Introduction
In acute aortic dissection (AD) in pregnancy,
increased cardiovascular stress due to pregnancy is an important
factor leading to an emergent aortic event (1,2). It is
very rare with an annual incidence rate of 5.5 per million
maternities in the US (among 6,566,826 pregnancies in 4,933,697
females) and 0.5 per million maternities in Europe (341,381 females
were followed up after 10 years) (3,4).
However, AD often results in a devastating event for both the
pregnant woman and the foetus (5–8). The
mortality of AD prior to admission is 21.4%, which rises to 60.7,
75.0 and 85.7% if the onset of symptoms occurs 1, 2 days and 1
week, respectively, prior to admission (9). Once a patient is diagnosed with a
Stanford type A dissection (10),
emergency surgery should be recommended according to the
gestational age in weeks, with the aortic repair and delivery
method decided prior to surgery (7,10,11). The
present report details two cases of acute AD (Stanford type A) in
pregnant women. Both patients were diagnosed by echocardiography,
and the diagnosis was confirmed by computed tomography (CT)
angiography prior to aortic surgery. The first patient underwent
aorta repair followed by caesarean section, and the second patient
underwent caesarean section followed by aorta repair. In the first
case, the mother survived, but the foetus succumbed. In the second
case, both the mother and infant survived. Up to 50% of ADs in
pregnancy occur in patients with fibrillin-1 (FBN1) gene mutations
(1,7,8,11). Marfan syndrome, aortic root
enlargement, bicuspid aortic valve disease and hypertension are
also risk factors for AD in pregnancy (1,3,7). The FBN1 gene was sequenced in both
patients, and notably, novel pathogenic mutations of FBN1 were
identified in both patients. The literature on available diagnostic
imaging, intervention and prognosis of AD in pregnancy was also
reviewed. These findings may have important clinical
implications.
Case report
Case one
A 31-year-old female (gravida 5, para 1; height, 165
cm; weight, 58 kg) presented in the 26th week of pregnancy to The
First Affiliated Hospital of Wenzhou Medical University (Wenzhou,
China) in August 2017 with frequent vomiting and epigastric pain
for 4 h and 30 min. The pain was described as continuous, 10/10 in
severity, without radiating pain, and with no association to
movement, diet or breathing. Associated symptoms included nausea,
frequent vomiting and sweating. The patient had no known risk
factors and no family history of aortic dissection.
The patient underwent a routine check-up, which
indicated a normal mental status and vital signs were within the
normal range on admission (temperature, 37°C; blood pressure,
118/55 mmHg; heart rate, 78 beats/min; respiratory rate, 20
breaths/min with 96% SpO2 in room air). Both lungs
sounded clear with no obvious rales, the heart sounded normal and
the epigastric district was tender, but without rebound pain. The
paediatrician reported that the foetus's vital signs were stable.
The laboratory data indicated the following: White blood cells,
16.72×109/l (normal range, 3.50–9.50×109/l);
D-Dimer: >20 mg/l (normal range, 0.00–0.50 mg/l); bicarbonate,
14.9 mmol/l (normal range, 21.4–27.3 mmol/l); and urine ketone
body, strong positive (normal range: Negative). Normal values were
described previously (12–14). Amylase, troponin I, aminotransferase,
creatinine, brain natriuretic peptide, blood pH and PaO2
were all normal. An electrocardiogram, abdominal ultrasound, and
chest and abdominal CT were all normal, except for the presence of
the foetus. The patient was diagnosed with acute gastritis and was
initially treated with 40 mg esomeprazole magnesium (AstraZeneca
PLC, Cambridge, UK) to protect the stomach, 4,000 mg ceftazidime
(GlaxoSmithKline plc, Brentford, UK) to treat the infection and a
fluid challenge. At 5 days following this prescription, the
patient's symptoms improved, and the laboratory data were almost
normal. The patient was planned to be discharged at this time.
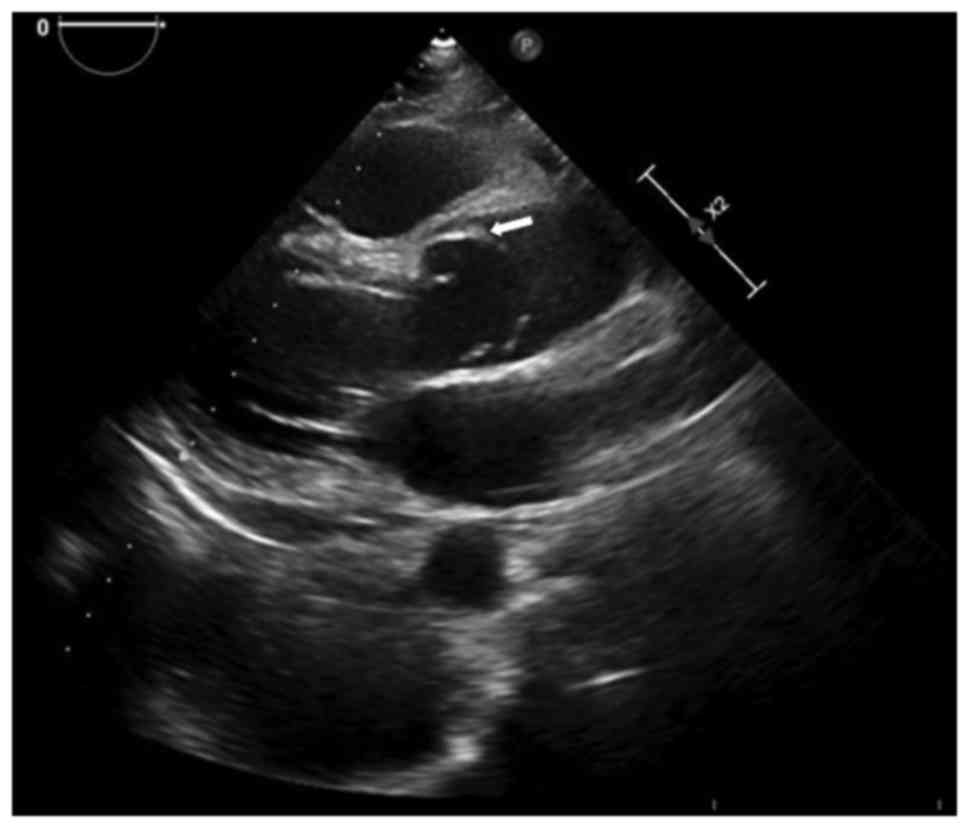
However, transthoracic echocardiography revealed an AD (Stanford
type A) affecting the entire thoracic aorta with torrential aortic
regurgitation, without mediastinal haematoma or pleural effusion
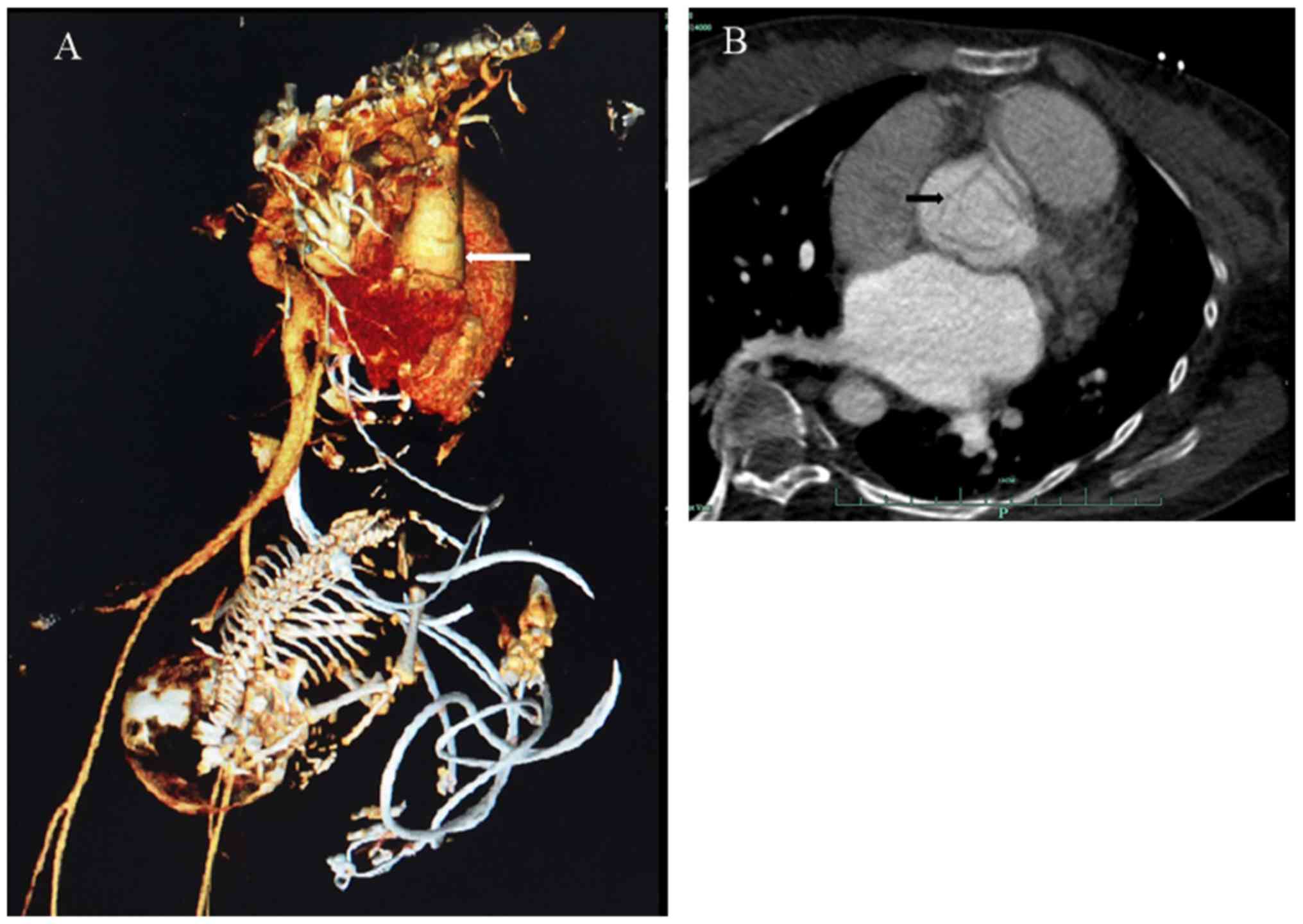
(Fig. 1). The chest CT angiography
revealed a Stanford type A AD (DeBakey type I) from the level of
the Valsalva sinuses to the distal ascending aorta involving the
right coronary orifice (Fig.
2A).
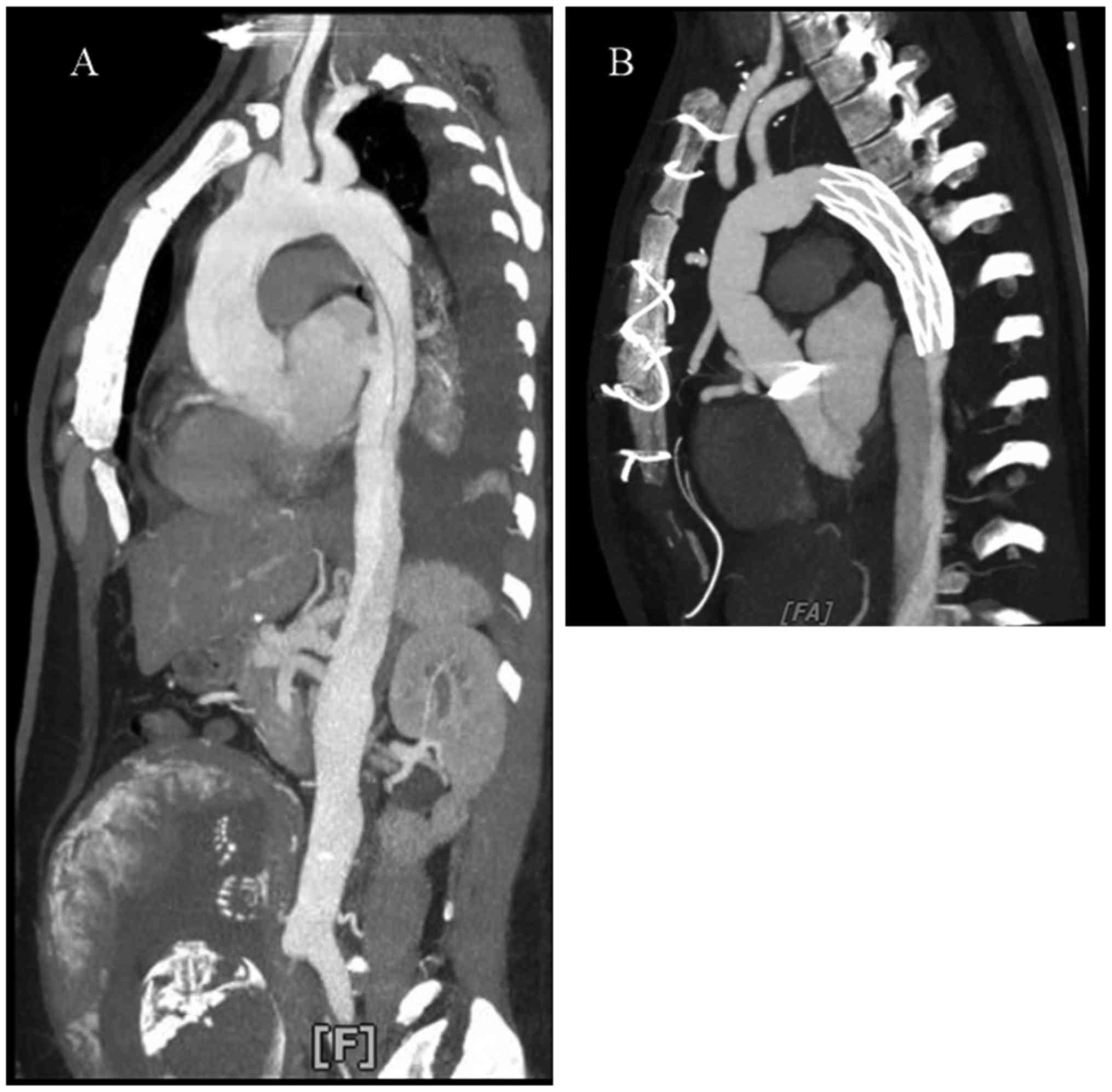
The patient received ascending and descending aorta
and total arch replacement with cardiopulmonary bypass (Fig. 2B) for AD followed by a caesarean
section, delivering a male infant who had succumbed in
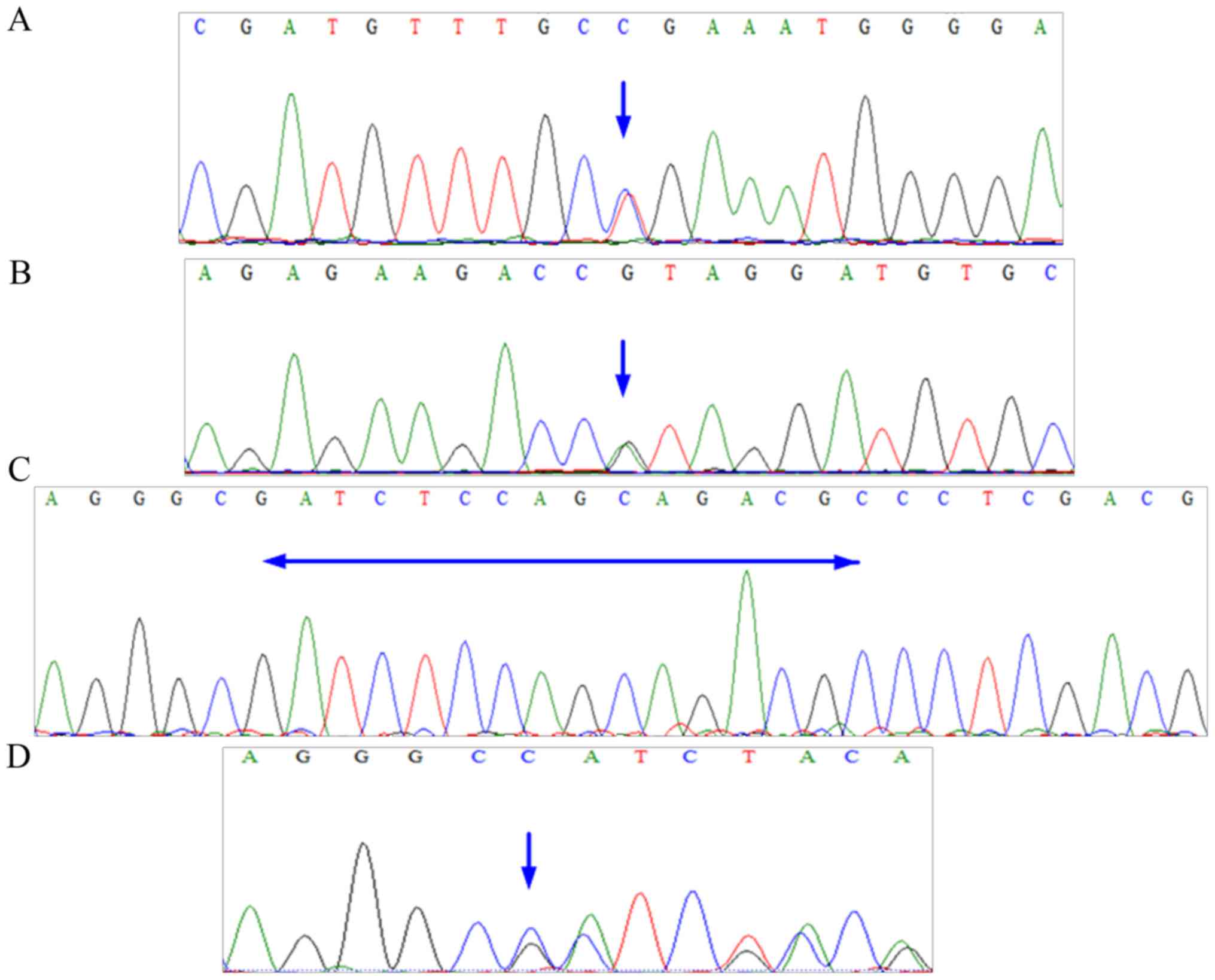
utero. A sequencing peak map for FBN1 is presented in Fig. 3, and the results of the genetic tests
were as follows: The outcome of actin, aortic smooth muscle (ACTA2)
was negative; FBN1 was mutated at exon 16, c. 1875 T > C (p.
Asn625Asn), exon 56, c. 6855 T > C (p. Asp2285Asp), and exon 59,
c. 7240 C > T (p. Arg2414 Termination codon) (Table I). Exon 59, c. 7240 C > T (p.
Arg2414 Termination codon) was the pathogenic mutation (Fig. 3A). Following 5 days in the intensive
care unit, the patient was transferred to the general ward and
uneventfully discharged on postoperative day 21.
 | Table I.Mutations in the FBN1 and ACTA2 genes
of the present patients. |
Table I.
Mutations in the FBN1 and ACTA2 genes
of the present patients.
| Patient | Age (years) | Gestation
(weeks) | Gene | Genetic sub
regions | Nucleotide
changes | Amino acid
changes |
|---|
| Case one | 31 | 26 | FBN1 | Exon 16 | c. 1875 T > C | p. Asn625Asn |
|
|
|
|
| Exon 56 | c. 6855 T > C | p. Asp2285Asp |
|
|
|
|
| Exon 59 | c. 7240 C > T | p. Arg2414 |
|
|
|
|
|
|
| Termination
codon |
|
|
|
| ACTA2 |
| None | None |
| Case two | 32 | 34 | FBN1 | Exon 56 | c. 6725 G > A | p. Arg2242His |
|
|
|
|
| Exon 02 | c. 12–27 del
GCGTCTGCTGGAGATC |
|
|
|
|
| ACTA2 |
| None | None |
Case two
A 32-year-old female (gravida 1, para 0) with a
gestational age of 34+1 weeks was referred to the emergency room of
Hangzhou First People's Hospital (Hangzhou, China) in September
2017 due to an acute onset of back pain for 1 h. The patient
described the pain as continuous, 10/10 in severity, without
radiating pain, with nausea, vomiting and dyspnoea, severe sweating
and hypotension. The pain occurred on the way home following a
prenatal examination. The patient's medical history included an
atrial septal defect repair at the age of 17 years and scoliosis
for 20 years; in addition, the patient's mother had succumbed to AD
at the age of 30 years.
On arrival the patient was conscious. The heart rate
was 115 beats/min, blood pressure was 78/45 mmHg, respiratory rate
was 24 breaths/min, oxygen saturation was 97% on room air, and
temperature was 36.0°C. The patient was 158 cm tall and weighed 52
kg. Both lungs sounded clear without obvious rales. The heartbeat
was weak, both lower limbs exhibited slight oedema, and the abdomen
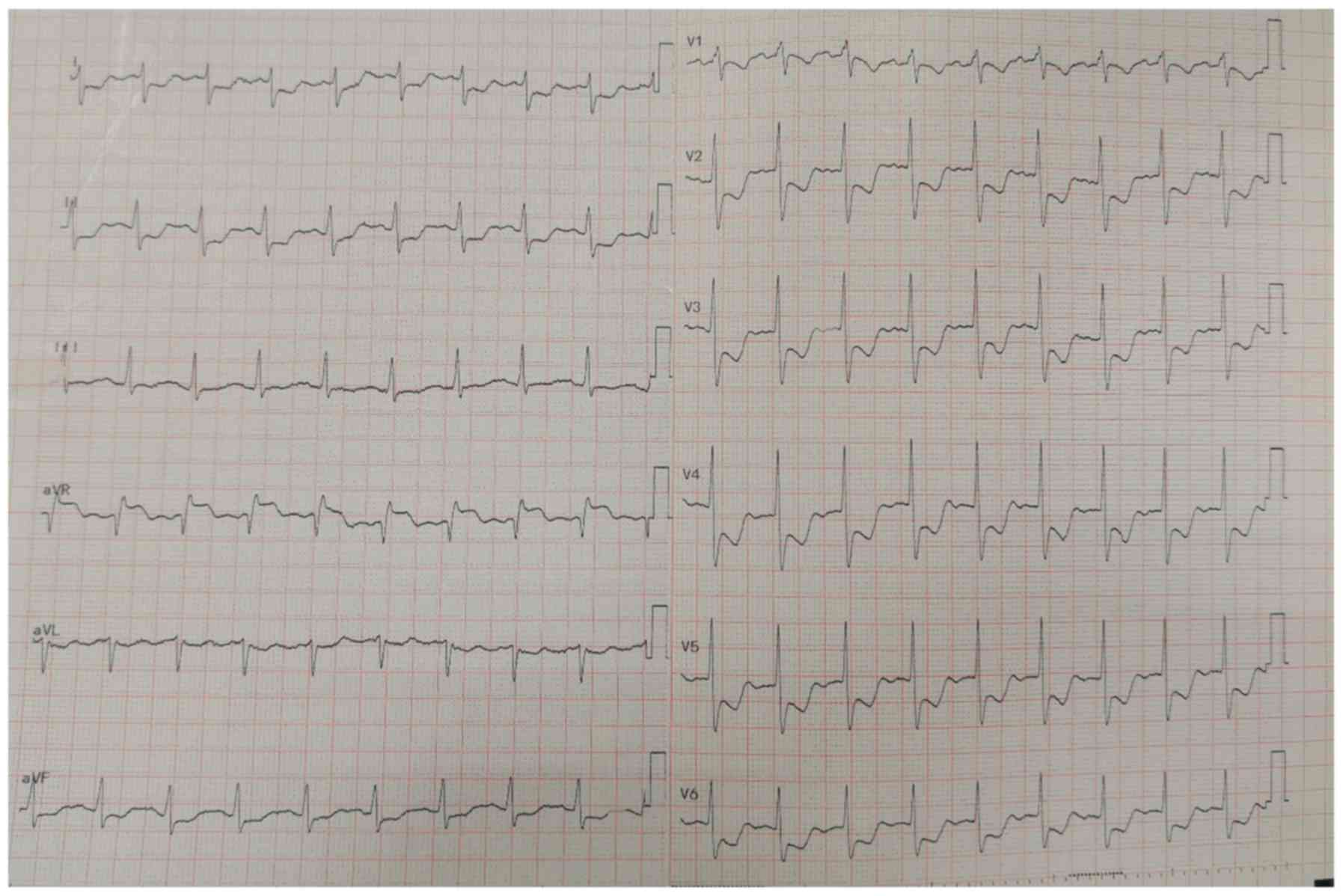
was protuberant due to the gravidity. An electrocardiogram
indicated a sinus rhythm with a rate of 115 beats/min and signs of
ischaemic changes (Fig. 4). The
laboratory data demonstrated the following: Serum lactate, 3.9
mmol/l (normal range, 1.0–2.5 mmol/l); D-Dimer, 1,400 µg/l (normal
range, 0.0–1,000.0 µg/l); and values within the normal ranges for
creatine kinase isozymes, troponin I, serum creatinine,
aminotransferase and bilirubin tests. The transthoracic
echocardiography was immediately examined. It revealed that the
diameter of the ascending aorta was 45 mm at the sinotubular
junction and a zonal echo in the ascending aorta involving the
coronary orifice with torrential aortic regurgitation, without
mediastinal haematoma or pleural effusion. The foetal ultrasound
was normal. The CT angiography was checked to confirm a Stanford
type A AD (DeBakey type II; Fig. 5).
The obstetrics, cardiac anaesthesiology and thoracic surgery teams
immediately performed an emergency caesarean section and a modified
Bentall procedure. The results of genetic tests were as follows:
The outcome of ACTA2 was negative; FBN1 was mutated at Exon 56, c.
6725 G > A (p. Arg2242His) and Exon 02, c. 12–27 del
GCGTCTGCTGGAGATC (Table I). Both
mutations were pathogenic (Fig.
3B-D). Finally, the healthy patient and infant male were
uneventfully discharged on postoperative day 36.
Discussion
Type A AD comprises the ascending aorta, De Bakey
type I (ascending plus descending) and De Bakey type II (ascending
only), and is a life-threatening but relatively rare complication
of pregnancy (1,7). Immer et al (1) previously reported that the incidence of
pregnancy Type A dissection was 0.34% at the Mayo Clinic
(Rochester, MN, USA) and 1.45% at University Hospital Berne (Berne,
Switzerland) in AD patients. Previous reports also indicated that
the annual incidence of AD was 5.5 per million maternities in the
US (among 6,566,826 pregnancies in 4,933,697 females) and 0.5 per
million maternities in Europe (341,381 females were followed up
after 10 years) (3,4). The mortality rate for untreated
proximal AD increases by 1–3% per h following presentation
(6). The mortality prior to
admission was 21.4% and up to 60.7% at 1 day, 75.0% at 2 days and
85.7% at 1 week (9).
Pregnancy was associated with a 4-fold risk of AD
compared with the control period within 1 year following delivery
(3). The ages ranged from 22 to 43
years with a mean age of 31.0 years, and the gestational age ranged
from 8–38 weeks with a mean gestation of 28.7 weeks [summarized
data from 30 cases presented in Table
II (2,4,6,8,11,15–21)].
ADs in pregnancy were predominantly Type A dissection (~80%)
(1,5,7,8) as the gravid uterus induces significant
compression of the aorta and iliac arteries, particularly in the
supine position. This possibly increases the outflow resistance of
the lower arterial tree. Hence, the upper aorta may be further
predisposed to initiate an intimal tear (1). AD in pregnancy occurred in the late
second or third trimester, which was correlated with increased
capacity (7,11). Hypertension was exhibited in <20%
of patients, but this was a risk factor for pregnancy-triggered AD
(3).
 | Table II.Summary of previously published cases
of aortic dissection in pregnancy. |
Table II.
Summary of previously published cases
of aortic dissection in pregnancy.
|
|
|
|
|
|
|
| Prognosis |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Author | Publication year | Age (years) | Gestation
(weeks) | Diagnostic
imaging | Type | Intervention | Mother | Foetus | (Refs.) |
|---|
| Kim et
al | 2014 | 31 | 24 | Echocardiography/CT
angiographic | Type A
dissection | Aortic repair with
the foetus in utero | S | M | (2) |
| Thalmann et
al | 2011 | 32 | 36 | CT | – | Caesarean delivery
prior to aortic repair | S | – | (4) |
|
|
| 34 | 32 | MRI | Type B
dissection | Caesarean delivery
prior to aortic repair | S | – |
|
| Jovic et
al | 2014 | 32 | 32 |
Echocardiography | Type A
dissection | Caesarean delivery
prior to aortic repair | S | S | (6) |
|
|
| 38 | 38 |
Echocardiography | Type A
dissection | Caesarean delivery
prior to aortic repair | S | S |
|
| Kim et
al | 2016 | 31 | 29 | Echocardiography/CT
angiographic | Type A
dissection | Caesarean delivery
prior to aortic repair | S | S | (8) |
| Yang et
al | 2015 | 27±4 (range from
22–31) | 8 | CT
angiographic | Type A
dissection | Delivery prior to
aortic repair in 2 stages | S | M | (11) |
|
|
|
| 22 | CT
angiographic | Type B
dissection | Caesarean delivery
prior to aortic repair in 2 stages | S | M |
|
|
|
|
| 22 | CT
angiographic | Type A
dissection | Caesarean delivery,
hysterectomy prior to aortic repair | M | M |
|
|
|
|
| 24 | MRI | Type B
dissection | Aortic repair prior
to caesarean delivery in 2 stages | S | S |
|
|
|
|
| 18 |
Echocardiography | Type B
dissection | Aortic repair prior
to caesarean delivery in 2 stages | S | M |
|
|
|
|
| 29 | CT
angiographic | Type A
dissection | Caesarean delivery
prior to aortic repair | S | M |
|
|
|
|
| 32 | CT
angiographic | Type A
dissection | Caesarean delivery
prior to aortic repair | S | S |
|
| Sakaguchi et
al | 2005 | 32 | 33 |
Echocardiography/CT | Type A
dissection | Caesarean delivery
prior to aortic repair | S | S | (15) |
|
|
| 33 | 26 |
Echocardiography/CT | Type A
dissection | Aortic repair with
the foetus in utero | M | M |
|
|
|
| 28 | 30 |
Echocardiography/CT | Type A
dissection | Aortic repair
following spontaneous delivery | S | S |
|
|
|
| 34 | 34 |
Echocardiography/CT | Type A
dissection | Caesarean delivery
prior to aortic repair | S | S |
| Master and Day | 2012 | 27 | 28 | Echocardiography/CT
angiographic | Type A
dissection | Caesarean delivery
prior to aortic repair | S | S | (16) |
| Kohli et
al | 2013 | 41 | 36 | CT
angiographic | Type A
dissection | Caesarean delivery
prior to aortic repair | S | S | (17) |
| Regalado et
al | 2014 | 30 | 28 | CT | Type A
dissection | Caesarean delivery,
hysterectomy prior to aortic repair | S | S | (18) |
|
|
| 35 | 28 |
Echocardiography | Type A
dissection | Caesarean delivery
prior to aortic repair | M | S |
|
|
|
| 37 | 31 | Cardiac
catheterization | Type A
dissection | Caesarean delivery
and failed repair | M | S |
|
|
|
| 43 | 37 |
Echocardiography/CT | Type A
dissection | Caesarean delivery
before aortic repair | S | S |
|
| Li et
al | 2017 | 30 | 38 |
Echocardiography/CT | Type A
dissection | Caesarean delivery,
hysterectomy prior to aortic repair | S | S | (19) |
|
|
| 34 | 23 |
Echocardiography/CT | Type A
dissection | Aortic repair with
the foetus in utero | S | M |
|
|
|
| 22 | 25 |
Echocardiography/CT | Type A
dissection | Aortic repair with
the foetus in utero | S | M |
|
|
|
| 30 | 32 |
Echocardiography/CT | Type A
dissection | Caesarean delivery,
hysterectomy prior to aortic repair | M | S |
|
|
|
| 26 | 32 |
Echocardiography/CT | Type A
dissection | Caesarean delivery,
hysterectomy prior to aortic repair | S | S |
|
| Barrus et
al | 2017 | 31 | 21 | Echocardiography/CT
angiographic | Type A
dissection | Aortic repair prior
to caesarean delivery in 2 stages | S | S | (20) |
| Patel et
al | 2018 | 31 | 32 |
Echocardiography/CT | Type A
dissection | Caesarean delivery
prior to aortic repair | S | – | (21) |
Due to the indeterminacy as to whether conventional
clinical imaging examination (such as X-ray and CT scans) causes
harm during pregnancy, echocardiography, with its non-invasive,
quick, safe, and highly sensitive characteristics and its specific
imaging capability, was the recommended method for diagnosing AD
(6,22). A literature review was performed
using the Pubmed database (https://www.ncbi.nlm.nih.gov/pubmed/). The key words
used in the search were ‘aortic dissection’ and ‘pregnant’. The
inclusion criterion was that the case report or the original study,
for which the author provided the clinic data, must have been
published in or after 2005. Studies were excluded if the personal
information, diagnostic imaging or intervention method were not
clear. The discrepancies between reviewers were solved by
discussing the study and voting. From the present review of the
literature demonstrated in Table
II, echocardiography was used in 19/30 patients for the
diagnosis of AD in pregnancy. The sensitivity and specificity of
transthoracic echocardiography were 59–85 and 63–96%, respectively
(22). It is notable that the
sensitivity and specificity of transesophageal echocardiography can
be up to 97–100 and 98–100%, respectively (22). Both of the present patients were
diagnosed by echocardiography. In addition, magnetic resonance
imaging (MRI) may be considered as a reasonable diagnostic tool
when echocardiography is negative. In Japan, abdominal pain
guidelines state that MRI can be recommended when ultrasounds are
negative for pregnancy (23). Review
of the literature revealed that 2/30 patients with AD in pregnancy
were diagnosed by MRI (Table II).
One meta-analysis of 7 studies revealed that the sensitivity and
specificity of MRI were 98 and 98%, respectively (24). At a minimum, CT angiography, a vital
imaging test for AD, can reveal true and false cavities and the
fracture position of the intima (10,25,26).
Hence, it is generally accepted that for AD in pregnancy, the
reasonable diagnostic imaging order is echocardiography, MRI and
then CT/CT angiography (7,11). For safety, certain patients,
including those described in the present study, when AD is
diagnosed by echocardiography, the diagnosis requires further
confirmation by CT angiography prior to aortic surgery.
Genetic screening can indicate whether a patient is
at risk of AD. Up to 50% of ADs in pregnancy occur in patients with
Marfan syndrome (1,7,8,11). Patients with Marfan syndrome have a
mutation in FBN1 on chromosome 15q21 (1,8). Both of
the present patients exhibited mutations in FBN1 that resulted in
amino acid changes. The first patient had a c. 7240 C > T (p.
Arg2414 Termination codon) mutation, and the second patient had a
c. 6725 G > A (p. Arg2242His) mutation. The second patient also
had a c. 12–27 del GCGTCTGCTGGAGATC. These mutations have not been
previously reported, to the best of our knowledge. Hence, these
were novel pathogenic mutations. Regalado et al (18) previously demonstrated that ACTA2
mutations were correlated with AD in pregnancy. The rate of
peripartum AD in women with ACTA2 mutations was much higher than
the population-based frequency of peripartum AD (20: 0.6%)
(18). Neither of the present
patients, however, had ACTA2 mutations.
In general, once a patient is diagnosed with a
Stanford type A dissection, emergency surgery should be the
recommendation. Mainly according to the gestational age in weeks,
aortic repair and delivery order should be decided, similar to the
present patients. The first patient underwent aorta repair followed
by caesarean section, and the second patient underwent caesarean
section followed by aorta repair.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, SC and GH designed the present study. YL, ZJ and
JC drafted the manuscript. YL, ZJ, JC and DW collected the clinical
and imaging data. YL, JC and DW performed the literature review.
YL, SC and GH were major contributors in writing and revising the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Both patients provided informed written consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Immer FF, Bansi AG, Immer-Bansi AS,
McDougall J, Zehr KJ, Schaff HV and Carrel TP: Aortic dissection in
pregnancy: Analysis of risk factors and outcome. Ann Thorac Surg.
76:309–314. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim SW, Kim D and Hong JM: Acute aortic
dissection in pregnancy with the marfan syndrome. Korean J Thorac
Cardiovasc Surg. 47:291–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamel H, Roman MJ, Pitcher A and Devereux
RB: Pregnancy and the risk of aortic dissection or rupture: A
cohort-crossover analysis. Circulation. 134:527–533. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thalmann M, Sodeck GH, Domanovits H,
Grassberger M, Loewe C, Grimm M and Czerny M: Acute type A aortic
dissection and pregnancy: A population-based study. Eur J
Cardiothorac Surg. 39:e159–e163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Banerjee A, Begaj I and Thorne S: Aortic
dissection in pregnancy in England: An incidence study using linked
national databases. BMJ Open. 5:e0083182015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jovic TH, Aboelmagd T, Ramalingham G,
Jones N and Nashef SA: Type A aortic dissection in pregnancy: Two
operations yielding five healthy patients. Aorta (Stamford).
2:113–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu JM, Ma WG, Peterss S, Wang LF, Qiao
ZY, Ziganshin BA, Zheng J, Liu YM, Elefteriades JA and Sun LZ:
Aortic dissection in pregnancy: Management strategy and outcomes.
Ann Thorac Surg. 103:1199–1206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim WH, Bae J, Choi SW, Lee JH, Kim CS,
Cho HS and Lee SM: Stanford type A aortic dissection in a patient
with Marfan syndrome during pregnancy: A case report. Korean J
Anesthesiol. 69:76–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mészáros I, Mórocz J, Szlávi J, Schmidt J,
Tornóci L, Nagy L and Szép L: Epidemiology and clinicopathology of
aortic dissection. Chest. 117:1271–1278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
JCS Joint Working Group, . Guidelines for
diagnosis and treatment of aortic aneurysm and aortic dissection
(JCS 2011): Digest version. Circ J. 77:789–828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang P, Zhang J, Li Y, Wang H and Zheng J:
Maternal and fetal outcomes with aortic dissection in pregnant
patients with Marfan syndrome. Zhonghua Fu Chan Ke Za Zhi.
50:334–340. 2015.(In Chinese). PubMed/NCBI
|
|
12
|
Shinya S, Masaru A, Akira H, Eisaku H and
Susumu O: Development of an assay of seven biochemical items,
HbA1c, and hematocrit using a small amount of blood collected from
the fingertip. Clin Chim Acta. 413:192–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei Y, Zheng MH, Huang W, Zhang J and Lu
Y: Wet beriberi with multiple organ failure remarkably reversed by
thiamine administration: A case report and literature review.
Medicine (Baltimore). 97:e00102018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lei Y, Xiao S, Chen S, Zhang H, Li H and
Lu Y: N,N-dimethylformamide-induced acute hepatic failure: A case
report and literature review. Exp Ther Med. 14:5659–5663.
2017.PubMed/NCBI
|
|
15
|
Sakaguchi M, Kitahara H, Seto T, Furusawa
T, Fukui D, Yanagiya N, Nishimura K and Amano J: Surgery for acute
type A aortic dissection in pregnant patients with Marfan syndrome.
Eur J Cardiothorac Surg. 28:280–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Master M and Day G: Acute aortic
dissection in pregnancy in a woman with undiagnosed marfan
syndrome. Case Rep Obstet Gynecol. 2012:4901692012.PubMed/NCBI
|
|
17
|
Kohli E, Jwayyed S, Giorgio G and Bhalla
MC: Acute type a aortic dissection in a 36-week pregnant patient.
Case Rep Emerg Med. 2013:3906702013.PubMed/NCBI
|
|
18
|
Regalado ES, Guo DC, Estrera AL, Buja LM
and Milewicz DM: Acute aortic dissections with pregnancy in women
with ACTA2 mutations. Am J Med Genet A. 164A:1–112. 2014.PubMed/NCBI
|
|
19
|
Li X, Zhang HY, Han FZ, Yu CJ, Fan XP, Fan
RX and Zhuang J: Surgical management of pregnancy-associated acute
Stanford type A aortic dissection: Analysis of 5 cases. Nan Fang Yi
Ke Da Xue Xue Bao. 37:1555–1558. 2017.(In Chinese). PubMed/NCBI
|
|
20
|
Barrus A, Afshar S, Sani S, LaBounty TG,
Padilla C, Farber MK, Rudikoff AG and Conte Hernandez A: Acute type
A aortic dissection and successful surgical repair in a woman at 21
weeks gestational pregnancy with maternal and fetal survival: A
case report. J Cardiothorac Vasc Anesth. 32:1487–1493. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel PA, Fernando RJ, MacKay EJ, Yoon J,
Gutsche JT, Patel S, Shah R, Dashiell J, Weiss SJ, Goeddel L, et
al: Acute type A aortic dissection in pregnancy-diagnostic and
therapeutic challenges in a multidisciplinary setting. J
Cardiothorac Vasc Anesth. 32:1991–1997. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y: The value of echocardiography in
diagnosis and treatment of aortic dissection. J Cardiovasc Surg
(Electron Ed). 2:61–63. 2013.
|
|
23
|
Mayumi T, Yoshida M, Tazuma S, Furukawa A,
Nishii O, Shigematsu K, Azuhata T, Itakura A, Kamei S, Kondo H, et
al: The practice guidelines for primary care of acute abdomen 2015.
Jpn J Radiol. 34:80–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shiga T, Wajima Z, Apfel CC, Inoue T and
Ohe Y: Diagnostic accuracy of transesophageal echocardiography,
helical computed tomography, and magnetic resonance imaging for
suspected thoracic aortic dissection: Systematic review and
meta-analysis. Arch Intern Med. 166:1350–1356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao H, Wen D, Duan W, An R, Li J and
Zheng M: Identification of CTA-based predictive findings for
temporary and permanent neurological dysfunction after repair in
acute type A aortic dissection. Sci Rep. 8:97402018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang S, Li X, Chao B, Wu L, Cheng Z, Duan
Y, Wu D, Zhan Y, Chen J, Liu B, et al: Abdominal aortic intimal
flap motion characterization in acute aortic dissection: Assessed
with retrospective ECG-gated thoracoabdominal aorta dual-source CT
angiography. PLoS One. 9:e876642014. View Article : Google Scholar : PubMed/NCBI
|