Introduction
Gastric cancer (GC), one of the most common types of
human cancer, results in high morbidity and mortality worldwide
(1,2). Although strategies involving surgery
combined with radiotherapy and chemotherapy have been extensively
investigated, patients with advanced GC present poor prognosis
(1–4). As the malignant progression of GC is a
multistep and multifactorial process, investigation on the
molecular mechanism underlying GC growth and metastasis may be
helpful for developing effective therapeutic strategies for this
disease (3,4).
MicroRNAs (miRs) are small non-coding RNAs
containing 22–25 ribonucleic acids, which directly bind to the
3′-untranslated region (3′UTR) of their target genes, causing
translation inhibition or mRNA degradation (5). Through negatively mediating the gene
expression, miRs participate in the regulation of a variety of
cellular biological processes. Certain miRs targeting oncogenes or
tumor suppressors have been demonstrated to serve key roles in
different types of human cancer (6–9). In
addition, previous studies have revealed that numerous miRs,
including miR-326 (9), miR-126
(10), miR-145 (11) and miR-506 (12), serve suppressive or oncogenic roles
in GC.
Deregulation of miR-153 has recently been observed
in several common human tumors, while miR-153 serves a promoting or
tumor suppressive role in different cancer types, including GC
(13–15). For instance, Zhang et al
(13) has reported that miR-153 was
downregulated in GC, which promoted the migration and invasion of
the GC cells, SGC-7901. Wang and Liu (16) also suggested that miR-153 suppression
in GC promoted Snail-mediated cancer metastasis. However, the
detailed regulatory mechanism of miR-153 in GC remains unclear.
Kruppel-like factor 5 (KLF5), a member of the KLF
subfamily of zinc finger proteins, is a transcriptional activator
that binds directly to a specific recognition motif in the
promoters of target genes (17).
Recent studies have identified that KLF5 is frequently upregulated
in certain human cancer types and functions as an oncogene
(14,17–19).
Furthermore, Chia et al (20)
reported a regulatory crosstalk between KLF5, GATA4 and GATA6 that
cooperatively promotes GC development. However, the molecular
mechanism underlying KLF5 expression in GC remains largely
unknown.
Therefore, the present study aimed to investigate
the molecular mechanism of miR-153 underlying the malignant
progression of GC, as well as examine the involvement of KLF5 in
GC.
Materials and methods
Clinical samples
The current study was approved by the Ethical
Committee of Xiangya Hospital, Central South University (Changsha,
China). A total of 83 GC tissues and their matched adjacent
non-tumor tissues were collected at the Department of
Gastrointestinal Surgery of Xiangya Hospital between March 2011 and
April 2012, and informed consent were obtained from the patients.
The 83 GC patients included 50 male and 33 female, with an age
range of 47–82 years and a mean age of 64.7 years. In addition, 42
of the patients with GC were at TNM stage I–II and 41 patients were
at TNM stage III–IV (21). These GC
patients were divided into low and high miR-153 expression groups
based on the mean expression value (1.03) as the cutoff value. The
tissues were immediately snap-frozen in liquid nitrogen following
surgery and stored at −80°C prior to use. The clinical information
of these patients is summarized in Table
I.
 | Table I.Association between miR-153 expression
and clinicopathological characteristics of gastric cancer
patients. |
Table I.
Association between miR-153 expression
and clinicopathological characteristics of gastric cancer
patients.
| Variables | Low miR-153
(n=43) | High miR-153
(n=40) | P-value |
|---|
| Age (years) |
|
| 0.511 |
| ≤65 | 21 | 23 |
|
|
>65 | 22 | 17 |
|
| Sex |
|
| 1.000 |
| Male | 26 | 24 |
|
|
Female | 17 | 16 |
|
| Tumor
differentiation |
|
| 0.080 |
| Well and
moderate | 16 | 23 |
|
|
Poor | 27 | 17 |
|
| Tumor size
(cm) |
|
| 0.275 |
| ≤5 | 18 | 22 |
|
|
>5 | 25 | 18 |
|
| Lymph node
metastasis |
|
| 0.045 |
|
Present | 31 | 20 |
|
|
Absent | 12 | 20 |
|
| Distant
metastasis |
|
| 0.022 |
|
Present | 12 | 3 |
|
|
Absent | 31 | 37 |
|
| TNM stage |
|
| 0.004 |
|
I–II | 15 | 27 |
|
|
III–IV | 28 | 13 |
|
Cell culture
Human GC cell lines, including KATO III, NCI-N87,
SNU-16 and SNU-5, and normal gastric mucosa epithelial GES-1 cells
were purchased from the Cell Bank of Central South University.
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified incubator containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol Reagent (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The total RNA was then converted into cDNA using an miRNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. The expression of mRNA was then
examined using a SYBR Green qPCR Assay kit (Thermo Fisher
Scientific, Inc.) on a thermocycler (ABI 7300 plus; Thermo Fisher
Scientific, Inc.), while the expression of miR was determined using
an miRNA qPCR Detection kit (GeneCopoeia, Inc., Rockville, MD,
USA), according to the manufacturer's protocol. GAPDH or U6 was
used as the internal reference for the determination of mRNA or miR
expression, respectively. The primers sequences used in qPCR were
as follows: KLF5 forward, 5′-CCTGGTCCAGACAAGATGTGA-3′, and reverse,
5′-GAACTGGTCTACGACTGAGGC-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′, and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The primers for miR-153 (cat. no.
SG-has-miR-153) and U6 (cat. no. SG-U6) were obtained from Shenzhen
Hua Anping Hong Biological Technology Co., Ltd. (Shenzhen, China).
The qPCR reaction was performed at 95°C for 5 min, followed by 45
cycles of denaturation at 95°C for 30 sec and annealing/elongation
step at 60°C for 30 sec. The relative expression was analyzed by
the 2−ΔΔCq method (22).
Cell transfection
SNU-5 cells were transfected with scrambled miR
mimic (miR-NC), miR-153 mimic, negative control (NC) inhibitor or
miR-153 inhibitor, or co-transfected with miR-153 inhibitor and
pcDNA3.1 vector, or with miR-153 inhibitor and pcDNA3.1-KLF5
plasmid (all generated from Yearthbio, Changsha, China).
Transfection was performed using Lipofectamine 2000 (Thermo Fisher
Scientific) at 37°C, according to the manufacturer's protocol.
Following transfection for 48 h, the mRNA or protein expression
levels of KLF5, miR-153 and the corresponding internal controls
were determined using RT-qPCR or western blot analysis,
respectively.
Cell proliferation analysis
An MTT assay was conducted for the analysis of cell
proliferation. Briefly, SNU-5 cells (5×104 cells per
well) were plated into a 96-well plate and cultured at 37°C with 5%
CO2 for 0, 12, 24, 48 or 72 h. Subsequently, 20 µl of
MTT (5 mg/ml; Sigma-Aldrich; Merck, Darmstadt, Germany) was added
and incubated at 37°C for 4 h, followed by addition of 150 µl of
dimethyl sulfoxide (Sigma-Aldrich; Merck). Following incubation at
room temperature for 10 min, formazan production was detected by
determining the optical density at 570 nm using an enzyme
immunoassay analyzer (Typhoon 8600; GE Healthcare, Chicago, IL,
USA).
Cell migration analysis
For the analysis of cell migration, a wound healing
assay was conducted. Briefly, SNU-5 cells (2×105 cells
per well) were seeded into a 24-well plate and cultured to full
confluence. Next, a wound was formed using a plastic cell scraper,
and then cells were washed using phosphate-buffered saline (PBS).
Subsequent to incubation in DMEM without FBS at 37°C for 24 h, the
medium was replaced with DMEM containing 10% FBS, and then cultured
at 37°C for a further 48 h. Finally, the wounds were observed under
a microscope (Nikon Corp., Tokyo, Japan).
Cell invasion analysis
For investigation of cell invasion, a Transwell
assay was performed. Briefly, the SNU-5 cell suspension
(1×105 cells per well) was added into the upper chamber
that was pre-coated with Matrigel (Chemicon; EMD Millipore,
Billerica, MA, USA), while 300 µl DMEM containing 10% FBS was added
into the lower chamber. Following incubation at 37°C for 24 h, the
cells on the interior of the inserts were removed using a
cotton-tipped swab. Cells on the lower surface of the membrane were
stained with gentian violet (Sigma-Aldrich; Merck), and then rinsed
by water, and dried in air. Invading cells were counted under a
microscope (Olympus Corp., Tokyo, Japan).
Western blot analysis
SNU-5 cells were lysed in cold
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.), and the protein concentration was determined using a
Bicinchoninic Acid Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein was separated by 10% SDS-PAGE and then
transferred to a polyvinylidene difluoride (PVDF) membrane (Thermo
Fisher Scientific, Inc.), which was then blocked in 5% non-fat milk
in PBS (Thermo Fisher Scientific, Inc.) containing 0.1% Tween-20
(Sigma-Aldrich; Merck) at room temperature for 3 h. Subsequently,
the PVDF membrane was incubated with rabbit anti-human polyclonal
KLF5 (1:50; cat. no. ab137676; Abcam, Cambridge, MA, USA) or rabbit
anti-human GAPDH (1:100; cat. no. ab9485; Abcam) primary antibodies
at room temperature for 3 h. Following washing with PBS for 10 min,
the PVDF membrane was incubated with a goat anti-rabbit secondary
antibody (1:5,000; cat. no. ab7090; Abcam) at room temperature for
1 h. After further washing with PBS for 10 min, the protein bands
were detected using an Enhanced Chemiluminescence Western Blotting
kit (Pierce; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols, and then quantified using Image Lab
analysis software version 3.1 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Bioinformatics prediction
In order to predict the potential target genes of
miR-153, the TargetScan (targetscan.org) and PicTar (pictar.mdc-berlin.de) online software were used,
according to the manufacturer's instructions. ‘MiR-153’ was
inserted and ‘human’ was selected. The putative target genes of
miR-153 were scanned.
Recombinant vector construction for
dual-luciferase reporter assay
The predicted miR-153 binding sites on the 3′UTR of
KLF5 were cloned into the pGL3 vector (Promega Corporation,
Madison, WI, USA) and termed wild type (WT)-KLF5-3′UTR. The
mutant-type (MT) miR-153 binding sites on the 3′UTR of KLF5 were
constructed using a QuikChange Site-Directed Mutagenesis kit
(Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA), in
accordance with the manufacturer's protocol. This was also inserted
into the pGL3 vector and termed MT-KLF5-3′UTR.
Dual-luciferase reporter gene
assay
SNU-5 cells were transfected with 100 nM
WT-KLF5-3′UTR or MT-KLF5-3′UTR plasmid, as well as with or without
100 nM miR-153 mimic. The luciferase activity was then measured at
48 h after transfection using the Dual-Luciferase Reporter Assay
System (Promega Corporation) on an Lmax II Luminometer (Molecular
Devices, LLC, Sunnyvale, CA, USA). The firefly luciferase activity
was then normalized to the Renilla luciferase activity.
Statistical analysis
The data in the present study are expressed as the
mean ± standard deviation. Statistical analysis was conducted by
Student t-test for comparison between 2 groups or by one-way
analysis of variance for comparison of >2 groups, using SPSS
version 19.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-153 is downregulated in GC, and
associated with malignant progression and poor prognosis
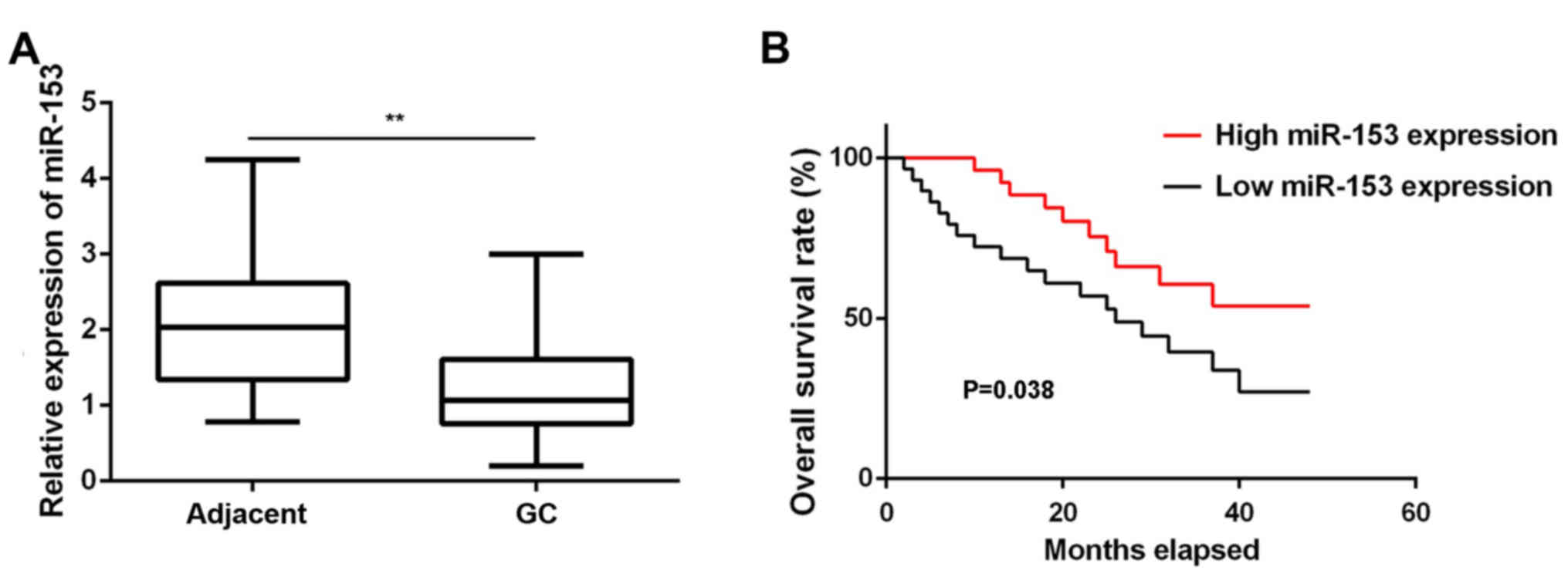
In the present study, RT-qPCR data demonstrated that
miR-153 was significantly downregulated in GC tissues compared with
the adjacent non-tumor tissues (Fig.
1A). These GC patients were divided into low and high miR-153
expression groups based on the mean expression value (1.03) as the
cutoff value. In addition, low expression of miR-153 was
significantly associated with lymph node metastasis, distant
metastasis and advanced TNM stage, although it was not correlated
with the patient age, sex, tumor differentiation or tumor size
(Table I). Further investigation
revealed that the GC patients with low miR-153 expression had a
shorter survival time, when compared with those presenting a high
miR-153 expression (Fig. 1B).
Accordingly, miR-153 downregulation may contribute to the GC
aggressiveness, as well as the poor prognosis of patients.
Overexpression of miR-153 inhibits GC
cell proliferation, migration and invasion
The miR-153 expression levels were then examined in
several common GC cell lines (KATO III, NCI-N87, SNU-16 and SNU-5),
using normal gastric epithelial GES-1 cells as the control. In
accordance with the findings in the GC tissues, RT-qPCR data
revealed that miR-153 was also significantly downregulated in the
four GC cell lines compared with the normal GES-1 cells (Fig. 2A). Since miR-153 expression was
downregulated to a greater extent in SNU-5 cells, this cell line
was used in subsequent experiments.
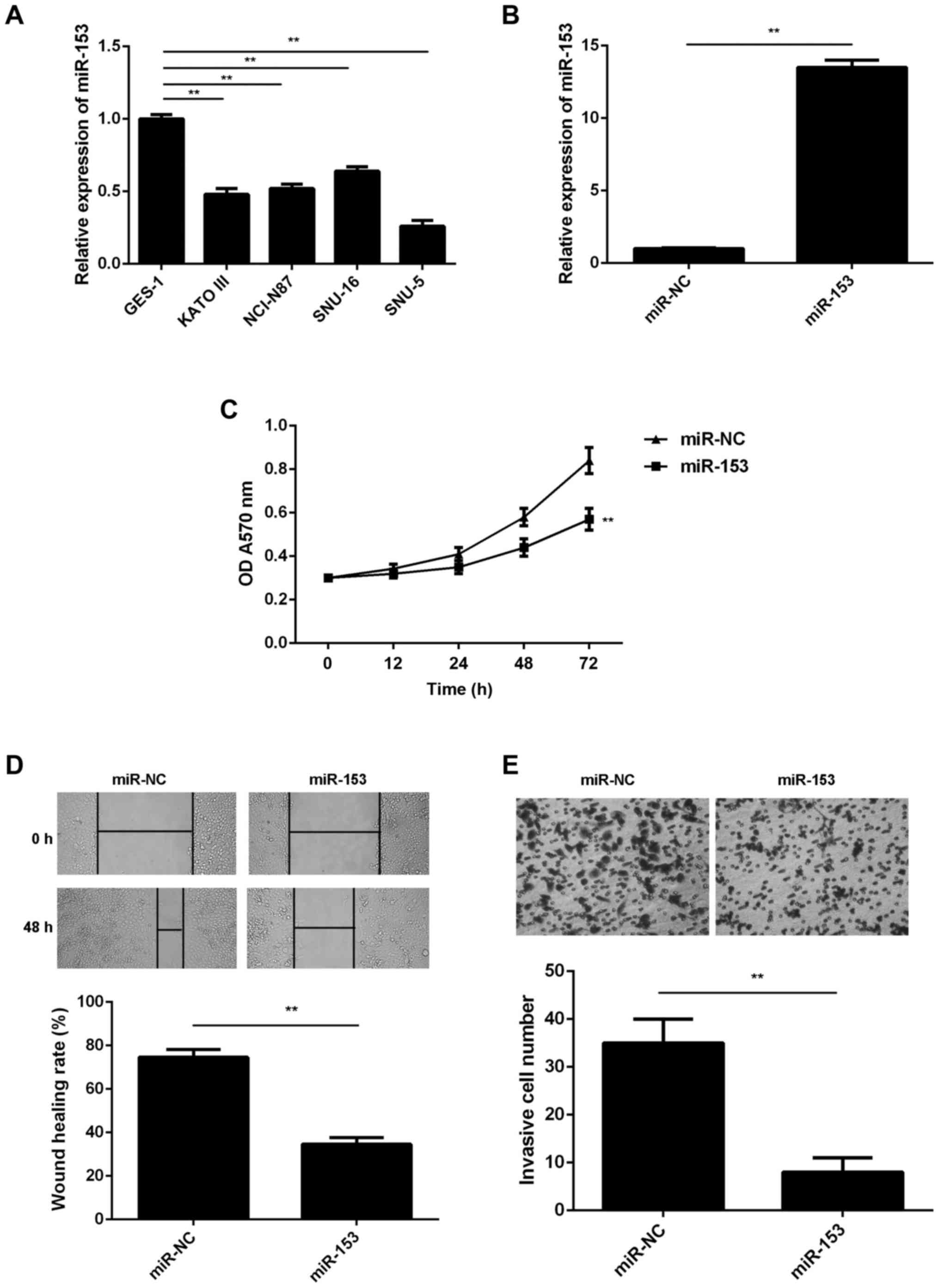 | Figure 2.miR-153 inhibits the malignant
phenotypes of GC cells. Reverse transcription-quantitative
polymerase chain reaction was conducted to examine the miR-153
expression in (A) four GC cell lines (KATO III, NCI-N87, SNU-16 and
SNU-5) and normal gastric mucosa epithelial GES-1 cells, and in (B)
SNU-5 cells transfected with miR-153 mimic or miR-NC. (C) MTT, (D)
wound healing and (E) transwell assays were conducted to examine
the proliferation, migration and invasion of SNU-5 cells,
respectively. Magnification: Wound healing assay, ×40; transwell
assay, ×400. **P<0.01. GC, gastric cancer; miR, microRNA; NC,
negative control; OD, optical density. |
SNU-5 cells were then transfected with miR-153 mimic
or miR-NC. As shown in Fig. 2B, the
miR-153 levels were significantly higher in the miR-153 group
compared with the miR-NC group. The study further demonstrated that
the overexpression of miR-153 significantly decreased the SNU-5
cell proliferation when compared with the miR-NC group, indicating
the suppressive effect of miR-153 on GC cell proliferation
(Fig. 2C). Overexpression of miR-153
also reduced the migration and invasion of SNU-5 cells (Fig. 2D and E), suggesting that miR-153 may
also inhibit GC metastasis.
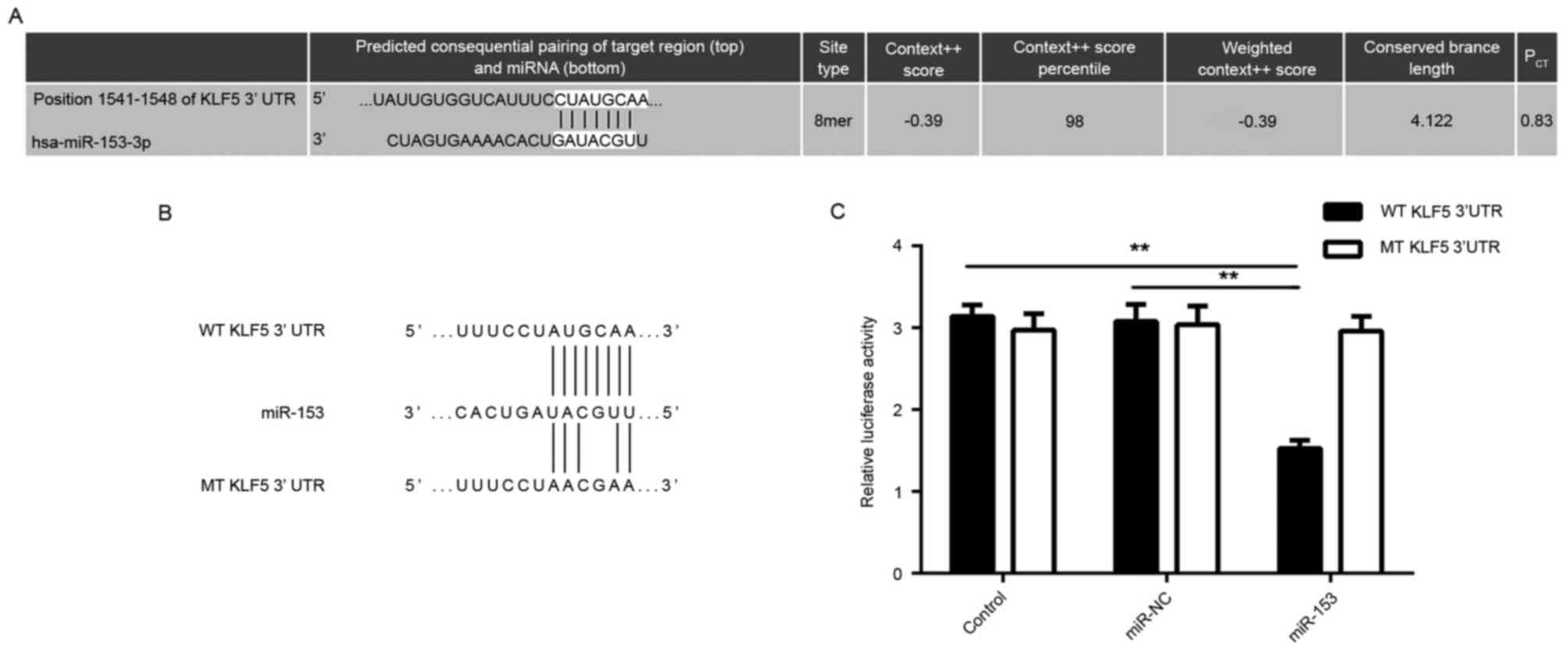
KLF5 is a target gene of miR-153 in GC
cells
The potential target genes of miR-153 were further
predicted using bioinformatics analysis. As indicated in Fig. 3A, KLF5 was predicted to be a putative
target gene of miR-153. To further verify this association,
luciferase reporter gene plasmids containing the WT or MT of KLF5
3′-UTR were constructed (Fig. 3B).
The dual-luciferase reporter gene assay data indicated that the
luciferase activity was significantly decreased following
co-transfection with miR-153 mimic and WT-KLF5-3′UTR plasmid, which
was eliminated by co-transfection with miR-153 mimic and
MT-KLF5-3′UTR plasmid (Fig. 3C).
This indicates that miR-153 was able to directly bind to the 3′-UTR
of KLF5 mRNA, and therefore, KLF5 is a target gene of miR-153 in GC
cells.
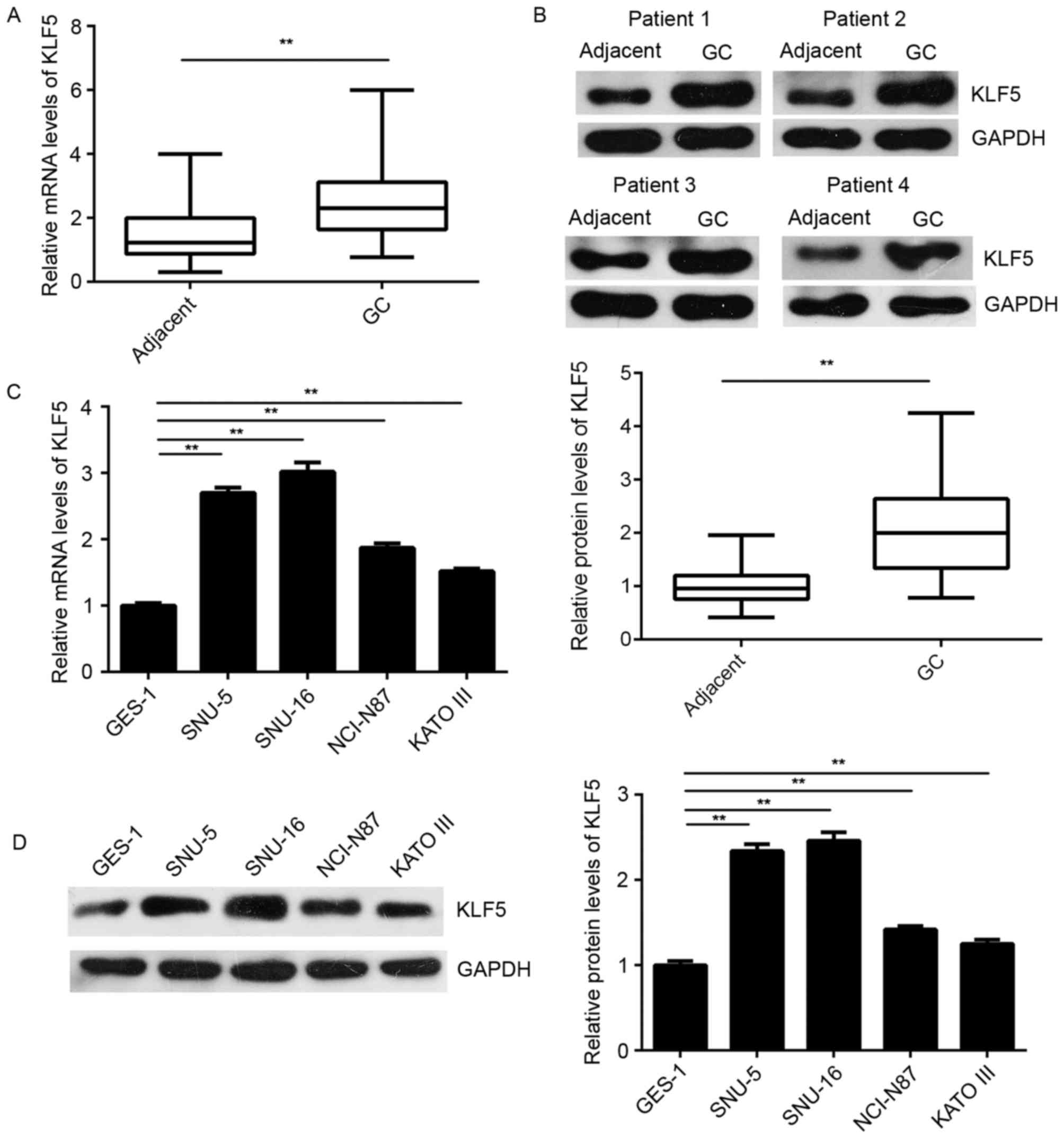
KLF5 is upregulated in GC tissues and
cells, but negatively regulated by miR-153 in SNU-5 cells
The expression of KLF5 was further examined in GC
tissues and cells. As shown in Fig. 4A
and B, the mRNA and protein levels of KLF5 were significantly
higher in GC tissues as compared with the levels in matched normal
adjacent tissues. In addition, these levels were higher in the GC
cell lines when compared with the normal gastric epithelial GES-1
cells (Fig. 4C and D).
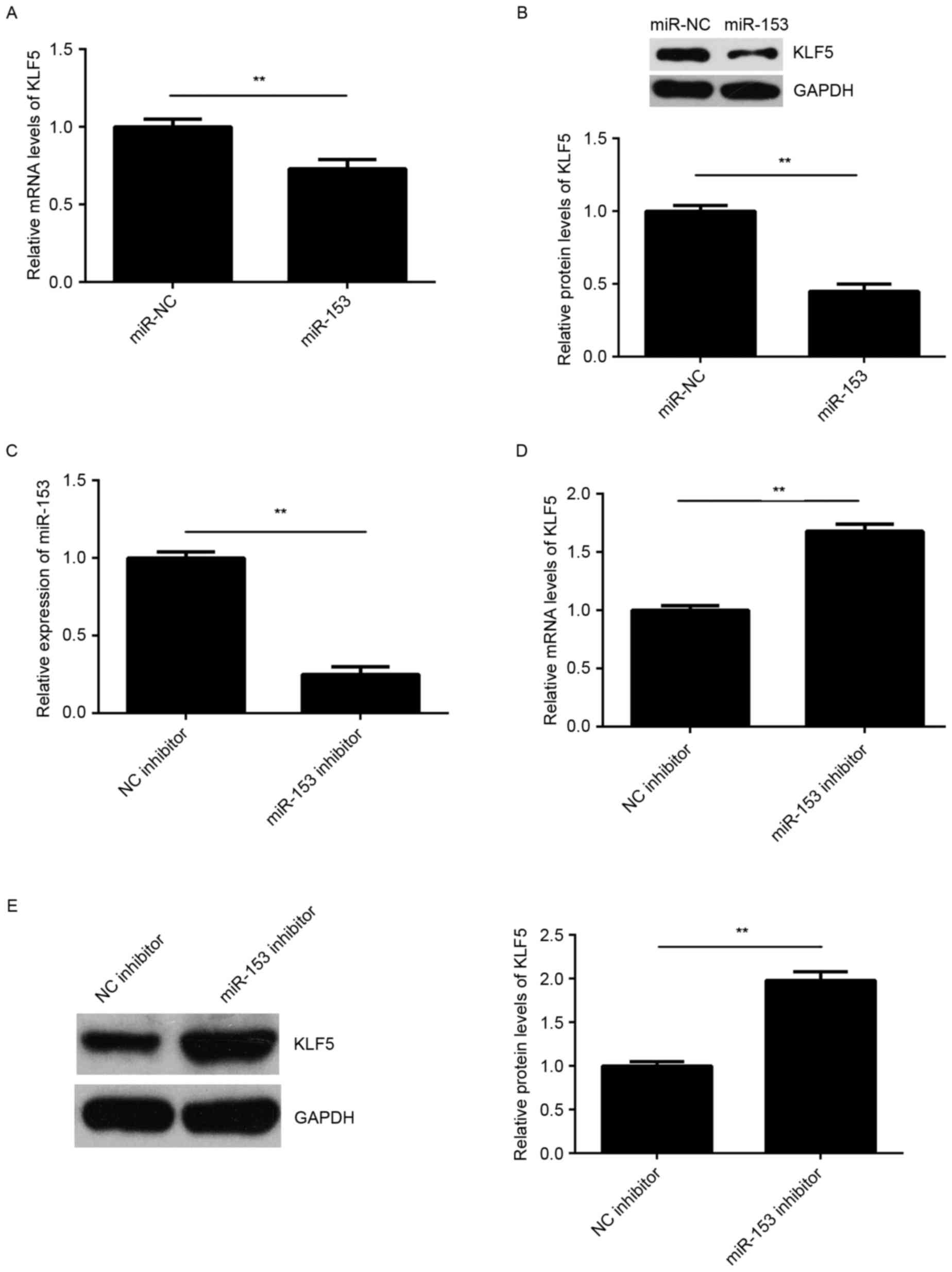
The regulatory effect of miR-153 on the expression
of KLF5 was then examined in SNU-5 cells. As shown in Fig. 5A and B, overexpression of miR-153
significantly reduced the mRNA and protein expression levels of
KLF5 in SNU-5 cells, suggesting that miR-153 negatively regulates
the expression of KLF5 in GC cells. To further confirm these
findings, SNU-5 cells were transfected with an miR-153 inhibitor or
NC inhibitor, respectively. Following transfection, the miR-153
levels were significantly reduced in the miR-153 inhibitor group
compared with NC inhibitor group (Fig.
5C). Furthermore, it was observed that knockdown of miR-153
promoted the mRNA and protein expression levels of KLF5 in SNU-5
cells (Fig. 5D and E). Accordingly,
KLF5 was suggested to be negatively regulated by miR-153 in GC
cells.
KLF5 is a downstream effector in the
miR-153-mediated malignant phenotypes of GC cells
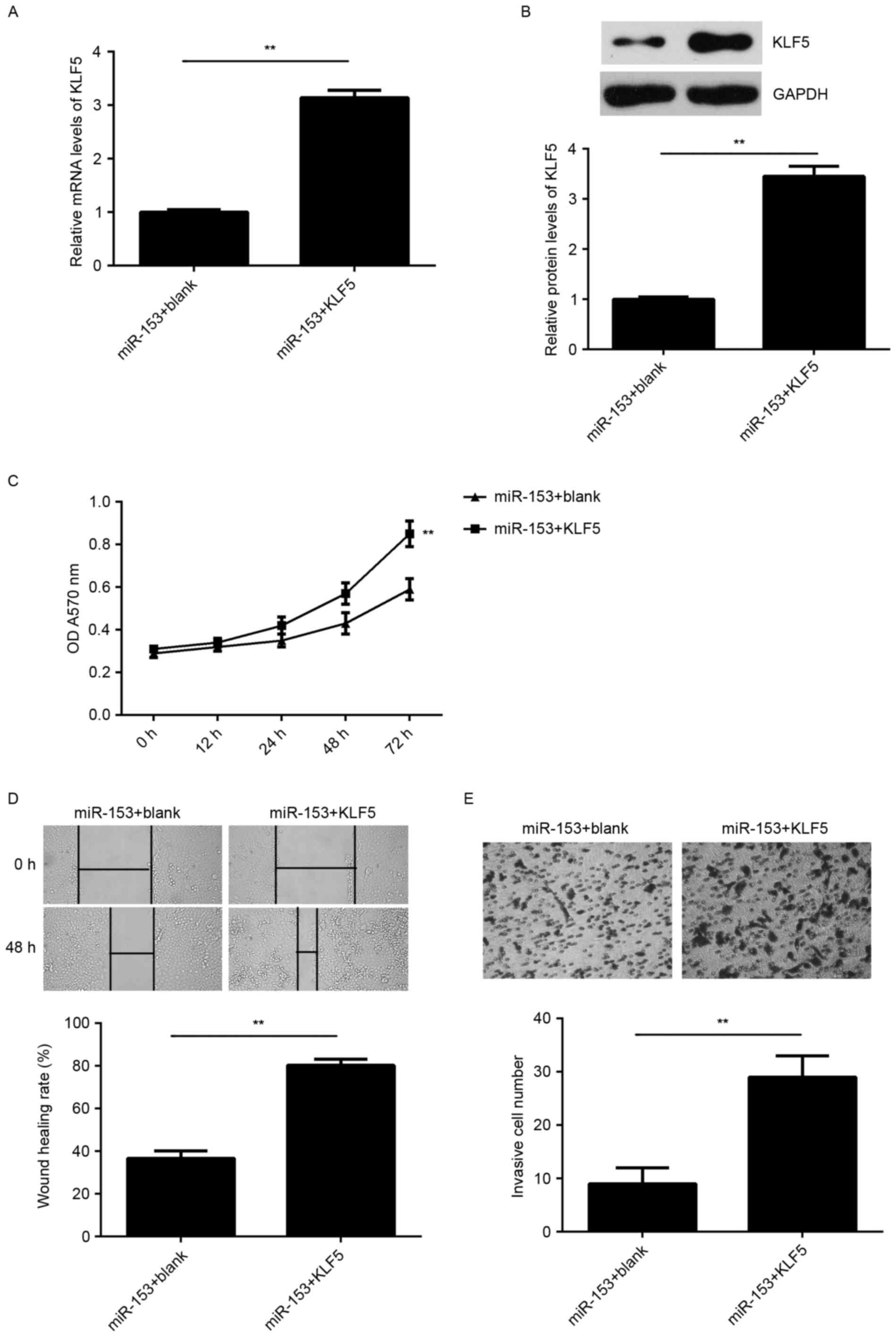
According to the aforementioned findings, KLF5 may
be involved in the miR-153-mediated malignant phenotypes of SNU-5
cells. To clarify this hypothesis, SNU-5 cells were co-transfected
with miR-153 mimic and pcDNA3.1-KLF5 plasmid. Cells that were
co-transfected with miR-153 inhibitor and blank pcDNA3.1 vector
served as the control group. As indicated in Fig. 6A and B, the mRNA and protein
expression levels of KLF5 were significantly increased in the
miR-153 + KLF5 group compared with those in the miR-153 + blank
group. In addition, the cell proliferation, migration and invasion
were significantly upregulated in the miR-153 + KLF5 group compared
with the miR-153 + blank group (Fig.
6C-E). These data indicate that the suppressive effects of
miR-153 on the proliferation, migration and invasion of SNU-5 cells
are possibly through directly targeting KLF5.
Discussion
miR-153 has been suggested to be involved in GC
progression (13); however, the
molecular mechanism underlying its function remains unclear. In the
present study, it was observed that miR-153 was significantly
downregulated in GC tissues and cell lines, and this low expression
was associated with the GC aggressiveness, as well as poor
prognosis of patients. Overexpression of miR-153 induced by mimic
transfection was observed to inhibit the SNU-5 cell proliferation,
migration and invasion. In addition, KLF5, which was significantly
upregulated in GC tissues and cells, was identified as a target
gene of miR-153, and its expression was negatively regulated by
miR-153 in SNU-5 cells. Furthermore, overexpression of KLF5
impaired the inhibitory effects of miR-153 on the malignant
phenotypes of SNU-5 cells.
In recent years, numerous miRs have been
demonstrated to be significantly deregulated and serve promoting or
tumor suppressive roles in GC (7,9,23). For instance, miR-23a promotes the
growth in GC cells via directly targeting metallothionein 2A
(24), while miR-29a suppresses the
growth and invasion of GC cells in vitro by targeting
vascular endothelial growth factor A (VEGF-A) (25). In the present study, it was observed
that miR-153 was significantly upregulated in GC tissues and cell
lines, when compared with matched adjacent non-tumor tissues or
normal gastric epithelial cells, respectively. This reduced
expression of miR-153 was significantly associated with lymph node
metastasis and advanced clinical stage in GC, as well as with
shorter survival time of GC patients, consistent with the
observations of a previous study (13). In addition, Zhang et al
(13) performed multivariate Cox
regression analysis and identified that miR-153 was an independent
prognostic marker in GC. Based on these previous findings and the
present study observations, it is suggested that miR-153
downregulation may contribute to GC progression and the poor
outcomes of treatment.
The current study also observed that overexpression
of miR-153 markedly reduced the proliferation, migration and
invasion of SNU-5 cells. Similarly, Zhang et al (13) reported that miR-153 upregulation was
able to inhibit the migration and invasion of GC MKN-45 cells,
while knockdown of miR-153 promoted GC SGC-7901 cell migration and
invasion. Besides, the authors reported that miR-153 overexpression
reduced the expression of SNAI1 and subsequently inhibited the
epithelial-mesenchymal transition of GC cells with upregulated
E-cadherin and downregulated vimentin (13).
As miRs function through the regulation of their
target genes, the potential targets of miR-153 in GC were further
investigated. Bioinformatics prediction and dual-luciferase
reporter gene assay data confirmed that KLF5 was a direct target
gene of miR-153. KLF5 is an important transcription factor,
localizing to the nucleus and binding to the epidermal growth
factor response element (17).
Additionally, KLF5 has been demonstrated to be regulated by
post-translational modification by miRs and to function downstream
of multiple signaling pathways associated with cell proliferation
and motility (19,26). In recent years, the oncogenic role of
KLF5 has gradually been revealed in certain common types of human
cancer (20,27). For instance, KLF5 promotes cell
migration and angiogenesis in bladder cancer by increasing the
expression of FYN and VEGF-A (28,29).
Besides, KLF5 promotes the proliferation, migration and invasion of
breast cancer cells by enhancing TNFAIP2 expression (27). Recently, Helicobacter pylori,
a pathogenic factor of GC, were found to promote KLF5 expression in
gastric epithelial cells in vitro and in vivo
(30). Furthermore, nuclear staining
of KLF5 expression was reported to be significantly associated with
a higher tumor grade, advanced clinical stage, lymph node status
and lower disease-free survival rate of GC patients (18). In the current study, the results
revealed that KLF5 was significantly upregulated in GC tissues and
cell lines, and was negatively regulated by miR-153 in GC cells,
which suggests that the reduced expression of miR-153 may
contribute to the increased expression of KLF5 in GC. It was
further observed that overexpression of KLF5 impaired the
suppressive effects of miR-153 on GC cell proliferation, migration
and invasion, suggesting that miR-153 has suppressive effects on GC
cell proliferation, migration and invasion, at least partly, via
targeting KLF5. A similar study also reported that miR-153
inhibited the proliferation and invasion of laryngeal squamous cell
carcinoma by targeting KLF5 (26).
In conclusion, the present study demonstrated for
the first time that miR-153, which is significantly downregulated
in GC, functions as a tumor suppressor, at least partly, via
targeting KLF5. Thus, the current study expanded the understanding
on the miR-153/KLF5 function in human cancer. Accordingly, these
findings suggest that miR-153 may be used as a promising
therapeutic candidate for GC treatment.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rocken C: Molecular classification of
gastric cancer. Expert Rev Mol Diagn. 17:293–301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tran P, Nguyen C and Klempner SJ:
Targeting the phosphatidylinositol-3-kinase pathway in gastric
cancer: Can omics improve outcomes? Int Neurourol J. 20(Suppl 2):
S131–S140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu K, He Y, Xia C, Yan J, Hou J, Kong D,
Yang Y and Zheng G: MicroRNA-15a inhibits proliferation and induces
apoptosis in CNE1 nasopharyngeal carcinoma cells. Oncol Res.
24:145–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang G, Fu Y, Liu G, Ye Y and Zhang X:
miR-218 inhibits proliferation, migration, and EMT of gastric
cancer cells by targeting WASF3. Oncol Res. 25:355–364. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Li J, Yu Z, Sun R and Kan Q:
MiR-935 promotes liver cancer cell proliferation and migration by
targeting SOX7. Oncol Res. 25:427–435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji S, Zhang B, Kong Y, Ma F and Hua Y:
MiR-326 inhibits gastric cancer cell growth through downregulating
NOB1. Oncol Res. 25:853–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng R, Chen X, Yu Y, Su L, Yu B, Li J,
Cai Q, Yan M, Liu B and Zhu Z: miR-126 functions as a tumour
suppressor in human gastric cancer. Cancer Lett. 298:50–63. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao P, Xing AY, Zhou GY, Zhang TG, Zhang
JP, Gao C, Li H and Shi DB: The molecular mechanism of microRNA-145
to suppress invasion-metastasis cascade in gastric cancer.
Oncogene. 32:491–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng J, Lei W, Xiang X, Zhang L, Yu F,
Chen J, Feng M and Xiong J: MicroRNA-506 inhibits gastric cancer
proliferation and invasion by directly targeting Yap1. Tumour Biol.
36:6823–6831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z, Sun J, Bai Z, Li H, He S, Chen R
and Che X: MicroRNA-153 acts as a prognostic marker in gastric
cancer and its role in cell migration and invasion. Onco Targets
Ther. 8:357–364. 2015.PubMed/NCBI
|
|
14
|
Liu R, Shi P, Nie Z, Liang H, Zhou Z, Chen
W, Chen H, Dong C, Yang R, Liu S and Chen C: Mifepristone
suppresses basal triple-negative breast cancer stem cells by
down-regulating KLF5 expression. Theranostics. 6:533–544. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu X, Li L, Li Y and Liu Z: MiR-153
promotes breast cancer cell apoptosis by targeting HECTD3. Am J
Cancer Res. 6:1563–1571. 2016.PubMed/NCBI
|
|
16
|
Wang Z and Liu C: MiR-153 regulates
metastases of gastric cancer through Snail. Tumour Biol 2015 [Epub
ahead of print].
|
|
17
|
Gao Y, Ding Y, Chen H and Zhou J:
Targeting Kruppel-like factor 5 (KLF5) for cancer therapy. Curr Top
Med Chem. 15:699–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soon MS, Hsu LS, Chen CJ, Chu PY, Liou JH,
Lin SH, Hsu JD and Yeh KT: Expression of Kruppel-like factor 5 in
gastric cancer and its clinical correlation in Taiwan. Virchows
Arch. 459:161–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Z, Zhang Y, Cao R, Li L, Zhong K,
Chen Q and Xiao J: MiR-5195-3p inhibits proliferation and invasion
of human bladder cancer cells by directly targeting oncogene KLF5.
Oncol Res. 25:1081–2587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chia NY, Deng N, Das K, Huang D, Hu L, Zhu
Y, Lim KH, Lee MH, Wu J, Sam XX, et al: Regulatory crosstalk
between lineage-survival oncogenes KLF5, GATA4 and GATA6
cooperatively promotes gastric cancer development. Gut. 64:707–719.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yegin EG and Duman DG: Staging of
esophageal and gastric cancer in 2014. Minerva Med. 105:391–411.
2014.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li C, Lu S and Shi Y: MicroRNA-187
promotes growth and metastasis of gastric cancer by inhibiting
FOXA2. Oncol Rep. 37:1747–1755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
An J, Pan Y, Yan Z, Li W, Cui J, Yuan J,
Tian L, Xing R and Lu Y: MiR-23a in amplified 19p13.13 loci targets
metallothionein 2A and promotes growth in gastric cancer cells. J
Cell Biochem. 114:2160–2169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen L, Xiao H, Wang ZH, Huang Y, Liu ZP,
Ren H and Song H: miR-29a suppresses growth and invasion of gastric
cancer cells in vitro by targeting VEGF-A. BMB Rep. 47:39–44. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu JY, Lu JB and Xu Y: MicroRNA-153
inhibits the proliferation and invasion of human laryngeal squamous
cell carcinoma by targeting KLF5. Exp Ther Med. 11:2503–2508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia L, Zhou Z, Liang H, Wu J, Shi P, Li F,
Wang Z, Wang C, Chen W, Zhang H, et al: KLF5 promotes breast cancer
proliferation, migration and invasion in part by upregulating the
transcription of TNFAIP2. Oncogene. 35:2040–2051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du C, Gao Y, Xu S, Jia J, Huang Z, Fan J,
Wang X, He D and Guo P: KLF5 promotes cell migration by
up-regulating FYN in bladder cancer cells. FEBS Lett. 590:408–418.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao Y, Wu K, Chen Y, Zhou J, Du C, Shi Q,
Xu S, Jia J, Tang X, Li F, et al: Beyond proliferation: KLF5
promotes angiogenesis of bladder cancer through directly regulating
VEGFA transcription. Oncotarget. 6:43791–43805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Noto JM, Khizanishvili T, Chaturvedi R,
Piazuelo MB, Romero-Gallo J, Delgado AG, Khurana SS, Sierra JC,
Krishna US, Suarez G, et al: Helicobacter pylori promotes the
expression of Krüppel-like factor 5, a mediator of carcinogenesis,
in vitro and in vivo. PLoS One. 8:e543442013. View Article : Google Scholar : PubMed/NCBI
|