Introduction
Liver ischemia-reperfusion injury (I/R) is an
important clinical complication after hemorrhagic shock, hepatic
trauma, resection surgery or transplantation (1,2). It has
a biphasic pattern: The first 6 h of reperfusion after ischemia is
the acute injury phase, which is characterized by activated massive
Kupffer cells and release of pre-inflammatory cytokines, including
tumor necrosis factor (TNF)-α and generation of reactive oxygen
species. The subsequent phase is the sub-acute pattern, in which
neutrophil infiltration and production of inflammatory mediators
prevail, as well as inhibition of the Kupffer cell function by
diminishing cytokine production and I/R injury (3–5). As
excessive inflammation is one important factor of I/R injury
(6), its regulation is a key
approach to prevent it.
Acupuncture is one of the most ancient treatments in
Traditional Chinese Medicine (TCM), which has potent effects in
treating numerous diseases, including tumors and digestive tract
diseases, or it may be applied for anesthesia, and previous studies
reported its capacity regulate the inflammatory response (7). However, the effect of
electroacupuncture (EA) and on liver I/R injury has remained to be
elucidated. Hence, the aim of the present study was to explore the
effect of pre-treatment by EA at the Hegu acupoint (LI-4) on I/R
and to investigate the underlying mechanisms.
Materials and methods
Establishment of the animal model of
liver I/R injury
All animal experiments complied with the National
Institute of Health Guide for the Care and Use of Laboratory
Animals (8,9). Ethical approval was obtained from the
ethics committee of Shanghai Public Health Clinical Center
(Shanghai, China). A total of 96 male Sprague Dawley (SD) rats
(Pharmacy College of Shanghai Jiaotong University, Shanghai, China;
6–7 weeks; 180–220 g) were acclimatized to the laboratory
environment for 7 days prior to surgery. The rats were divided into
two equal groups (each n=48) at random, one of which received I/R
surgery and the sham group received the same surgery but without
hepatic portal vessel occlusion. Animals in the two groups were
further divided into four sub-groups (each n=12), namely the no
treatment (NT), non-point acupuncture (NPA), 1,1-dimethyl-4-phenyl
L-piperazinium iodide (DMPPI) and EA at LI-4 (EA) group. The
protocol of I/R surgery was in accordance with that of previous
studies (10–12). Rats were anesthetized with sodium
pentobarbital (50 mg/kg) by intraperitoneal (i.p.) injection. A
midline laparotomy was performed, the portal circulation to the
median and left lateral lobes of the liver was carefully dissected,
and a 3-cm bending atraumatic vascular clip (Surgical Instruments
Factory, Shanghai, China) was placed on the vessels to obstruct the
portal venous and hepatic arterial blood supply to these lobes. A
total of 0.2 ml sterile saline was dripped into the abdomen to
avoid the organs to dry and a simple suture was applied to prevent
contamination. After 90 min of partial liver ischemia, the clamp
was removed and the reperfusion began. Rats were euthanized by
CO2 asphyxiation (100% CO2 at a fill rate of
20% cv/min) followed by cervical dislocation to ensure sacrifice
following 4, 8 or 24 h of reperfusion. Blood samples as well as
hepatic left lateral lobes were collected as described below for
analysis.
Vagus block
In an additional experiment, 72 SD rats were divided
into Sham, MH and SV groups (n=24). Rats in MH and SV groups were
subjected to vagus nerve block prior to I/R surgery through i.p.
injections of mecamylamine hydrochloride (MH) and subphrenic
vagotomy (SV) respectively (13,14).
Rats in these 3 groups were further divided into 2 subgroups
(n=12), NPA and EA, and pretreated with NPA and EA prior to I/R or
Sham surgeries as aforementioned. Following 8 h reperfusion, serum
alanine aminotransferase (ALT), liver myeloperoxidase (MPO), serum
TNF-α, interleukin (IL)-6 and IL-10 levels in MH and SV groups were
determined and compared with those in Sham group.
EA administration
The rats in the EA and NPA groups were subjected to
EA at 2/100 Hz and 3 mA for 30 min (Hans electro-stimulator;
Nanjing Jisheng Medical Technology Co., Ltd., Nanjing, China). The
current intensity and duration of EA were selected based on a
preliminary experiment. The LI-4 is located in the forelimb,
between the first and second metacarpals and needles were inserted
at a straight angle at a depth of 1 mm. The non-acupoint was
located at a similar site but between the fourth and fifth
metacarpals (15).
Drug administration
The rats received i.p. administration of the
nonselective nicotinic acetylcholine receptor (AChR) agonist DMPPI
(2 mg/kg) at 15 min prior to ischemia (16). The rats received i.p. injection of
noncompetitive nicotinic AChR antagonist MH (2 mg/kg) at 15 min
prior to EA (16,17).
Blood sampling and collection of
hepatic left lateral lobes
A total of 5 ml peripheral blood was obtained by
inferior vena cava puncture. The blood was collected in a sterile
plastic tube with coagulant, left to stand for 30 min and then
centrifuged at a speed of 1,500 × g for 20 min at 4°C to obtain the
serum. The serum samples were stored at −80°C until use for ALT
assays. A sample from the hepatic lateral lobe was obtained for
analysis. A portion of tissue was fixed in 10% buffered formalin at
room temperature for 36–48 h and then embedded in paraffin for
H&E staining, the procedure of which is described in the
‘Histopathology’ section. Other part of the tissue was immediately
placed into liquid nitrogen and then stored at −80°C until use for
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR).
Assessment of ALT
Serum ALT was determined using an auto-bioassay at
the Inspection division of Eastern Hepatobiliary Surgery Hospital
(Shanghai, China). The value was expressed in international units
per liter.
Histopathology
The liver tissue was prepared for H&E staining
by a pathologist (Pathology of Eastern Hepatobiliary Surgery
Hospital, Shanghai, China) according to the protocol described
elsewhere (18), who was blinded to
the all experimental conditions. Embedded tissue was sliced into
4-µm-thick sections. Sections were then mounted on regular glass
slides, deparaffinized in xylene at 37°C for ~15 min, rehydrated in
decreasing concentrations of ethanol (100, 95, 85 and 75%, each for
5 min) at room temperature. After being rinsed with distilled
water, the sections were stained with hematoxylin for 5 min at 37°C
and 0.5% eosin for 1 min successively. Photomicrographs were
captured under an Olympus BX51 microscope (Olympus, Tokyo, Japan)
at an original magnification of ×100.
Liver neutrophil accumulation
MPO is an index of neutrophil accumulation, which
was assessed by a sandwich ELISA kit (cat. no. orb411170; Wuhan
Booute Biotechnology Co., Ltd, Wuhan, China).
Assessment of serum cytokines
Serum TNF-α, IL-6 and IL-10 was determined in a
96-well micro-plate using a sandwich ELISA kit (cat. nos. PRTA00,
PR6000B and PR1000, respectively; R&D Systems, Inc.,
Minneapolis, MN, USA). All serum samples were tested in
duplicate.
RT-qPCR
Total RNA was isolated from the liver samples using
a TRIzol RNA isolation system (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). An aliquot of 500 ng total RNA
was reverse-transcribed to complementary cDNA, using the
PrimeScript™ RT reagent kit (Takara Bio Inc., Otsu, Japan).
Real-time PCR was performed using SYBR® Premix Ex Taq™
(Takara Bio Inc.) in an ABI 7300 (Applied Biosystems; Thermo Fisher
Scientific, Inc.). All reactions were performed in a 50-µl reaction
system in duplicate following the manufacturer's protocol using the
following conditions: 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec and 60°C for 30 sec. Each of the samples was tested
for TNF-α, IL-6 and IL-10 expression levels. A housekeeping gene,
β-actin, was used to verify equal loading of RNA and cDNA in the RT
and real-time PCR reactions. The threshold cycle (Cq) values were
determined and used to determine the relative copy number via the
2−ΔΔCq formula (19). The
PCR primer sequences were as follows: β-actin sense,
5′-CCACACCCGCCACCAGTTCG-3′ and antisense,
5′-CTTGCTCTGGGCCTCGTCGC-3′; TNF-α sense, 5′-TCAGTTCCATGGCCCAGAC-3′
and antisense, 5′-GTTGTCTTTGAGATCCATGCCATT-3′; IL-6 sense,
5′-TCTGCTCTGGTCTTCTGGAGTTCCG-3′ and antisense,
5′-TGGATGGTCTTGGTCCTTAGCCACT-3′; IL-10 sense,
5′-GCTGCGACGCTGTCATCGATTTCT-3′ and antisense,
5′-TGTCCTGCAGTCCAGTAGACGCC-3′.
Statistical analysis
All values are expressed as the mean ± standard
error of the mean. Differences between multiple groups were
analyzed by one-way analysis of variance, followed by two-group
comparison via the least significant differences test. P≤0.05 was
considered to indicate a statistically significant difference. All
data were analyzed using PASW 18.0 statistics software for windows
(IBM Corp., Armonk, NY, USA).
Results
Effect of EA on serum ALT
Previous studies reported that 90 min of ischemia
effectively cause severe hepatocellular injury and serum ALT is
significantly elevated in a time-dependent manner after
reperfusion. According to the two patterns of I/R injury, the
effects of EA on I/R injury were assessed after reperfusion for 4,
8 and 24 h.
As presented in Fig.
1, in the sham groups, no liver injury was encountered in the
NT and different pre-treatment groups (NPA, DMPPI or EA).
Subsequently, the effects of EA on I/R-associated increases in
serum ALT levels were assessed after 4, 8 and 24 h of reperfusion.
Compared with those in the sham group, the serum ALT levels were
significantly increased in the I/R groups (NT, NPA, DMPPI or EA;
n=12; P<0.05). The EA or DMPPI groups had lower ALT levels than
the NT or NPA groups at 4, 8 and 24 h. A maximum in ALT levels was
observed at 8 h (Fig. 1). The ALT
levels in the NT group (n=12; P>0.05) reached a normal level at
48 h, while they were still elevated at 24 h (n=12; P<0.05; data
not shown). Compared with the NT group, the EA group (n=12;
P>0.05) had similar serum ALT levels to those in sham group.
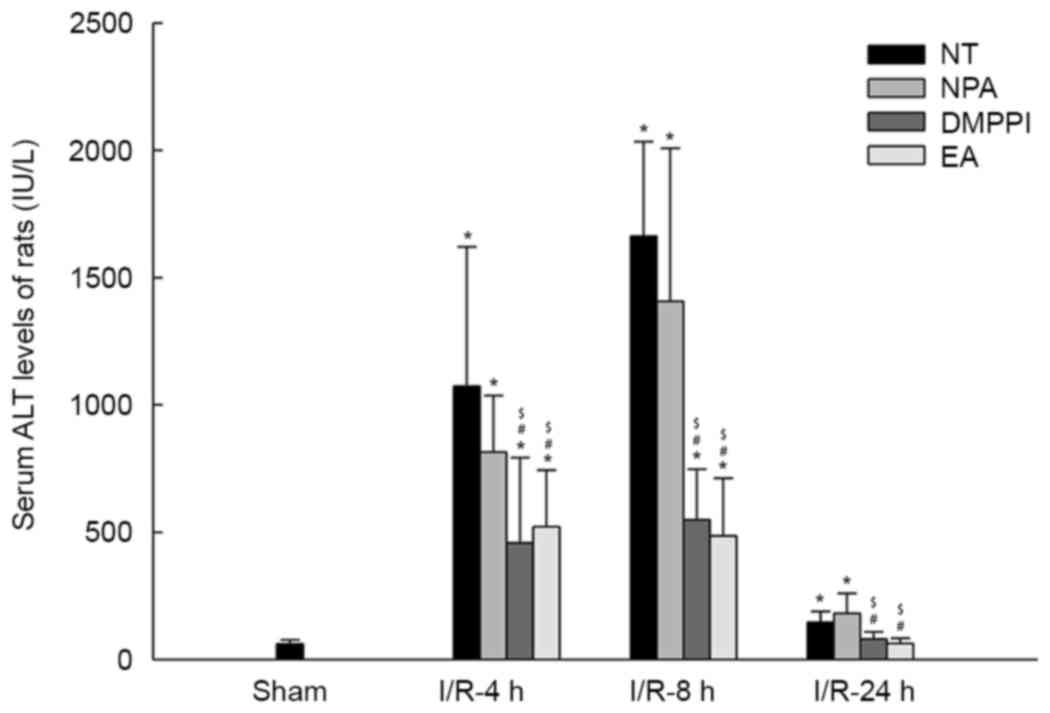 | Figure 1.EA inhibits rat serum ALT following
liver I/R. Rats remained untreated (NT group) or were pre-treated
with NPA, DMPPI or EA prior to the onset of 90 min ischemia,
followed by 4, 8 or 24 h of reperfusion. Values are expressed as
the mean ± standard error of the mean (n=12 per group). *P<0.05
vs. sham group; #P<0.05 vs. NT group;
$P<0.05 vs. NPA group. ALT, alanine aminotransferase;
EA, electroacupuncture; I/R, ischemia/reperfusion; NT, no
treatment; NPA, non-point acupuncture; DMPPI, 1,1-dimethyl-4-phenyl
L-piperazinium iodide. |
EA reduces I/R-associated liver tissue
injury
Liver tissue injury was determined via
histopathology and assessment of neutrophil accumulation. The
histopathology results were obtained by evaluating the extent of
hepatocellular necrosis, cytoplasmic vacuolization and sinusoidal
congestion. In the sham groups, the NPA, EA or DMPPI treatments had
no obvious effects on the histopathological results (Fig. 2, first row), which was in line with
the absence of effects on the ALT levels. In the NT group, the
liver tissue contracted increasingly severe injury with time,
displaying as hepatocellular necrosis, cytoplasmic vacuolization
and sinusoidal congestion. However, in the EA and DMPPI groups, the
injury was reduced after both 8 and 24 h reperfusion (Fig. 2), indicating EA prevented I/R induced
liver injury.
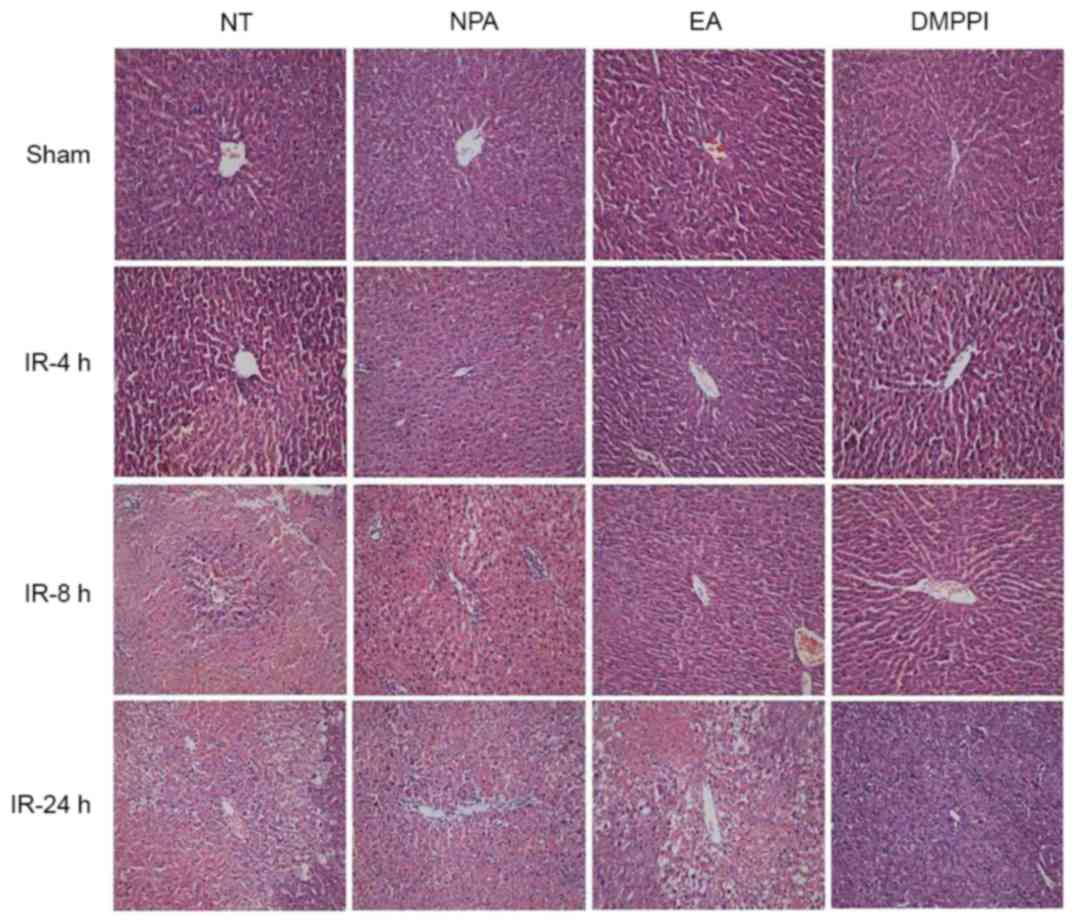 | Figure 2.Pre-treatment by EA reduces
I/R-induced rat liver injury-associated histopathological changes.
Rats remained untreated (NT group) or were pre-treated with NPA,
DMPPI or EA prior to the onset of 90 min ischemia, followed by 4, 8
or 24 h of reperfusion. Histopathology was evaluated through
H&E staining, original magnification, ×100. EA,
electroacupuncture; I/R, ischemia/reperfusion; NT, no treatment;
NPA, non-point acupuncture; DMPPI, 1,1-dimethyl-4-phenyl
L-piperazinium iodide. |
Neutrophil accumulation is also an index of liver
tissue injury, which was determined via the MPO concentration. The
concentration of MPO in the sham group was lower than that in the
other groups at 4, 8 and 24 h (Fig.
3). However, after 4 h of reperfusion, the MPO levels in the EA
and DMPPI groups were merely different from those in the NT and NPA
groups (P>0.05), which was inconsistent with the histopathology
results. After 8 h of reperfusion, the concentration of MPO in the
NT and NPA groups was much higher than that in the EA and DMPPI
groups (n=6; P<0.05) and it displayed a maximum at this
time-point. After 24 h of reperfusion, the concentrations of MPO in
the EA group did not decline further. In addition, levels in NT and
NPA groups were similar to those in the EA group at 8 h, but
remained higher than those after 4 h of reperfusion (Fig. 3).
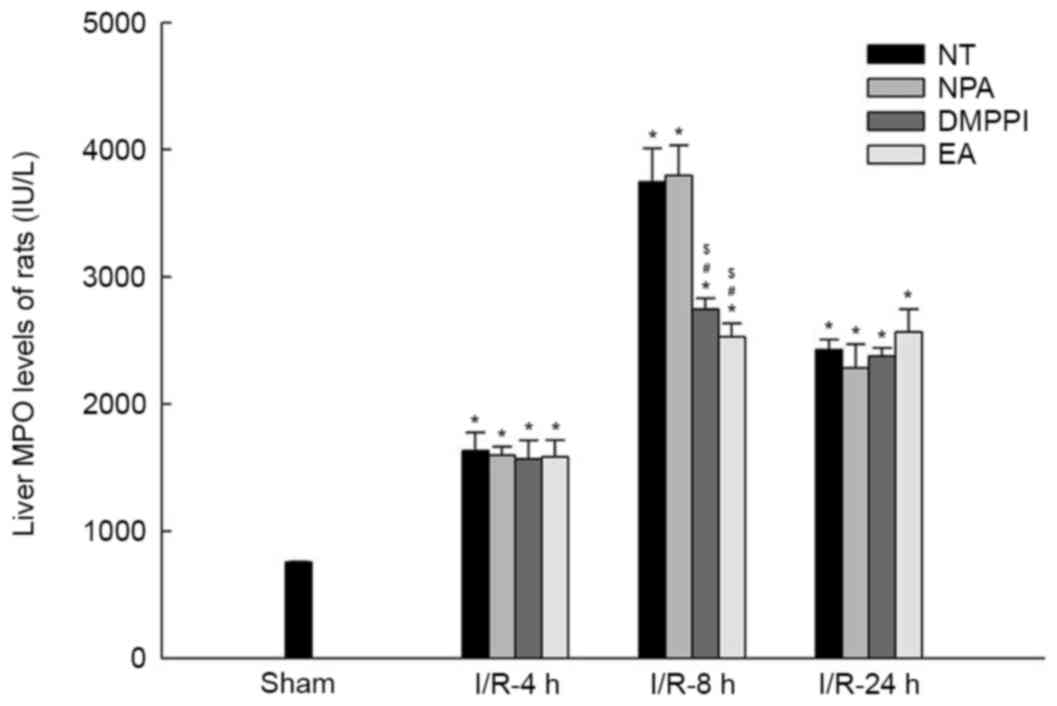 | Figure 3.EA inhibits I/R-associated neutrophil
accumulation in rat livers. Rats remained untreated (NT group) or
were pre-treated with NPA, DMPPI or EA prior to the onset of 90 min
ischemia, followed by 4, 8 or 24 h of reperfusion. MPO in liver
tissue was assessed using ELISA and indicted neutrophil
accumulation. Values are expressed as the mean ± standard error of
the mean (n=3 in sham group; n=6 in I/R group). *P<0.05 vs. sham
group; #P<0.05 vs. NT group; $P<0.05
vs. NPA group. EA, electroacupuncture; I/R, ischemia/reperfusion;
NT, no treatment; NPA, non-point acupuncture; DMPPI,
1,1-dimethyl-4-phenyl L-piperazinium iodide; MPO,
myeloperoxidase. |
Effect of EA on serum cytokines after
liver I/R
Inflammation is a key factor associated with the
pathophysiology of I/R; therefore, the serum levels of inflammatory
cytokines, including TNF-α and IL-6 and the anti-inflammatory
cytokine IL-10 were assessed. There were no increases in the levels
of TNF-α, IL-6 and IL-10 in the sham groups (Fig. 4). Compared with that in the sham
groups, an increase in cytokines was detected after 4, 8 and 24 h
of reperfusion in the NT and NPA groups. After 4 h of reperfusion,
the inflammatory cytokines (TNF-α, IL-6) in the serum reached a
maximum, while EA treatment had a significantly inhibitory effect
on the secretion of cytokines (Fig. 4A
and B), but had no effect on the secretion of the
anti-inflammatory cytokine IL-10 (Fig.
4C). However, while the levels of inflammatory cytokines TNF-α
and IL-6 in the NT an NPA groups declined rapidly after 8 and 24 h
of reperfusion, they were still higher than those in the EA group.
However, there were no significant differences in the levels of
IL-10 among the NT, NPA and EA groups (Fig. 4A-C).
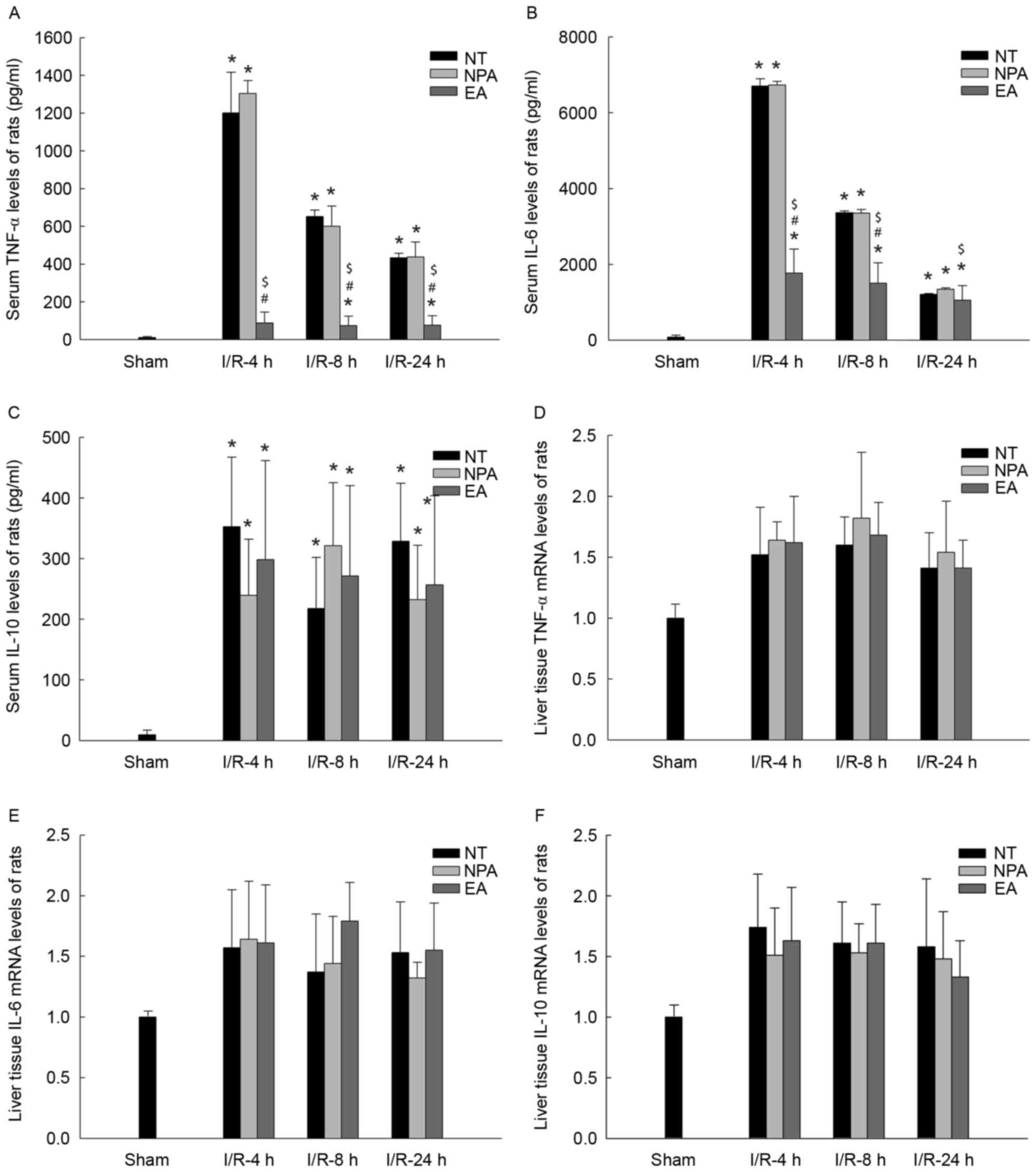 | Figure 4.The rat serum cytokines and hepatic
mRNA expression levels after I/R. Rats remained untreated (NT
group) or were pre-treated with NPA or EA prior to the onset of 90
min ischemia, followed by 4, 8 or 24 h of reperfusion. Serum levels
of cytokines (A) TNF-α, (B) IL-6 and (C) IL-10 were assessed using
ELISA. *P<0.05 vs. sham group; #P<0.05 vs. NT
group; $P<0.05 vs. NPA group. (D-F) mRNA expression
levels of (D) TNF-α, (E) IL-6 and (F) IL-10 in liver tissues.
Values are expressed as mean ± standard error of the mean (n=10 in
A-C and n=6 in D-F). TNF, tumor necrosis factor; IL, interleukin;
EA, electroacupuncture; NPA, non-point acupuncture; I/R,
ischemia/reperfusion; NT, not treated. |
Effect of EA on the mRNA levels of
cytokines in liver tissue after I/R
To investigate the suppressive effect of EA on
cytokine production, the levels of inflammatory cytokines TNF-α,
IL-6 and anti-inflammatory cytokine IL-10 mRNA in liver tissue were
assessed by RT-qPCR in relativity to those in the sham group. In
all I/R groups, it was demonstrated that cytokine mRNA levels were
higher than those in the Sham group. No significant differences in
the levels of TNF-α mRNA and IL-6 mRNA were identified between the
EA and NT/NPA groups after 4, 8 or 24 h of reperfusion (Fig. 4D and E). Furthermore, the levels of
IL-10 mRNA did not display any differences among these groups
(Fig. 4F).
Effect of EA on vagus blocking
To explore the mechanisms of EA, rat vagus nerves
were blocked by SV or by MH injection. Following these treatments,
the rats were divided into 2 subgroups (NPA or EA) at random and
all received I/R surgery. The levels of ALT, liver MPO, serum
TNF-α, IL-6 and IL-10 were significantly higher in SV and MH groups
when compared to Sham group (all P<0.05; Fig. 5). No significant difference was
identified in serum ALT, liver MPO and serum cytokines between NPA
and EA subgroups (Fig. 5),
suggesting that the protective effect of EA was abolished by vagus
nerve block.
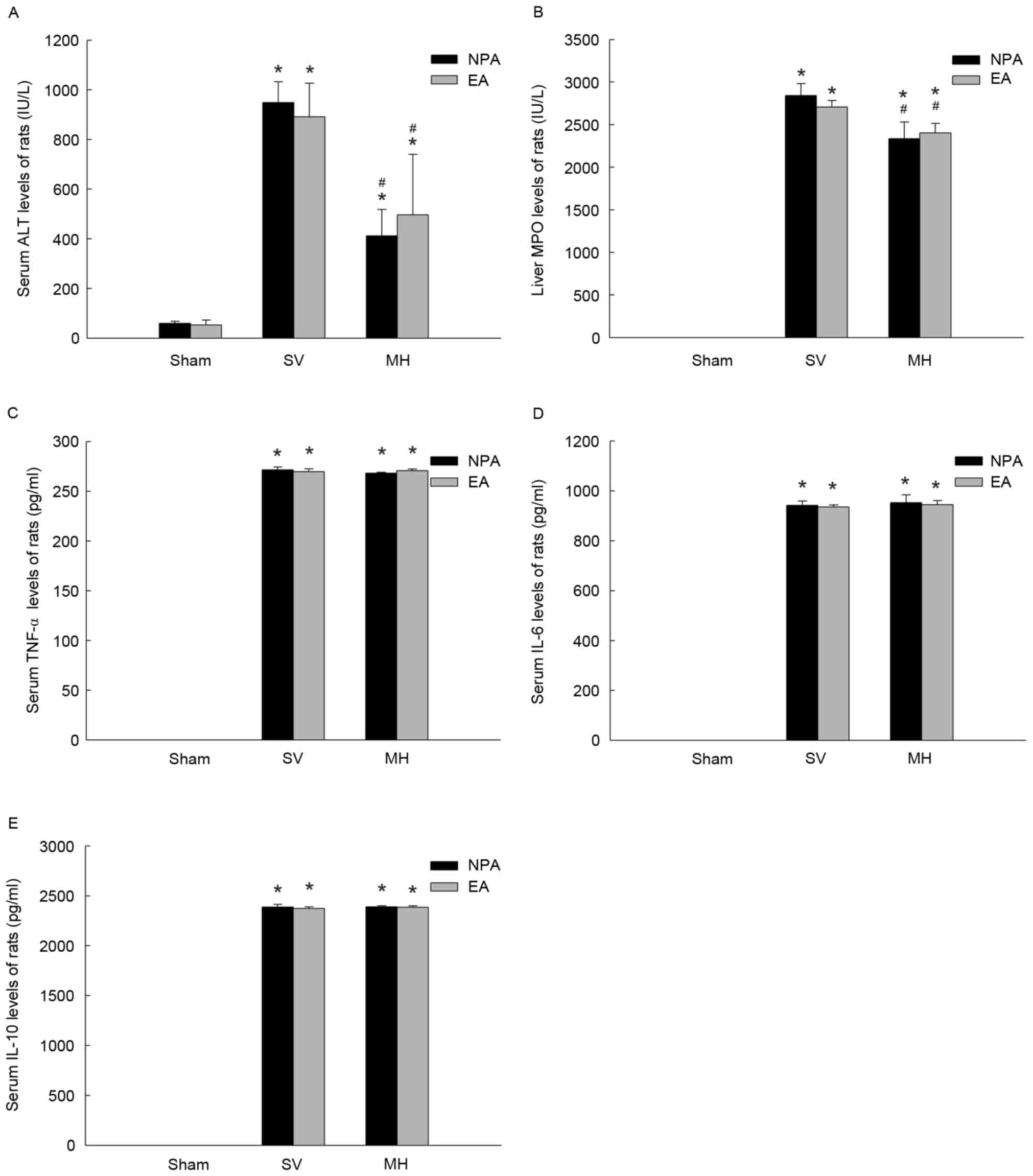 | Figure 5.Vagus block disrupts the effects of EA
on inflammatory cytokines after ischemia/reperfusion. Rats were
pre-treated with NPA or EA prior to the onset of 90 min of
ischemia, followed by 8 h of reperfusion and the animal model was
pre-treated by SV or MH. Effects on the serum levels of (A) ALT,
(B) MPO, (C) TNF-α, (D) IL-6 and (E) IL-10 were assessed. Values
are expressed as the mean ± standard error of the mean (SV, n=10;
MH, n=6). *P<0.05 vs. sham group; #P<0.05 vs. SV
group. ALT, alanine aminotransferase; MPO, myeloperoxidase; TNF,
tumor necrosis factor; IL, interleukin; SV, subphrenic vagotomy;
MH, mecamylamine hydrochloride; EA, electroacupuncture; NPA,
non-point acupuncture. |
Discussion
To the best of our knowledge, no previous studies
have reported on the protective effects of EA on liver I/R, which
was the aim of the present study. The results demonstrated that EA
significantly attenuated liver injury, as indicated by the levels
of ALT and MPO as well as histopathology. However, when the vagus
nerve was blocked, EA lost its potency (16). The inflammatory cytokines assessed
are correlated with the cholinergic anti-inflammatory pathway. NPA
used as the negative control therapy illustrated that only EA at
the specific acupoint was responsible for the specific effect.
Acupuncture is a distinctive therapy in TCM and has thousands of
years of history. It is increasingly prevalent to treat numerous
diseases, but the mechanism has not been clarified, as it does not
provide a specific cure for certain diseases and too many sites of
action are present (7,15,20).
LI-4 is one of the most useful acupoints in the human body and is
the source point belonging to the hand yangming large intestine
meridian, which is subjected to acupuncture for treating pain or
inflammatory conditions (15).
Hence, acupuncture at the LI-4 acupoint was performed in the
present study to assess its protective effect against liver I/R
injury in rats.
As in previous studies (6,16,21), the
levels of ALT and MPO were assessed and liver tissues were
subjected to histopathological examination following H&E
staining. ALT is an index to reflect liver function and it is
released into the peripheral blood upon hepatocyte injury. In the
present study, the release of ALT was time-dependent, displaying a
peak level at 8 h of reperfusion following liver ischemia with
restoration to basal levels at 48 h without any interventional
treatment of the rats. However, in the EA group, this restoration
was reduced to 24 h. To the best of our knowledge, the present
study was the first to demonstrate the time-dependent effect of EA
on liver I/R. The results indicated that EA obviously inhibited the
increase in ALT levels compared with that in the NT group in the
acute phase after 4 and 8 h of reperfusion, which was similar to
previously published results. However, the result that after 24 h
of reperfusion (sub-acute phase), ALT levels in the EA group were
still inhibited compared with those in the NT group was
inconsistent with that of a previous study (16). The underlying mechanisms of action of
acupuncture remain elusive, but in TCM, its long-term effects on
diseases compared with those of other treatments are
well-established. In the present study, H&E staining was
performed to visualize tissue injury following I/R. No injury was
observed in the sham operation group, while in the NT group
subjected to I/R, hepatocellular breakage caused liver injury,
which became increasingly severe from 4 to 24 h of reperfusion,
which was in accordance with a previous study (21). EA treatment had a therapeutic effect
on I/R, but at 24 h, the difference between the EA and NT groups
was less obvious. This meant that to a certain extent, EA inhibited
the injury in the acute phase, but it did not protect against
hepatocellular damage in the sub-acute phase. MPO is another index
of I/R injury and reflects neutrophil accumulation (21). A difference in MPO levels between the
EA and NT groups was only observed at 8 h, which may be explained
by the model of the acute and sub-acute stages of I/R-induced liver
injury and was in accordance with the results of the H&E
staining. However, there were certain inconsistencies in the
time-course of changes in the ALT and MPO levels as well as H&E
staining results. This might be due to the fact that ALT is a
matrix enzyme and may be gradually degraded when it is released
into the blood. But the hepatocellular breaking was not a
reversible procedure which was proved by H&E staining. MPO
level was relatively lower after 24 h of reperfusion compared to
that after 8 h of reperfusion because it was also an enzyme which
would be metabolized in body.
Inflammation is one of the most complex problems in
surgery. According to TCM, the central nervous system engages in
cross-talk with the immune system. However, it is a slow response
that does not provide any efficient protection from acute injury,
including liver I/R promoted by excessive inflammation. Certain
studies reported on the involvement of the cholinergic
anti-inflammatory pathway (CAIP), which has shorter response times
than the humoral anti-inflammatory pathways (22). The stimulated vagus releases Ach,
which inhibits cytokines released from macrophages, but this effect
is diminished or eliminated when the α-7 receptor of nicotine is
blocked (17). Another study
reported that early-phase I/R-associated liver injury in rats was
mitigated when the nicotine receptor of the vagus nerve was
stimulated by certain drugs (16).
However, the drug has various known and unknown side effects and
vagus electro-stimulation is likely to be harmful for the human
body (23).
In addition, inflammation is the key promoting
factor of I/R-associated injury and has important roles in the
whole process (24,25). However, the acute phase features a
massive release of cytokines, particularly pro-inflammatory
cytokines including TNF-α or IL-6, which are later degraded
(26). In the present study, the
cytokines were assessed in the serum by ELISA and in liver tissues
at the mRNA level, but they were not in parallel. No significant
difference was present in the liver mRNA levels of cytokines
between the EA and NT groups. However, the I/R-associated increases
in the serum levels of these cytokines were effectively inhibited
by EA (16). Therefore, the
inhibitory effect of EA on I/R-associated liver injury may be due
to the attenuation of the release of inflammatory cytokines into
the serum, particularly of TNF-α, which is the core cytokine to
stimulate various cell types, including Kupffer cells or
hepatocytes, to produce various other cytokines. The response to EA
was displayed in the ALT and MPO levels, as well as in the
histopathology results, indicating that I/R-induced injury was
reduced. Another cytokine, IL-10, which is considered to be an
anti-inflammatory index, was assessed in the present study
(27,28). However, the results indicated that EA
had no evident effect on it at the serum or tissue mRNA level,
which was similar to the results of previous studies. It was
therefore demonstrated that EA did not inhibit I/R-induced liver
injury through the anti-inflammatory system (26).
The effect of EA on the inflammatory system attracts
numerous interests. The CAIP has been in the focus of research
since 2000. Studies have indicated that direct electro-stimulation
of the vagus nerve antagonized inflammation caused by sepsis and
inhibited macrophages incubated with cytokines in vitro
(13). Another study revealed that
EA at the Zusanli (ST36) point had an anti-sepsis effect via the
CAIP. It was reported that activation of the vagus CAIP protected
against I/R at the early phase (29). The present study therefore
hypothesized that EA protects the liver from I/R-associated injury
through the CAIP. To prove this, the vagus potential was first
tested under EA and the potential pulse was more obvious with a
stronger current (data not shown). Subsequently, DMPPI, a
nonselective nicotinic AChR agonist, was used as a positive control
treatment for comparison with the EA group, which was proved to
effectively activate vagus CAIP (16). The results indicated no differences
between the EA group and the DMPPI group, indicating that the
mechanism of EA to protect the liver from I/R was similar to that
of DMPPI, namely via CAIP; however, this mechanism remains to be
fully confirmed. Following this, the vagus was blocked by SV or
i.p. injection of MH prior to I/R and liver injury and serum
cytokines were determined after 8 h of reperfusion (13). The protective effect of EA against
I/R induced liver injury was ameliorated following vagus block,
indicating the primary role of the vagus in EA. A limitation of the
present study is that the effect of SV and MH on liver injury was
not assessed in Sham rats. As relatively little information exists
regarding the effect of SV or MH on liver injury, it is
hypothesized that SV and MH themselves may have little effect on
liver injury and serum cytokine levels, which requires further
experimental verification.
To demonstrate that only EA performed at specific
acupoints, including LI-4, had a protective effect, the
experimental design of the present study included an NPA group. All
indexes of the NPA group were indifferent from those of the NT
group, while those in the EA group exhibited a marked difference.
This was supported by the ancient concept of TCM that a point with
a non-meridian location, known as the ‘ah-shi’ point, is considered
to only treat pain that does not concentrate in one area but does
not have any specific curative effect as acupuncture performed at a
meridian point such as LI-4 (15).
In TCM, LI-4 is an important acupoint, as is ST-36
and the two are always combined. Thus, as acupuncture on LI-4
affects ST-36; the LI-4 acupoint was selected for the present
study. LI-4 belongs to the hand yangming large intestine meridian,
which is connected with the foot yangming stomach meridian, on
which ST-36 is located. Therefore, acupuncture on LI-4 has part of
the effect of that on ST-36. ST-36 is always used to exert
anti-inflammatory effects and a previous study reported that the
CAIP constituted the major mechanism of the effect of acupuncture
on ST-36 against lipopolysaccharide-induced sepsis in rats
(15). This study demonstrated the
power of acupuncture, but it only determined that it reduced the
sepsis, but did not fully prevent its occurrence. In the present
study, the anti-inflammatory function of EA at LI-4, an important
acupoint with strong therapeutic efficacy, was investigated in
association with its protective effect against liver I/R. EA is
safer for humans than drugs or direct electro-stimulation of the
vagus nerve, as it has barely any side effects and has been used
for thousands of years in China, while drugs are currently not
approved for clinical use and direct vagus stimulation is obviously
dangerous.
In conclusion, the present study indicated that the
EA effectively protects the liver from I/R injury and the major
mechanism of action was to reduce pro-inflammatory cytokines
through activation of the CAIP. However, anti-inflammatory
cytokines did not have any important role. Acupuncture is an
ancient TCM treatment with significant efficacy, although the
underlying mechanisms remain elusive. The function of acupuncture
was explored and further details require to be assessed in-depth in
the future.
Acknowledgements
The data of this study were previously presented at
the 2013 meeting of the Americas Hepato-Pancreato-Biliary
Association from 27th to 30th March, Shanghai, China (30).
References
|
1
|
Nastos C, Kalimeris K, Papoutsidakis N,
Tasoulis MK, Lykoudis PM, Theodoraki K, Nastou D, Smyrniotis V and
Arkadopoulos N: Global consequences of liver ischemia/reperfusion
injury. Oxid Med Cell Longev. 2014:9069652014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mourad MM, Algarni A, Liossis C and
Bramhall SR: Aetiology and risk factors of ischaemic cholangiopathy
after liver transplantation. World J Gastroenterol. 20:6159–6169.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ji H, Shen XD, Zhang Y, Gao F, Huang CY,
Chang WW, Lee C, Ke B, Busuttil RW and Kupiec-Weglinski JW:
Activation of cyclic adenosine monophosphate-dependent protein
kinase a signaling prevents liver ischemia/reperfusion injury in
mice. Liver Transpl. 18:659–670. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu A, Fang H, Dirsch O, Jin H and Dahmen
U: Early release of macrophage migration inhibitory factor after
liver ischemia and reperfusion injury in rats. Cytokine.
57:150–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang JW and Lee SM: Melatonin inhibits
type 1 interferon signaling of toll-like receptor 4 via heme
oxygenase-1 induction in hepatic ischemia/reperfusion. J Pineal
Res. 53:67–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yun N, Kang JW and Lee SM: Protective
effects of chlorogenic acid against ischemia/reperfusion injury in
rat liver: Molecular evidence of its antioxidant and
anti-inflammatory properties. J Nutr Biochem. 23:1249–1255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pfab F, Schalock PC, Napadow V,
Athanasiadis GI, Huss-Marp J and Ring J: Acupuncture for allergic
disease therapy-the current state of evidence. Expert Rev Clin
Immunol. 10:831–841. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaughn SE: Review of the third edition of
the guide for the care and use of agricultural animals in research
and teaching. J Am Assoc Lab Anim Sci. 51:298–300. 2012.PubMed/NCBI
|
|
9
|
Institute of Laboratory Animal Resources
CoLS, National Research Council: Guide for the care and use of
laboratory animals. National Academy Press; Washington, DC:
1996
|
|
10
|
Chan YY, Lo WY, Li TC, Shen LJ, Yang SN,
Chen YH and Lin JG: Clinical efficacy of acupuncture as an adjunct
to methadone treatment services for heroin addicts: A randomized
controlled trial. Am J Chin Med. 42:569–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ha LJ, Cui JJ, Wang FC, Jing XH and Bai
WZ: The expression of calcitonin gene-related peptide in the
sensory and motor neurons associated with ‘Hegu’ (LI 4) in the rat.
Zhen Ci Yan Jiu. 39:112–116. 2014.(In Chinese). PubMed/NCBI
|
|
12
|
Zhang J, Wang X and Lü R: Analgesic effect
of acupuncture at hegu (LI 4) on transvaginal oocyte retrieval with
ultrasonography. J Tradit Chin Med. 33:294–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirshenbaum AP, Jackson ER, Brown SJ,
Fuchs JR, Miltner BC and Doughty AH: Nicotine-induced impulsive
action: Sensitization and attenuation by mecamylamine. Behav
Pharmacol. 22:207–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rivera RE, Eagon JC, Soper NJ,
Klingensmith ME and Brunt LM: Experience with laparoscopic gastric
resection: Results and outcomes for 37 cases. Surg Endosc.
19:1622–1626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scognamillo-Szabó MV, Bechara GH, Ferreira
SH and Cunha FQ: Effect of various acupuncture treatment protocols
upon sepsis in Wistar rats. Ann N Y Acad Sci. 1026:251–256. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crockett ET, Galligan JJ, Uhal BD, Harkema
J, Roth R and Pandya K: Protection of early phase hepatic
ischemia-reperfusion injury by cholinergic agonists. BMC Clin
Pathol. 6:32006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He YQ, Li Y, Wang XY, He XD, Jun L, Chuai
M, Lee KK, Wang J, Wang LJ and Yang X: Dimethyl phenyl piperazine
iodide (DMPP) induces glioma regression by inhibiting angiogenesis.
Exp Cell Res. 320:354–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gujral JS, Bucci TJ, Farhood A and
Jaeschke H: Mechanism of cell death during warm hepatic
ischemia-reperfusion in rats: Apoptosis or necrosis? Hepatology.
33:397–405. 2010. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng CH, Zhang J, Wu J and Zhang MM: The
effect of transcutaneous electrical acupoint stimulation on
pregnancy rates in women undergoing in vitro fertilization: A study
protocol for a randomized controlled trial. Trials. 15:1622014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuboki S, Shin T, Huber N, Eismann T,
Galloway E, Schuster R, Blanchard J, Zingarelli B and Lentsch AB:
Peroxisome proliferator-activated receptor-gamma protects against
hepatic ischemia/reperfusion injury in mice. Hepatology.
47:215–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiang H, Hu B, Li Z and Li J:
Dexmedetomidine controls systemic cytokine levels through the
cholinergic anti-inflammatory pathway. Inflammation. 37:1763–1770.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong J, Xue FS, Yuan YJ, Wang Q, Liao X
and Wang WL: Cholinergic anti-inflammatory pathway: A possible
approach to protect against myocardial ischemia reperfusion injury.
Chin Med J (Engl). 123:2720–2726. 2010.PubMed/NCBI
|
|
24
|
Manhas A, Khanna V, Prakash P, Goyal D,
Malasoni R, Naqvi A, Dwivedi AK, Dikshit M and Jagavelu K: Curcuma
oil reduces endothelial cell mediated inflammation in post
myocardial ischemia/reperfusion in rats. J Cardiovasc Pharmacol.
64:228–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dvoriantchikova G, Santos AR, Saeed AM,
Dvoriantchikova X and Ivanov D: Putative role of protein kinase C
in neurotoxic inflammation mediated by extracellular heat shock
protein 70 after ischemia-reperfusion. J Neuroinflammation.
11:812014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cámara-Lemarroy CR, Guzmán-de la Garza FJ,
Alarcón-Galván G, Cordero-Pérez P, Muñoz-Espinosa L,
Torres-González L and Fernández-Garza NE: Hepatic
ischemia/reperfusion injury is diminished by atorvastatin in wistar
rats. Arch Med Res. 45:210–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tukhovskaya EA, Turovsky EA, Turovskaya
MV, Levin SG, Murashev AN, Zinchenko VP and Godukhin OV:
Anti-inflammatory cytokine interleukin-10 increases resistance to
brain ischemia through modulation of ischemia-induced intracellular
Ca2+ response. Neurosci Lett. 571:55–60. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zuo Z, Wang C, Carpenter D, Okada Y,
Nicolaidou E, Toyoda M, Trento A and Jordan SC: Prolongation of
allograft survival with viral IL-10 transfection in a highly
histoincompatible model of rat heart allograft rejection.
Transplantation. 71:686–691. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang CL, Tsai PS, Wang TY, Yan LP, Xu HZ
and Huang CJ: Acupuncture stimulation of ST36 (Zusanli) attenuates
acute renal but not hepatic injury in lipopolysaccharide-stimulated
rats. Anesth Anal. 104:646–654. 2007. View Article : Google Scholar
|
|
30
|
Xu F, Li YS, Lv X, et al: Protective
effect of electroacupuncture on liver ischemiareperfusion injury in
rats. Official J Int Hepato Pancreato Biliary Assoc.
15:1222013.
|



















