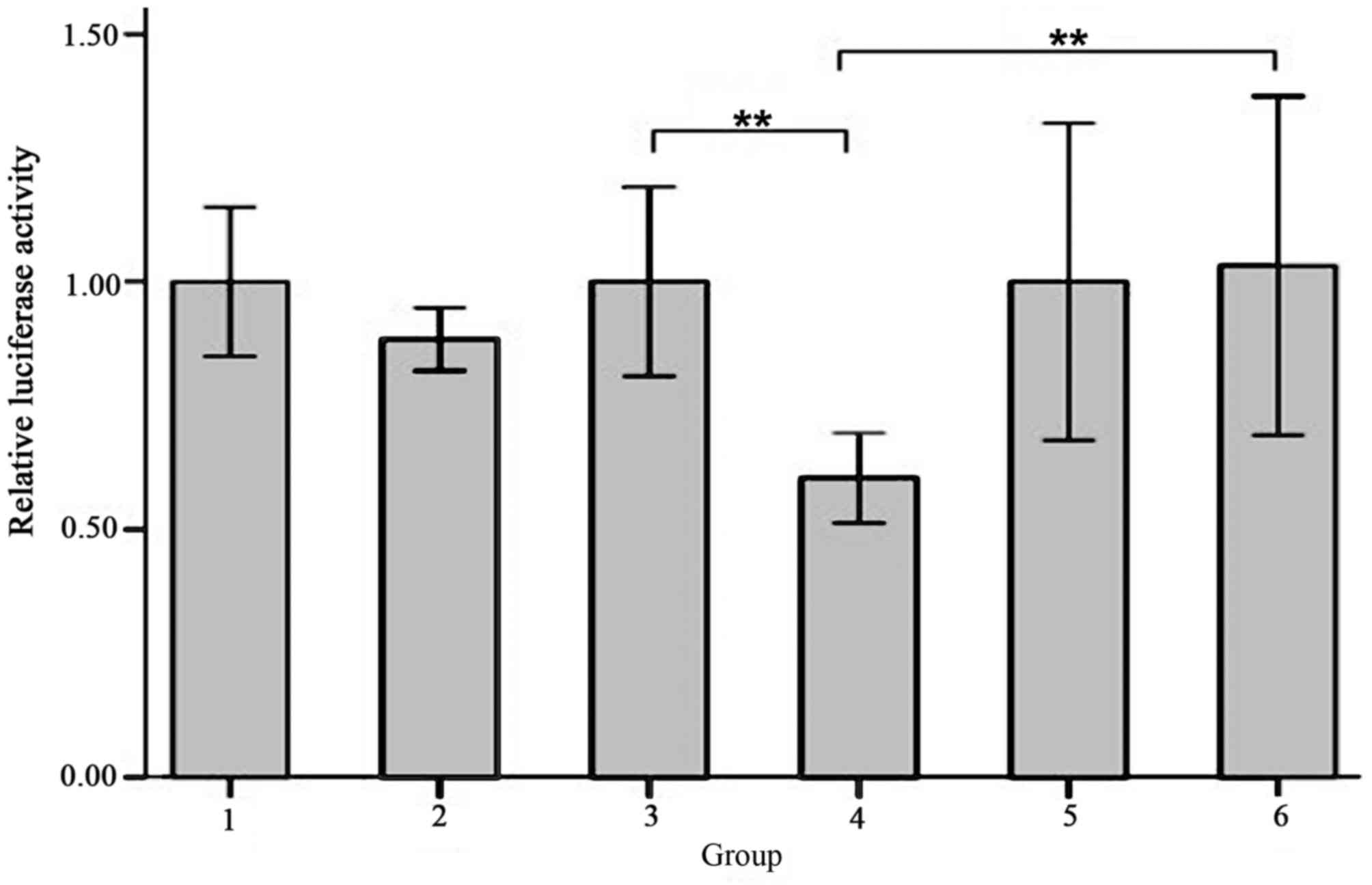
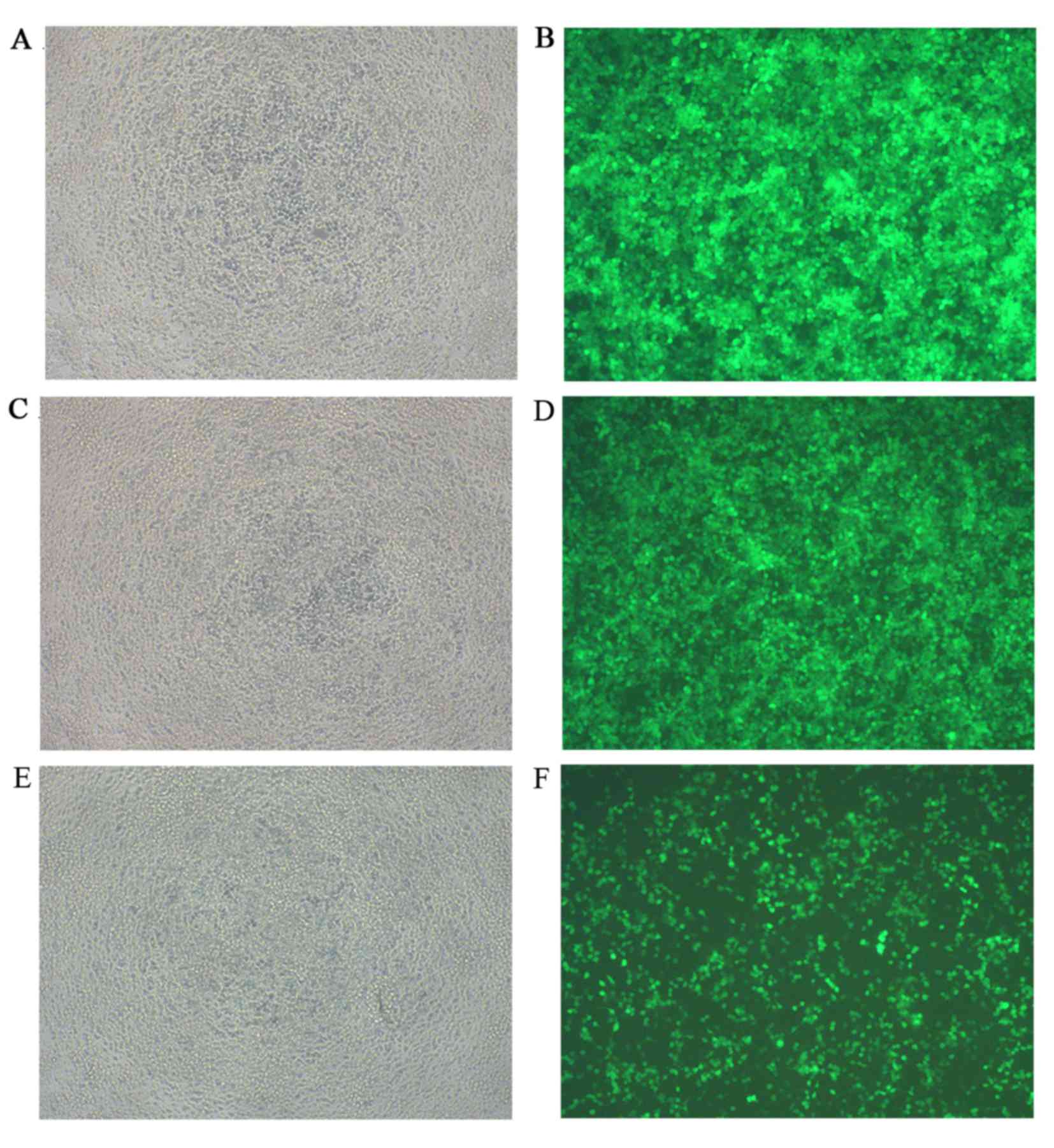
Co-transfection efficiency and
luciferase activity evaluation
None of the co-transfection plasmids contained green
fluorescent protein (GFP) in order to avoid the effects of
luciferase activity, whereas the reference group, which used an
empty plasmid was marked with GFP. The green fluorescence
expression profile of all experiments including the reference group
was then evaluated. Successful co-transfection was defined as 80%
of GFP expression rate, as indicated by fluorescence microscopy
(Fig. 6). The results of the
luciferase activity test indicated that there was a significantly
decreased fluorescence value for the cells co-transfected with
SIRT1-3′-UTR recombinant plasmid and miR-34a over-expression
plasmid (Group 4) compared with cells co-transfected with
SIRT1-3′-UTR recombinant plasmid and miRNA empty plasmids (Group 3;
0.60±0.04 vs. 1.00±0.08, t=−8.062, P=0.001) and the cells
co-transfected with SIRT1-3′-UTR mutant plasmid and miR-34a
over-expression plasmid (Group 6; 0.60±0.04 vs. 1.03±0.14,
t=−5.211; P=0.006; Fig. 7). The
differences between the other groups were not significant (Fig. 7).
Titer measurement of hsa-miR-34a
over-expressing lentivirus
Transfection was performed with 293 cells using
miR-34a over-expressing lentiviral vector marked with GFP. The
decrease in the number of fluorescent cells indicated an increase
in virus dilution fold (Fig. 8).
According to the equation virus titer (Tu/ml)=(fluorescent cell
number × virus dilution fold)/0.1, the titer of the miR-34a
over-expressing lentiviral system was calculated and the result was
4×108 Tu/ml. The recombinant lentiviruses were then
transfected into SGC-7901 cells in the experimental group and
cultured for 3 days. Subsequent experiments were performed when
culture plates were filled with transfected cells.
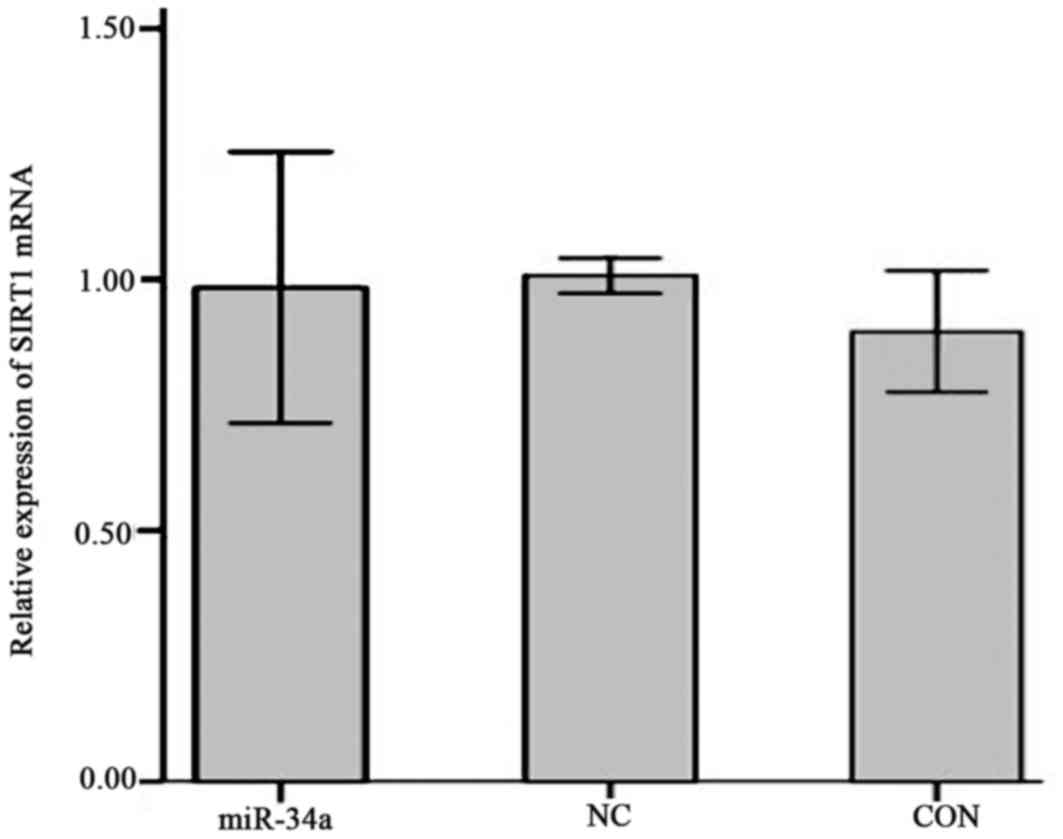
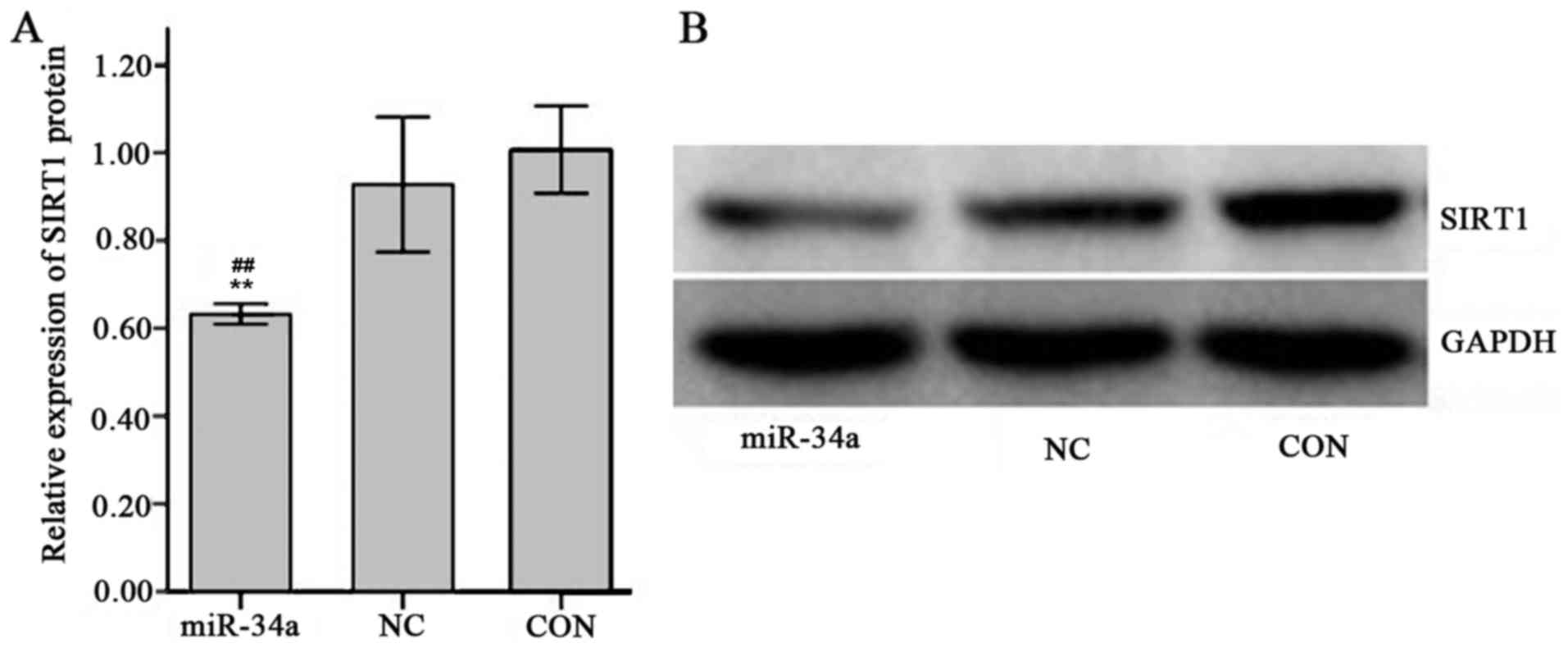
Effect of miR-34a on SIRT1 mRNA and
protein expression
The expression of SIRT1 mRNA in SGC-7901 cells in
the miR-34a group was decreased compared with the NC group, however
this result was not significant (0.98±0.11 vs. 1.01±0.01; Fig. 9). The expression of SIRT1 protein was
significantly decreased in the miR-34a group compared with the NC
group (0.63±0.01 vs. 0.93±0.06, t=−8.17, P<0.01; Fig. 10).
Inhibition of SGC-7901 cell
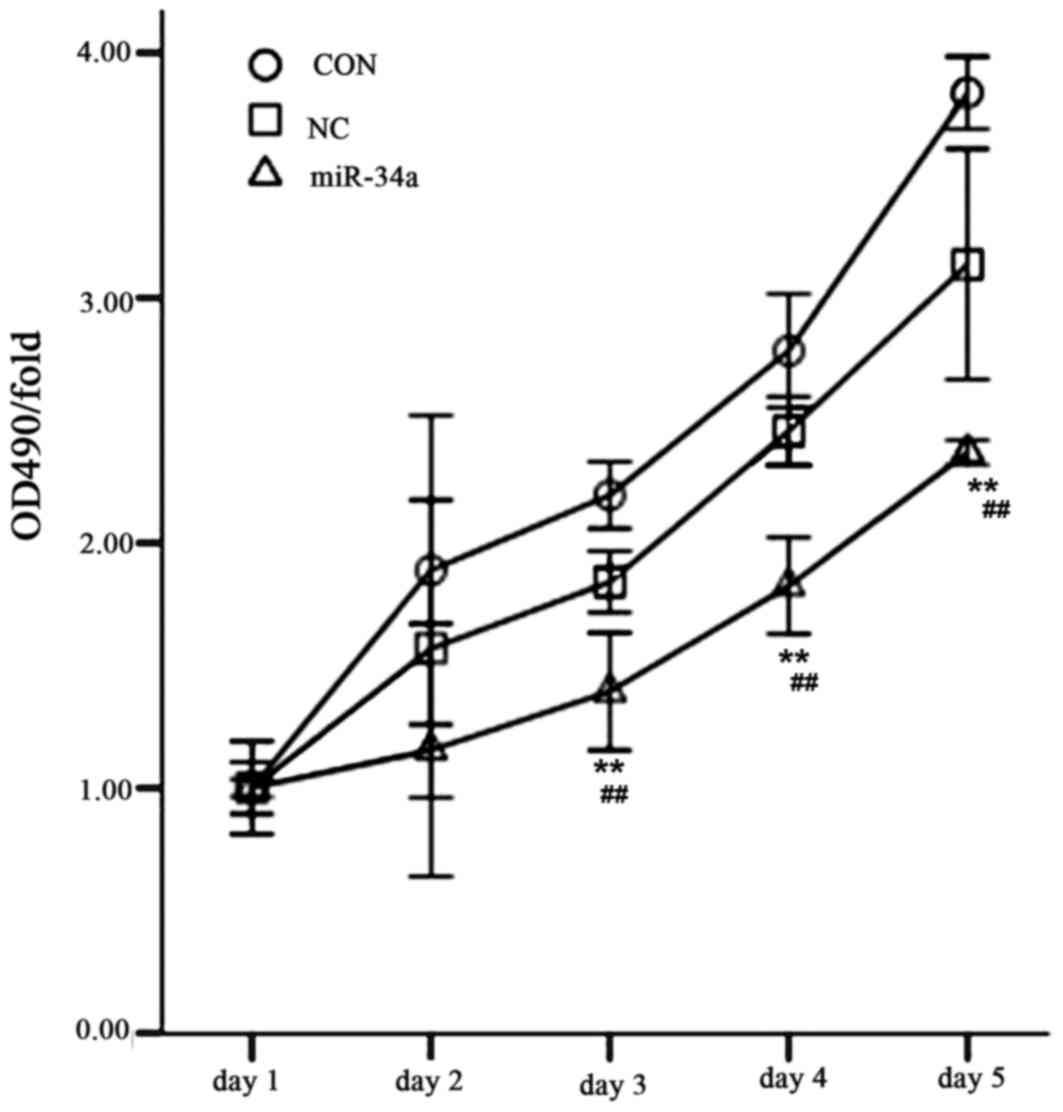
proliferation by miR-34a
The MTT method was used to determine the cell
proliferation of all experimental groups over 5 days. The results
indicated that SGC-7901 cell proliferation was significantly
decreased in the miR-34a group compared with the NC group between
days 3 and 5 (day 3, 1.39±0.19 vs. 1.84±0.10, t=4.567; day 4,
1.82±0.16 vs. 2.46±0.11, t=7.265; day 5, 2.37±0.04 vs. 3.14±0.38,
t=4.500; all P<0.01; Fig.
11).
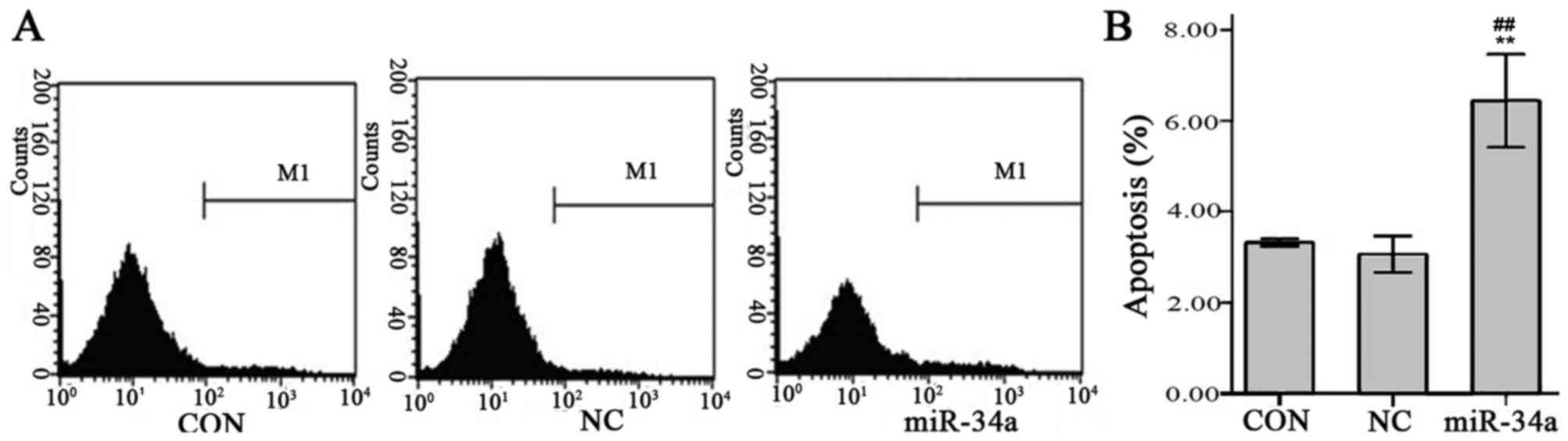
miR-34a promotes SGC-7901 cell
apoptosis
Results of flow cytometry indicated that the
SGC-7901 cell apoptosis rate was significantly increased in the
miR-34a group compared with the NC group (6.44±0.41 vs. 3.06±0.16,
t=−13.226, P<0.01, Fig. 12).
Discussion
miR-34a is an endogenous, non-coding regulatory
small RNA containing 22 base pairs (15). Its coding gene is located on
chromosome 1p36.22 and is associated with regulation of cell
growth, proliferation and metabolism. Previous studies have
demonstrated that there is abnormal expression of miR-34a due to
the lack of 1p36 in certain tumors and metabolic-associated
diseases (16,17). Furthermore, abnormal expression of
miR-34a caused by an abnormal methylation promoter region and
mutation of p53 widely exists in various tumor-associated diseases
(18,19). Therefore, miR-34a is studied due to
its vital role in tumor development. It has been previously
demonstrated that there is a low expression level of miR-34a in
gastric cancer cells (20). To
further investigate the association between miR-34a and gastric
cancer, the results of the present study indicated that the
expression of miR-34a was significantly lower in gastric cancer
tissue compared with pericarcinomatous tissue and the expression
level was associated with tumor differentiation degree and
lymphatic metastasis. The significant decrease in the expression of
miR-34a in various gastric cancer cell strains implied that there
was an association between miR-34a and gastric cancer development.
The 3′-UTR region of miR-34a is able to identify and bind to the
3′-UTR of its targeting sequence, which is able to induce the
degradation of the target gene and inhibit transcription (15). Therefore, the expression of the
downstream protein is inhibited, which leads to various biological
effects, including DNA damage repair, cell proliferation and
apoptosis (21,22). Bioinformatics was used to overlap the
search results from different data banks to ensure that target gene
prediction was correct in the present study. Furthermore, the seed
sequences were analyzed and screened according to the high tumor
correlation biological function, high thermodynamic stability of
binding and high conservatism of the predicted target gene. The
miR-34a specific targeting gene SIRT1 was subsequently selected as
the focus of the present study.
SIRT1 is a member of the Sirtuin protein family,
which is associated with cell stress reaction regulation, DNA
repair, chromatin recombination, metabolism, aging and apoptosis
due to the acetyl enzyme function of co-enzyme NAD+ to
SIRT1 (23,24). The function of SIRT1 in cancer is
unclear. It has previously been indicated that SIRT1 blocks certain
cancer pathways and serves a role as a cancer suppressor (25). However, it has also been demonstrated
that SIRT1 undergoes de-acetylation to transcription factors,
including the p53 ‘gene guardian’, which weakens its mediation of
cell proliferation inhibition, cell cycle arrest and apoptosis
process (26). In addition, SIRT1
has an oncogenic function via the direct inhibition of
pro-apoptotic proteins, including Bax and caspase (27). Yamakuchi previously demonstrated that
human HCT116 cells exhibited p53/miR-34a/SIRT1 positive feedback
regulation (20). miR-34a is the
downstream activating receptor, which serves an important role in
elevating the acetylation level of p53 by inhibiting SIRT1
expression to recover the activity of miR-34a and subsequently
promoting apoptosis and the anti-tumor function of p53 (20). However, the de-acetylation function
of SIRT1 decreases the activity of p53 due to the interruption of
positive feedback loop regulation when the expression of miR-34a is
decreased or exogenously inhibited (20). Previous studies have suggested that
the association between miR-34a and SIRT1 was the key regulatory
mechanism of damaged DNA double-strand break repair (28). It has also been demonstrated that the
expression of SIRT1 is increased in gastric cancer (29). In order to determine whether miR-34a
targets SIRT1 to regulate and control cell proliferation and
apoptosis, a SIRT1-3′-UTR luciferase reporter plasmid was
successfully constructed in the present study and it was
demonstrated that miR-34a specifically binds to the SIRT1-3′-UTR.
The results of the luciferase reporter assay demonstrated that
SIRT1 was the target gene of miR-34a. Therefore, an miR-34a
over-expression lentiviral vector system was constructed and then
transfected into human gastric cancer SGC-7901 cells to determine
the regulatory role served by miR-34a on SIRT1 using RT-qPCR.
Western blot analysis was then used to determine the expression of
SIRT1 protein. The results indicated that miR-34a inhibits SIRT1
protein expression. Cell proliferation was inhibited and apoptosis
was promoted in the group with cells transfected with miR-34a,
therefore it is suggested that miR-34a serves a role in gastric
cancer inhibition via its targeting of SIRT1.
In conclusion, the expression of miR-34a was
decreased in human gastric cancer tissues and cells compared with
normal tissues. The expression level was associated with gastric
cancer differentiation degree and lymphatic metastasis, suggesting
that the development of gastric cancer was associated with miR-34a
expression disorder and dysfunction. The results also demonstrated
that miR-34a specifically binds to the SIRT1-3′-UTR to inhibit
SIRT1 protein expression. Therefore, miR-34a serves a role in cell
proliferation inhibition and apoptosis promotion of gastric cancer,
which suggests that miR-34a is a tumor suppressor. The level of
SIRT1 mRNA was not significantly decreased in the miR-34a group and
this may be due to the more than 16 miRNAs that regulate SIRT1
expression and activity (30).
Therefore, further study is required to investigate the pathway
network and concrete downstream regulation of miR-34a. The aim of
the present study was to determine the role of miR-34a in gastric
cancer and the results indicated its tumor suppression mechanism
via the regulation of the expression of SIRT1. Therefore, the
present study identified miR-34a as a potential target for the
diagnosis and treatment of gastric cancer.
The current first line therapy for gastric cancer is
combination chemotherapy, which consists of a multi-agent
chemotherapy regimen combining fluoropyrimidines and platinum
derivatives (31). In addition,
irinotecan, 5-fluorouracil and leucovorin are acceptable
alternatives to the platinum-based ECX (epirubicin, cisplatin and
capecitabine) regimen (31). Second
line therapy includes cytotoxic agents and biological agents such
as Ramucirumab, Lapatinib, Gefitinib and Everolimus (32). Gastric cancer treatment is now
shifting from disease-specific novel drug development to
biomarker-oriented investigations (19). Biomarker-oriented investigations may
improve the prognosis of patients with gastric cancer.
Acknowledgements
The present study was supported by grants from the
Anhui Natural Science Foundation of China (grant no. KJ2015A177)
and The Natural Science Foundation of China (grant nos. 81372899
and 81172087).
References
|
1
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Szász AM, Lánczky A, Nagy Á, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu HH, Lin W and Tsai KW: Advances in
molecular biomarkers for gastric cancer: miRNAs as emerging novel
cancer markers. Expert Rev Mol Med. 16:e12014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lan H, Lu H, Wang X and Jin H: MicroRNAs
as Potential Biomarkers in Cancer: Opportunities and Challenges.
Biomed Res Int. 2015:1250942015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Costa FF: Epigenomics in cancer
management. Cancer Manag Res. 2:255–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taby R and Issa JP: Cancer epigenetics. CA
Cancer J Clin. 60:376–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Yan C, Xin M, Han L, Zhang Y and
Sun M: Sirtuin 1 (Sirt1) overexpression in BaF3 cells contributes
to cell proliferation promotion, apoptosis resistance and
pro-inflammatory cytokine production. Med Sci Monit. 23:1477–1482.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmid G, Notaro S, Reimer D, Abdel-Azim
S, Duggan-Peer M, Holly J, Fiegl H, Rössler J, Wiedemair A, Concin
N, et al: Expression and promotor hypermethylation of miR-34a in
the various histological subtypes of ovarian cancer. BMC Cancer.
16:1022016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Welch C, Chen Y and Stallings RL:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuroblastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li XJ, Ren ZJ and Tang JH: MicroRNA-34a: A
potential therapeutic target in human cancer. Cell Death Dis.
5:e13272014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng Y, Guo JJ, Liu YM and Wu XL:
MicroRNA-34A inhibits the growth, invasion and metastasis of
gastric cancer by targeting PDGFR and MET expression. Biosci Rep.
34:pii. e001122014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB and Compton CC: The american joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pourianfar HR, Javadi A and Grollo L: A
colorimetric-based accurate method for the determination of
enterovirus 71 titer. Indian J Virol. 23:303–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lal A, Thomas MP, Altschuler G, Navarro F,
O'Day E, Li XL, Concepcion C, Han YC, Thiery J, Rajani DK, et al:
Capture of microRNA-bound mRNAs identifies the tumor suppressor
miR-34a as a regulator of growth factor signaling. PLoS Genet.
7:e10023632011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Firestein R, Blander G, Michan S,
Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S,
de Cabo R, Fuchs C, et al: The SIRT1 deacetylase suppresses
intestinal tumorigenesis and colon cancer growth. PLoS One.
3:e20202008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng Y, Yi R and Cullen BR: MicroRNAs and
small interfering RNAs can inhibit mRNA expression by similar
mechanisms. Proc Natl Acad Sci USA. 100:9779–9784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji Q, Hao X, Meng Y, Zhang M, Desano J,
Fan D and Xu L: Restoration of tumor suppressor miR-34 inhibits
human p53-mutant gastric cancer tumorspheres. BMC Cancer.
8:2662008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Leeuwen IM, Higgins M, Campbell J,
McCarthy AR, Sachweh MC, Navarro AM and Laín S: Modulation of p53
C-terminal acetylation by mdm2, p14ARF, and cytoplasmic SirT2. Mol
Cancer Ther. 12:471–480. 2013. View Article : Google Scholar
|
|
20
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu M, Lu L, Mao B, Lü X, Wu XS, LI L and
Liu DP: Mutual inhibition between miR-34a and SIRT1 contributes to
regulation of DNA double-strand break repair. Chin Sci Bulletin.
58:979–985. 2013. View Article : Google Scholar
|
|
22
|
Ma W, Xiao GG, Mao J, Lu Y, Song B, Wang
L, Fan S, Fan P, Hou Z, Li J, et al: Dysregulation of the
miR-34a-SIRT1 axis inhibits breast cancer stemness. Oncotarget.
6:10432–10444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Price NL, Gomes AP, Ling AJY, Duarte FV,
Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro
JS, et al: SIRT1 is required for AMPK activation and the beneficial
effects of resveratrol on mitochondrial function. Cell Metab.
15:675–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tulino R, Benjamin AC, Jolinon N, Smith
DL, Chini EN, Carnemolla A and Bates GP: SIRT1 activity is linked
to its brain region-specific phosphorylation and is impaired in
huntington's disease mice. PLoS One. 11:e01454252016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mvunta DH, Miyamoto T, Asaka R, Yamada Y,
Ando H, Higuchi S, Ida K, Kashima H and Shiozawa T: SIRT1 regulates
the chemoresistance and invasiveness of ovarian carcinoma cells.
Transl Oncol. 10:621–631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lynch CJ, Shah ZH, Allison SJ, Ahmed SU,
Ford J, Warnock LJ, Li H, Serrano M and Milner J: SIRT1 undergoes
alternative splicing in a novel auto-regulatory loop with p53. PLoS
One. 5:e135022010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riquelme I, Saavedra K, Espinoza JA, Weber
H, García P, Nervi B, Garrido M, Corvalán AH, Roa JC and Bizama C:
Molecular classification of gastric cancer: Towards a
pathway-driven targeted therapy. Oncotarget. 6:24750–24779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng L, Yuan Z, Li Y, Ling H, Izumi V,
Fang B, Fukasawa K, Koomen J, Chen J and Seto E: Ubiquitinated
sirtuin 1 (SIRT1) function is modulated during DNA damage-induced
cell death and survival. J Biol Chem. 290:8904–8912. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bilici A: Treatment options in patients
with metastatic gastric cancer: Current status and future
perspectives. World J Gastroenterol. 20:3905–3915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang G, Wang Y, Xiong Y, Chen XC, Ma ML,
Cai R, Gao Y, Sun YM, Yang GS and Pang WJ: Sirt1 AS lncRNA
interacts with its mRNA to inhibit muscle formation by attenuating
function of miR-34a. Sci Rep. 6:218652016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aoyagi K, Kouhuji K, Kizaki J, Isobe T,
Hashimoto K and Shirouzu K: Molecular targeting to treat gastric
cancer. World J Gastroenterol. 20:13741–13755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Vita F, Di Martino N, Fabozzi A,
Laterza MM, Ventriglia J, Savastano B, Petrillo A, Gambardella V,
Sforza V, Marano L, et al: Clinical management of advanced gastric
cancer: The role of new molecular drugs. World J Gastroenterol.
20:14537–14558. 2014. View Article : Google Scholar
|