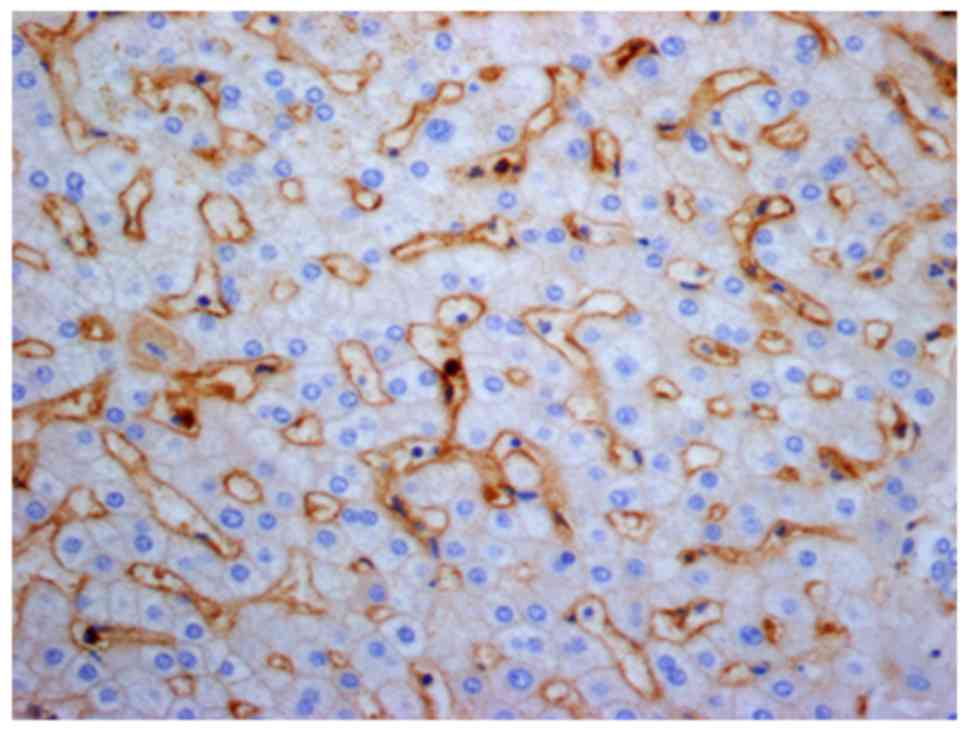
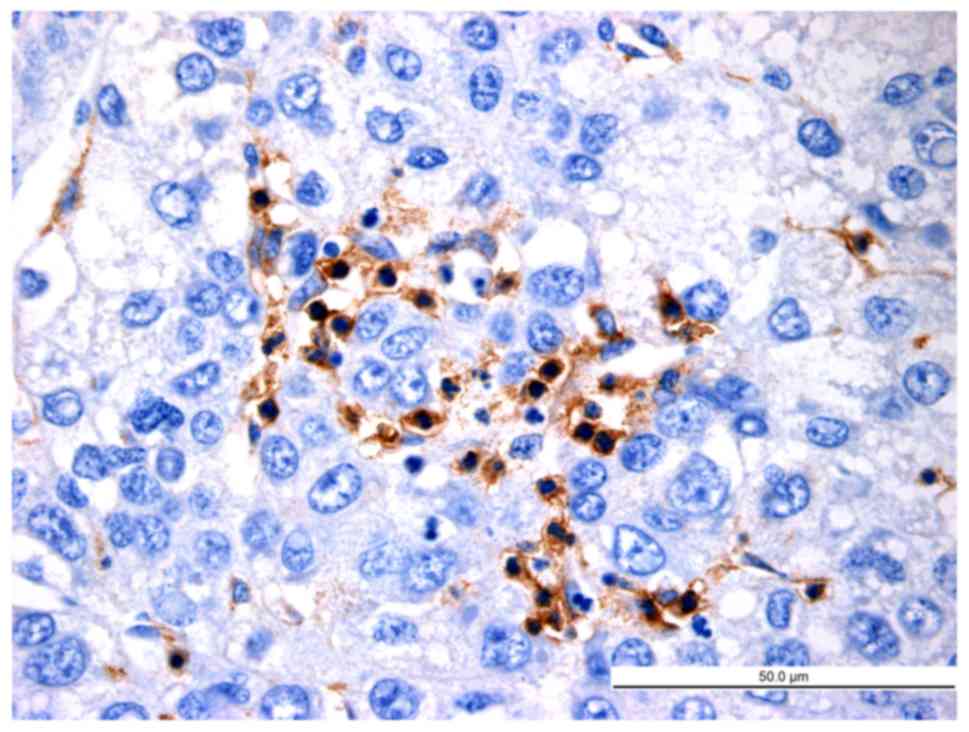
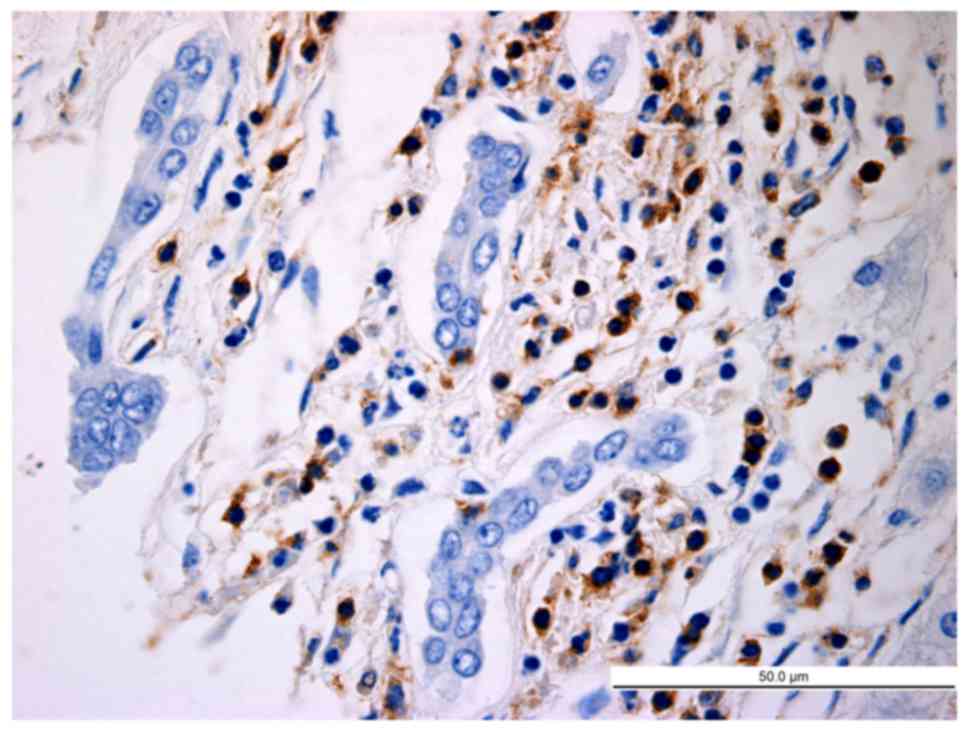
|
1
|
Sayour EJ, Mclendon P, Mclendon R, de Leon
G, Reynolds R, Kresak J, Sampson JH and Mitchell DA: Increased
proportion of FoxP3+ regulatory T cells in tumor infiltrating
lymphocytes is associated with tumor recurrence and reduced
survival in patients with glioblastoma. Cancer Immunol Immunother.
64:419–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan CH, Sun XM, Zhu CL, Liu SP, Wu L,
Chen H, Feng MH, Wu K and Wang FB: Amphiregulin activates
regulatory T lymphocytes and suppresses CD8+T cell-mediated
anti-tumor response in hepatocellular carcinoma cells. Oncotarget.
6:32138–32153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bluestone JA and Abbas AK: Natural versus
adaptive regulatory T cells. Nat Rev Immunol. 3:253–257. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weiner HL: Oral tolerance for the
treatment of autoimmune diseases. Annu Rev Med. 48:341–351. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roncarolo MG and Levings MK: The role of
different subsets of T regulatory cells in controlling
autoimmunity. Curr Opin Immunol. 12:676–683. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiner HL, da Cunha AP, Quintana F and Wu
H: Oral tolerance. Immunol Rev. 241:241–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Filaci G, Fenoglio D and Indiveri F:
CD8(+) T regulatory/suppressor cells and their relationships with
autoreactivity and autoimmunity. Autoimmunity. 44:51–57. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong CH and Kubes P: Imaging natural
killer T cells in action. Immunol Cell Biol. 91:304–310. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen ML, Yan BS, Bando Y, Kuchroo VK and
Weiner HL: Latency-associated peptide identifies a novel CD4+CD25+
regulatory T cell subset with TGFbeta-mediated function and
enhanced suppression of experimental autoimmune encephalomyelitis.
J Immunol. 180:7327–7337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan W, So T, Mehta AK, Choi H and Croft
M: Inducible CD4+LAP+Foxp3-regulatory T cells suppress allergic
inflammation. J Immunol. 187:6499–6507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ilan Y, Maron R, Tukpah AM, Maioli TU,
Murugaiyan G, Yang K, Wu HY and Weiner HL: Induction of regulatory
T cells decreases adipose inflammation and alleviates insulin
resistance in ob/ob mice. Proc Natl Acad Sci USA. 107:9765–9770.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahalingam J, Lin YC, Chiang JM, Su PJ,
Fang JH, Chu YY, Huang CT, Chiu CT and Lin CY: LAP+CD4+ T cells are
suppressors accumulated in the tumor sites and associated with the
progression of colorectal cancer. Clin Cancer Res. 18:5224–5233.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakaguchi S, Sakaguchi N, Asano M, Itoh M
and Toda M: Immunologic self-tolerance maintained by activated T
cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a
single mechanism of self-tolerance causes various autoimmune
diseases. J Immunol. 155:1151–1164. 1995.PubMed/NCBI
|
|
14
|
Sakaguchi S: Naturally arising
Foxp3-expressing CD25+CD4+ regulatory T cells in immunological
tolerance to self and non-self. Nat Immunol. 6:345–352. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang CT, Workman CJ, Flies D, Pan X,
Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, et
al: Role of LAG-3 in regulatory T cells. Immunity. 21:503–513.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyazono K, Ichijo H and Heldin CH:
Transforming growth factor-beta: Latent forms, binding proteins and
receptors. Growth Factors. 8:11–22. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keski-Oja J, Koli K and von Melchner H:
TGF-beta activation by traction? Trends Cell Biol. 14:657–659.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McMahon GA, Dignam JD and Gentry LE:
Structural characterization of the latent complex between
transforming growth factor beta 1 and beta 1-latency-associated
peptide. Biochem J. 313:343–351. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura K, Kitani A and Strober W: Cell
contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T
cells is mediated by cell surface-bound transforming growth factor
beta. J Exp Med. 194:629–644. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oida T, Zhang X, Goto M, Hachimura S,
Totsuka M, Kaminogawa S and Weiner HL:
CD4+CD25− T cells that express
latency-associated peptide on the surface suppress
CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent
mechanism. J Immunol. 170:2516–2522. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamura K, Kitani A, Fuss I, Pedersen A,
Harada N, Nawata H and Strober W: TGF-beta 1 plays an important
role in the mechanism of CD4+CD25+ regulatory
T cell activity in both humans and mice. J Immunol. 172:834–842.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ochi H, Abraham M, Ishikawa H, Frenkel D,
Yang K, Basso AS, Wu H, Chen ML, Gandhi R, Miller A, et al: Oral
CD3-specific antibody suppresses autoimmun encephalomyelitis by
inducing CD4+ CD25-LAP+ T cells. Nat Med. 12:627–635. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu HY, Maron R, Tukpah AM and Weiner HL:
Mucosal anti-CD3 monoclonal antibody attenuates collagen-induced
arthritis that is associated with induction of LAP+ regulatory T
cells and is enhanced by administration of an emulsome-based
Th2-skewing adjuvant. J Immunol. 185:3401–3407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu HY, Quintana FJ and Weiner HL: Nasal
anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting
CD4+ CD25-LAP+ regulatory T cell and is associated with
down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T
cells. J Immunol. 181:6038–6050. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishikawa H, Ochi H, Chen ML, Frenkel D,
Maron R and Weiner HL: Inhibition of autoimmune diabetes by oral
administration of anti-CD3 monoclonal antibody. Diabetes.
56:2103–2109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akiba J, Yano H, Ogasawara S, Higaki K and
Kojiro M: Expression and function of interleukin-8 in human
hepatocellular carcinoma. Int J Oncol. 18:257–264. 2001.PubMed/NCBI
|
|
27
|
Gandhi R, Farez MF, Wang Y, Kozoriz D,
Quintana FJ and Weiner HL: Cutting edge: Human latency-associated
peptide+ T cells: A novel regulatory T cell subset. J Immunol.
184:4620–4624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Y, Wang FM, Wang T, Wang YJ, Zhu ZY,
Gao YT and Du Z: Tumor-infiltrating FoxP3+Tregs and
CD8+T cells affect the prognosis of hepatocellular
carcinoma patients. Digestion. 86:329–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brunner SM, Itzel T, Rubner C, Kesselring
R, Griesshammer E, Evert M, Teufel A, Schlitt HJ and Fichtner-Feigl
S: Tumor-infiltrating B cells producing antitumor active
immunoglobulins in resected HCC prolong patient survival.
Oncotarget. 8:71002–71011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Y, Wang B, Wu J, Zhang C, Zhou Y,
Yang X, Zhou J, Guo W and Fan J: Association of preoperative EpCAM
circulating tumor cells and peripheral treg cell levels with early
recurrence of hepatocellular carcinoma following radical hepatic
resection. BMC Cancer. 16:5062016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Jiang G, Yao F, Liang G, Wang F,
Xu H, Wu Y, Yu X and Liu H: Osthole promotes anti-tumor immune
responses in tumor-bearing mice with hepatocellular carcinoma.
Immunopharmacol Immunotoxicol. 37:301–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyara M and Sakaguchi S: Natural
regulatory T cells: Mechanisms of suppression. Trends Mol Med.
13:108–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong W, Jiang ZY, Zhang L, Huang JH, Wang
SJ, Liao C, Cai B, Chen LS, Zhang S, Guo Y, et al: Role of
lap+cd4+t cells in the tumor microenvironment of colorectal cancer.
World J Gastroenterol. 23:455–463. 2017. View Article : Google Scholar : PubMed/NCBI
|