|
1
|
Ungvari Z, Podlutsky A, Sosnowska D,
Tucsek Z, Toth P, Deak F, Gautam T, Csiszar A and Sonntag WE:
Ionizing radiation promotes the acquisition of a
senescence-associated secretory phenotype and impairs angiogenic
capacity in cerebromicrovascular endothelial cells: Role of
increased DNA damage and decreased DNA repair capacity in
microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci.
68:1443–1457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yentrapalli R, Azimzadeh O, Barjaktarovic
Z, Sarioglu H, Wojcik A, Harms-Ringdahl M, Atkinson MJ, Haghdoost S
and Tapio S: Quantitative proteomic analysis reveals induction of
premature senescence in human umbilical vein endothelial cells
exposed to chronic low-dose rate gamma radiation. Proteomics.
13:1096–1107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young EF and Smilenov LB: Impedance-based
surveillance of transient permeability changes in coronary
endothelial monolayers after exposure to ionizing radiation. Radiat
Res. 176:415–424. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gabryś D, Greco O, Patel G, Prise KM,
Tozer GM and Kanthou C: Radiation effects on the cytoskeleton of
endothelial cells and endothelial monolayer permeability. Int J
Radiat Oncol Biol Phys. 69:1553–1562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park MT, Oh ET, Song MJ, Lee H and Park
HJ: Radio-sensitivities and angiogenic signaling pathways of
irradiated normal endothelial cells derived from diverse human
organs. J Radiat Res. 53:570–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson MA: Maintaining a proper
perspective of risk associated with radiation exposure. J Nucl Med
Technol. 29:137–142; quiz 148–150. 2001.PubMed/NCBI
|
|
7
|
Doi H, Matsumoto S, Odawara S, Shikata T,
Kitajima K, Tanooka M, Takada Y, Tsujimura T, Kamikonya N and
Hirota S: Pravastatin reduces radiation-induced damage in normal
tissues. Exp Ther Med. 13:1765–1772. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
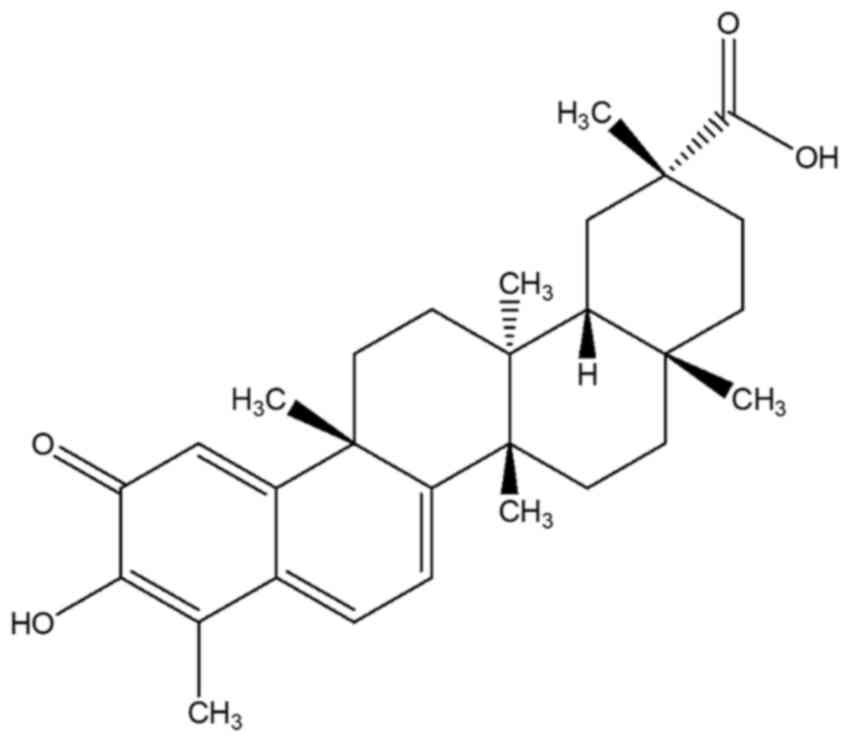
Kuchta K, Xiang Y, Huang S, Tang Y, Peng
X, Wang X, Zhu Y, Li J, Xu J, Lin Z and Pan T: Celastrol, an active
constituent of the TCM plant Tripterygium wilfordii Hook.f.,
inhibits prostate cancer bone metastasis. Prostate Cancer Prostatic
Dis. 20:156–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stankova K, Ivanova K, Nikolov V, Aneva N,
Georgieva R and Boteva R: Proteasome inhibition protects human
peripheral blood mononuclear cells from radiation-induced oxidative
stress. Int J Radiat Biol. 89:493–500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang M, Zha Q, Zhang C, Lu C, Yan X, Zhu
W, Liu W, Tu S, Hou L, Wang C, et al: Predicting and verifying
outcome of Tripterygium wilfordii Hook F. based therapy in
rheumatoid arthritis: From open to double-blinded randomized trial.
Sci Rep. 5:97002015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv QW, Zhang W, Shi Q, Zheng WJ, Li X,
Chen H, Wu QJ, Jiang WL, Li HB, Gong L, et al: Comparison of
Tripterygium wilfordii Hook F with methotrexate in the
treatment of active rheumatoid arthritis (TRIFRA): A randomised,
controlled clinical trial. Ann Rheum Dis. 74:1078–1086. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun J, Shen X, Dong J, Wang H, Zuo L, Zhao
J, Zhu W, Li Y, Gong J and Li J: Tripterygium wilfordii Hook
F as maintenance treatment for Crohn's disease. Am J Med Sci.
350:345–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu W, Li Y, Gong J, Zuo L, Zhang W, Cao
L, Gu L, Guo Z, Li N and Li J: Tripterygium wilfordii Hook.
f. versus azathioprine for prevention of postoperative recurrence
in patients with Crohn's disease: A randomized clinical trial. Dig
Liver Dis. 47:14–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Venkatesha SH and Moudgil KD: Celastrol
and its role in controlling chronic diseases. Adv Exp Med Biol.
928:267–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Allison AC, Cacabelos R, Lombardi VR,
Alvarez XA and Vigo C: Celastrol, a potent antioxidant and
anti-inflammatory drug, as a possible treatment for Alzheimer's
disease. Prog Neuropsychopharmacol Biol Psychiatry. 25:1341–1357.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cleren C, Calingasan NY, Chen J and Beal
MF: Celastrol protects against MPTP- and 3-nitropropionic
acid-induced neurotoxicity. J Neurochem. 94:995–1004. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Venkatesha SH, Yu H, Rajaiah R, Tong L and
Moudgil KD: Celastrus-derived celastrol suppresses autoimmune
arthritis by modulating antigen-induced cellular and humoral
effector responses. J Biol Chem. 286:15138–15146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu X, Tao W, Jiang F, Li C, Lin J and Liu
C: Celastrol attenuates hypertension-induced inflammation and
oxidative stress in vascular smooth muscle cells via induction of
heme oxygenase-1. Am J Hypertens. 23:895–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bakar Abu MH, Sarmidi MR, Tan JS and Rosdi
Mohamad MN: Celastrol attenuates mitochondrial dysfunction and
inflammation in palmitate-mediated insulin resistance in C3A
hepatocytes. Eur J Pharmacol. 799:73–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Lee J, Hernandez Salazar MA,
Mazitschek R and Ozcan U: Treatment of obesity with celastrol.
Cell. 161:999–1011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Avilla J, Teixidò A, Velázquez C,
Alvarenga N, Ferro E and Canela R: Insecticidal activity of
Maytenus species (Celastraceae) nortriterpene quinone methides
against codling moth, Cydia pomonella (L.) (Lepidoptera:
Tortricidae). J Agric Food Chem. 48:88–92. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Sethi G, Loke WK and Sim MK:
Des-aspartate-angiotensin i attenuates mortality of mice exposed to
gamma radiation via a novel mechanism of action. PLoS One.
10:e01380092015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Howard BJ, Fesenko S, Balonov M, Pröhl G
and Nakayama S: A comparison of remediation after the Chernobyl and
Fukushima Daiichi accidents. Radiat Prot Dosimetry. 173:170–176.
2017.PubMed/NCBI
|
|
24
|
Ghosh SP, Kulkarni S, Perkins MW, Hieber
K, Pessu RL, Gambles K, Maniar M, Kao TC, Seed TM and Kumar KS:
Amelioration of radiation-induced hematopoietic and
gastrointestinal damage by Ex-RAD(R) in mice. J Radiat Res.
53:526–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ran XZ, Ran X, Zong ZW, Liu DQ, Xiang GM,
Su YP and Zheng HE: Protective effect of atorvastatin on
radiation-induced vascular endothelial cell injury in vitro. J
Radiat Res. 51:527–533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Desouky W, Hanafi A and Abbas MM:
Radioprotective effect of green tea and grape seed extracts mixture
on gamma irradiation induced immune suppression in male albino
rats. Int J Radiat Biol. 93:433–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lata M, Prasad J, Singh S, Kumar R, Singh
L, Chaudhary P, Arora R, Chawla R, Tyagi S, Soni NL, et al: Whole
body protection against lethal ionizing radiation in mice by
REC-2001: A semi-purified fraction of Podophyllum hexandrum.
Phytomedicine. 16:47–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sankhwar S, Gupta ML, Gupta V, Verma S,
Suri KA, Devi M, Sharma P, Khan EA and Alam MS: Podophyllum
hexandrum-mediated survival protection and restoration of other
cellular injuries in lethally irradiated mice. Evid Based
Complement Alternat Med. 2011:1751402011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dutta A, Verma S, Sankhwar S, Flora SJ and
Gupta ML: Bioavailability, antioxidant and non toxic properties of
a radioprotective formulation prepared from isolated compounds of
Podophyllum hexandrum: A study in mouse model. Cell Mol Biol
(Noisy-le-grand). 58 Suppl:OL1646–OL1653. 2012.PubMed/NCBI
|
|
30
|
Saini R, Verma S, Singh A and Gupta Lata
M: Role of active principles of podophyllum hexandrum in
amelioration of radiation mediated lung injuries by reactive
oxygen/nitrogen species reduction. CellBio. 2:pp105–116. 2013.
View Article : Google Scholar
|
|
31
|
Sandeep D and Nair CK: Protection of DNA
and membrane from γ-radiation induced damage by the extract of
Acorus calamus Linn.: An in vitro study. Environ Toxicol
Pharmacol. 29:302–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sandeep D and Nair CK: Protection from
lethal and sub-lethal whole body exposures of mice to γ-radiation
by Acorus calamus L.: Studies on tissue antioxidant status
and cellular DNA damage. Exp Toxicol Pathol. 64:57–64. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan H, Ashour A, Katakura Y and Shimizu K:
A structure-activity relationship study on antiosteoclastogenesis
effect of triterpenoids from the leaves of loquat (Eriobotrya
japonica). Phytomedicine. 22:498–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang WJ, Wang J, Tong X, Shi JB, Liu XH
and Li J: Design and synthesis of celastrol derivatives as
anticancer agents. Eur J Med Chem. 95:166–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Z, Zhai Z and Du X: Celastrol
inhibits migration and invasion through blocking the NF-κB pathway
in ovarian cancer cells. Exp Ther Med. 14:819–824. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schultz-Hector S and Trott KR:
Radiation-induced cardiovascular diseases: Is the epidemiologic
evidence compatible with the radiobiologic data? Int J Radiat Oncol
Biol Phys. 67:10–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hwang S, Lee DH, Lee IK, Park YM and Jo I:
Far-infrared radiation inhibits proliferation, migration, and
angiogenesis of human umbilical vein endothelial cells by
suppressing secretory clusterin levels. Cancer Lett. 346:74–83.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sinha M, Das DK, Manna K, Datta S, Ray T,
Sil AK and Dey S: Epicatechin ameliorates ionising
radiation-induced oxidative stress in mouse liver. Free Radic Res.
46:842–849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu J, Zhu X, Qi X, Che J and Cao B:
Paeoniflorin protects human EA.hy926 endothelial cells against
gamma-radiation induced oxidative injury by activating the
NF-E2-related factor 2/heme oxygenase-1 pathway. Toxicol Lett.
218:224–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu J, Piao BK, Pei YX, Qi X and Hua BJ:
Protective effects of tetrahydropalmatine against gamma-radiation
induced damage to human endothelial cells. Life Sci. 87:55–63.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu S, Gao Y, Zhou H, Kong F, Xiao F, Zhou
P and Chen Y: New insight into mitochondrial changes in vascular
endothelial cells irradiated by gamma ray. Int J Radiat Biol.
93:470–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cervelli T, Panetta D, Navarra T,
Andreassi MG, Basta G, Galli A, Salvadori PA, Picano E and Del
Turco S: Effects of single and fractionated low-dose irradiation on
vascular endothelial cells. Atherosclerosis. 235:510–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Olteanu D, Baldea I, Clichici S, Bolfa P,
Cenariu M, Schrepler-Perde M, Alupei M, Muresan A and Filip A: In
vitro studies on the mechanisms involved in chemoprevention using
Calluna vulgaris on vascular endothelial cells exposed to
UVB. J Photochem Photobiol B. 136:54–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gu L, Bai W, Li S, Zhang Y, Han Y, Gu Y,
Meng G, Xie L, Wang J, Xiao Y, et al: Celastrol prevents
atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS
One. 8:e654772013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guan Y, Cui ZJ, Sun B, Han LP, Li CJ and
Chen LM: Celastrol attenuates oxidative stress in the skeletal
muscle of diabetic rats by regulating the AMPK-PGC1α-SIRT3
signaling pathway. Int J Mol Med. 37:1229–1238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shaker ME, Ashamallah SA and Houssen ME:
Celastrol ameliorates murine colitis via modulating oxidative
stress, inflammatory cytokines and intestinal homeostasis. Chem
Biol Interact. 210:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jin HZ, Hwang BY, Kim HS, Lee JH, Kim YH
and Lee JJ: Antiinflammatory constituents of Celastrus orbiculatus
inhibit the NF-kappaB activation and NO production. J Nat Prod.
65:89–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jung HW, Chung YS, Kim YS and Park YK:
Celastrol inhibits production of nitric oxide and proinflammatory
cytokines through MAPK signal transduction and NF-kappaB in
LPS-stimulated BV-2 microglial cells. Exp Mol Med. 39:715–721.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sassa H, Takaishi Y and Terada H: The
triterpene celastrol as a very potent inhibitor of lipid
peroxidation in mitochondria. Biochem Biophys Res Commun.
172:890–897. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ameziane-El-Hassani R, Talbot M, de Souza
Dos Santos MC, Al Ghuzlan A, Hartl D, Bidart JM, De Deken X, Miot
F, Diallo I, de Vathaire F, et al: NADPH oxidase DUOX1 promotes
long-term persistence of oxidative stress after an exposure to
irradiation. Proc Natl Acad Sci USA. 112:5051–5056. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shaban NZ, Zahran Ahmed AM, El-Rashidy FH
and Kodous Abdo AS: Protective role of hesperidin against
γ-radiation-induced oxidative stress and apoptosis in rat testis. J
Biol Res (Thessalon). 24:52017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Divya T, Dineshbabu V, Soumyakrishnan S,
Sureshkumar A and Sudhandiran G: Celastrol enhances Nrf2 mediated
antioxidant enzymes and exhibits anti-fibrotic effect through
regulation of collagen production against bleomycin-induced
pulmonary fibrosis. Chem Biol Interact. 246:52–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang C, Shi C, Yang X, Yang M, Sun H and
Wang C: Celastrol suppresses obesity process via increasing
antioxidant capacity and improving lipid metabolism. Eur J
Pharmacol. 744:52–58. 2014. View Article : Google Scholar : PubMed/NCBI
|