Introduction
Skin is an extremely sensitive nerve-dependent
tissue, which is recently considered as the largest organ of the
body. Therefore, surface wound injury is often accompanied by nerve
damage, and nerve repair ensues through the whole process of wound
healing (1). Peripheral nerve damage
in wounds can affect the wound-healing rate, and may cause
paresthesia, affecting the patients life quality, especially in
case of chronic refractory healing wounds. Nerve regeneration is
limited by damage, and if the duration of damage is more than 4
weeks, this may lead to the loss of functional recovery and
regeneration (2).
Skin regeneration medical technology (namely moist
medical technology) encompasses moist exposed burn ointment (MEBO)
which is a new technique practiced in regenerative medicine
(3). In recent years, this
technology has been widely used in the treatment of chronic healing
wounds, as an integral part of traditional Chinese medicine (TCM),
and its efficacy has been recognized by many clinicians and medical
professionals (4). After many years
of improvement and development, MEBO has become a routine technical
method that is widely used in many clinics. MEBO is mainly used to
treat burns and a variety of body surface wounds with good results
(5,6), and is a currently recognized technique
in the academic community (7–9).
However, the molecular mechanisms of MEBO in the process of burn
treatment have not been studied. It is currently understood that
this technology launches four major biological reactions of
hydrolysis, enzymatic hydrolysis, saponification and esterification
via MEBO to keep the wound moist and create a suitable
physiological environment for the healing process. While retaining
healthy and repairing tissues, it liquefies necrotic tissue and
discharges it layer by layer. This removes the necrotic barrier to
healing and gives situ epidermal stem cells access to the wound bed
to allow physiological regeneration and wound repair (10). At the same time, MEBO promotes
activation and proliferation of epidermal stem cells and enhances
the expression of the epidermal stem cells marker CK19 (11).
Many factors are involved in the natural wound
healing process. Nerve growth factor (NGF), the first member of the
family of neurotrophic factor discovered, can promote the
development and differentiation of the central and peripheral
nervous system, and is identified as one of the important factors
in nerve injury repair. It plays a key role in axonal growth, cell
differentiation, cell migration, promotion and control of nerve
regeneration (12,13). NGF acts as a signaling molecule by
binding two types of trans-membrane protein receptors, namely the
neurotrophic factor receptor (p75NTR) and the tyrosine kinase A
(TrkA) receptor (14,15). These trans-membrane proteins exhibit
different binding efficiency, showing low affinity by the p75NTR
and a higher affinity by TrkA (16).
NGF combines with TrkA receptor to play a positive regulation by
activating a series of phosphorylation cascades that promote
neuronal growth, survival and differentiation (17). NGF binds with P75NTR to promote
endothelial cell apoptosis. It was showed that the growth rate and
quality of axons can be improved by these neurotrophic factors
(18). Therefore, we hypothesized
that NGF may be involved in MEBO burn therapy.
Substance P (SP) is a common neuropeptide composed
of 11 amino acids that belongs to the tachykinin family. As a
neurotransmitter or neuromodulator, it plays an important role in
the nervous system. Neurokinin 1 (NK1) receptor is the specific
receptor for SP (19,20). Exogenous SP can stimulate the
proliferation and differentiation of fibroblasts on the wound
surface, accelerate synthesis and secretion of angiogenesis factors
and collagen fibers, and play a significant role in wound healing
and scar hyperplasia (21). On
damage skin, the sensory nerve endings release SP causing a
long-term neuroendocrine response aiding the cell repair, which may
be the reason for the high levels of SP found at injury sites. In
scar tissue, fibroblasts simultaneously up-regulate fibroblast
growth factors and receptors in the granulation tissue, further
amplifying the reparative effects of SP. A previous study revealed
that the SP released by nerve endings could induce and elevate the
levels of SP released from scar fibroblasts (22). Therefore, we hypothesized that SP may
be associated with the burn recovery process of MEBO.
This study was aimed to investigate the effects of
MEBO on the expression of NGF, TrkA and SP in chronic refractory
granulation tissue of rats, and to unravel the possible mechanisms
of MEBO in the promotion of nerve regeneration and repair in
chronic refractory wounds.
Materials and methods
Reagents
MEBO was obtained from Shantou Special Economic Zone
MEBO Pharmaceutical Co., Ltd. (Shantou, China), Beijing Guangming
Chinese Medicine Burns and Urology Institutem [Beijing, China;
State Food and Drug Administration (SFDA), approval no. Z20000004].
Recombinant bovine basic fibroblast growth factor (rb-bFGF) was
obtained from Essex Bio-Technology Co., Ltd. (Zhuhai, China; SFDA,
approval no. S19991021). Chloral hydrate was obtained from Haoshen
Chemical Trading Co., Ltd. [Taixing, China), Chemical Abstracts
Service (CAS), no. 302-17-0]; the Hydrocortisone acetate injection
was obtained from Shanghai General Pharmaceutical Co., Ltd.,
(Shanghai, China; SFDA, approval no. H31021290). The Rat TNF-α
ELISA kit and Rat IL-6 ELISA kit were from RapidBio (West Hills,
CA, USA); the anti-NGF antibody and anti-TrkA antibody were from
Abcam (Cambridge, UK); the anti-SP antibody was from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA); the horseradish peroxidase
labeled goat anti-rabbit antibody and β-actin antibody were from
Zhongshan Jinqiao Biotechnology, (Beijing, China); the Real-Time
quantitative PCR kit, reverse transcription kit and RNA extraction
kit were from Takara Bio, Inc. (Otsu, Japan).
Animals
Adult Wistar male rats of specific-pathogen-free
(SPF) grade (12 weeks old, weighing between 220-250 g) were
provided by the Youjiang National Medical College Animal
Experimental Center. The rats were kept in the SPF grade laboratory
of the animal experiment center, Youjiang National Medical College
with controlled humidity of 55-75%, and an indoor temperature of
20-25°C with good ventilation. After 1-week acclimatization, the
rats were used for generating the experimental models, which was
approved by the Ethics Committee of the Youjiang National Medical
College and adhered to the National Laboratory for Experimental
Animal Care published by the National Institutes of Health (NIH
publication no. 85-23, 1996).
Chronic refractory wound model
The animal models created in this study adopted the
whole layer skin defection method established by Zhao et al
(23) and the quantitative method of
plastic ring granulomamodified by Shen (24). We further improved these methods and
created the rat model of chronic refractory wounds. Rats were
anesthetized with intraperitoneal injection of 7% chloral hydrate
at 280 mg/kg. The rats back hair was shaved with an electric razor,
and then Nair hair removal cream was used to ensure complete hair
removal. The rats were then washed with water and their back skin
was dried and placed at 28-30°C. When recovering naturally, the
rats were given free access to diet for more than 24 h to eliminate
the effect of hair removal agent on the skin damage. The rats were
then anesthetized again in the same manner. We marked with a 1.5 cm
diameter circular stamp to ensure a consistent model area. Under
sterile conditions, two whole layer skin wounds were made along the
direction of the spine using surgical methods, which were deep in
the fascia and 1.5 cm in diameter. The two wounds were covered with
two layers of dry gauze and fixed with tape in a ‘丰’ shape. After
modeling, hydrocortisone acetate was immediately injected
intraperitoneally (6 mg/100 g body weight). At this point, a
chronic refractory wound model was established.
Experimental animal grouping and
treatment
A total of 90 rats were divided into control group
(18 rats), sham operation group (18 rats) and chronic refractory
wound group (54 rats). The rats in the control group were treated
without damage. The rats in the sham operation group were treated
following the basis of the above model, where they underwent the
whole layer skin resection at the model site, but without
hydrocortisone injection. The rats in the chronic refractory wound
group were randomly sub-divided into the wound group, MEBO group
and rb-bFGF group (n=18 in each group). After the wounds were
created, we immediately provided treatment. 1/5,000 nitrofurazone
liquid was used to clean wounds before the first dressing, and
after that saline was applied to clean the wound before each
dressing. Each group underwent the corresponding dressing treatment
twice daily (morning and evening).
In the MEBO group, wounds were covered with two
layers of gauze that contained MEBO. The preparation of MEBO gauze
(0.2 g/cm2) was based on the Clinical Application
Specification of Burned Skin Regenerative Medical Technology
(25). rb-bFGF, as an exogenous
growth factor was selected as the control drug, which can improve
the wound's lack of endogenous growth factors and can induce
vascular endothelial cells and fibroblast proliferation to promote
wound healing. rb-bFGF is now widely used in the treatment of
trauma, burns and other wounds (26). In the rb-bFGF group, the wound
surface was covered with two layers of gauze (60 U/cm2)
impregnated with rb-bFGF. In the wound group and sham operation
group, wound surfaces were covered with two layers of gauze that
contained saline. In the control group, the skin was covered over
the corresponding site with two layers of saline gauze. The gauze
was cut into small pieces according to the size of the wound and
stuck to the wound, and then covered with another two layers of dry
gauze and fixed with tape.
Specimen preparation
In a preliminary experiment, we found that the
duration of wound healing in rats was approximately 14 days. Six
rats were randomly selected from each group at three time points,
namely 3, 7 and 14 days and were culled by cervical dislocation.
The entire wound granulation tissue (0.5 cm from the wound margin)
was collected under the wound deep fascia from both sides of the
spine. The tissues were then carefully cut into small pieces,
rinsed with saline, and placed in a cryopreservation tube then
transferred to a −80°C freezer for storage in a liquid nitrogen
tank.
Wound healing rate
Wound healing in all 5 groups was observed. The
wound area was measured and recorded daily to calculate the
wound-healing rate. Wound healing rate=(area before
administration-non-healing wound area)/area before administration ×
100%.
ELISA test
The levels of tumor necrosis factor α (TNF-α) and
interleukin-6 (IL-6) were measured by enzyme-linked immunosorbent
assay (ELISA), and the procedure was carried out according to the
manufacturer's instructions.
Quantitative PCR experiment
RNA extraction and cDNA preparation were performed
according to the experimental method provided by the TAKARA kit.
The extracted cDNA was used as template to conduct amplification
and expression determination according to the kit instructions.
Primers were designed and synthesized by Takara Biotechnology Co.,
Ltd., Dalian, China (Table I). The
amplification expression rate of detected Cq values was calculated
by 2−ΔΔCq method.
 | Table I.Sequences of primers used for
quantitative PCR. |
Table I.
Sequences of primers used for
quantitative PCR.
| Name of the
gene | Forward Primer
sequence | Reverse Primer
sequence | Size (bp) |
|---|
| GAPDH |
5′-GGCACAGTCAAGGCTGAGAATG-3′ |
5′-ATGGTGGTGAAGACGCCAGTA-3′ | 143 |
| SP |
5′-CGCCGATGTTTCAGTCCATTC-3′ |
5′-GACGTATTCAGTCCGTGTTGGTTG-3′ | 96 |
| TrkA |
5′-TGCTCAACAAATGTGGACAGAGG-3′ |
5′-TGTCATGAAGTGTAGGGACATGG-3′ | 98 |
| NGF |
5′-TGCCAAGGACGCAGCTTTC-3′ |
5′-TGAAGTTTAGTCCAGTGGGCTTCAG-3′ | 171 |
Western blotting
The tissues were homogenized by grinding manually in
liquid nitrogen. After protein extraction, protein concentrations
were determined and then adjusted to equal amount of protein levels
for all samples before protein denaturation and storage in at −20°C
freezer. Protein concentrations were assessed using the Bradford
method. 30 µg protein was separated by 12% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and then
transferred to polyvinylidene difluoride (PVDF) membrane followed
by blocking the PVDF membrane with 5% skim milk for 1 h. The PVDF
membranes were then incubated with the anti-NGF antibody (1:1,000),
anti-SP antibody (1:1,000) and anti-TrkA antibody (1:1,000),
respectively overnight at 4°C. The membranes were washed with TBST
for 4 times (5 min each time). Subsequently the membranes were
incubated with an appropriate secondary antibody for 1 h at room
temperature. After washing with TBST, the membranes were exposed
using chemiluminescence system and the gel image system was used to
analyze the gray value of target protein. β-actin (1:1,000) was
selected as internal reference. The protein content of the sample
was expressed as the ratio of the absorbance of the target band to
the internal control band.
Statistical analysis
SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. The data were expressed as mean ±
standard deviation and the means of two groups were compared by t
test. The comparison between the means of three or more independent
groups was based on one-way analysis of variance (ANOVA) and
comparison between two means among all the groups was done by
Fisher's least significant difference (LSD) test. P<0.05 was
considered to indicate a statistically significant difference.
Results
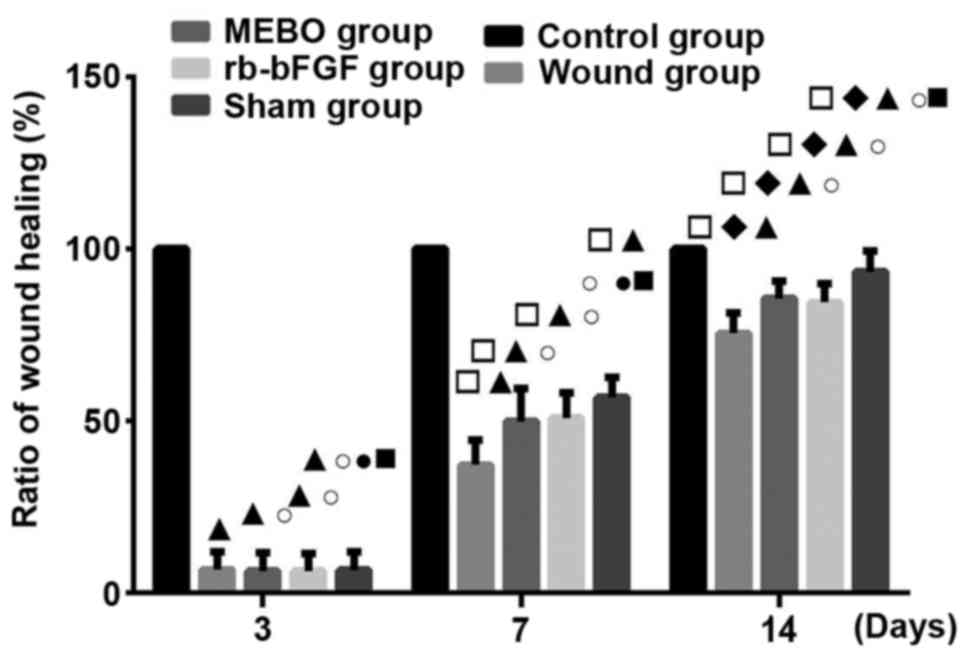
Rats in MEBO and rb-bFGF treatment
groups showed a higher healing rate
All experimental groups showed wound healing over
time. The wound healing rate in each group was as follows: Control
group>sham operation group>MEBO group=rb-bFGF group>wound
group (Fig. 1). The results showed
that the rats in MEBO and rb-bFGF groups could benefit by
significantly increased wound healing from MEBO and rb-bFGF
treatment compared to the wound group (P<0.01).
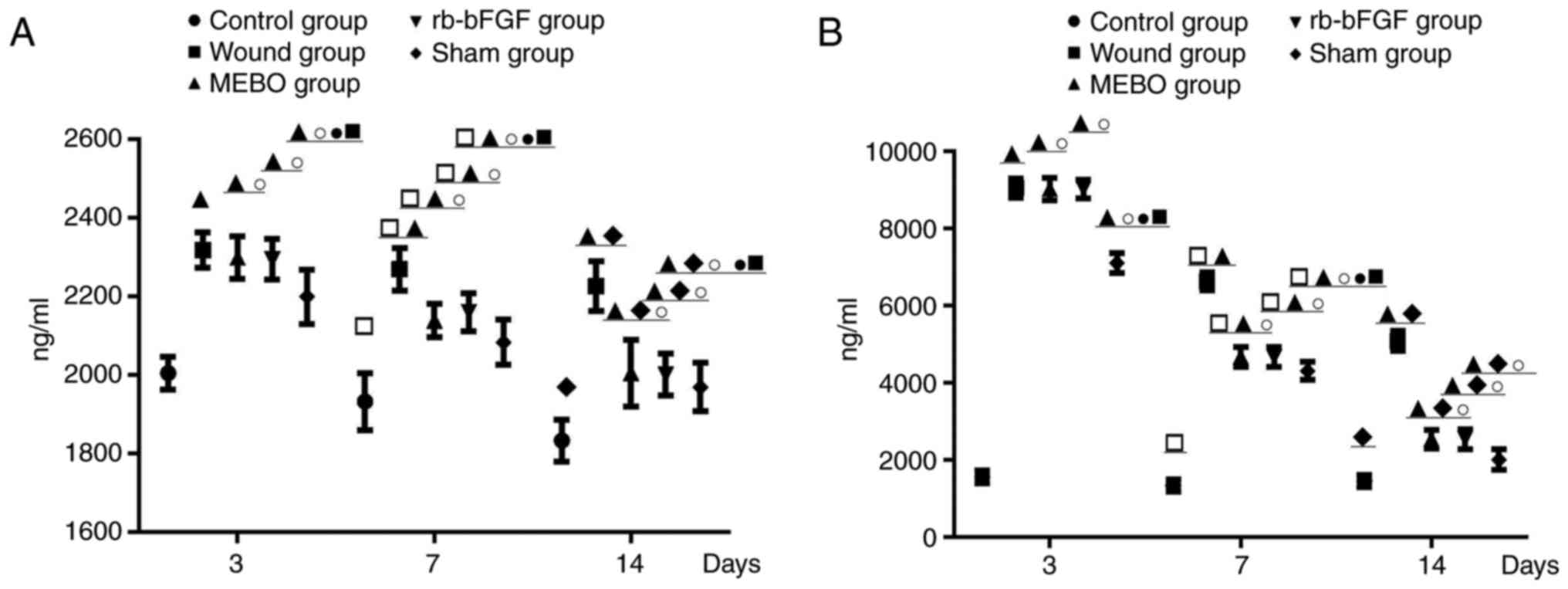
MEBO and rb-bFGF treatment
significantly down-regulated the expression of TNF-α and IL-6
Compared with the control group, the levels of TNF-α
and IL-6 in wound tissue homogenate decreased with time in the
wound group, MEBO group, rb-bFGF group and sham operation group.
The levels of TNF-α and IL-6 in the sham operation group showed the
highest significant decrease (P<0.01) while those in the wound
group showed the lowest decrease (P<0.01). The levels of TNF-α
and IL-6 in rats within each group were as follows: Wound
group>MEBO group=rb-bFGF group>sham operation
group>control group (Fig. 2), and
the difference was statistically significant (P<0.01). The
results showed that MEBO and rb-bFGF treatment in MEBO and rb-bFGF
groups could significantly decrease the expression of TNF-α and
IL-6 compared with the wound group (P<0.01).
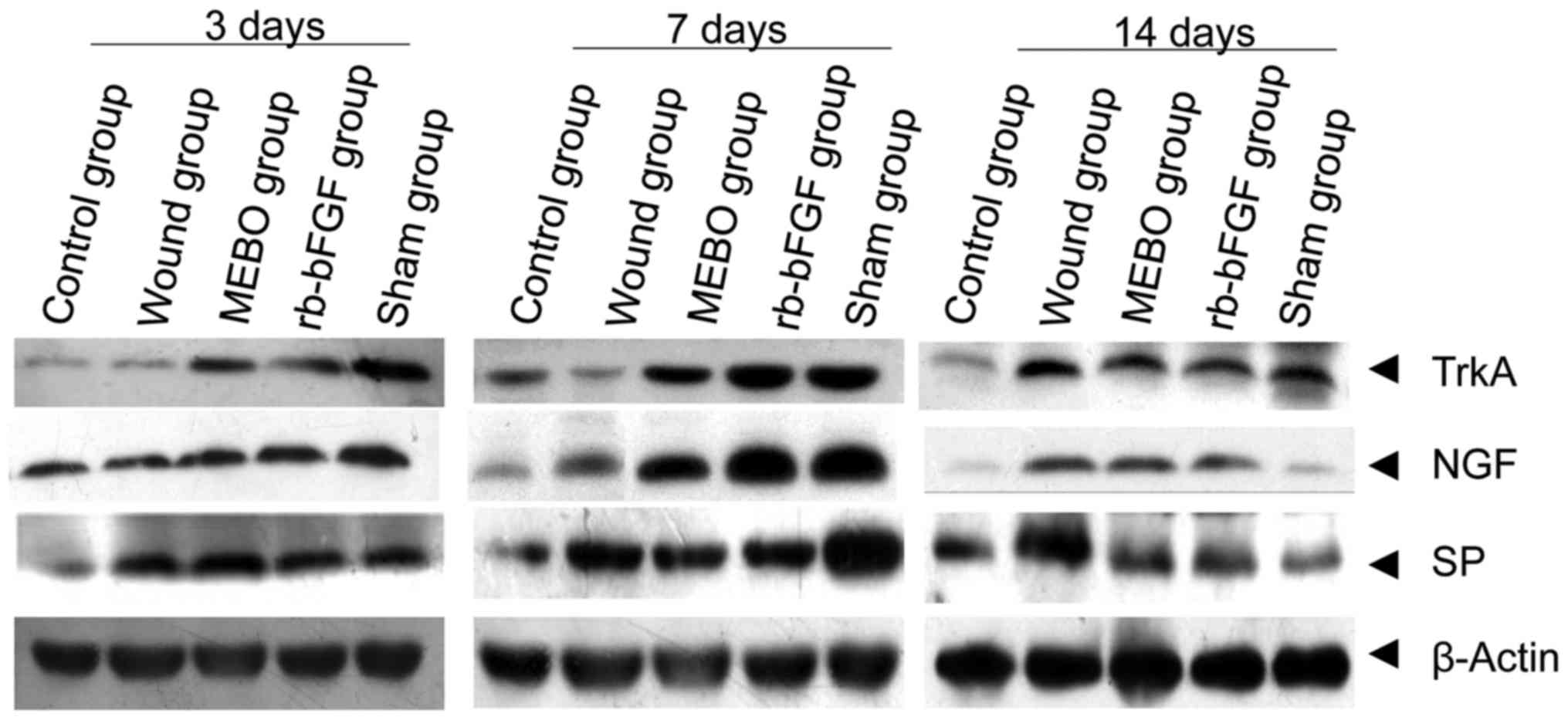
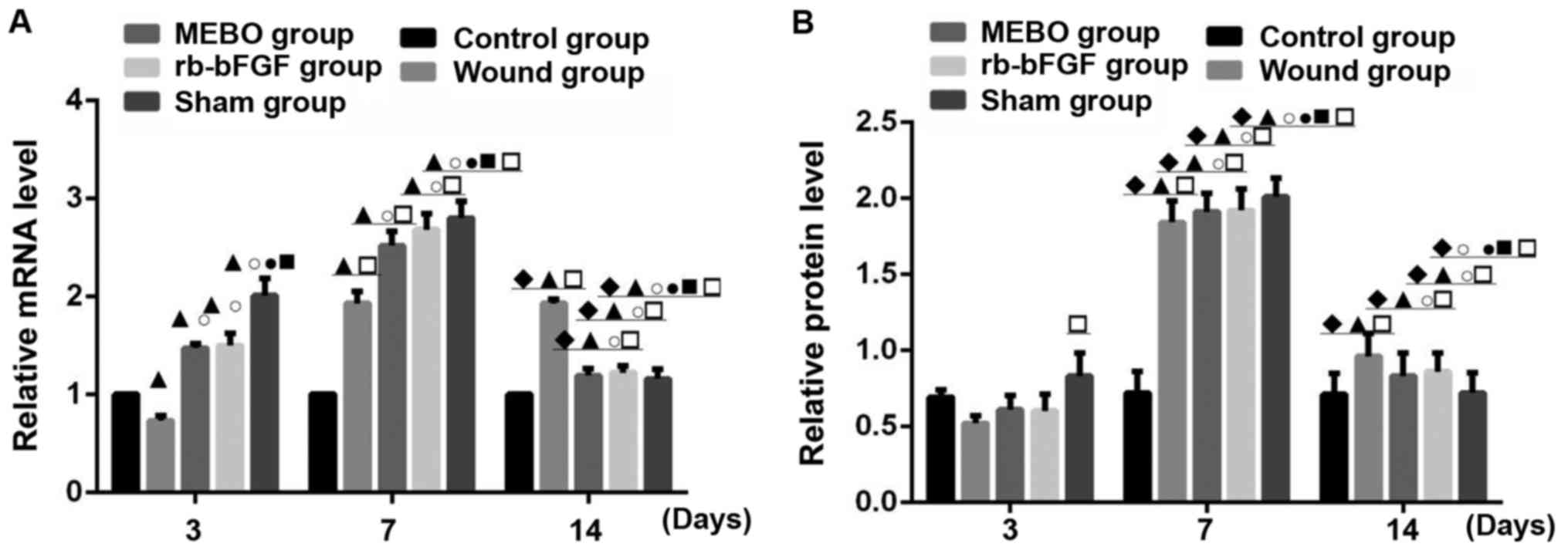
Comparison of NGF mRNA transcription
and protein expression
Compared with the control group, the protein
expression and mRNA transcription level of NGF in rats wound tissue
of MEBO group, rb-bFGF group and sham operation group showed an
upward-downward trend at 3 time points, and reached its highest
level at day 7 and decreased at day 14 (Figs. 3 and 4). However, rats in the wound group showed
no decrease in NGF mRNA on day 14 (P<0.01; Figs. 3 and 4). The rats in control group displayed
constant levels of NGF mRNA and protein (P>0.05; Figs. 3 and 4). On day 3, the expression of NGF mRNA in
the wound tissue of the 5 groups was as follows: Sham operation
group>rb-bFGF group=MEBO group>control group>wound group
(Fig. 4A), while difference in the
expression level of NGF protein was not statistically significant
between groups (P>0.05; Figs. 3
and 4B). On day 7, the expression of
NGF mRNA in wound tissue of the 5 groups was as follows: Sham
operation group>rb-bFGF group=MEBO group>wound
group>control group (Fig. 4A),
and the expression of NGF protein was consistent with the change in
mRNA (Figs. 3 and 4B). On day 14, the expression of NGF mRNA
in wound tissue of 5 groups were as follows: Wound group>rb-bFGF
group=MEBO group=sham operation group=control group (Fig. 4A) and the expression of NGF protein
was consistent with the changes of mRNA (Figs. 3 and 4B). The above results suggested that NGF
was directly involved in the MEBO and rb-bFGF-mediated wound repair
processes.
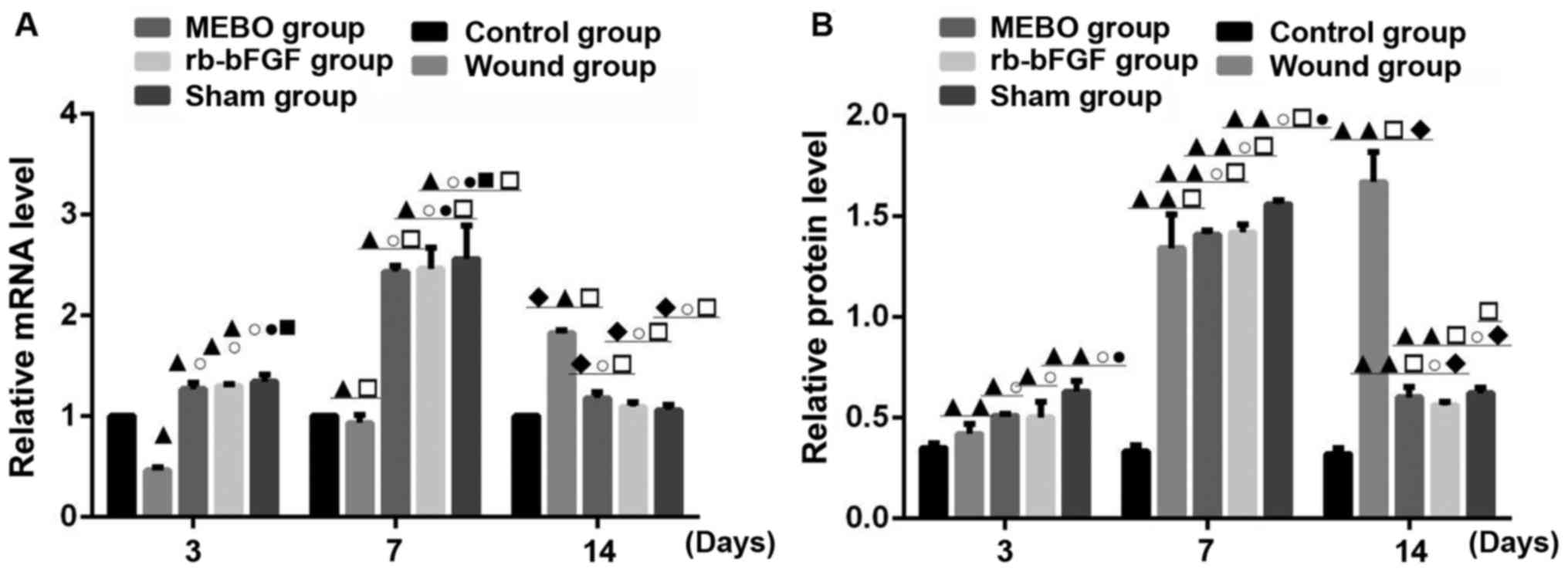
Comparison of TrkA mRNA transcription
and protein expression
TrkA expression was examined in all groups on days
3, 7 and 14 (Figs. 3 and 5). Compared with the control group, the
protein expression and mRNA transcription level of TrkA in wound
tissue of the MEBO group, rb-bFGF group and sham operation group
had an upward-downward trend at three time points, reaching their
highest levels on day 7 and decreasing on day 14 (Figs. 3, and 5CA
and B). The levels of TrkA in the wound group showed a
continually increasing trend with the highest levels on day 14. The
difference among groups was statistically significant (P<0.01;
Fig. 5A and B). The rats in the
control group showed no significant difference in TrkA protein and
mRNA transcription expression levels at the 3 time points
(P>0.05; Figs. 3 and 5A). On day 3, the expression of TrkA
protein in the wound tissue of the 5 groups were as follows: Sham
operation group>rb-bFGF group=MEBO group>control
group>wound group (Fig. 5A), and
the expression of TrkA protein was consistent with the changes in
mRNA levels (Figs. 3 and 5B). On day 7, the expression of TrkA
protein in the wound tissue of the 5 groups was as follows: Sham
operation group>rb-bFGF group=MEBO group>wound group>blank
group (Figs. 3 and 5A), and the protein expression of TrkA was
consistent with the changes in mRNA levels (Figs. 3 and 5B). On day 14, the protein expression of
TrkA in the wound tissue of the 5 groups was as follows: Wound
group>rb-bFGF group=MEBO group=sham operation group=blank group
(Figs. 3 and 5A), and the expression of TrkA protein was
consistent with the changes in mRNA levels (Figs. 3 and 5B). The results suggested that TrkA was
involved in MEBO and rb-bFGF-mediated wound repair processes.
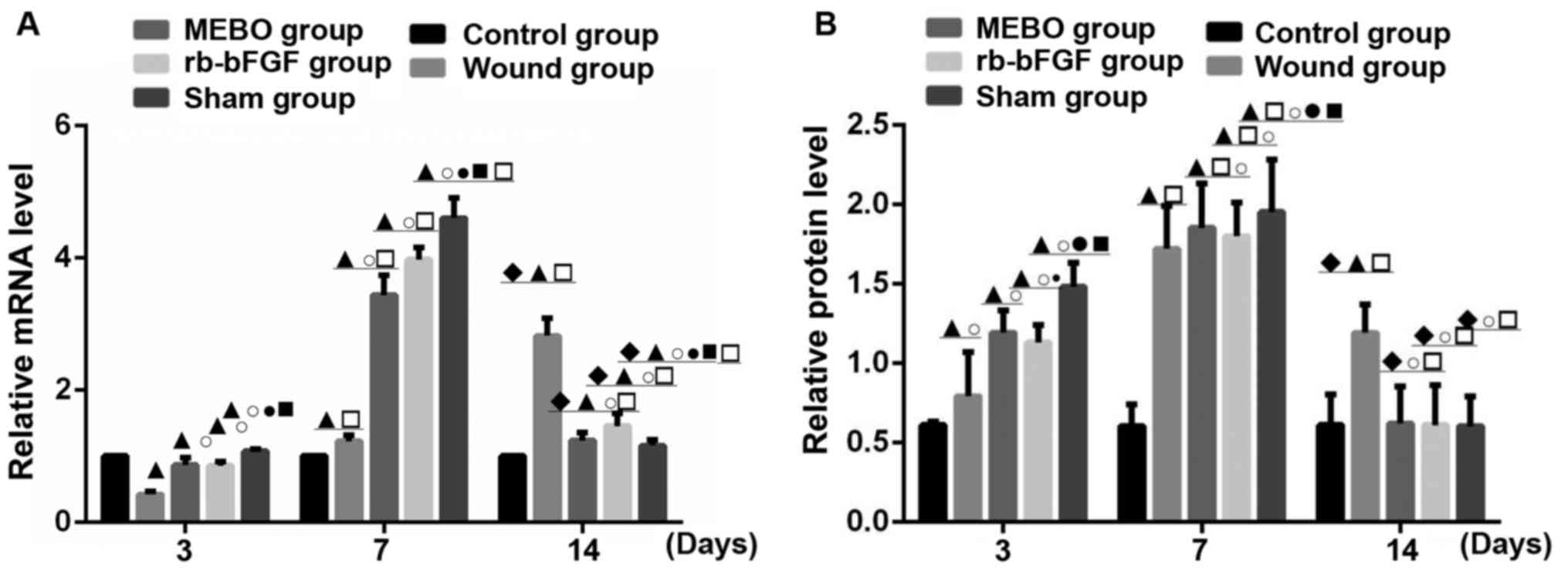
Comparison of mRNA transcription and
protein expression of SP
SP expression was examined in all groups on days 3,
7 and 14 (Figs. 3 and 6). Compared with control group, the protein
expression and mRNA transcription level of SP in the MEBO group,
rb-bFGF group and sham operation group presented an upward-downward
trend at 3 time points, and reached the peak level at 7 days and
decreased on the 14th day (P<0.01; Figs. 3, and 6A
and B). The mRNA levels in the wound group showed an upward
trend with the highest expression level on day 14. The protein
levels showed only a slight decrease, and was much less than the
decrease in the other experimental groups, and the difference
between groups was statistically significant (P<0.01; Figs. 3 and 6A). The rats in the control group showed no
significant difference in SP protein and mRNA transcription
expression levels between the 3 time points (P>0.05; Figs. 3 and 6A). On day 3, the mRNA expression level of
SP in the wound tissue of the 5 groups was as follows: Sham
operation group>rb-bFGF group=MEBO group>control
group>wound group (Fig. 6A) and
the expression of SP protein was consistent with the changes in
mRNA levels (Fig. 6B). On day 7, the
mRNA expression levels of SP in the wound tissue of the 5 groups
was as follows: Sham operation group>rb-bFGF group=MEBO
group>trauma group>control group (Fig. 6A) and the protein expression levels
of SP was consistent with the changes in mRNA levels (Fig. 6B). On day 14, the mRNA expression of
SP in the wound tissue of the 5 groups was as follows: Sham
operation group>rb-bFGF group=MEBO group>wound group
>control group (Fig. 6A) and the
expression of SP protein was consistent with the changes in mRNA
levels (Fig. 6B). The results
suggested that SP was involved in MEBO and rb-bFGF-mediated wound
repair processes.
Discussion
The aim of this study was to investigate the effects
of MEBO on the expression of NGF, TrkA and SP in chronic refractory
granulation tissue of rats to unravel the possible mechanisms of
therapeutic benefits associated with wound-healing. This is the
first report to suggest that MEBO may promote wound healing by
mediating NGF, TrkA, SP mRNA and protein expression, providing a
novel insight into wound healing.
The initial inflammatory response is important for
effective wound healing, but if the initial response is too high it
can delay the healing process (27).
The results of this study showed that intervention with either MEBO
or rb-bFGF increased wound-healing rates and reduced TNF-α and IL-6
overexpression in wound homogenate tissue, especially on the 7th
day during treatment, and MEBO showed a more obvious effect. The
improvement in wound healing was consistent with similar previous
studies conducted with MEBO (5–9). Because
MEBO and rb-bFGF do not contain anti-inflammatory drugs, we
speculated that MEBO indirectly reduces the expression of some
inflammatory factors, during wound-healing and improves the wound's
microenvironment, thereby creating a positive physiological
environment for wound cells to speed up the wound healing
process.
In order to understand the potential mechanism of
MEBO, we investigated factors that have been shown to have
important roles in wound healing (12–22). The
results showed that the levels of NGF, TrkA, SP protein and mRNA in
the wounds of rats had an upward trend until day 7 and then
decreased again on day 14. Contrarily, in the control group, their
levels remained constant and in the wound group it either continued
to increase till day 14, or did not decrease as rapidly as in the
other experimental groups. So, these results suggest that the
healing rate in chronic refractory wounds treated with either MEBO
or rb-bFGF was close to that of the normal wound physiological
healing level seen in the sham operation group. Rats in the wound
group did not receive any drug intervention, so wound healing was
slow, resulting in higher protein and mRNA levels of NGF, TrkA and
SP at 3 time points.
However, this study has some limitations. Due to the
complexity of the signaling pathways involved, this study has
revealed only a part of the mechanism involved in MEBO promoting
chronic refractory wound healing. With regard to the specific
mechanism much remains to be investigated including the complete
signaling pathways and the multi-channel interactions. In addition,
we have evaluated the wound-healing process by collecting the
parameters during three time points only, which may not fully
explain the entire wound healing process. As MEBO is a TCM
technique with complex interactions between many different factors,
the details of the active factors involved in wound healing still
need to be evaluated and investigated.
In conclusion, the results of this study showed that
MEBO promoted wound repair and accelerated the wound healing in a
similar way as application of rb-bFGF. The mechanism may involve
the regulation of inflammatory response and the expression of NGF,
TrkA and SP. However, more specific details of the mechanism remain
to be investigated to understand the complete signaling pathways
and multi-channel interactions involved.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81560776).
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
IL-6
|
interleukin-6
|
|
LSD
|
least square difference
|
|
MEBO
|
moist exposed burn ointment
|
|
NGF
|
nerve growth factor
|
|
NK1
|
neurokinin 1
|
|
PVDF
|
polyvinylidene difluoride
|
|
rb-bFGF
|
recombinant bovine basic fibroblast
growth factor
|
|
SDS-PAGE
|
sodium dodecyl sulphate-polyacrylamide
gel electrophoresis
|
|
SP
|
substance P
|
|
TCM
|
traditional Chinese medicine
|
|
TNF-α
|
tumor necrosis factor α
|
|
TrkA
|
tyrosine kinase A
|
References
|
1
|
Huang Y, Qi SH, Shu B, Mao RX, Xie JL and
Xu YB: In vivo study on the effect of Skin-derived precursors
(SKPs) on neural repair in skin wounds. Chin J Nerv Mental Dis.
37:531–534. 2011.(In Chinese).
|
|
2
|
Sulaiman W and Dreesen TD: Effect of local
application of transforming growth factor-β at the nerve repair
site following chronic axotomy and denervation on the expression of
regeneration-associated genes. Laboratory investigation. J
Neurosurg. 121:859–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu RQ: Theory and practice of regenerative
medicine: A speech at the 7th National Academic conference on Burns
and Wounds. Chin J Burns Wound Surf Ulcers. 14:201–215. 2002.(In
Chinese).
|
|
4
|
Mabrouk A, Boughdadi NS, Helal HA, Zaki BM
and Maher A: Moist occlusive dressing (Aquacel(®) Ag)
versus moist open dressing (MEBO(®)) in the management
of partial-thickness facial burns: A comparative study in Ain Shams
University. Burns. 38:396–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang QL, Han SS, Feng J, Di JQ, Qin WX, Fu
J and Jiang QY: Moist exposed burn ointment promotes cutaneous
excisional wound healing in rats involving VEGF and bFGF. Mol Med
Rep. 9:1277–1282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang QL, Huang X and Wang Y:
Ultrastructural Pathology and the Mechanism of Expression of MAPKK
mRNA & c-myc mRNA of Chronic Refractory Wound Treated by
MEBT/MEBO. Chin Gen Pract. 18:294–299. 2015.(In Chinese).
|
|
7
|
Li NQ: New Century National College of
Integrative Medicine clinical medicine professional planning
teaching materials ‘integrated traditional Chinese and Western
medicine’. China Traditional Chinese Medicine Press; Bejing:
2005
|
|
8
|
He QH: National traditional chinese
medicine industry higher education ‘12th five-year’ national
planning textbook ‘integrative medicinesurgery’. 2nd edition. China
Traditional Chinese Medicine Press; Bejing: 2014
|
|
9
|
He QH: National traditional chinese
medicine industry higher education ‘13th five-year’ national
planning textbook ‘integrative medicine surgery’. 3rd edition.
China Traditional Chinese Medicine Press; Bejing: 2016
|
|
10
|
Xu RQ and Xiao M: The mechanism of burn
regenerative therapy and wound healing. Chin J Burns Wounds Surface
Ulcers. 15:253–261. 2003.(In Chinese).
|
|
11
|
El-Hadidy MR, El-Hadidy AR, Bhaa A, Asker
SA and Mazroa SA: Role of epidermal stem cells in repair of
partial-thickness burn injury after using moist exposed burn
ointment (MEBO(®)) histological and immunohistochemical
study. Tissue Cell. 46:144–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muir D: The potentiation of peripheral
nerve sheaths in regeneration and repair. Exp Neurol. 223:102–111.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Griffin JW, Hogan MV, Chhabra AB and Deal
DN: Peripheral nerve repair and reconstruction. J Bone Joint Surg
Am. 95:2144–2151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schecterson LC and Bothwell M:
Neurotrophin receptors: Old friends with new partners. Dev
Neurobiol. 70:332–338. 2010.PubMed/NCBI
|
|
15
|
Xu YF: Effects of FGF19-FGFR4 and NGF-TrkA
Signaling Pathways on Progression and Prognosis of
Cholangiocarcinoma. PhD dissertationShandong University 2014
|
|
16
|
Yang XQ: The preliminary study of
expression and interaction of EGFR family and NGF/TrkA in
cholangiocarcinoma. PhD dissertationShandong University 2014
|
|
17
|
Ji YX: Expression of Nerve Growth Factor
in Diabetic Foot Wound Tissue. PhD dissertationHuazhong University
of Science and Technology 2011
|
|
18
|
Fowler JR, Lavasani M, Huard J and Goitz
RJ: Biologic strategies to improve nerve regeneration after
peripheral nerve repair. J Reconstr Microsurg. 31:243–248.
2015.PubMed/NCBI
|
|
19
|
Hosli E and Hosli L: Receptors for
neurotransmitters on astrocytes in the mammalian central nervous
system. Prog Neurobiol. 40:477–506. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hamke M, Herpfer I, Lieb K, Wandelt C and
Fiebich BL: Substance P induces expression of the
corticotropin-releasing factor receptor 1 by activation of the
neurokinin-1 receptor. Brain Res. 1102:135–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu G: Investigation on the Interaction
Mechanism of Nerve Growth Factor NGF and Neuropeptide SP in
Hypertrophic Scar. PhD dissertationSouth China University 2015
|
|
22
|
Jia Y: The relationship between exogenous
substance p and focal adhesion kinase, protein kinase B and nuclear
factor in scar formation. Shanxi Medical University; 2012
|
|
23
|
Zhao JY, Fu XB and Lei YH: Preparation of
small area whole layer skin defect wound model in rats. Infect
Inflamm Rep. 9:642008.
|
|
24
|
Shen DX: A Quantitative Method for the
Study of Anti-inflammatory Effects of Chinese and Western Medicine.
Chin J IntegTradit West Med. 3:491983.(In Chinese).
|
|
25
|
Tang QL: Burn skin regenerative medical
technology clinical application specification (Chinese Edition).
Chin Trad Chinese Med Press; Beijing: 2013
|
|
26
|
Ma FS: Accumulative eschar after burn.
Clin Case Rep. 4:151–153. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakallioglu AE, Basaran O, Karakayali H,
Ozdemir BH, Yucel M, Arat Z and Haberal M: Interactions of systemic
immune response and local wound healing in different burn depths:
An experimental study on rats. J Burn Care Res. 27:357–366. 2006.
View Article : Google Scholar : PubMed/NCBI
|