Introduction
Oral squamous cell carcinoma (OSCC) is one of the
most common head and neck malignancies, comprising ~90% of all oral
malignancies (1,2). Multiple factors including genetic
alterations, environmental risk factors and viral infections are
the primary causes for OSCC development and progression. Alongside
the progress in therapeutic technology, radiotherapy, chemotherapy,
traditional surgery and targeted therapy are used in the treatment
of OSCC (3). Although much progress
has occurred in previous years in the treatment of OSCC, the
overall 5-year survival rate of patients with OSCC remains
unsatisfactory, at ~50% (4).
Therefore, understanding the molecular mechanisms underlying OSCC
development and progression is necessary for improving the survival
rate of patients.
Forkhead box protein C1 (FOXC1) belongs to the FOX
family of transcription factors and functions as an important
regulator in ocular development during the embryonic stage
(5). Previous studies have
demonstrated the role of FOXC1 as an oncogene in a diverse range of
cancer types: Hypoxic stresses significantly induced FOXC1
expression in lung cancer and FOXC1 promoted the proliferation,
migration, invasion, angiogenesis and epithelial-mesenchymal
transition (EMT) of lung cancer cells (6). High levels of FOXC1 expression were
identified in human cervical cancer tissues and was significantly
associated with advanced clinical stages, a high degree of
malignancy and a poor outcome (7).
Furthermore, FOXC1 knockdown suppressed cell growth and induced
apoptosis (7). In nasopharyngeal
carcinoma (NPC), FOXC1 expression was upregulated and positively
associated with lymph node metastasis, distant metastasis and an
advanced clinical stage in patients with NPC (8). Knockdown of FOXC1 in NPC cells also
inhibited the migratory and invasive abilities of cells (8). Collectively, FOXC1 may be an effective
therapeutic target for cancer. However, the expression and
biological function of FOXC1 in OSCC remains unclear. In the
present study, FOXC1 expression was detected in human OSCC tissues
using immunohistochemical (IHC) staining and the potential
biological role of FOXC1 in OSCC cell lines was investigated. The
results suggested that silencing FOXC1 inhibits the growth and
migration of OSCC, which may provide experimental evidence for the
role of FOXC1 in the diagnosis and treatment of OSCC.
Materials and methods
Clinical samples
All of the experiments in the present study were
approved by the Medical Ethics Committee of the Stomatological
Hospital, Southern Medical University (Guangzhou, China). Informed
consent was provided by the patients, who agreed to the use of
their tissues in the present study. A total of 42 archival cases
that were diagnosed and treated from January 2014 to August 2016 at
the Stomatological Hospital, Southern Medical University, of which
15 cases (age range, 51–68 years; 7 females and 8 males) were
healthy controls and 27 were cases of OSCC (age range, 50–74 years;
12 females and 15 males), were included. The collected tissues were
embedded by paraffin following dehydration with an ethanol series
(70, 80, 90, 95 and 100%) and 100% xylene treatment at room
temperature, and then stored at 4°C until use.
IHC staining
For IHC staining, 4-µm thick paraffin-embedded
tissue sections were deparaffinized at room temperature with
xylene, and then rehydrated in a descending ethanol series (100, 95
and 80% ethanol, then PBS) at room temperature. Then, 3%
H2O2 in methanol (v/v) was added to block the endogenous
peroxidase activity at room temperature for 10 min. Following
blocking with 5% bovine serum albumin (Beyotime Institute of
Biotechnology, Haimen, China) in PBS for 30 min at room
temperature, the slides were incubated overnight with the primary
anti-FOXC1 antibody (1:100; cat. no. DF3252; Affinity Biosciences,
Cincinnati, OH, USA) at 4°C overnight. Subsequent to incubation
with the horseradish peroxidase-conjugated goat anti-rabbit
horseradish peroxidase-conjugated immunoglobulin (Ig)G (1:200; cat.
no. S0001; Affinity Biosciences) for 2 h at room temperature, the
DAB substrate kit (Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China)
was used to detect positively stained cells. The slide was
photographed by a fluorescence microscope (Olympus CX31-32RFL;
Olympus Corporation, Tokyo, Japan) at a magnification of ×40. The
number of positive and total cells in each frame were counted and
analyzed. The IHC score was quantified as follows: 0, 0–20%
positive cells; 1, 20–40%; 2, 40–60%; 3, 60–80%; 4, 80–100%.
Cell lines and cell cultures
The UM-SCC-1 (SCC-1) and UM-SCC-23
(SCC-23) cell lines were obtained from Dr Li Cui (University
of California Los Angeles School of Dentistry, Los Angeles, CA,
USA). These cells were maintained in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) containing 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 mg/ml streptomycin (Gibco-BRL; Thermo
Fisher Scientific, Inc) at 37°C with 5% CO2 in a humidified
incubator.
Lentivirus infection
The lentivirus-based short hairpin (sh)RNA
(5′-GGGAAACTGTATTAATCTTAT-3′) targeting FOXC1 and negative control
(shNC; 5′-GATCCGACTTCATAAGGCTTC-3′), which was a scrambled sequence
with no homology to human genes were purchased from GeneCopoeia,
Inc., (Rockville, MD, USA). The lentiviral particles were generated
by following a standardized protocol using the QIAGEN Plasmid Mini
kit (Qiagen GmbH, Hilden, Germany), and EndoFectin-Lenti™ and
TiterBoost™ reagents (both GeneCopoeia, Inc., Rockville, MD, USA).
The lentiviral transfer vector was co-transfected into 293Ta cells
(cat. no. CLv-PK-01) with Lenti-Pac™ HIV packaging mix (cat. no.
HPK-LvTR-20). The lentivirus particles were purified using
centrifugation (3,500 × g, 4°C, 25 min) and stored at −80°C in
aliquots. The lenti-shFOXC1 and lenti-shNC were used to infect
SCC-1 and SCC-23 cell lines at a multiplicity of infection (MOI) of
20. For each well, 0.5 ml lenti-shFOXC1 or lenti-shNC suspension
diluted in DMEM (Gibco; Thermo Fisher Scientific, Inc.) with
Polybrene (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at a
final concentration of 5–8 µg/ml. After a 72-h infection at 37°C in
a 5% CO2 atmosphere, puromycin (2 µg/ml; Beyotime
Institute of Biotechnology, Haimen, China) was added to select the
stably infected cells at 37°C with 5% CO2 in a
humidified incubator.
Purification of total RNA and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Quick-RNA MiniPrepKit (Zymo Research Corp., Irvine,
CA, USA) was used to extract the total RNA from OSCC cells,
according to the manufacturer's protocol. cDNA was synthesized
using the Prime-Script RT reagent kit (cat. no. RR140A) and the
qPCR using the SYBR Premix DimerEraser kit (cat. no. RR840A) (both
from; Takara Bio, Inc., Otsu, Japan), following the protocol of
manufacturer. RT was performed using 1 µg total RNA in 2 µl water
at 65°C for 5 min, 30°C for 10 min, 42°C for 10–30 min and 2°C for
3 min. The qPCR conditions were as follows: Denaturation at 94°C
for 2 min, then denaturation for 30 cycles at 94°C for 30 sec,
annealing at 58°C for 30 sec and extension at 72°C for 30 sec,
followed by a terminal elongation step at 72°C for 10 min. The
following qPCR primers were used: FOXC1: Forward,
5′-CATTTTGGTCTAGGGTGGTTTC-3′; reverse, 5′-TCTGATTGGCAGGGCAGAT-3′
and GAPDH: Forward, 5′-CCAGGTGGTCTCCTCTGACTTC-3′; reverse,
5′-GTGGTCGTTGAGGGCAAT-3′. (Sangon Biotech Co., Ltd., Shanghai,
China). Relative mRNA expression was calculated using
2−ΔΔCq (9). The qPCR
reaction was performed using the ABI ViiA 7 real-time PCR system
(Thermo Fisher Scientific, Inc.). GAPDH was used as an internal
control.
Western blot analysis
Western blot analysis was performed as described
previously (10). Total protein was
extracted from SSC1 and SCC23 cells infected with lenti-shNC or
lenti-shFOXC1 with RIPA lysis buffer (Beyotime Institute of
Biotechnology). The concentration of total protein was measured
with a BCA kit (Beyotime Institute of Biotechnology). Protein (10
µg/lane) was separated using 10% SDS-PAGE gel and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA) at 200 mA for 2 h. The blots were then blocked with 5% skimmed
milk in TBS/T buffer for 1 h at room temperature, incubated
overnight at 4°C with the primary anti-FOXC1 (cat. no. 8758;
1:500), anti-matrix metalloproteinase (MMP)-2 (cat. no. 40994;
1:800), anti-MMP-9 (cat. no. 13667; 1:500), anti-cyclin B1 (cat.
no. 12231; 1:1,000), anti-cyclin D1 (cat. no. 2922; 1:1,000) and
anti-GAPDH (cat. no. 51332, 1:5,000; all Cell Signaling Technology,
Inc., Danvers, MA, USA) antibodies, and incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:3,000) for 2 h at
room temperature. Immunoreactive proteins were visualized using ECL
Plus reagent (Beyotime Institute of Biotechnology). ImageJ software
(version 1.8.0; National Institutes of Health, Bethesda, MD, USA)
was used for densitometric analysis. Each assay was repeated 3
times.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
The EdU detection kit (Guangzhou Ribobio Co., Ltd.,
Guangzhou, China) was used to evaluate cell proliferation.
According to the manufacturer's protocol, cells were treated with
25 mmol/l EdU for 6 h at 37°C and fixed with 4% paraformaldehyde at
room temperature for 30 min. Following incubation with 2 mg/ml
glycine for 5 min, cells were treated with 0.5% Triton X-100
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 20 min and
stained with 1X Apollo reaction cocktail (Guangzhou Ribobio Co.,
Ltd.) for 30 min at room temperature. Following washing with 0.5%
TritonX-100 in PBS, 1X Hoechst dye (Beyotime, Beijing, China) was
used to incubate cells at room temperature for 30 min. Images were
captured using a confocal laser scanning microscope (magnification,
×100). Following the merging of the images, the ratios of Apollo
staining-positive cells to Hoechst staining-positive ones were
calculated using ImageJ software (version 1.8.0; National
Institutes of Health, Sacaton, AZ, USA). The assay was repeated in
triplicate.
Cell counting kit-8 (CCK-8) assay
SCC-1 and SCC-23 cells that were stably infected
with lenti-shFOXC1 or lenti-shNC were starved for 24 h and then
seeded into a 96-well plate at a density of 4,000 cells/well in 100
µl culture medium. Following cell plating for 0, 24, 48 and 72 h,
10 µl CCK-8 (Sigma-Aldrich; Merck KGaA) was added to each well.
Following incubation for 2 h at 37°C, the absorbance (OD 450 nm)
was measured using a microculture plate reader (Tecan Group, Ltd.,
Männedorf, Switzerland). All experiments were repeated three
times.
Colony formation assay
SCC-1 and SCC-23 (2,000) cells that were stably
infected with lenti-shFOXC1 or lenti-shNC were plated in 6-well
plated with 2 ml DMEM containing 10% FBS. Following cell culturing
for 14 days at 37°C in a 5% CO2 atmosphere, the
cells were fixed in 100% methanol at room temperature for 10 min
and stained with 0.1% crystal violet for 10 min at room
temperature. The colonies in each well were counted using an
inverted microscope (Olympus IX51; Olympus Corporation;
magnification, ×40) and relative colony numbers were calculated as
follows: The colony numbers of shFOXC1/the colony numbers of
shNC.
Migration assay
SCC-1 and SCC-23 cells that were stably infected
with lenti-shFOXC1 or lenti-shNC were starved for 24 h. Cells were
then harvested with Trypsin (Gibco; Thermo Fisher Scientific,
Inc.), centrifuged at 200 × g for 5 min at room temperature,
resuspended in DMEM containing 0.1% FBS and added to the upper
chamber of Transwell chambers (8 µm pore size; Corning
Incorporated, Corning, NY, USA), while DMEM containing 10% FBS was
added to the lower chambers. Following culturing at 37°C in a 5%
CO2 atmosphere for 48 h, the cells were fixed at room
temperature for 10 min in 100% methanol and stained for 10 min at
room temperature with 0.1% crystal violet. Cells that had moved
through the membrane were counted from 3 random fields using an
inverted microscope (Olympus IX51; magnification, ×40).
Statistical analysis
Data are expressed as mean ± standard deviation. The
paired sample t-test was used to compare the results between two
groups and a one-way analysis of variance followed by Tukey's
post-hoc test was performed to analyze the difference between
≥three groups using SPSS v21.0 software (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of FOXC1 in OSCC and
adjacent normal tissues
To evaluate the expression of FOXC1 in human tissue
samples, 27 OSCC tissue samples and 15 normal tissue samples
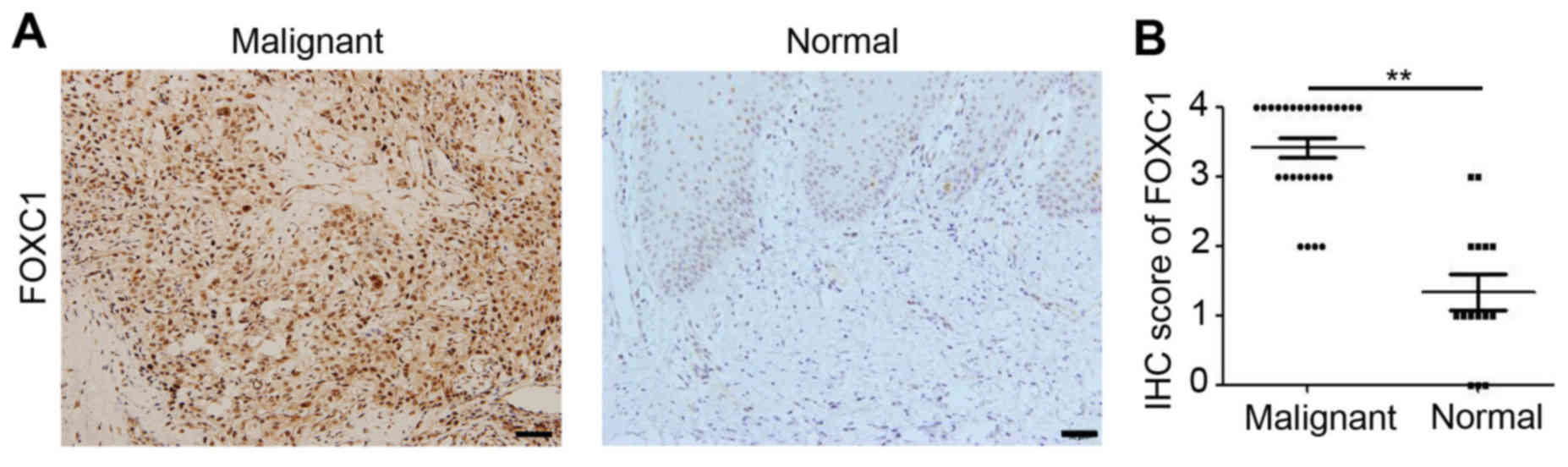
healthy controls were collected for IHC staining. As indicated in
Fig. 1A, positive staining of
epithelial cells was identified as the presence of yellow-brown
granules. Positive staining was observed in the cell nuclei of OSCC
tissues throughout the epithelium and the tumor (Fig. 1A). Whereas positive staining in
normal tissues was almost absent and detected only in the most
active parabasal and basal layers (Fig.
1A). Additional analysis suggested that the density of FOXC1
staining in OSCC tissues was increased compared with that in
adjacent normal tissues (Fig. 1B).
These results demonstrated the upregulation of FOXC1 in OSCC.
Knockdown of FOXC1 inhibits OSCC
growth in vitro
To investigate the potential biological effects of
FOXC1 in OSCC, shRNA targeting the FOXC1 gene was used to inhibit
FOXC1 expression in the OSCC cell lines, SCC-1 and SCC-23. As
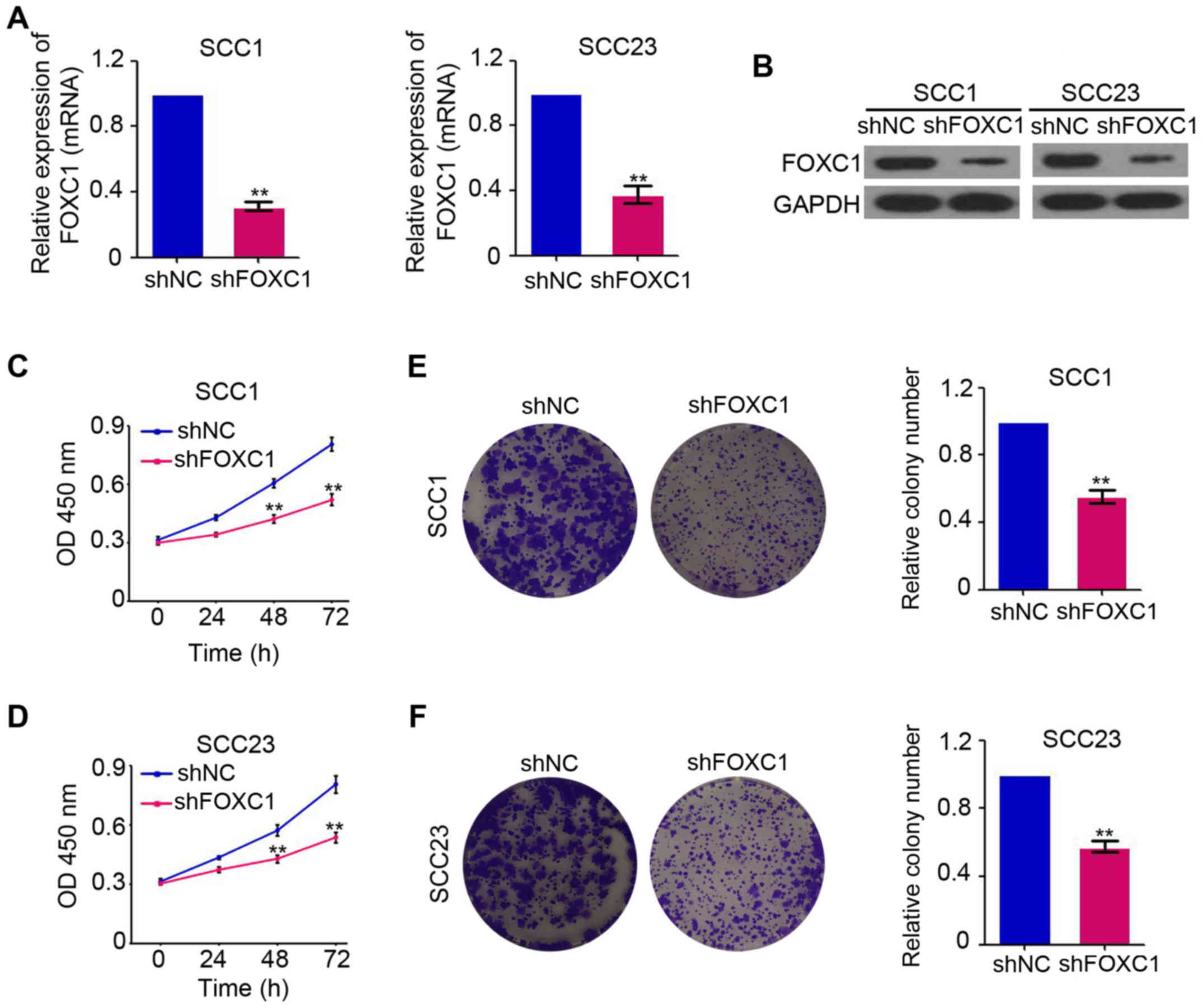
indicated in Fig. 2A, FOXC1 mRNA
expression was efficiently downregulated by lenti-shFOXC1 in SCC-1
and SCC-23 cells. Western blot analysis also confirmed the
inhibition of FOXC1 protein production by shFOXC1 in the SCC-1 and
SCC-23 cell lines (Fig. 2B). The
CCK-8 assay was performed to determine the role of FOXC1 in the
growth of OSCC and the results indicated that there was significant
inhibition of cell growth in FOXC1-knockdown SCC-1 and
SCC-23 cells at 48 and 72 h post-infection (Fig. 2C and D). Additional colony formation
assay results also confirmed the inhibition of cell growth in
FOXC1-knockdown SCC-1 and SCC-23 cells (Fig. 2E and F). Collectively, these data
confirmed the oncogenic role of FOXC1 in OSCC.
Downregulation of FOXC1 inhibits the
proliferative capacity of OSCC cells
To investigate the role of FOXC1 in the
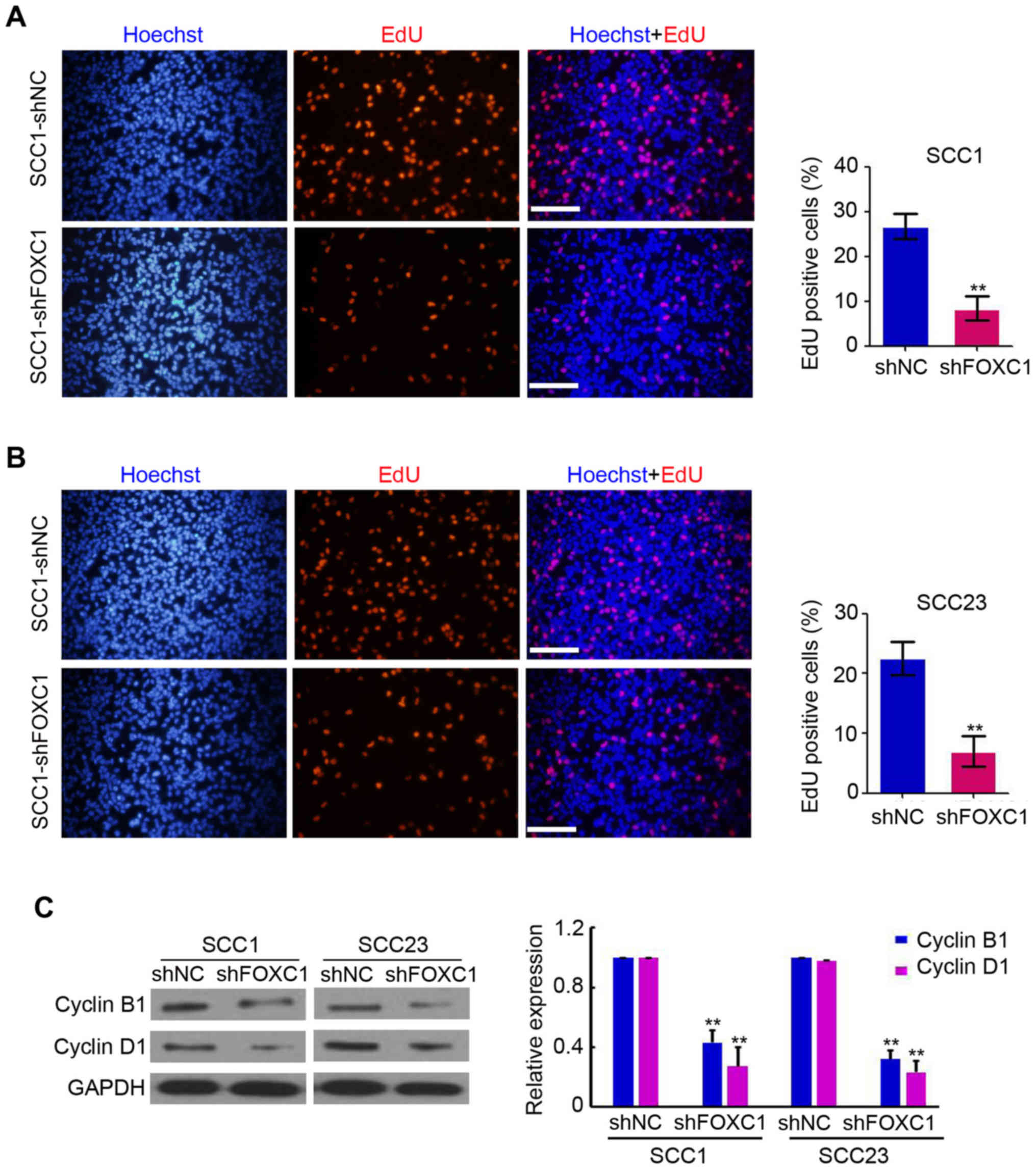
proliferation of OSCC, EdU assays were performed in the OSCC SCC-1
and SCC-23 cell lines, which were infected with lentiviruses
expressing shRNA targeting FOXC1 or the NC. As demonstrated in
Fig. 3A and B, proliferative cells
were observed in shFOXC1 and shNC-infected cells. However, there
were fewer proliferative SCC-1 and SCC-23 cells following
FOXC1 knockdown compared with the paired shNC cells
(Fig. 3A and B). Furthermore,
western blot analysis indicated a marked downregulation of cyclin
B1 and cyclin D1 levels in SCC-1 and SCC-23 cells following
lenti-shFOXC1 infection, as compared with that in
lenti-shNC-infected cells (Fig. 3C).
These results suggested that the downregulation of FOXC1 suppressed
the proliferative capacity of OSCC cells through the regulation of
cyclin levels.
Downregulation of FOXC1 inhibits the
migration capacity of OSCC cells
To determine the potential regulatory role of FOXC1
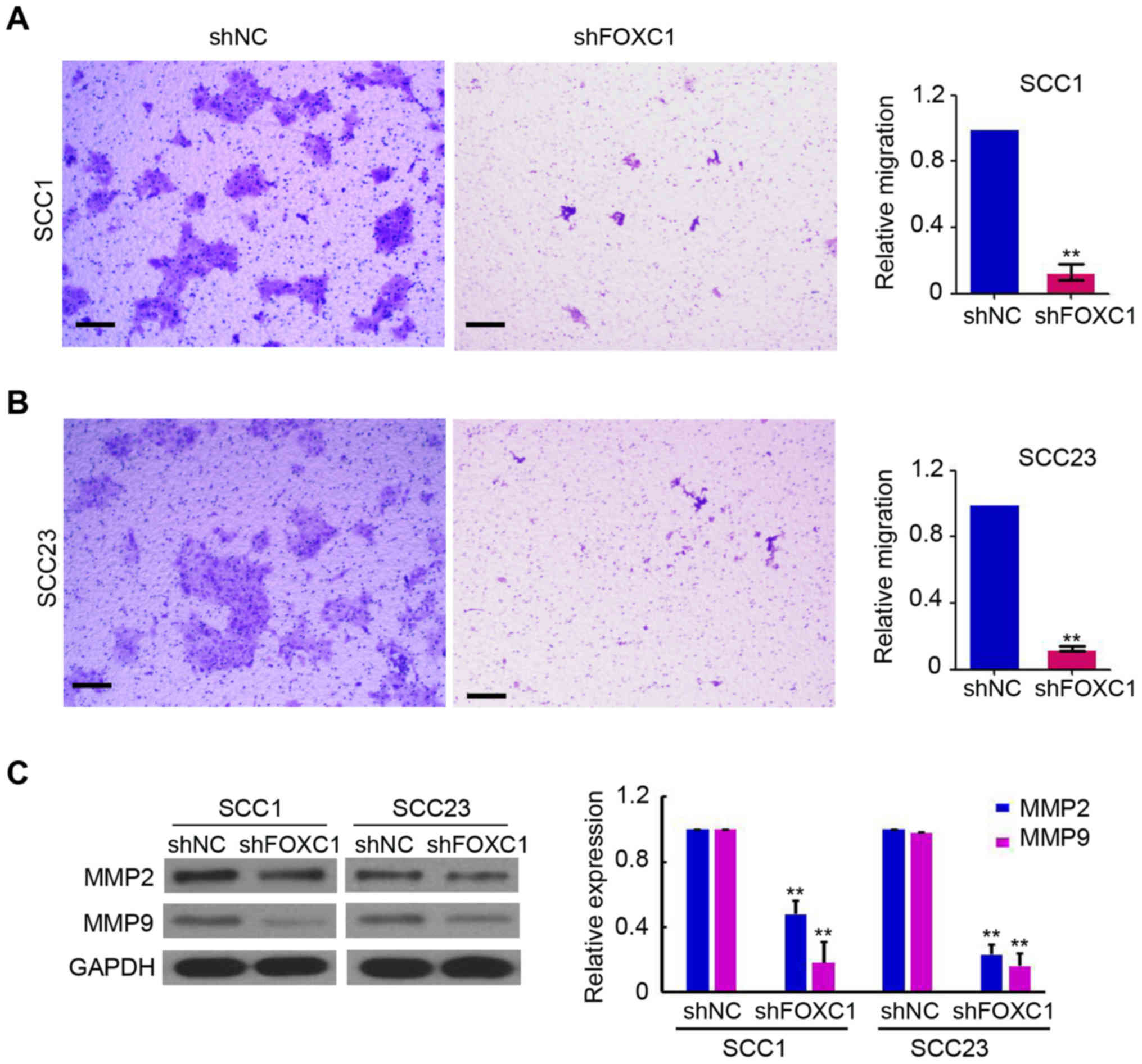
in the migration of OSCC cells, Transwell chamber-based cell
migration assays were performed. Microscopic images of the
Transwell chamber assays are presented in Fig. 4A and B. The migration of SCC-1 and
SCC-23 cells in shFOXC1-infected groups were suppressed by 52.7±4.3
and 61.2±3.7%, respectively, as compared with that in the
corresponding shNC groups (Fig. 4A and
B). Accordingly, the expression of MMP-2 and MMP-9 in SCC-1 and
SCC-23 cells was significantly decreased following the silencing of
FOXC1 (Fig. 4C). These
results demonstrated a stimulatory role of FOXC1 in the
migration of OSCC cells.
Discussion
FOXC1 is a 3,500 bp transcription factor, which is
located on chromosome 6p25 (11).
FOXC1 protein consists of active domain 1, a forkhead domain and
active domain 2 (11). The present
study demonstrated the upregulation of FOXC1 in OSCC tissues.
Additional experimental results suggested that silencing
FOXC1 inhibited OSCC cell growth and migration through the
regulation of cyclin and MMP protein levels.
Previous studies have demonstrated the deregulation
of FOXC1 in a diverse range of cancer types (12–16).
Increased expression of FOXC1 was identified in hepatocellular
carcinoma cell (HCC) lines, partly contributing to the induction of
interleukin 8 (IL-8) (14).
Furthermore, patients with HCC who exhibited positive FOXC1
expression demonstrated decreased survival and higher recurrence
rates compared with those who exhibited negative FOXC1 expression
(12). FOXC1 mRNA and protein levels
were overexpressed in pancreatic ductal adenocarcinoma tissue as
compared with corresponding normal tissue, and the overexpression
of FOXC1 was significantly associated with the clinical stage,
histological differentiation and presence of lymph node metastases
(15). FOXC1 mRNA was also
upregulated in gastric cancer and high FOXC1 expression was
associated with shorter overall survival of patients compared with
that in patients exhibiting low expression (16). In the present study, it was firstly
demonstrated that the immunoreactive intensity of FOXC1 was
consistently increased in OSCC tissues as compared with that in
adjacent normal tissues. This suggested that FOXC1 may be
associated with the onset and progression of this type of cancer.
However, additional investigation is required to determine the
predictive role of FOXC1 in the survival of patients with OSCC.
FOXC1 has been demonstrated to be correlated with
Axenfeld-Rieger syndrome and embryonic development (17,18).
Various studies have suggested that FOCX1 serves a stimulatory role
in cancer development and progression: A previous study by Hayashi
and Kume (19) indicated that there
was a marked interaction between FOXC1 and Notch signals,
accompanied with vascular endothelial growth receptor regulating
the expression of vascular genes and inducing tumor angiogenesis.
Furthermore, Ray et al (20)
demonstrated that patients with high FOXC1 mRNA expression
exhibited a lower survival rate. In addition, ectopic FOXC1
expression increased tumor invasion by EMT, during which tumor
cells undergo distal migration and invasion (21,22). The
results of the present study first identified that the suppression
of FOXC1 markedly inhibited cell growth and migration of OSCC cells
in vitro, which indicated that FOXC1 functions as an
oncogene in OSCC. Additional mechanistic investigations indicated
that cyclin and MMP family proteins were involved in the
proliferation and migration regulated by FOXC1 in OSCC.
Although the function of FOXC1 in regulating normal
biological processes and tumor progression is well known (23), the underlying mechanisms involved in
the regulation of tumorigenesis and development remain unclear.
Several microRNAs (miR), including miR-639 and miR-548I, are
involved in modulating FOXC1 expression via post-transcriptional
gene regulation during tumor progression (24,25).
Furthermore, in basal-like breast cancer, FOXC1 transcription was
activated by epidermal growth factor receptor through the
extracellular signal-regulated kinase and protein kinase B pathways
(26). In addition, FOXC1 expression
may be also induced by inflammation, as demonstrated by the
induction of FOXC1 expression by IL-8 through activation of
phosphoinositide 3-kinase signaling (14). In early osteogenic differentiation,
bone morphogenic protein 4 was identified to regulate FOXC1
expression (27). In the present
study, it was demonstrated that knockdown of FOXC1 suppressed
cyclin B1, cyclin D1, MMP-2 and MMP-9 expression in OSCC. However,
additional studies are required to understand the mechanism
underlying FOXC1-mediated regulation of OSCC formation and
prognosis.
In conclusion, FOXC1 functions as an oncogene
in OSCC cells, increasing their proliferation and migration
abilities. The results of the present study suggest that FOXC1 is a
potential therapeutic target for the treatment of OSCC.
Acknowledgements
The authors would like to thank the kind support
from Dr. Cui (University of California Los Angeles School of
Dentistry and Jonsson Comprehensive Cancer Center).
Funding
The present study was supported by grants from the
Natural Science Foundation of Guangdong Province (grant no.,
2015A030313810), Scientific research start-up project of Southern
Medical University in 2017 (grant no., PY2017N038) and Start-up
project research of Southern Medical University Dental Hospital
(grant no., PY2017009).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
ZL and SX were involved in the acquisition of the
data. HC, YL and PY were involved in the analysis and
interpretation of the data. XZ was involved in the conception and
design of the present study and obtaining the funding.
Ethics approval and consent to
participate
All of the experiments in the present study were
approved by the Medical Ethics Committee of the Stomatological
Hospital, Southern Medical University (Guangzhou, China). Informed
consent was provided by the patients who agreed to the use of their
tissues in the present study.
Patient consent for publication
Patient provided consent for their data to be
published.
Competing interests
All authors declare that there are no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: Implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abram MH, van Heerden WF, Rheeder P,
Girdler-Brown BV and van Zyl AW: Epidemiology of oral squamous cell
carcinoma. SADJ. 67:550–553. 2012.PubMed/NCBI
|
|
4
|
Fuller CD, Wang SJ, Thomas CR Jr, Hoffman
HT, Weber RS and Rosenthal DI: Conditional survival in head and
neck squamous cell carcinoma: Results from the SEER dataset
1973–1998. Cancer. 109:1331–1343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aldinger KA, Lehmann OJ, Hudgins L,
Chizhikov VV, Bassuk AG, Ades LC, Krantz ID, Dobyns WB and Millen
KJ: FOXC1 is required for normal cerebellar development and is a
major contributor to chromosome 6p25.3 Dandy-Walker malformation.
Nat Genet. 41:1037–1042. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin YJ, Shyu WC, Chang CW, Wang CC, Wu CP,
Lee HT, Chen LJ and Hsieh CH: Tumor hypoxia regulates forkhead box
C1 to promote lung cancer progression. Theranostics. 7:1177–1191.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Chai L, Ji Q, Cheng R, Wang J and
Han S: Forkhead box protein C1 promotes cell proliferation and
invasion in human cervical cancer. Mol Med R. 17:4392–4398.
2018.
|
|
8
|
Ou-Yang L, Xiao SJ, Liu P, Yi SJ, Zhang
XL, Ou-Yang S, Tan SK and Lei X: Forkhead box C1 induces
epithelial-mesenchymal transition and is a potential therapeutic
target in nasopharyngeal carcinoma. Mol Med Rep. 12:8003–8009.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai L, Cui X, Zhang X, Cheng L, Liu Y,
Yang Y, Fan P, Wang Q, Lin Y, Zhang J, et al: SARI inhibits
angiogenesis and tumour growth of human colon cancer through
directly targeting ceruloplasmin. Nat Commun. 7:119962016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berry FB, Saleem RA and Walter MA: FOXC1
transcriptional regulation is mediated by N- and C-terminal
activation domains and contains a phosphorylated transcriptional
inhibitory domain. J Biol Chem. 277:10292–10297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia L, Huang W, Tian D, Zhu H, Qi X, Chen
Z, Zhang Y, Hu H, Fan D, Nie Y and Wu K: Overexpression of forkhead
box C1 promotes tumor metastasis and indicates poor prognosis in
hepatocellular carcinoma. Hepatology. 57:610–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu ZY, Ding SM, Zhou L, Xie HY, Chen KJ,
Zhang W, Xing CY, Guo HJ and Zheng SS: FOXC1 contributes to
microvascular invasion in primary hepatocellular carcinoma via
regulating epithelial-mesenchymal transition. Int J Biol Sci.
8:1130–1141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang W, Chen Z, Zhang L, Tian D, Wang D,
Fan D, Wu K and Xia L: Interleukin-8 induces expression of FOXC1 to
promote transactivation of CXCR1 and CCL2 in hepatocellular
carcinoma cell lines and formation of metastases in mice.
Gastroenterology. 149:1053–1067.e1014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Gu F, Liu CY, Wang RJ, Li J and Xu
JY: High level of FOXC1 expression is associated with poor
prognosis in pancreatic ductal adenocarcinoma. Tumour Biol.
34:853–858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Shao QS, Yao HB, Jin Y, Ma YY and
Jia LH: Overexpression of FOXC1 correlates with poor prognosis in
gastric cancer patients. Histopathology. 64:963–970. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishimura DY, Searby CC, Alward WL, Walton
D, Craig JE, Mackey DA, Kawase K, Kanis AB, Patil SR, Stone EM and
Sheffield VC: A spectrum of FOXC1 mutations suggests gene dosage as
a mechanism for developmental defects of the anterior chamber of
the eye. Am J Hum Genet. 68:364–372. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanwar M, Kumar M, Dada T, Sihota R and
Dada R: MYOC and FOXC1 gene analysis in primary congenital
glaucoma. Mol Vis. 16:1996–2006. 2010.PubMed/NCBI
|
|
19
|
Hayashi H and Kume T: Forkhead
transcription factors regulate expression of the chemokine receptor
CXCR4 in endothelial cells and CXCL12-induced cell migration.
Biochem Biophys Res Commun. 367:584–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ray PS, Wang J, Qu Y, Sim MS, Shamonki J,
Bagaria SP, Ye X, Liu B, Elashoff D, Hoon DS, et al: FOXC1 is a
potential prognostic biomarker with functional significance in
basal-like breast cancer. Cancer Res. 70:3870–3876. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou M, Ye Z, Gu Y, Tian B, Wu B and Li J:
Genomic analysis of drug resistant pancreatic cancer cell line by
combining long non-coding RNA and mRNA expression profling. Int J
Clin Exp Pathol. 8:38–52. 2015.PubMed/NCBI
|
|
22
|
Strizzi L, Bianco C, Normanno N, Seno M,
Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y,
Sanicola M and Salomon DS: Epithelial mesenchymal transition is a
characteristic of hyperplasias and tumors in mammary gland from
MMTV-Cripto-1 transgenic mice. J Cell Physiol. 201:266–276. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lambers E, Arnone B, Fatima A, Qin G,
Wasserstrom JA and Kume T: Foxc1 regulates early cardiomyogenesis
and functional properties of embryonic stem cell derived
cardiomyocytes. Stem Cells. 34:1487–1500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin Z, Sun L, Chen W, Liu B, Wang Y, Fan
S, Li Y and Li J: miR-639 regulates transforming growth factor
beta-induced epithelial-mesenchymal transition in human tongue
cancer cells by targeting FOXC1. Cancer Sci. 105:1288–1298. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Medina-Trillo C, Aroca-Aguilar JD,
Ferre-Fernandez JJ, Méndez-Hernández CD, Morales L, García-Feijoo J
and Escribano J: The role of hsa-miR-548l dysregulation as a
putative modifier factor for glaucoma-associated FOXC1 mutations.
Microrna. 4:50–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin Y, Han B, Chen J, Wiedemeyer R,
Orsulic S, Bose S, Zhang X, Karlan BY, Giuliano AE, Cui Y and Cui
X: FOXC1 is a critical mediator of EGFR function in human
basal-like breast cancer. Ann Surg Oncol. 21 Suppl 4:S758–S766.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hopkins A, Mirzayans F and Berry F: Foxc1
expression in early osteogenic differentiation is regulated by
BMP4-SMAD activity. J Cell Biochem. 117:1707–1717. 2016. View Article : Google Scholar : PubMed/NCBI
|