Introduction
With the increase in arterial pressure, hypertension
is a disease usually accompanied by severe complications in
vascular system of heart, brain and kidney, severely threatening
the life and health of human beings. As the life quality has been
continuously improved, an increasing trend on a year-by-year basis
has been identified in the incidence rate of cardiovascular
diseases; in addition, the incidence rate of complications induced
by hypertension is gradually increased; considering all these
factors, researchers have paid great attention to the pathogenesis
of hypertension and vascular lesions (1–3). In
recent years, although the pathogenesis and pathological process of
hypertension remains poorly understood, literature has reported
that damaged vascular endothelial cells, thickened vascular wall
and non-specific inflammatory responses are the major factors
contributing to the onset of hypertension (4–6).
Dynamin-related protein 1 (Drp1) plays a key role in the regulation
of mitochondrial division; according to the study of Hu et
al (7), they found that
mitochondrial fusion/division imbalance greatly contributes to the
ischemia-reperfusion injuries and sepsis, in which Drp1 has a
regulatory role (8). Tanwar et
al (9) indicated that inhibition
on Drp1 can block cell apoptosis caused by the tumor necrosis
factor. However, there is no literature reporting the role of Drp1
in hypertension and its effect on inflammatory factors. In this
study, we investigated how variations induced by inhibition of Drp1
in inflammatory responses in endothelial cells of hypertension
could ameliorate the vessels.
Spontaneous hypertension rat (SHR) models were
established for exploring the variations caused by Drp1 in vascular
endothelial cells, the effect of Drp1 on inflammatory factors, and
the effect of inhibition on Drp1 on inflammatory factors in
vascular endothelial cells and the vessels in spontaneous
hypertension, aiming to provide new ideas for elucidating the
pathogenesis of spontaneous hypertension and developing effective
clinical treatment methods.
Materials and methods
Instruments and materials
Mdivi-1 (Selleck, Houston, TX, USA); TRIzol kit, rat
anti-Drp1 antibody, enzyme-linked immunosorbent assay (ELISA) kit
for interleukin-6 (IL-6) and ELISA kit for tumor necrosis factor-α
(TNF-α) (all from BD Biosciences, Franklin Lakes, NJ, USA);
secondary antibody against rat (Beijing Zhongshan Golden Bridge
Biotech Co., Ltd., Beijing, China); rabbit anti-IL-6 (1:500; cat.
no. 12153), rabbit anti-TNF-α (1:500; cat. no. 8184), rabbit
anti-MCP-1 (1:500; cat. no. 39091), horseradish peroxidase-labeled
secondary antibody against rabbit (1:1,000; cat. no. 7074) (all
from Cell Signaling Technology Co., Ltd.); electrochemiluminescence
(ECL) solution and color development powder (both from Invitrogen,
Carlsbad, CA, USA); pipette (Eppendorf, Hamburg, Germany);
electronic scale (BP121S; Sartorious, Goettingen, Germany); −80°C
refrigerator and low-temperature centrifuge (Thermo Fisher
Scientific, Dreieich, Germany); other instruments and reagents are
indicated in the corresponding part in this study.
Experimental animals and grouping
In this study, we selected a total of 10 male,
healthy, Sprague-Dawley rats and 20 spontaneous hypertension rats
(SHR) with the weight ranging from 220 to 250 g. All these animals
were purchased from Guangdong Medical Laboratory Animal Center, and
the qualification number of experimental animals was SCXK
(Guangdong) 2013–0015. The 20 SHR rats were randomly divided into
two groups, i.e. the SHR group (n=10) and the inhibition group (MD
group; n=10). Rats in the MD group received Mdivi-1 (25 mg/kg)
every day, while rats in the normal control group (C group) and the
SHR group were given normal saline (10 ml/kg/day) every day. The
study was approved by the Ethics Committee of Guizhou Provincial
People's Hospital (Guiyang, China).
Sample collection and preparation
Preparation of the serum samples
After 4 weeks of drug administration, blood was
drawn from the abdominal aorta of rats in each group after the
blood pressure and weight had been measured. Blood samples were
then centrifuged at 2060 × g for 15 min, and the supernatant was
divided into several pieces and placed into Eppendorf (EP) tubes.
The EP tubes were at −80°C.
Preparation of abdominal aorta
After blood collection, the thoracic artery at 0.5
cm below the aortic arch was immediately isolated, some of which
were then preserved at −80°C, while the remaining artery was rinsed
with Hanks solution and fixed in 4% paraformaldehyde. For the
resected thoracic aorta, routine paraffin embedding, serial
sectioning and hematoxylin eosin staining were sequentially
performed followed by observation of variations in vascular media
under the microscope, in which 5 visions were randomly selected for
each rat, and the images were loaded into the IPP 6.0 image
analytic system to measure the medial thickness of the vascular
wall.
Detection of C-reaction protein (CRP)
content in serum
Radioimmunoassay (RIA) was performed to assay the
content of CRP in serum. The serum samples that were centrifuged
and frozen were preserved at −20°C, and then thawed at room
temperature. In the numbered polystyrene tubes, 100 µl
125I-CRP and standard substance of CRP were added and
mixed well. Then the tubes were transferred into a dark room for
incubation at 37°C for 1 h. Thereafter, 500 µl separating agent was
dropped into the tubes that were later shaken and placed at room
temperature for 20 min. Then the tubes were centrifuged at 2800 × g
for 15 min, and the supernatant was discarded. Standard curve was
prepared with the counts per minute as the vertical coordinate and
the corresponding standard substance of CRP as the horizontal
coordinate, and, accordingly, the concentration of CRP in serum
samples of each rat in all groups was calculated.
Detection of content of inflammatory
factors in serum via ELISA
Procedures were carried out in strict accordance
with the instruction of ELISA kit: standard substances of
interleukin-6 and tumor necrosis factor-α were prepared for assay
of standard curve. In each well, 100 µl sample or standard
substance was added for 90 min of reaction at 37°C after the plate
was sealed using a membrane. Then, we added the 100 µl
biotin-labeled anti-rat TNF-α and IL-6 antibodies for 60 min of
reaction at 37°C. Then on the plate, 300 µl washing buffer was
added, and after the mixture was soaked for 1 min, the buffer was
discarded. In each well, 90 µl color development solution was
added, and the plate was sealed using membrane and placed in the
dark for 30 min of reaction at 37°C. Thereafter, 100 µl termination
solution was added, and the color of solution turned from blue to
yellow. With a microplate reader, the optical density in each well
at wavelength of 450 nm was detected, and accordingly, the standard
curve was prepared using CurveExpert 1.4 software. Then the
concentration of inflammatory factors in each well was calculated,
and the actual concentration of inflammatory factors in the sample
was the production result and the dilution ratio.
Detection of mRNA levels of Drp1 and
MCP-1 via semi-quantitative polymerase chain reaction (PCR)
RNA in serum of rats in all groups was extracted
with TRIzol kit to measure the D260/D280
ratio, and the results suggested that ratio was between 1.8 and
2.0. The RNA integrity was confirmed through agarose gel
electrophoresis, and the results showed that at 28S, 18S and 5S,
and the brightness of he stripe at 28S was approximately twice of
that at 18S. All these results indicated that the RNA was integral
and could be used in following experiments. Reverse transcription
was performed for preparation of cDNA using the relevant kit, and
with GAPDH as internal reference, the mRNA expression levels of
MCP-1 and CCR-2 were detected using semi-quantitative PCR in
following reaction conditions: initial denaturation at 95°C for 5
min; 95°C for 30 sec, 64°C for 25 sec, 72°C for 30 sec, for a total
of 35 cycles; extension at 72°C for 5 min. Primers were synthesized
by Tiangen Biotech (Beijing) Co., Ltd. (Beijing, China), and the
sequences are shown in Table I.
After reaction, agarose gel electrophoresis was performed for the
products followed by observation through ultraviolet imaging
system. The mRNA expression levels of MCP-1 and Drp1 in each group
are indicated by MCP-1/GAPDH and Drp1/GAPDH ratios.
 | Table I.Primers for PCR. |
Table I.
Primers for PCR.
| Gene | Primer sequence |
|---|
| MCP-1 | F:
5′-TGTCCCAAAGAAGCTGTAGTATTTGT-3′ |
|
| R:
5′-TTCTGATCTCACTTGGTTCTGTCC-3′ |
| Drp1 | F:
5′-GGAATCTTCTTCATTCCTGAC-3′ |
|
| R:
5′-CCAGTGCAGGGTCCGAGGT-3′ |
| GAPDH | F:
5′-GATGATTGGCATGGCTTT-3′ |
|
| R:
5′-CACCTTCCGTTCCAGTTT-3′ |
Detection of the expression levels of
relevant proteins via western blot assay
After quantitative assay using bicinchoninic acid
(BCA) protein quantification kit (Invitrogen) for serum samples in
each group, samples were boiled at 95°C water bath for 10 min of
degeneration, and the denatured samples were loaded for sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Thereafter, the proteins were transferred to the polyvinylidene
fluoride (PVDF) membrane. After 2 h of blocking, the target stripe
was then cut off for incubation using the primary antibodies
(1:1000) against Drp1, IL-6, TNF-α and MCP-1 overnight at 4°C. With
Tris-buffer saline + Tween-20 (TBST), the membrane was washed 3
times, and then compressed in the ECL solution (mixture of solution
A and solution in 1:1) in the dark, during which the compression
time was ascertained according to the fluorescent strength of
protein stripes. Then, color development and fixation were
performed for the membrane, and the stripe was then scanned and was
analysed for gray value using ImageJ software.
Statistical analysis
In this study, the data are presented as mean ±
standard deviation, and processed using SPSS 19.0 (SPSS Inc.,
Chicago, IL, USA) for analysis. t-test was performed for intergroup
comparison, and analysis of variance (ANOVA) for comparisons among
several groups. After homogeneity test of variance, Bonferronic
method was adopted for pairwise comparison if the variance was
equal; otherwise the Welch method was adopted. For multiple
comparisons, Dunnett's T3 method was used. P<0.05 was considered
to indicate a statistically significant difference.
Results
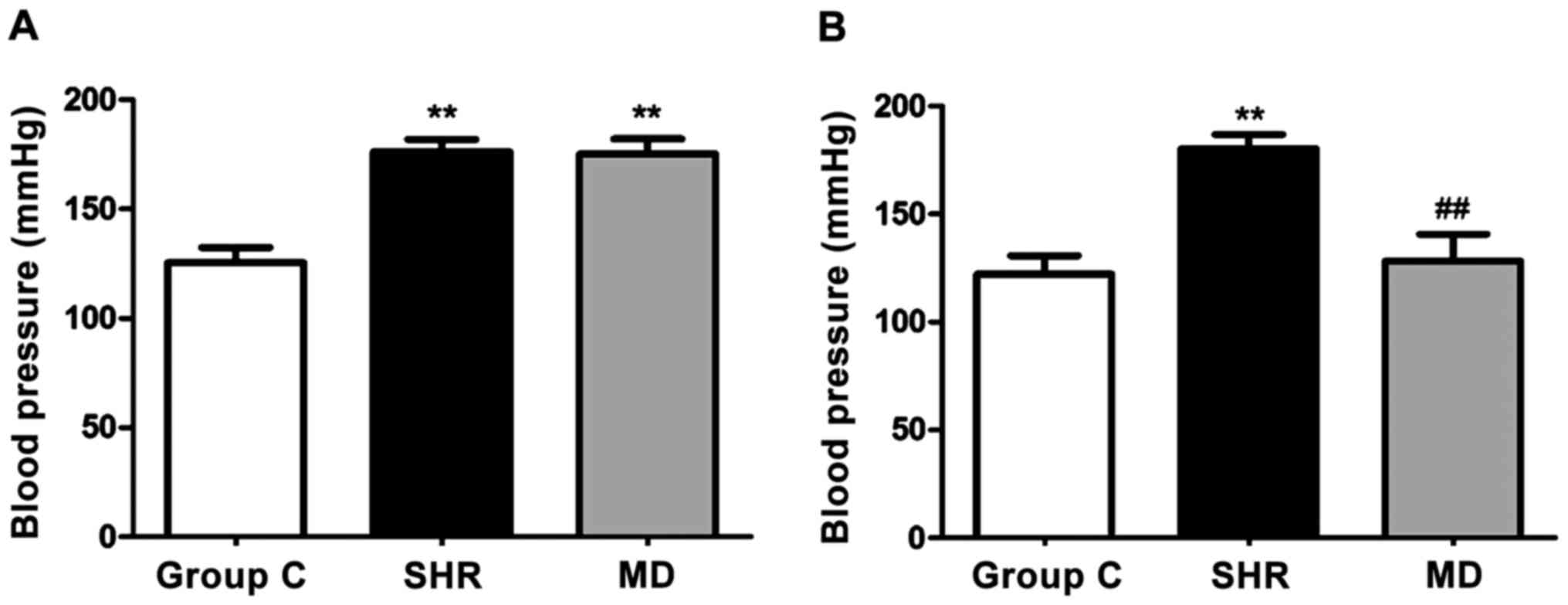
Variations in blood pressure of
rats
Before and at 4 weeks after drug administration of
rats in each group, we detected variations in blood pressure, and
the results are shown in Fig. 1.
Before drug administration, the blood pressure of rats in the SHR
group and the MD group was significantly higher than that in the C
group (P<0.01); after 4 weeks of drug administration, a
significant decrease was identified in blood pressure in the rats
of the MD group compared with the SHR group (P<0.01).
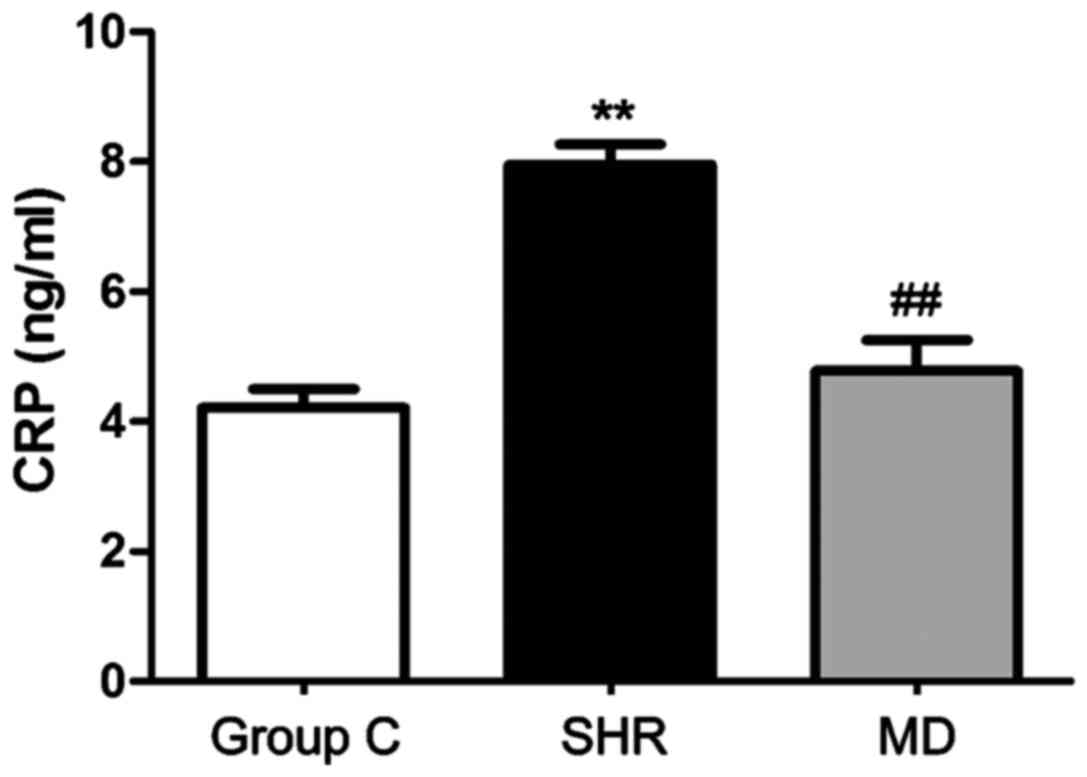
CRP level in serum
RIA was applied to detect the concentration of CRP
in serum, and the results are shown in Fig. 2. The concentration of CRP in serum of
rats in the C group was significantly lower than those in the SHR
group and the MD group (P<0.01); the level of CRP in serum of
rats in the MD group was lower than that in the SHR group, and the
difference had statistical significance (P<0.01).
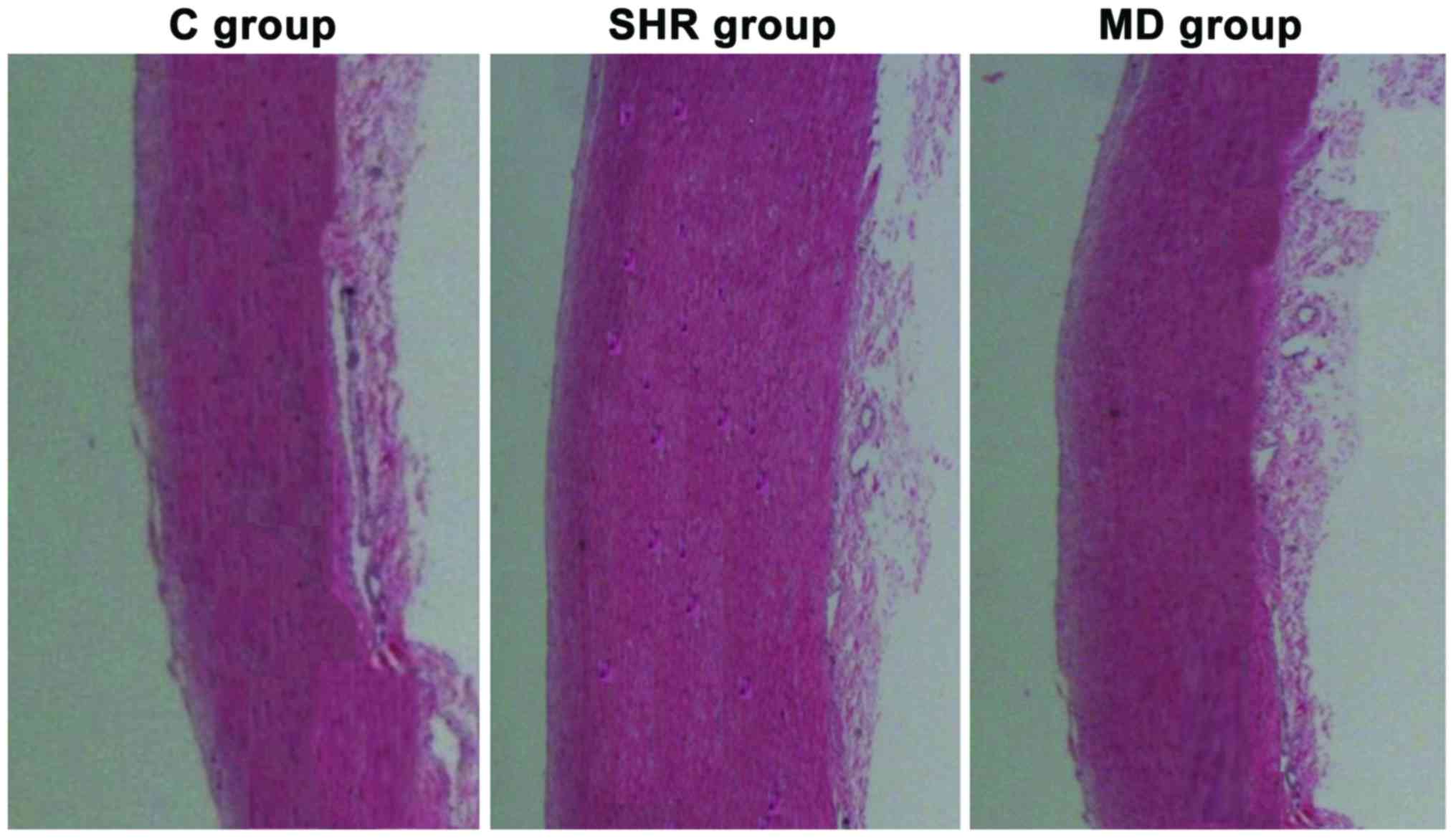
Variations in media of thoracic aorta
of rats in each group
Under the microscope, we observed the variations in
media of thoracic aorta of rats in each group that received the
hematoxylin and eosin (H&E) staining, and the results are shown
in Fig. 3. The smooth muscle cells
in media of thoracic aorta of rats in the C group were in
alignment; in the SHR group, the smooth muscle cells were in
malalignment with the increase in stained cells and thickened
media; but after the administration of Mdivi-1, compared with the
rats in the SHR group, the smooth muscle cells of thoracic aorta in
rats in the MD group were in regular alignment, and attenuation was
observed in the thickened media in thoracic aorta.
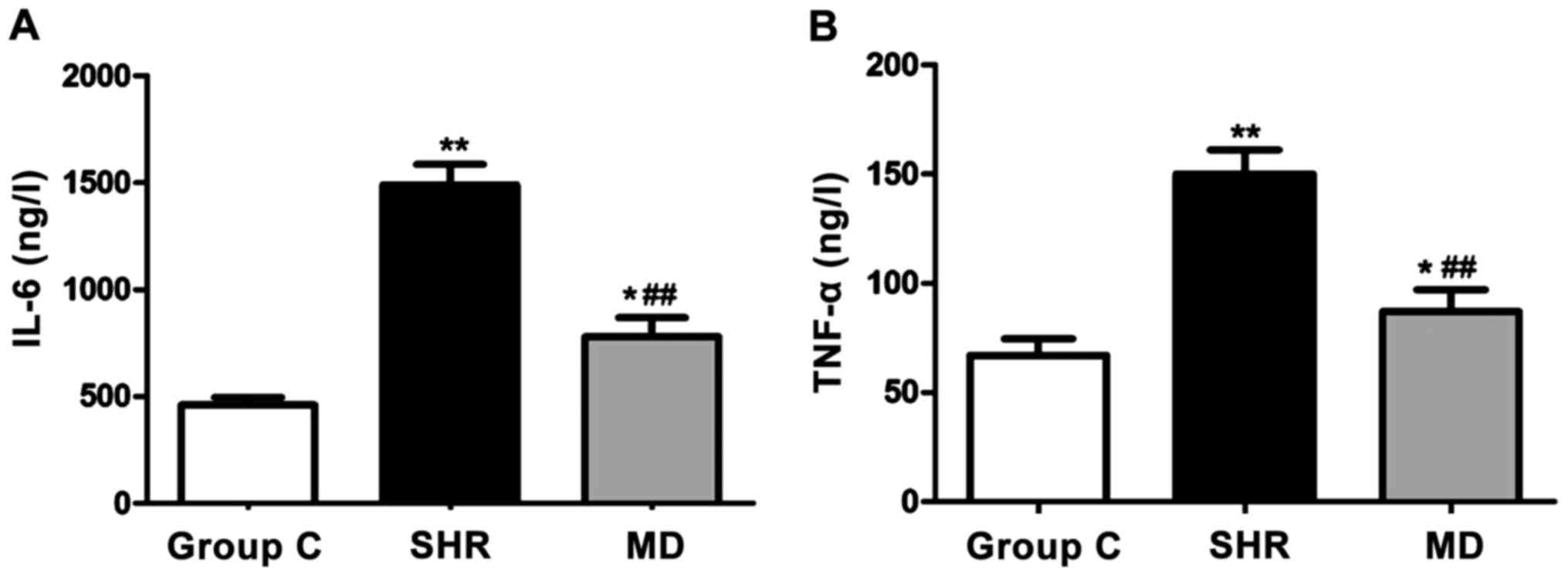
Detection of the expression levels of
inflammatory factors via ELISA
With the ELISA kit, we detected the expression
levels of IL-6 and TNF-α of rats in each group, and the results are
shown in Fig. 4. Compared with the C
group, the levels of IL-6 and TNF-α of rats in the SHR group and
the MD group were significantly elevated, and the differences had
statistical significance (P<0.01, P<0.05); the levels of IL-6
and TNF-α in serum of rats in the MD group were lower than those in
the MD group (P<0.01).
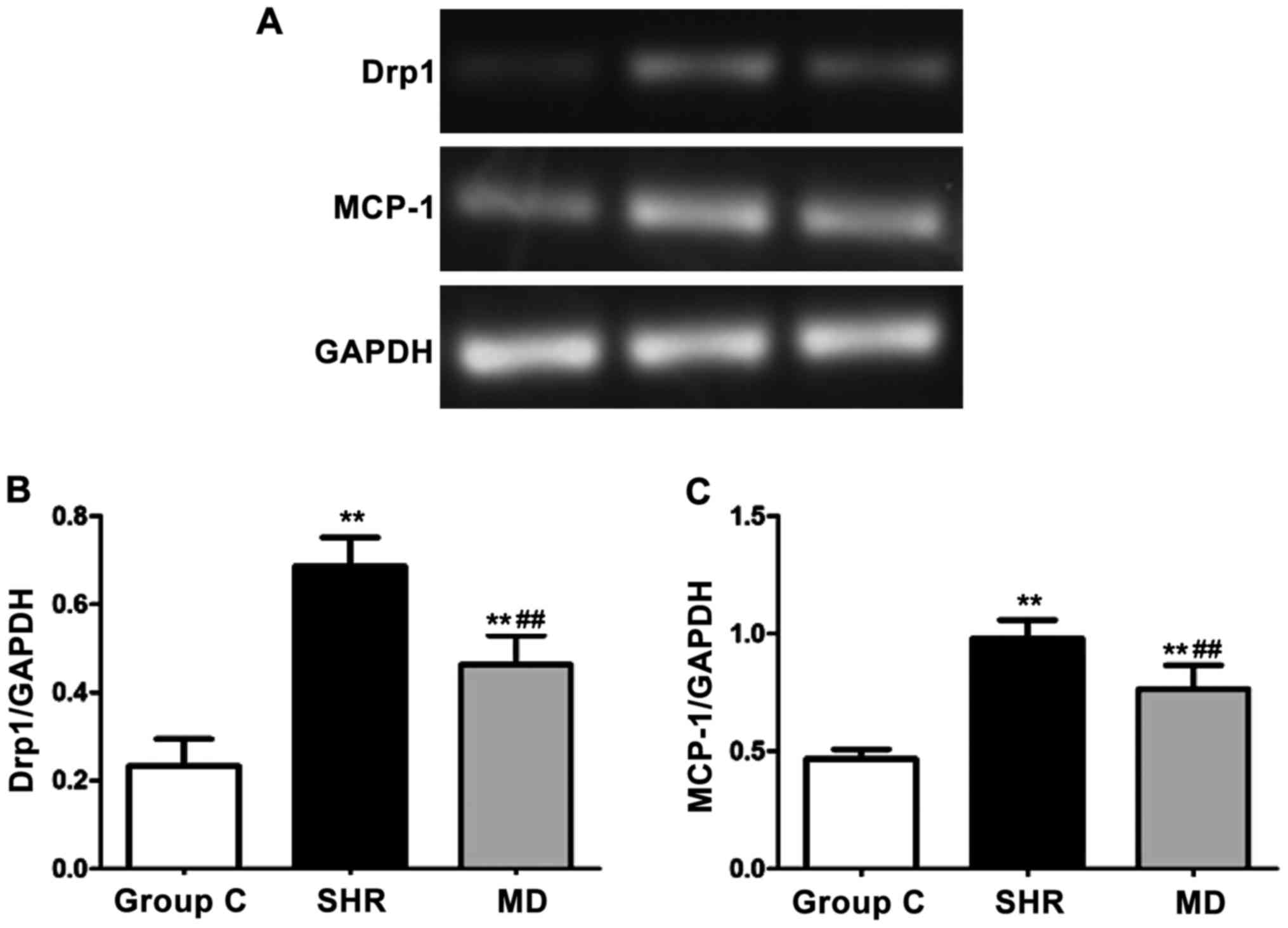
Detection of expression levels of Drp1
and MCP-1 via semi-quantitative PCR
We detected the mRNA expression levels of Drp1 and
MCP-1 via semi-quantitative PCR, and the results are shown in
Fig. 5. Compared with the C group,
the mRNA expression levels of Drp1 and MCP-1 of rats in the SHR
group and the MD group were significantly elevated, and the
differences had statistical significance (P<0.01); in comparison
with the SHR group, the mRNA expression levels of Drp1 and MCP-1 of
rats in the MD group were decreased (P<0.01).
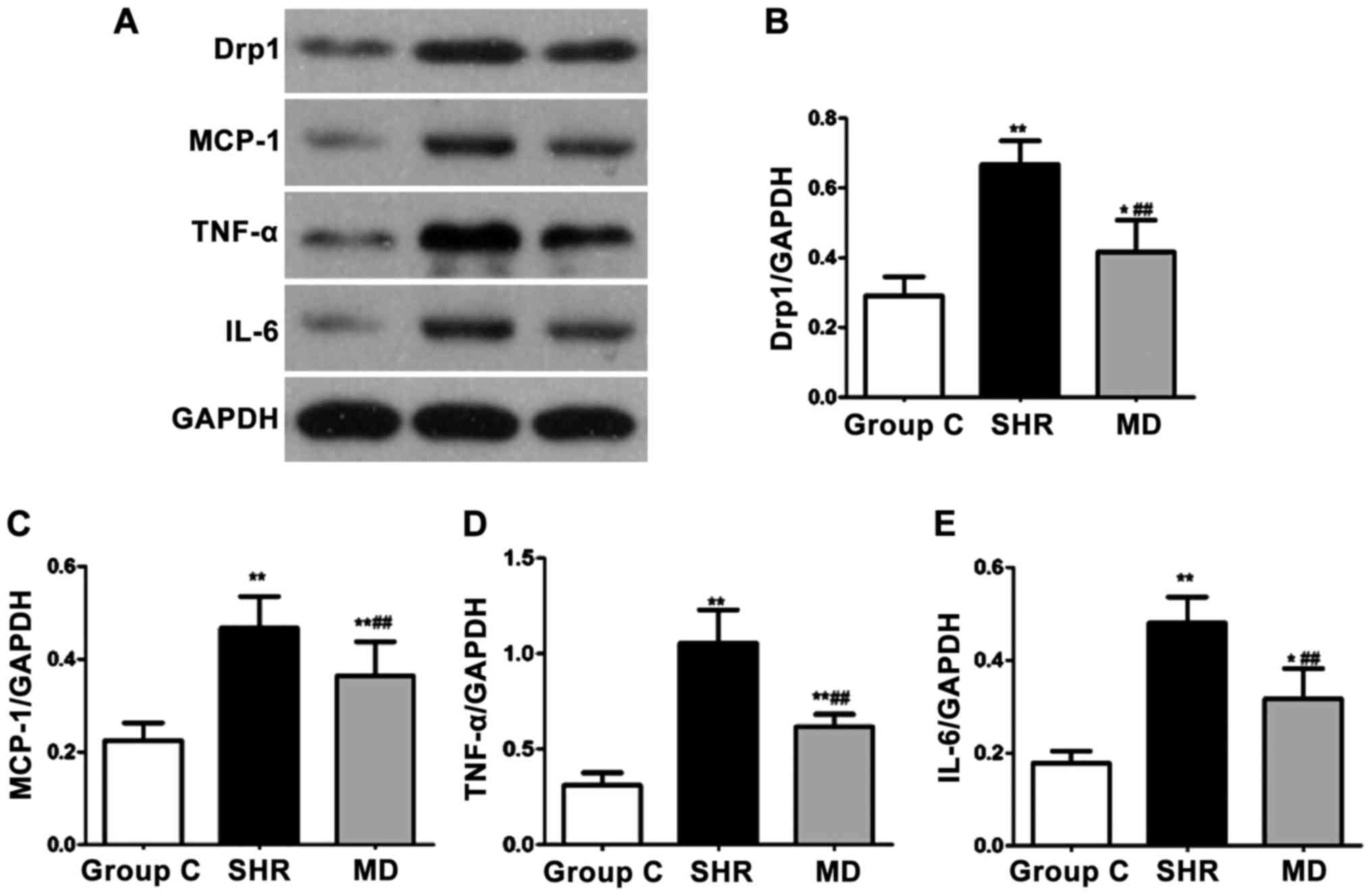
Detection of expression levels of
proteins via western blot assay
The protein expression levels of Drp1, MCP-1, IL-6
and TNF-α were detected through western blot assay, and the results
are shown in Fig. 6. Compared with
the C group, the protein expression levels of Drp1, MCP-1, IL-6 and
TNF-α of rats in the SHR group and the MD group were significantly
increased (P<0.01, P<0.05); in comparison with the SHR group,
significant decreases were identified in the protein expression
levels of Drp1, MCP-1, IL-6 and TNF-α of rats in the MD group, and
the differences had statistical significance (P<0.01).
Discussion
Hypertension patients usually suffer from vascular
lesions, and long-term chronic hypertension will generate
variations in vascular structure and function, thereby causing
vascular remodeling, and increment in thickness of vascular wall,
in which the vascular media are thickened and invade into the
lumen, clinically called as the vascular hypertrophic remodeling
(10). Vascular lesions are the
major factors inducing coronary heart disease and stroke, severely
affecting the life quality and clinical efficacy of patients
(11). The study of Hua (12) revealed that the inflammation of
vascular endothelium is one of the key causes for vascular
remodeling, and the dysfunction in vascular endothelial cells will
lead to increment in release of inflammatory factors. Castellano
et al (13) reduced the
vascular inflammatory responses in thoracic aorta in rat, which
could effectively ameliorate the injuries of hypertension to
vessels, and improve the vascular remodeling. The pathologic
conditions of inflammation include the activation of
pro-inflammatory transcription factor and damage to the
hypertension vessels (14).
Currently, researchers, through multiple aspects of
mitochondrial dysfunction, have confirmed that in patients with
hypertension complicated with vascular lesions usually suffer
metabolic dysfunction, damage to energy generation and excessive
production of reactive oxygen species in mitochondria, which are of
great significance for vascular lesions in hypertension (15). Research has shown that Drp1-mediated
mitochondrial division is associated with cell apoptosis and
necrosis, and decreasing the mitochondrial division induced by Drp1
can protect the vessels (16).
In this study, SHR rats were used for investigating
the correlation between Drp1 in the vascular endothelial cells and
inflammatory factors. SHR rat is a kind of spontaneous hypertension
rat model developed from Wistar rat in 1963 with the features of
stability and high incidence rate of hypertension. At 16 weeks,
significant elevation in blood pressure can be seen in SHR rats,
thereby inducing hypertension. Besides, the pathogenesis and
post-onset vascular and systemic pathologic changes of SHR rats are
coincident with the spontaneous hypertension in human beings, and
SHR rats have been recognized as the animal model that can mimic
the onset of spontaneous hypertension. In addition, SHR rats are
usually used for fundamental research on spontaneous hypertension
and screening the anti-hypertensive drugs (17).
In this study, we found that in SHR rats, the
expression of Drp1 was significantly elevated in the vascular
endothelial cells, the media of thoracic aorta were thickened, and
the administration of Mdivi-1 for 4 weeks could effectively
decrease the blood pressure in SHR rats, reduce the expression of
Drp1 to a certain extent, and attenuate the thickness of media in
thoracic aorta caused by the hypertension. All these results
suggested that hypertension can increase the expression of Drp1 in
vascular endothelial cells, while suppression on expression of Drp1
can decrease the blood pressure and protect the inner wall of the
vessel. Disorder in functions of vascular endothelial cells can
affect the generation and release of the substances that can
invigorate the circulation of blood, thereby attenuating the
vasodilatation, and enhancing the vasoconstriction. This will
contribute to the increase in hypertension and thickness of
vascular wall, which in turn will reduce the vascular compliance,
narrow down the lumen, increase the blood resistance and augment
the blood pressure (18,19). The expression levels of inflammatory
factors in serum of rats in each group were detected via ELISA and
western blot assay, and we found that the levels of IL-6 and TNF-α
in serum of SHR rat were remarkably elevated, and that the mRNA and
protein expression levels of MCP-1 and Drp1 were increased, but
after the administration of inhibitor, the expression levels of
IL-6, TNF-α and MCP-1 in serum of SHR rat could be effectively
decreased. High expression of Drp1 in vascular endothelium in
hypertension can induce the pro-inflammatory factors, and further
damage the vessels (20). Through
inhibition on Drp1 expression, the expression of inflammatory
factors can be effectively decreased, and, therefore, the vessels
are protected. In this study, we did not investigate the mechanism
that Drp1 affected in the release of inflammatory factors in
vessels, but the relevant mechanism could better explain the effect
and the mechanism of Drp1, which is one of the shortages in this
study. We anticipate to perform more studies on this mechanism.
In the vascular endothelium of hypertension, Drp1 is
highly expressed, and the excessive production of inflammatory
factors is also induced, which can lead to the damage to vascular
endothelium and increment in thickness of media in vascular wall.
Mdivi-1 can decrease the expression of Drp1, thereby ameliorating
the inflammatory responses and protecting the vessels in SHR
rats.
Acknowledgements
Not applicable.
Funding
This study was supported by the Foundation of
Science and Technology Bureau of Guiyang [zhukehetong
(20151001)she60hao].
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XinL contributed to the conception and design of the
study. HT collected the data and revised the manuscript for
important intellectual content. XiaL and QW analyzed and
interpreted the data, and drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Guizhou Provincial People's Hospital (Guiyang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Song CL, Zhang X, Liu YK, Yue WW and Wu H:
Heart rate turbulence in masked hypertension and white-coat
hypertension. Eur Rev Med Pharmacol Sci. 19:1457–1460.
2015.PubMed/NCBI
|
|
2
|
Misárková E, Behuliak M, Bencze M and
Zicha J: Excitation-contraction coupling and
excitation-transcription coupling in blood vessels: Their possible
interactions in hypertensive vascular remodeling. Physiol Res.
65:173–191. 2016.PubMed/NCBI
|
|
3
|
Kai H, Kudo H, Takayama N, Yasuoka S, Aoki
Y and Imaizumi T: Molecular mechanism of aggravation of
hypertensive organ damages by short-term blood pressure
variability. Curr Hypertens Rev. 10:125–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubattu S, Stanzione R and Volpe M:
Mitochondrial dysfunction contributes to hypertensive target organ
damage: lessons from an animal model of human disease. Oxid Med
Cell Longev. 2016:10678012016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, Fallahzadeh MK and McCullough PA:
Aging male spontaneously hypertensive rat as an animal model for
the evaluation of the interplay between contrast-induced acute
kidney injury and cardiorenal syndrome in humans. Cardiorenal Med.
7:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahmeda AF and Alzoghaibi M: Factors
regulating the renal circulation in spontaneously hypertensive
rats. Saudi J Biol Sci. 23:441–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu C, Huang Y and Li L: Drp1-dependent
mitochondrial fission plays critical roles in physiological and
pathological progresses in mammals. Int J Mol Sci. 18:1442017.
View Article : Google Scholar
|
|
8
|
Huang P, Sun Y, Yang J and Chen S:
Dynamin-related protein 1 (Drp1) promotes structural intermediates
of membrane division. J Biol Chem. 206:26–32. 2016.
|
|
9
|
Tanwar DK, Parker DJ, Gupta P, Spurlock B,
Alvarez RD, Basu MK and Mitra K: Crosstalk between the
mitochondrial fission protein, Drp1, and the cell cycle is
identified across various cancer types and can impact survival of
epithelial ovarian cancer patients. Oncotarget. 7:60021–60037.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu
X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, et al:
Dynamin-related protein 1-mediated mitochondrial mitotic fission
permits hyperproliferation of vascular smooth muscle cells and
offers a novel therapeutic target in pulmonary hypertension. Circ
Res. 110:1484–1497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Li R, Liu S and Zhang J: Novel
role for the regulation of mitochondrial fission by HIF-1α in the
control of smooth muscle remodeling and progression of pulmonary
hypertension. BioMed Res Int. 4:577–590. 2017.
|
|
12
|
Hua JN: Inflammation and hypertension: the
interplay of interleukin-6, dietary sodium and the
renin-angiotensin system in humans. PLoS One. 36:7591–7598.
2014.
|
|
13
|
Castellano G, Melchiorre R and Loverre A:
Interleukin-6 underlies angiotensin II-induced hypertension and
chronic renal damage. Am J Pathol. 8:435–447. 2016.
|
|
14
|
Park SY, Shrestha S, Youn YJ, Kim JK and
Kim SY: Deletion of interleukin-6 prevents cardiac inflammation,
fibrosis and dysfunction without affecting blood pressure in
angiotensin II-high salt-induced hypertension. Asian Pac J Trop
Med. 9:2–9. 2016.
|
|
15
|
Brands MW, Banes-Berceli AKL, Inscho EW,
Al-Azawi H, Allen AJ and Labazi H: Interleukin-6 knockout prevents
angiotensin II hypertension: Role of renal vasoconstriction and
JAK2/STAT3 activation. Hypertension. 56:879–884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Zhu M, Xu H, Cui L, Liu W, Wang X,
Shen S and Wang DH: Role of the monocyte chemoattractant
protein-1/C-C chemokine receptor 2 signaling pathway in transient
receptor potential vanilloid type 1 ablation-induced renal injury
in salt-sensitive hypertension. Exp Biol Med (Maywood).
240:1223–1234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y and Liu S: Effect of the
antihypertensive drug enalapril on oxidative stress markers and
antioxidant enzymes in kidney of spontaneously hypertensive rat.
Med Sci Monit. 53:25–32. 2016.
|
|
18
|
Batinic-Haberle I, Tovmasyan A and Emily
RH: Blockade of CCR2 reduces macrophage influx and development of
chronic renal damage in murine renovascular hypertension. Antioxid
Redox Signal. 30:266–271. 2014.
|
|
19
|
Zhang L, Gan W and An G: IL-10
supplementation increases Tregs and decreases hypertension in the
RUPP rat model of preeclampsia. Neural Regen Res. 20:97–110.
2016.
|
|
20
|
Nevers T, Kalkunte S and Sharma S: Uterine
regulatory T cells, IL-10 and hypertension. Am J Reprod Immunol. 66
Suppl 1:88–92. 2011. View Article : Google Scholar : PubMed/NCBI
|