Introduction
An estimated 185 million individuals worldwide are
infected with Hepatitis C virus (1,2). Most of
the mortalities associated with hepatitis C virus infection are due
to cirrhosis and liver cancer (3).
Combination therapy with pegylated interferon-α (peg IFN) and
ribavirin is the first line of treatment (4). On average, 55% of patients consistently
used these drugs (5). peg IFN in
combination with ribavirin is the currently recommended treatment
for 70–80% of genotype 2 or 3 and is also used for 40–50% of
genotype 1 patients with HCV infection, and conventional interferon
were used for other patients (6,7). peg IFN
and ribavirin are successful antiviral treatment for numerous
adverse effects, including fatigue, mood disorders, anxiety,
irritability, emotional lability, agitation, apathy, anhedonia,
anorexia, retardation, sleep disturbance, sexual dysfunction and
cognitive deficits have been reported during and after the
treatment of hepatitis C (8–10). Various studies have reported
psychiatric side effects in ~84.5% of patients during treatment
with peg IFN and in 42.6% of patients at 6 months after completion
of treatment (7,11). In terms of psychiatric side effects,
a relatively large body of literature documents high rates of
psychiatric symptoms, the definition of which varies between
studies, but all of them indicated that IFN induced psychiatric
side effects ranging from 30–70% for depression, 39–80% for
fatigue, 18–45% for sleep disturbances, 16–50% for irritability,
11–45% for anxiety, 0.0–3.2% for mania, 0.0–0.6% for psychosis,
3.5–10% for suicidal ideation and 0.0–0.2% for suicidal attempts
Previous studies have demonstrated that non-specific symptoms occur
early and that certain psychiatric side effects may occur following
the onset of therapy; most of these events occur after 3 weeks
(12,13). These side effects were evaluated in
patients treated with peg IFN and ribavirin in order to determine
whether it is reasonable to reduce these effects by replacing those
drugs. The present meta-analysis study assessed the psychiatric
side effects of peg IFN and ribavirin in Iranian patients with
chronic hepatitis C infection.
Materials and methods
Data source
In the present study, all of the studies published
in electronic format from January 2000 up to December 2016 were
included. Articles were searched using various international and
Persian databases, including Scopus, PubMed, Institute for
Scientific Information, Science Direct, Google Scholar, Magiran and
SID (sid.ir). The search strategy was performed using the following
major key words: ‘Prevalence’, ‘HCV’, ‘Iran’, ‘psychiatry’,
‘ribavirin’ and ‘interferon’. ‘AND’/‘OR’ operators were used to
identify the articles, e.g. (interferon alpha OR pegylated
interferon) AND (HCV OR Hepatitis C virus OR hepatitis C) AND (Iran
OR Islamic Republic of Iran). In addition, all of the studies with
a different aim to the present study were removed using the ‘NOT’
operator. For increasing the sensitivity of the present study, the
references of retrieved articles were also considered. Two
reviewers performed random searches in order to retrieve all of the
relevant studies.
Study selection
Retrieval of articles
During the advanced search, all articles retrieved
were collected, and duplicates and irrelevant articles were removed
after reviewing the titles and abstracts. In addition,
re-publication bias was prevented via excluding the any studies
containing duplicated data. The articles were evaluated by two
reviewers (T.M. and M.M.) independently and their results were
compared with each other. Any disagreements were resolved by
consultation with another researcher.
Quality assessment
In the present study, the quality of the selected
articles was assessed with a STROBE checklist. This checklist
comprised 22 questions based on the Strengthening the Reporting of
Observational Studies in Epidemiology statement (14). These questions contain all of the
aspects of the methodology, including data collection methods and
tools, type of the study, definition of the variables, statistical
analysis tests, study objectives, sample size estimation selection
and study population. Each question was assigned to score one
point. In this checklist, the minimum and maximum scores are 0 and
44, respectively. Based on the results of the quality assessment,
studies were divided into three categories: Low (<15.5), average
(15.5–29.5) and high quality (>30) and studies with low-quality
scores were excluded from the final meta-analysis.
Inclusion criteria
All of the articles in English and Persian language
with sufficient quality scores were included in meta-analysis if
they met the following inclusion criteria: i) HCV patients using
interferon or ribavirin or a combination of these two drugs during
treatment; ii) the studies reported on psychiatric side effects
among patients receiving IFN or ribavirin or their combination;
iii) cross-sectional (descriptive-analytic) studies or cohort
studies.
Exclusion criteria
The exclusion criteria applied in the present study
were as follows: i) Case reports, author replies, animal studies,
case series, reviews, commentaries, studies presented at
conferences, studies that did not achieve the desired scores or
that were unpublished; ii) duplicated studies and iii) studies
reporting on psychiatric side effects among HCV patients that
received other drugs.
Data extraction
The following information was extracted from the
selected the articles: Title, author names, publication date, study
location, population group, population size, age, study language,
drug use and factors of psychiatric side effects. The information
was recorded in an excel spreadsheet.
Statistical analysis
The standard error for primary studies was
calculated with a binomial distribution formula: (Se=P.(1-P)n). The degree of heterogeneity
among the results was estimated by Cochrane's test (Q) and
I2 indexes. For detection of heterogeneity, a forest
plot was used to indicate the mean frequency of occurrence and
estimates with 95% confidence intervals (CIs; horizontal lines).
Furthermore, publication biases were evaluated with Egger's test
(with a significance level of <0.01). All statistical analyses
were performed using the Stata SE, v.11 software (StataCorp LP,
College Station, TX, USA).
Results
Study selection
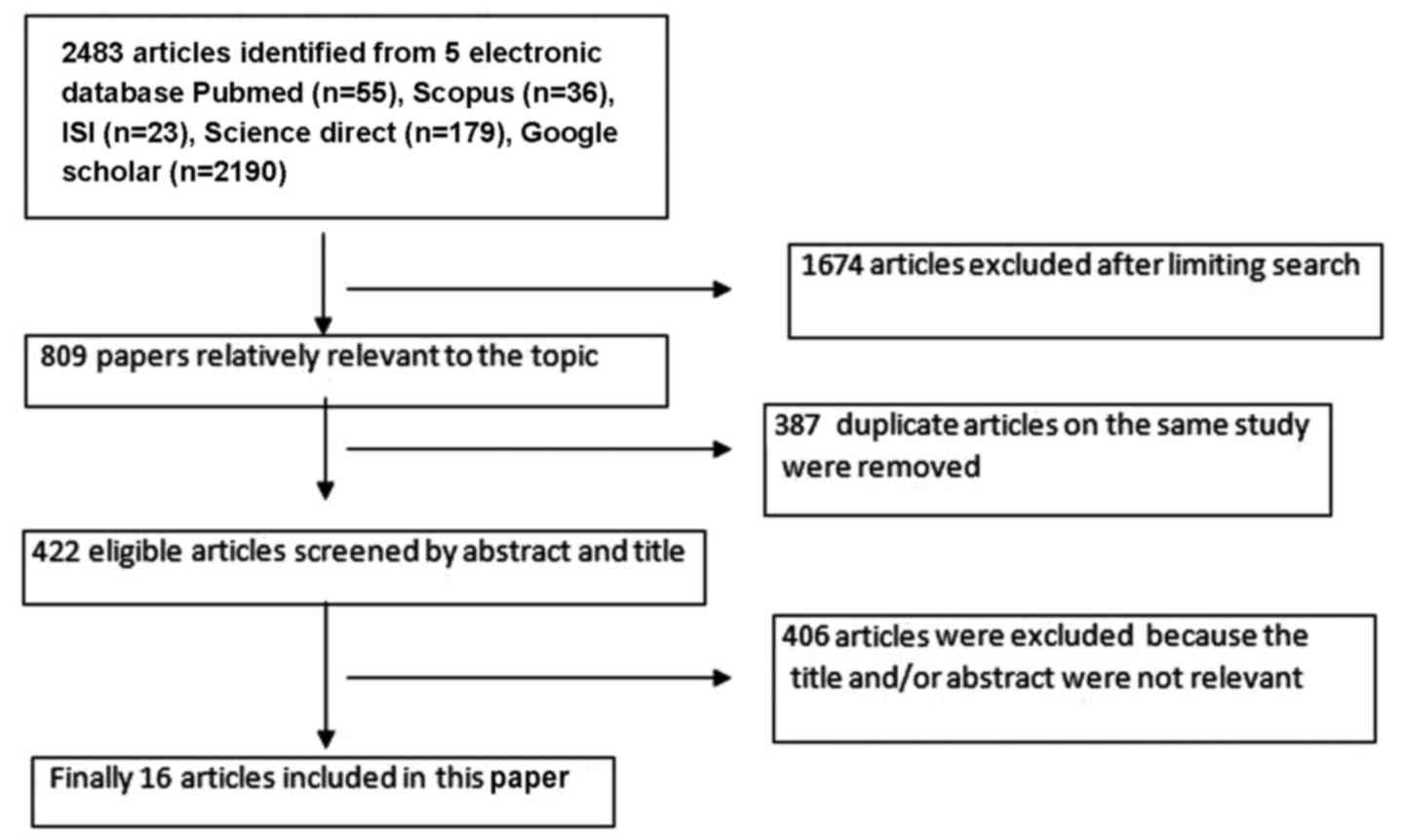
In the initial search, 2,483 articles in the field
of interest were identified. After the first limitation, 1,674
articles were preliminarily selected according to the search
strategy. Subsequently, 387 duplicated articles on the same study
were excluded and thereafter, 406 articles were removed due to
having an irrelevant title and/or abstract. Finally, 16 eligible
studies were retained (15–30), which were included in the
meta-analysis (Fig. 1; Table I).
 | Table I.Characteristics of primary
studies. |
Table I.
Characteristics of primary
studies.
| First author,
year | Sample size | age, years | Drug treatment | Duration
(months) | Nerv. | Fat. | Dep. | Morning Ha. | Ha. | Dizzi. | Lethargy | Somn. | Anxiety | Exc. | Mood changes | Weak. | Irrit. | Ins. | An. | Scorea | (Refs.) |
|---|
| Namazee, 2012 | 100 | 42.0 |
Interferon+ribavirin | 48 |
| 74 | 39 |
| 38 |
|
|
|
|
|
|
|
| 7 |
| 18 | (26) |
| Alavian, 2006 | 176 | 38.9 |
Interferon+ribavirin | 48 | 34.7 | 21.6 |
|
|
|
|
|
|
|
|
| 0.5 |
|
|
| 19 | (19) |
| Mirmomen, 2004 | 32 | 24.1 | Interferon | 48 |
|
|
|
| 16.3 |
|
| 22.5 |
| 16.1 | 29 | 32.2 |
|
|
| 17 | (24) |
| Alavian, 2004 | 52 | 38.5 |
Interferon+ribavirin | 48 |
|
| 5.8 |
|
|
|
|
|
|
|
|
|
|
|
| 18 | (16) |
| Alavian, 2010 | 367 | 30.0 |
Interferon+ribavirin | 48 |
| 60 | 19 |
| 36 | 20 | 21 |
| 41 |
|
|
| 24 | 26 |
| 18 | (17) |
| Alavian, 2009 | 51 | 25.0 | Interferon | 48 |
| 74 |
|
|
|
|
|
|
|
|
|
|
|
|
| 20 |
(18) |
| Bafandeh, 2007 | 118 | 37.47 |
Interferon+ribavirin | 24 |
|
| 15 |
|
|
|
|
|
|
|
|
|
|
|
| 16 | (20) |
| Forootan, 2005 | 97 | 35.1 |
Interferon+ribavirin | 12 |
|
|
|
| 13.6 |
|
|
|
|
| 36 |
|
| 4.9 | 34 | 18 | (21) |
| Jabbari, 2010 | 108 | 39.0 | Interferon | 24 |
| 73.5 |
|
|
|
|
|
|
|
|
|
|
|
| 66.2 | 17 | (22) |
| Mirmomen, 2003 | 29 | 25.24 | Interferon | 12 |
|
|
| 3.4 | 10.3 |
|
| 13.7 |
|
| 27.5 | 17.2 |
|
|
| 17 | (25) |
| Merat, 2004 | 37 | 30.1 |
Interferon+ribavirin | 48 |
|
|
|
|
|
|
|
|
|
| 31.4 |
|
|
|
| 16 | (23) |
| Zali, 2004 | 57 | 41 |
Interferon+ribavirin | 48 |
|
|
|
|
|
|
|
|
|
| 29.4 |
|
|
|
| 17 | (30) |
| Sandoughdaran,
2015 | 21 | 29.7 |
Interferon+ribavirin | 72 |
|
| 23.8 |
| 71.4 |
|
|
|
|
|
|
|
| 9 |
| 16 | (29) |
| Pouresmaeeli,
2015 | 247 | 39.2 |
Interferon-2a+ribavirin |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 32.8 | 25.9 | 17 | (28) |
| Pouresmaeeli,
2015 | 42 | 37.7 |
Interferon-2b+ribavirin |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 9.5 | 0 | 17 | (28) |
| Nasser Ebrahimi,
2004 | 23 | 45.43 |
Interferon+ribavirin | 48 |
|
|
|
|
|
|
|
|
|
| 48 |
|
| 26 |
| 15 | (27) |
| Alavian, 2006 | 48 | 39.8 |
Interferon+ribavirin |
|
|
| 6.25 |
|
|
|
|
|
|
|
|
|
|
|
| 19 | (15) |
Quality evaluation
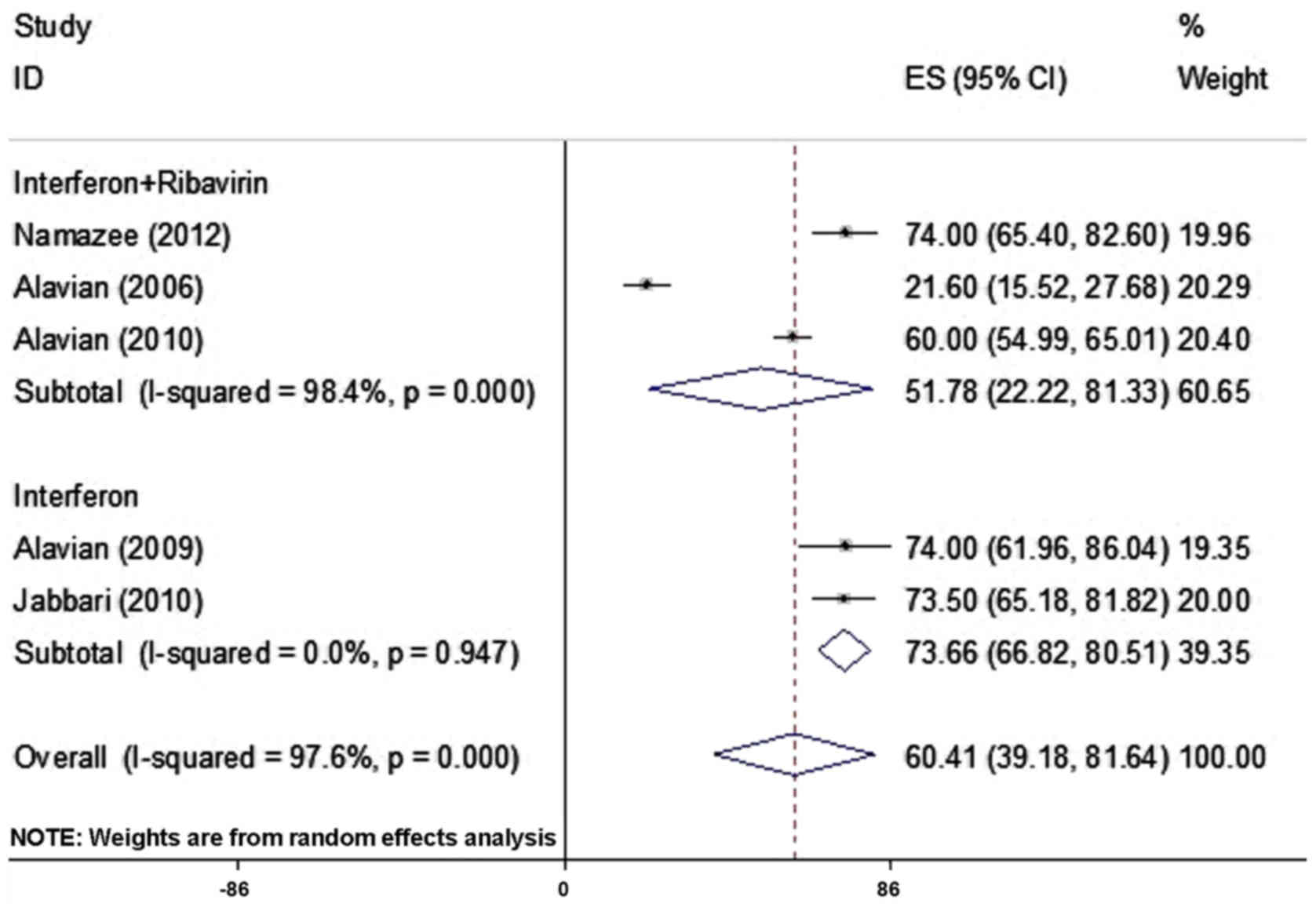
Among the selected studies, the prevalence of
fatigue was reported in 5 articles comprising 802 patients and
ranged from 21.6–74.0% (Table I). As
there was heterogeneity between the results for fatigue, the
random-effects model was used for assessment. By combining the
results of these 5 studies, the frequency of fatigue was determined
to be 60.41% (95% CI; 39.18–81.64%; Fig.
2; Table II).
 | Table II.Results of meta-analysis and
publication bias (Egger's test). |
Table II.
Results of meta-analysis and
publication bias (Egger's test).
|
|
|
| Frequency | Heterogeneity | Publication bias
(Egger's test) |
|---|
|
|
|
|
|
|
|
|---|
| Complication
type | Number of
studies | Sample size | % | CI 95% | I-squared | Q | P-value | β | P-value |
|---|
| Fatigue | 5 | 802 | 60.41 | 39.18–81.62 |
97.6% | 166.9 | <0.001 | 8.9 | 0.460 |
| Depression | 6 | 706 | 17.31 |
8.88–25.75 | 91.5 |
42.8 | <0.001 | 1.2 | 0.743 |
| Headache | 6 | 646 | 29.61 | 16.44–42.77 | 92.2 |
63.9 | <0.001 | 0.1 | 0.979 |
| Mood disorders | 6 | 275 | 32.97 | 27.45–38.49 | 0 |
3.54 | 0.617 | 0.3 | 0.849 |
| Insomnia | 7 | 897 | 16.21 |
6.61–25.80 | 93.6 |
93.2 | <0.001 | 0.7 | 0.877 |
| Anorexia | 4 | 494 | 31.29 |
3.65–58.94 | 99.1 | 347.7 | <0.001 | 10.4 | 0.043 |
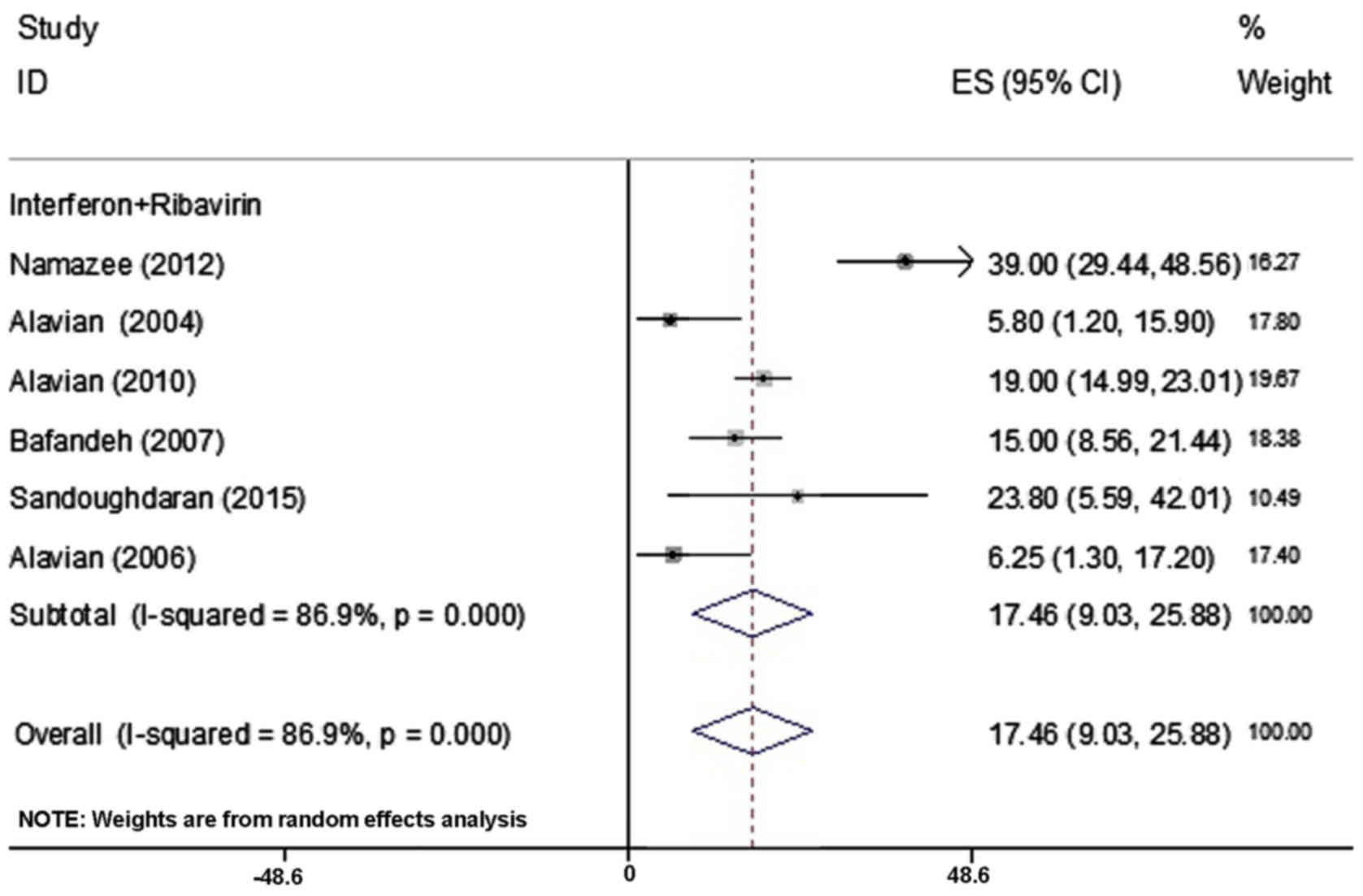
In the present study, the prevalence of depression
was reported in 6 articles comprising 706 patients and ranged
between 5.8 and 39% (Table I).
Combination of these 6 studies in the same manner as for fatigue,
using the random effect model revealed that the frequency of
depression was 17.46% (95% CI; 9.03–25.88%; Fig. 3).
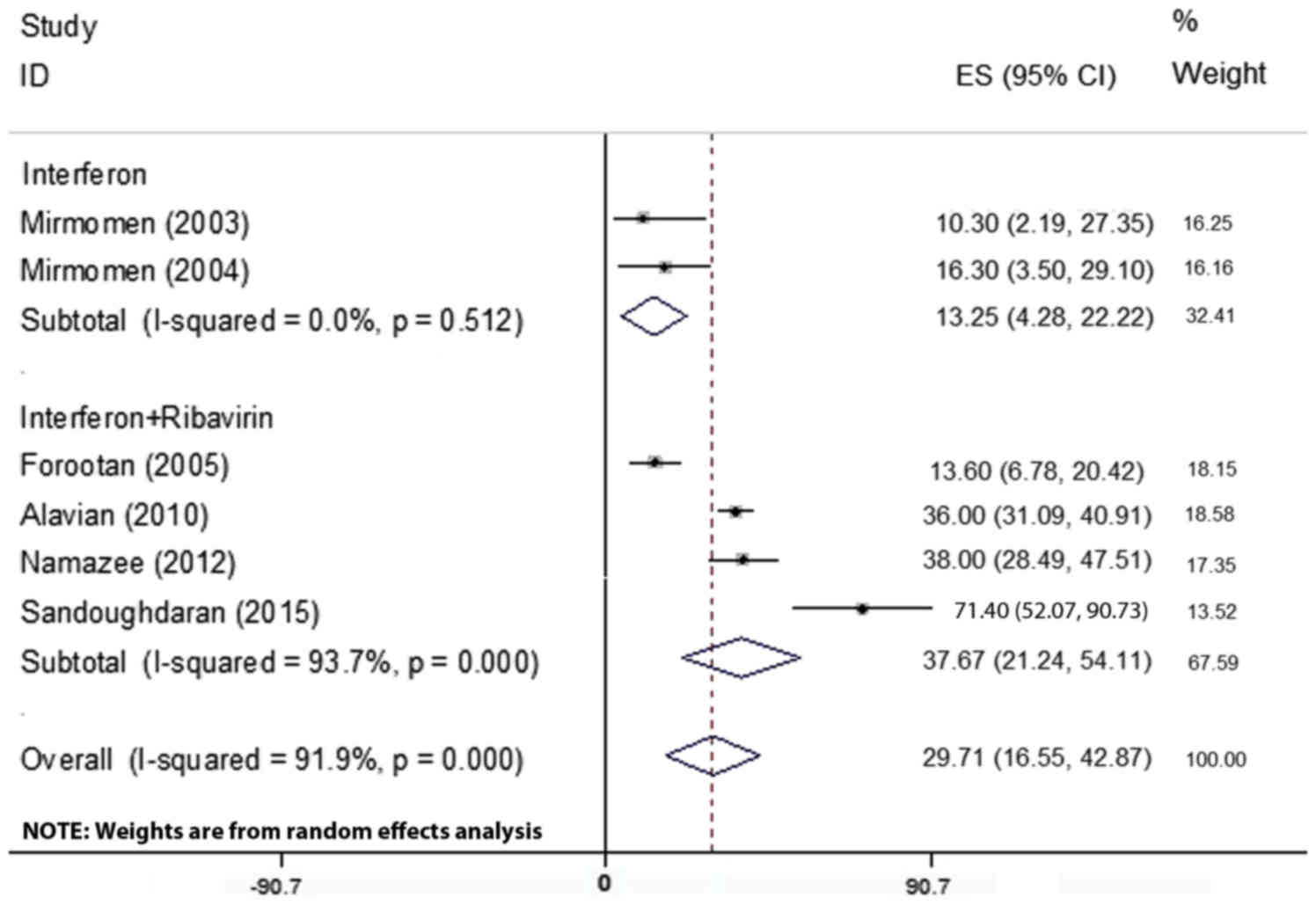
A total of 6 articles including 646 patients
reported on the prevalence of headache, which ranged from
10.3–71.40% (Table I). Due to the
heterogeneity in the results of the primary studies (Table II), the random-effects model used
for assessment. By combining these 6 articles, it was revealed that
headache had a prevalence of 29.71% (95% CI; 16. 55–42.87%;
Fig. 4).
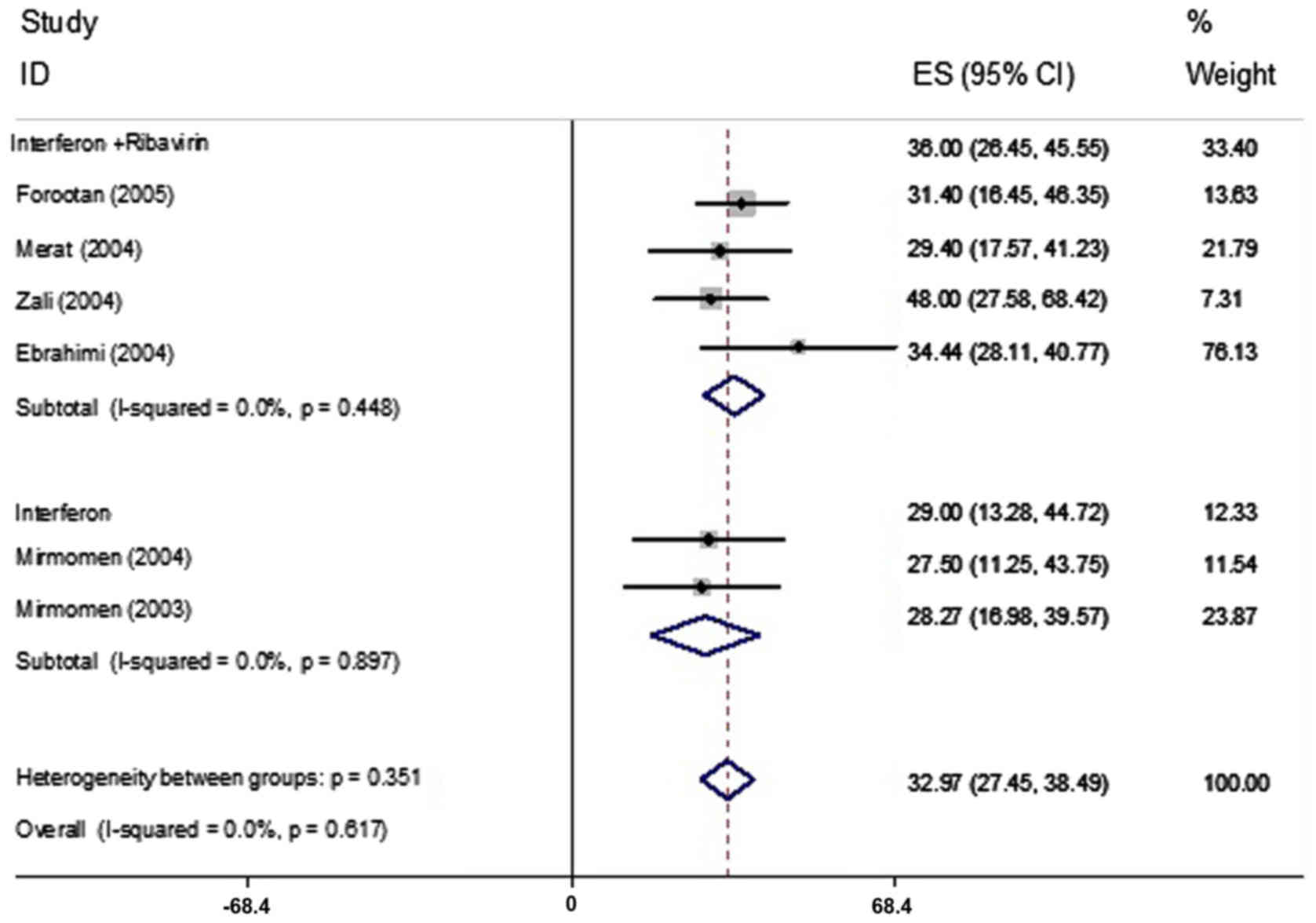
The prevalence of mood disorders was reported in 6
articles comprising 275 patients and ranged from 27.5–48%
respectively (Table I). Based on the
heterogeneity between the results of the primary studies (Table II), the random-effects model was
used for assessment. By combining of these 6 articles, mood
disorders were determined to have a prevalence of 32.97% (95% CI;
27.45–38.49; Fig. 5).
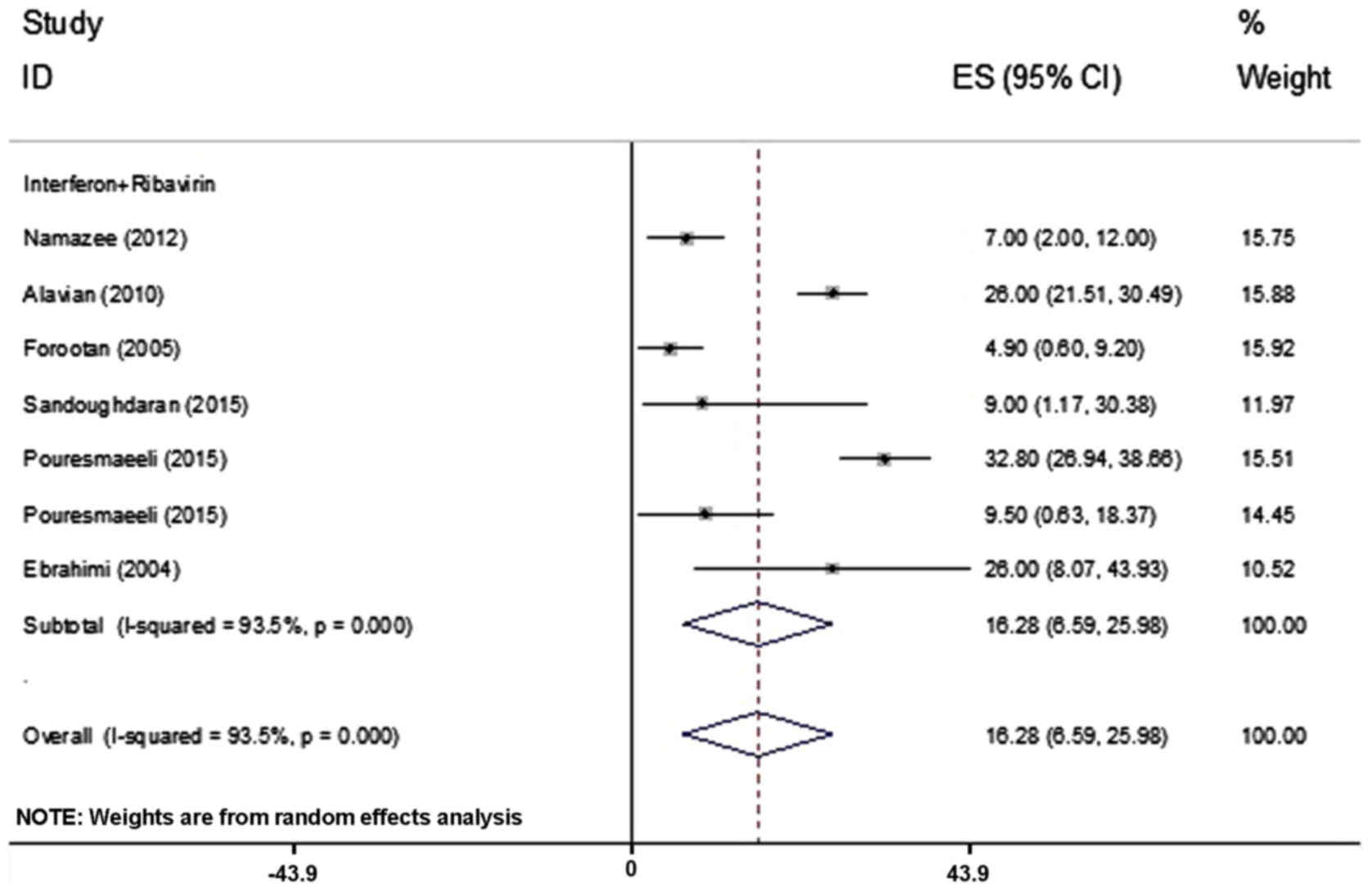
The prevalence of insomnia was assessed in 7
articles including 897 patients and the rate of insomnia varied
between 4.9 and 32.8% (Table I)
(21,28). Based on the heterogeneity between the
results of the primary studies (Table
II), the random-effects model was used for assessment,
revealing a frequency of insomnia in 16.28% (95% CI; 6.59–25.98%)
of patients among the 7 studies (Fig.
6).
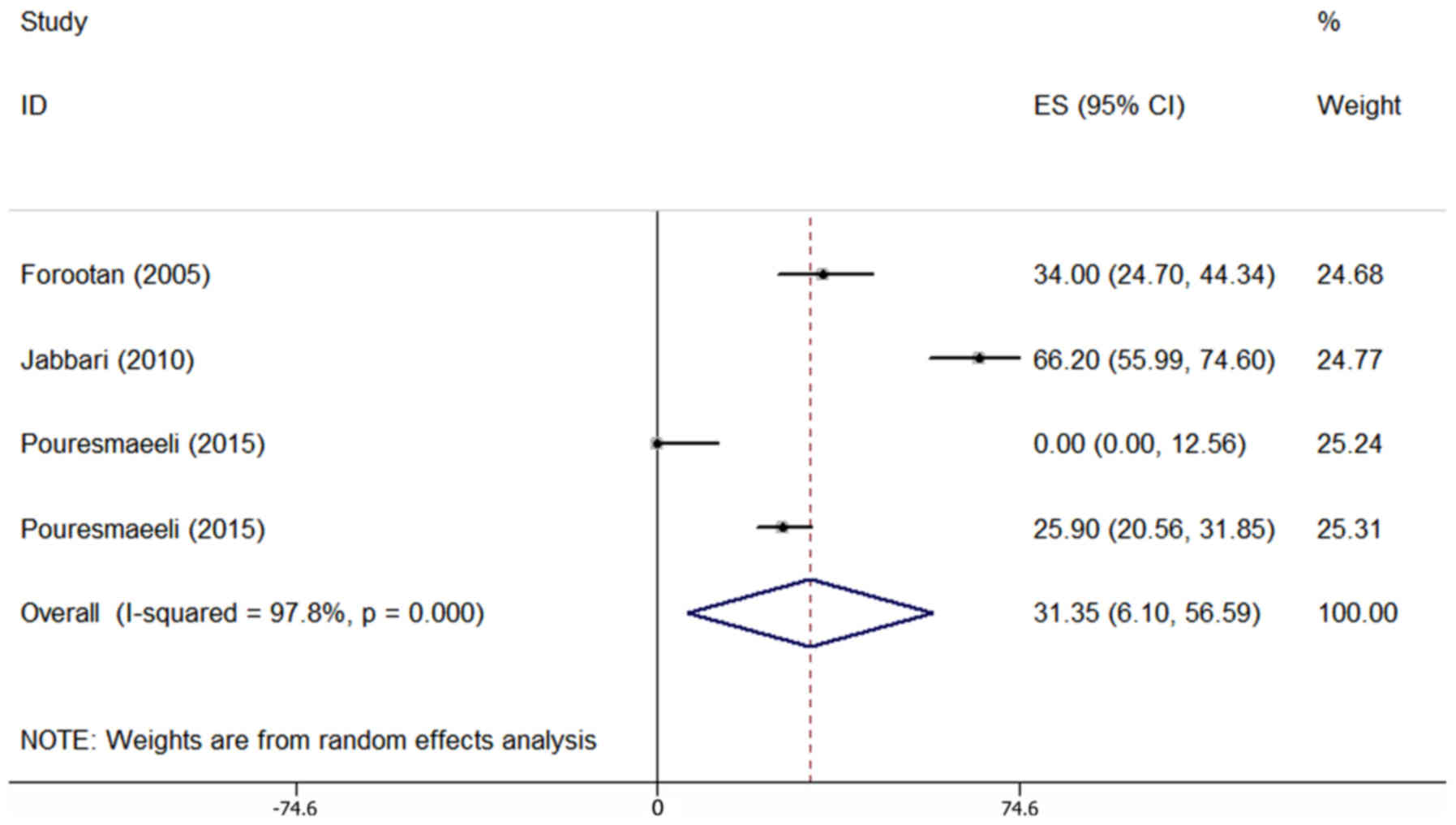
The prevalence of anorexia was assessed in 4
articles comprising 494 patients and was reported to range from
0–66.2% (Table I). Due to the
heterogeneity in the results of the primary studies (Table II), the random-effects model was
employed. Combined analysis of these 4 articles revealed that
anorexia occurred in 31.35% of patients (95% CI; 6.10–56.59%;
Fig. 7).
In the present meta-analysis, adverse events of
nervousness, dizziness, lethargy and irritability as well as
morning headache were reported to be 34.7, 20, 21, 24 and 3.4%,
respectively. Two studies reported on the prevalence of somnolence,
which was 22.5 and 13.7%, respectively. Anxiety and excitability
rates were reported as 41 and 16.1%, respectively. Weakness was
reported in 3 studies with a prevalence of 0.5, 32.2 and 17.2%,
respectively.
Egger's test was performed to assess the publication
bias and the results are presented in Tables II. This test indicated that
publication bias was not statistically significant (except for the
outcome of anorexia). In addition, the age of the patients,
duration of drug use and publication year were considered as
variables associated with heterogeneity in the Meta regression
(Table III). The publication year
had a significant role regarding the heterogeneity in the results
for fatigue, depression and headache among the initial studies. Due
to the small amount of data, subgroup analysis was not performed.
Furthermore, due to the insufficient amount of evidence, it was not
possible to consider the factors determining the heterogeneity
regarding the outcome of anorexia.
 | Table III.Factors associated with heterogeneity
between primary studies in the Meta regression analysis. |
Table III.
Factors associated with heterogeneity
between primary studies in the Meta regression analysis.
|
| Age | Duration of drug
use | Publication
year |
|---|
|
|
|
|
|
|---|
| Adverse event | β | P-value | β | P-value | β | P-value |
|---|
| Fatigue | −0.7 | 0.735 | −0.7 | 0.598 | 8.9 | 0.058 |
| Depression |
0.06 | 0.963 |
0.2 | 0.690 | 2.9 | 0.033 |
| Headache |
0.8 | 0.661 |
0.8 | 0.032 | 3.9 | 0.002 |
| Insomnia |
0.2 | 0.832 | 0.1 | 0.639 | 0.2 | 0.875 |
The present study demonstrated that the majority of
the psychiatric side effects, including weakness, anorexia, mood
change, irritability, headache, fatigue, depression, nervousness,
insomnia, anxiety, lethargy and dizziness were detected in patients
who received ribavirin and interferon. Conversely, somnolence,
morning headache and excitability were demonstrated in patients who
ribavirin treatment. These results suggest that taking ribavirin
alone is the most effective for treatment of patients with HCV,
particularly in individuals suffering from mental illness.
Discussion
The present meta-analysis study assessed the
neuropsychiatric side effects among Iranian patients with hepatitis
C that received peg IFN and ribavirin treatments for the first
time, to the best of our knowledge. In all groups, the
neuropsychiatric side effects with the highest and lowest frequency
were fatigue 60.41% (95% CI; 39.18–81.64%) and insomnia 16.28% (95%
CI; 6.59, 25.98%).
In the present study, fatigue was the most common
neuropsychiatric symptom during IFN plus ribavirin or IFN therapy
60.41% (95% CI, 39.18–81.64%). It has been indicated that fatigue
is associated with decreased adherence to treatment and reduced
viral response (17,18,22).
Certain patients receiving IFN plus ribavirin or IFN only develop
fatigue without necessarily suffering from any somatic symptoms of
depression or depressive emotional complaints, including sadness,
hopelessness, guilt or anhedonia. Although patients receiving
IFN-based therapy may have fatigue without fulfilling the criteria
for clinical depression, fatigue may be considered to be an
important symptom of depression (15,18,20–22).
For the above reasons, patients receiving
IFN-ribavirin therapy who present with fatigue as well as sleep
and/or appetite disturbance should be evaluated for clinical
depression. In certain studies, headache is reported as one of the
most frequent neuropsychiatric side effects of IFN-based treatment
(occurrence rate, 29.6%) (20–25),
which was similar to the results reported in the meta-analysis in
the present study 29.61% (95% CI; 16.44–42.77%).
An important neuropsychiatric side effect, which is
most frequently associated with IFN plus ribavirin or IFN
monotreatment, is depression (16,17). In
this context, depression occurs in a spectrum from minor to
clinical depression in the range of 5.80% (95% CI, 1.20–15.90%) and
39% (95% CI, 29.44–48.56%). Certain studies have diagnosed
depression based on certain symptoms (16,26,29),
while others have defined depression as a syndrome characterized by
a defined set of emotional and physical symptoms (15,17,20).
The prevalence of depression was determined to be
17.46% (95% CI, 9.03–25.88%) in the present meta-analysis. Although
this is not a very high rate, it is important, as depression
occurring during the treatment of hepatitis C may be associated
with a reduced quality of life and also interfere with the patient
pursuing to receive health care (20). Of note, as certain adverse effects,
including fatigue as well as sleep and appetite disturbances may
overlap with depression, patients should be evaluated for major
depression.
Numerous patients treated with IFN plus ribavirin or
IFN only for hepatitis C experienced mood disorders. Of note, in
the present meta-analysis the prevalence of mood disorders was
determined to be 32.97% (95% CI, 27.45–38.49%). Irritability in
patients receiving IFN plus ribavirin was reported by one study
(17) and excitability was reported
by one study on IFN (24). Certain
patients have been suffered from sleep disturbances as a side
effect of hepatitis treatment (26).
In the present meta-analysis, the rate of insomnia was determined
to be 16.28% (95% CI, 6.59–25.98%). Only two studies reported on
the prevalence of somnolence, providing rates of 22.5 and 13.7%
(24,25). Appetite disturbances were more
frequent and anorexia was determined in 31.35% (95% CI,
6.10–56.59%) of patients according to the present meta-analysis.
Nervousness (34%) (15), dizziness
(20%) (17), lethargy (21%)
(17) and anxiety (41%) (17) were reported in only one study for
each. In the present meta-analysis, immune suppression and the
length of treatment with peg IFN and ribavirin in chronic patients
were not taken into account, which is a limitation of the present
study.
In conclusion, the present meta-analysis indicated
that treatment with IFN plus ribavirin or IFN only is associated
with a wide range of neuropsychiatric side effects. Fatigue is the
most common single neuropsychiatric symptom during IFN-based
therapy. Other common neuropsychiatric side effects were mood
disorders and depression, headache, anorexia and sleep
disturbances. Certain patients who were treated with IFN plus
ribavirin or IFN only experienced nervousness, dizziness, lethargy,
irritability and anxiety. Finally, based on the results of the
present meta-analysis, it is recommended that psychiatrists are
increasingly involved in the care of such patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LD, TM and BM conceived and designed the study and
wrote the manuscript. MRH and HJ performed the literature search
and collected the data. MM performed the statistical analysis, and
all authors read and approved the final version of the
manuscript.
Ethical approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing of
interests.
References
|
1
|
Shepard CW, Finelli L and Alter MJ: Global
epidemiology of hepatitis C virus infection. Lancet Infect Dis.
5:558–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu JY, Shadbolt B, Teoh N, Blunn A, To C,
Rodriguez-Morales I, Chitturi S, Kaye G, Rodrigo K and Farrell G:
Influence of psychiatric diagnosis on treatment uptake and
interferon side effects in patients with hepatitis C. J
Gastroenterol Hepatol. 29:1258–1264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Teoh NC, Farrell GC and Chan HL:
Individualisation of antiviral therapy for chronic hepatitis C. J
Gastroenterol Hepatol. 25:1206–1216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spennati A and Pariante CM: Withdrawing
interferon-α from psychiatric patients: clinical care or
unjustifiable stigma? Psychol Med. 43:1127–1132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hauser P, Morasco BJ, Linke A, Bjornson D,
Ruimy S, Matthews A, Rifai A, Indest DW and Loftis JM: Antiviral
completion rates and sustained viral response in hepatitis C
patients with and without preexisting major depressive disorder.
Psychosomatics. 50:500–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fried MW, Shiffman ML, Reddy KR, Smith C,
Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G,
Dhumeaux D, et al: Peginterferon alfa-2a plus ribavirin for chronic
hepatitis C virus infection. N Engl J Med. 347:975–982. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manns MP, McHutchison JG, Gordon SC,
Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M
and Albrecht JK: Peginterferon alfa-2b plus ribavirin compared with
interferon alfa-2b plus ribavirin for initial treatment of chronic
hepatitis C: A randomised trial. Lancet. 358:958–965. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loftis JM and Hauser P: The phenomenology
and treatment of interferon-induced depression. J Affect Disord.
82:175–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asnis GM, de La Garza R II, Miller AH and
Raison CL: Ribavirin may be an important factor in IFN-induced
neuropsychiatric effects. J Clin Psychiatry. 65:581–582. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asnis GM, de La Garza R, Rego SA,
Henderson MA and Reinus JF: Interferon for hepatitis C patients
with psychiatric disorders. Am J Psychiatry. 161:2332–2334. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huckans M, Fuller B, Wheaton V, Jaehnert
S, Ellis C, Kolessar M, Kriz D, Anderson JR, Berggren K, Olavarria
H, et al: A longitudinal study evaluating the effects of
interferon-alpha therapy on cognitive and psychiatric function in
adults with chronic hepatitis C. J Psychosom Res. 78:184–192. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Masip M, Tuneu L, Pagès N, Torras X,
Gallego A, Guardiola JM, Faus MJ and Mangues MA: Prevalence and
detection of neuropsychiatric adverse effects during hepatitis C
treatment. Int J Clin Pharm. 37:1143–1151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coman HG, HERŢA DC and Nemeş B:
Psychiatric adverse effects of interferon therapy. Clujul Med.
86:318–320. 2013.PubMed/NCBI
|
|
14
|
Von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP: Iniciativa STROBE: The
strengthening the reporting of observational studies in
epidemiology [STROBE] statement: Guidelines for reporting
observational studies. Gac Sanit. 22:144–150. 2008.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alavian SM, Kabir A, Hajarizadeh B,
Nayebpour M, Dorodi T and Baralle FE: Preliminary report of
interferon α2b in combination with ribavirin for 48 weeks for
treatment of iranian patients with chronic hepatitis C: A
quasi-experimental study. Shiraz E Med J. 7:1–8. 2006.
|
|
16
|
Alavian SM, Hajarizadeh B, Hajibeigi B,
Doroudi T, Hamadanizadeh AK and Abar K: Efficacy and safety of
pegylated interferon alfa-2a plus ribavirin for treatment of
chronic hepatitis C and cirrhosis in Iranian. Hepat Mon. 4:53–58.
2004.
|
|
17
|
Alavian SM, Tabatabaei SV, Keshvari M,
Behnava B, Miri SM, Elizee PK and Lankarani KB: Peginterferon
alpha-2a and ribavirin treatment of patients with haemophilia and
hepatitis C virus infection: A single-centre study of 367 cases.
Liver Int. 30:1173–1180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alavian SM, Abolghasemi H, Miri SM,
Keshvari M, Karimi Elizee P, Behnava B, Tabatabaei SV, Hajibeigi B
and Lankarani KB: Safety and Efficacy of Pegylated Interferon
Alfa-2a for the Treatment of Hepatitis C in Patients with Major
Thalassemia. Iranian J Biood Cancer. 1:129–137. 2009.
|
|
19
|
Alavian SM, Ahmadzad M, Keshvari M,
Behnava B and Hajibeigi B: Efficacy and safety of Interferon-alpha
(Dferon B®and Ribavirin combination therapy in patients
with chronic hepatitis C in Iran. Hep Mon. 6:11–18. 2006.
|
|
20
|
Bafandeh Y, Saberi FM and BAGHERI LK:
Evaluation of combination therapy with interferon and ribavirin in
patients with chronic hepatitis C. A genotype based study. 2007
|
|
21
|
Forootan H, Sharifi A, Mirmomen SH,
Daryani NE, Ghofrani H, Farahvash M, Nasiri M, Talebi M, Ghavidel
A, Vosoghinia H, et al: A multicenter study to evaluate the safety
and efficacy of heber on (interferon alfa-2b) in combination with
ribavirin for the treatment of chronic hepatitis C in iran. Med J
Islam Repub Iran. 19:7–12. 2005.
|
|
22
|
Jabbari H, Bayatian A, Sharifi AH,
Zaer-Rezaee H, Fakharzadeh E, Asadi R, Zamini H, Shahzamani K,
Merat S and Nassiri-Toosi M: Safety and efficacy of locally
manufactured pegylated interferon in hepatitis C patients. Arch
Iran Med. 13:306–312. 2010.PubMed/NCBI
|
|
23
|
Merat S, Sohrabpour AA, Khaleghi S,
Sohrabi MR, Samimi-Rad K, Radmard AR and Malekzadeh R:
Peginterferon alfa-2a and ribavirin in patients with chronic
hepatitis C and inherited bleeding disorders. Hepat Mon. 4:59–64.
2004.
|
|
24
|
Mirmomen S, Ebrahimi Daryani N, Malekzadeh
R, Zali MR, Haghpanah B, Poursamimi P, Hashemi S and Alavian SM:
The efficacy and safety of peginterferon alpha-2a (PEGASYS)
monotherapy in the treatment of chronic hepatitis C infected
subjects with transfusion dependent thalassemia. Hepat Mon.
4:65–70. 2004.
|
|
25
|
Mirmomen S, Ghofrani H, Forootan Pishbuary
H, Ebrahimi Daryani N, Jafar Farahvash M, Sharifian R, Azmodeh F
and Malekzadeh R: Safety and efficacy of interferon alfa for the
treatment of chronic hepatitis C infected subjects with transfusion
dependent thalassemia in Iran. Med J Islam Repub Iran. 17:87–95.
2003.
|
|
26
|
Namazee N, Sali S, Asadi S, Shafiei M,
Behnava B and Alavian SM: Real response to therapy in chronic
hepatitis C virus patients: A study from iran. Hepat Mon.
12:e61512012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nasser Ebrahimi D, Babak H, AliReza S, Ali
Asad H, Mohammad B, Parisa P and Nikbin M: The efficacy and side
effects of therapy with peginterferon alpha-2 a (PEGASYS) combined
with ribavirin in chronic hepatitis C patients: An open label
clinical trial. Hepat Mon. 2004:71–74. 2004.
|
|
28
|
Pouresmaeeli M, Alavian SM, Keshvari M,
Salimi S and Mehrnoush L: Efficacy and tolerability of
peginterferon alpha-2a and peginterferon alpha-2b in Iranian
patients with chronic hepatitis C. Hepat Mon. 15:e307802015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sandoughdaran S, Alavian SM, Sharafi H,
Behnava B, Salimi S, Mehrnoush L, Karimi Elizee P and Keshvari M:
Efficacy of prolonged treatment with pegylated interferon (Peg-IFN)
and ribavirin in thalassemic patients with hepatitis C who relapsed
after previous peg-IFN-based therapy. Hepat Mon. 15:e235642015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zali MR, Shalmani HM, Norouzinia M,
Alizadeh AH, Nowroozi A and Berouz N: Peginterferon Alfa-2a
(Pegasys) and Ribavirin in the treatment of chronic hepatitis C.
Hepat Mon. 4:75–78. 2004.
|