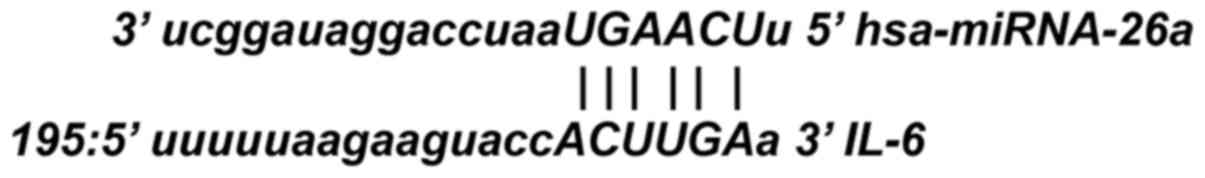Introduction
Neonatal sepsis refers to a severe systematic
infectious disease in neonates induced by a variety of pathogens
(such as bacteria, fungi, and viruses). These pathogens invade into
the blood circulation, which grow, proliferate, and metabolize in
the blood, body cells, organs, and tissues, synthesizing various
toxins to cause infection. It has been reported that, the incidence
of neonatal sepsis is 1–5‰ in developed countries, while it is as
high as 49–179‰ (1). In 2010, 7.6
million children under the age of 5 years have died from infection
throughout the world, in which neonates have accounted for 40%
(2). Due to the fact that neonatal
sepsis is caused by infection, in the inflammatory and
anti-inflammatory responses, all the tissues and organs in the body
might be involved, and the system functions might have disorders.
It has been widely accepted that the activation of neutrophils,
lymphocytes, and mononuclear macrophages, as well as the release of
endogenous mediators, play important roles in the pathogenesis and
development of neonatal sepsis (3–5).
Interleukin-6 (IL-6) is an important factor in the
immune response. Lymphokines produced by the activated monocytes
and macrophages could transform the B-cell precursors into the
antibody-generating cells. IL-6 could also cooperate with colony
stimulating factors to promote the growth and differentiation of
primary bone marrow-derived cells, enhancing the lysis function of
natural killer cells (6–8). There have been numerous studies
concerning the regulatory role of IL-6 in the pathogenesis of
neonatal sepsis (4,9,10).
Moreover, there has been research development in the regulating
mechanism of IL-6, concerning various mRNAs and microRNA (miRNAs).
It has been shown that miRNA-365 could negatively regulate the
expression of IL-6 in the HEK293 and HELA cells (11). However, the regulating effect of
miRNA-26a on IL-6 in the blood mononuclear cells in neonates with
sepsis has not yet been reported.
In this study, the role of miRNA-26a in the
pathogenesis of neonatal sepsis and its relationship with IL-6 were
investigated. The mRNA and protein expression levels of IL-6 in the
blood monocytes and serum in neonatal sepsis were detected by the
quantitative real-time PCR, western blot analysis, and
enzyme-linked immunosorbent assay (ELISA). The interaction between
IL-6 and miRNA-26a was predicted and confirmed by the
bioinformatics analysis and dual-reporter assay.
Materials and methods
Study subjects
Totally 28 cases of neonates with sepsis, 16 males
and 12 females, weighing 2.2±2.8 kg, were included in this study,
who were admitted to our hospital, from December 2012 to March
2017. The blood samples were collected. Moreover, 32 cases of
normal neonates were used as control, 19 males and 13 females,
weighing 2.1±3.0 kg. These neonatal sepsis cases and control
subjects were pathologically confirmed by the blood test (12). Prior written and informed consent for
each subject were obtained and the study was approved by the Ethics
Committee of the Second Hospital of Shandong University (Jinan,
China).
Sample preparation
For the collection of peripheral blood serum, the
gradient centrifugation combined with adherent separation method
was used. Totally 1–5 ml peripheral venous blood was harvested and
stored at 4°C for 1–2 h. Then the upper serum was collected and
centrifuged at 400 × g for 10 min, which was stored at −70°C.
For the collection of peripheral blood mononuclear
cells, based on the previous work, the blood cytosolic fraction was
equally diluted with Hanks solution. Totally 5 ml lymphocyte
separation solution was added into the 15-ml BD tube. The
heparin-anticoagulated venous blood was equally mixed with IMDM
without bovine serum (1:1), which was slowly superimposed on the
stratifying solution layer with a dropper, followed by gentle
addition of 8 ml equally diluted cytosolic fraction. After
centrifugation at 400 × g for 30 min, the mononuclear cells in the
narrow white cloud layer at the interface between the upper and
middle cloud layers were collected with the capillary pipette.
These cells were washed twice with the D-HANK's solution, followed
by centrifugation at 300 × g for 10 min. The cells were then seeded
onto the 9-cm2 cell culture dish, at the density of
3×106 cells/dish, and cultured in a 37°C, 5%
CO2 incubator for 1–2 h. The adherent cells were the
mononuclear cells.
Reverse transcription-quantitative
polymerase chain reaction
The mRNA expression levels of IL-6 were detected
with the quantitative real-time PCR. Total RNA was extracted with
the TRIzol. Totally 1 µg total RNA was used for the reverse
transcription PCR to obtain the cDNA template. Quantitative
real-time PCR was performed with the miRcute miRNA quantitative
real-time PCR detection kit (FP401; Tiangen, Beijing, China) on the
PCR-iQ5 machine (Bio-Rad, Hercules, CA, USA). The primer sequences
were as follows: IL-6 forward, 5′-GGCACTGGCAGAAAACAACC-3′ and
reverse, 5′-GCAAGTCTCCTCATTGAATCC-3′; GAPDH forward,
5′-GGGAAACTGCGGCGTGAT-3′ and reverse, 5′-AAAGGTGGAGGAGTGGGT-3′. The
25-µl reaction system included 1 µl cDNA, 12.5 µl SYBR Premix
EXTaqTM, 10 µM primer each, and 0.5 µl ddH2O. The PCR
conditions were as follows: 95°C for 30 sec; 95°C for 5 sec, 57°C
for 30 sec, for totally 45 cycles. The additional lysis curve
analysis conditions were as follows: 95°C for 15 sec, 60°C for 23
sec, and 95°C for 15 sec. The target expression levels were
determined with the 2−ΔΔCt method. GAPDH was used as
internal reference.
For the detection of miRNA-26a, the following primer
sequences were used: miRNA-26a forward, 5′-CTGTCAACGATACGCTAC-3′
and reverse, 5′-GTAATCCAGGATAGGCTG-3′; and U6 forward,
5′-CTTCGGCAGCACATATAC-3′ and reverse, 5′-GAACGCTTCACGAATTTGC-3′.
The PCR conditions were as follows: 90°C for 60 sec; 95°C for 15
sec, 60°C for 30 sec, for totally 40 cycles. U6 was used as
internal reference.
Western blot analysis
The protein expression levels of IL-6 in the blood
mononuclear cells were detected by the western blot analysis. Cells
were lysed with the lysis. Protein concentration was determined
with the BCA method (RTP7102; RealTimes, Beijing, China). Totally
20 µg protein sample was separated by the 10% SDS-PAGE, and then
electronically transferred onto the membrane. The membrane was
blocked by 5% non-fat milk at room temperature for 1 h, and then
incubated with rabbit anti-human anti-IL-6 primary antibody
(ab6672; 1:1,000 dilution), or rabbit anti-human anti-β-actin
primary antibody (ab129348; 1:5,000 dilution; both Abcam,
Cambridge, MA, USA), at 4°C overnight. The membrane was then
incubated with goat anti-rabbit secondary antibody (ab6721; 1:3,000
dilution; Abcam) at room temperature for 1 h. Color was developed
with the ECL method (ab65623; Abcam), and the protein band images
were analyzed with the ImageLab software (version 3.0). β-actin was
used as internal reference.
ELISA
The serum IL-6 contents were detected with the ELISA
kits (ab178013; Abcam). Briefly, totally 10 µl serum sample was
added into the detection well, while 50 µl standard samples were
added into the standard wells, followed by 40 µl sample diluting
solution. Except for the blank wells, 100 µl HRP-conjugated
detection antibody was added into the standard and sample wells.
The plate was sealed and incubated for 1 h. After washing,
substrates A and B (50 µl each) were added into the wells, followed
by incubation at 37°C for 15 min. Totally 50 µl stop solution was
added into each well, and the OD value at 450 nm was determined
within 15 min.
Bioinformatics analysis
The upper regulating miRNAs of IL-6 were predicted
with the bioinformatics analysis. Based on the literature mining,
the regulating genes for IL-6 were predicted with the following
prediction software: miRanda (http://www.microma.org/rnicroma/home.do), TargetSean
(www.targetscan.org), PiTa (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/),
and PICTA (http://pictar.mdc-berlin.de/).
In vitro sepsis model
establishment
In intro sepsis model was established with the
lipopolysaccharide (LPS) induction in Human monocytic leukemia
THP-1 cells (TCHu 57; Chinese Academy of Sciences Cell Bank,
Shanghai, China) THP-1 cells were transfected with l µg/ml LPS for
24 h to simulate the sepsis environment. The expression levels of
miRNA-26a and IL-6 were detected.
Dual-luciferase reported assay
The wild-type and mutant IL-6 3′-UTR fragments for
miRNA-26a were synthesized, with the Spe-1 and HindIII restriction
sites on each end (Sangon Biotech, Shanghai, China). These two
fragments were cloned into the pMIR-REPORT luciferase reporter
plasmid (E1980; Promega Corporation, Madison, WI, USA). These
plasmids containing wild-type and mutant 3′-UTR (each 0.8 µg) were
transfected into the 293T cells with the liposome, followed by the
transfection of agomiRNA-26a (100 nM; Sangon Biotech) for 24 h. The
cells were lysed, and the luciferase was detected with the GloMax
20/20 luminometer (Promega Corporation). Renilla was used as
internal reference.
Statistical analysis
Data were expressed as mean ± standard deviation.
SPSS 18.0 software was used for statistical analysis. After the
normality test, one-way ANOVA was performed for the multiple
comparisons, with the LSD and SNK methods for the homogeneous
variance, or the Tamhane's T2 or Dunnett's T3 method for the
heterogeneous variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Changed expression levels of IL-6 in
neonatal sepsis
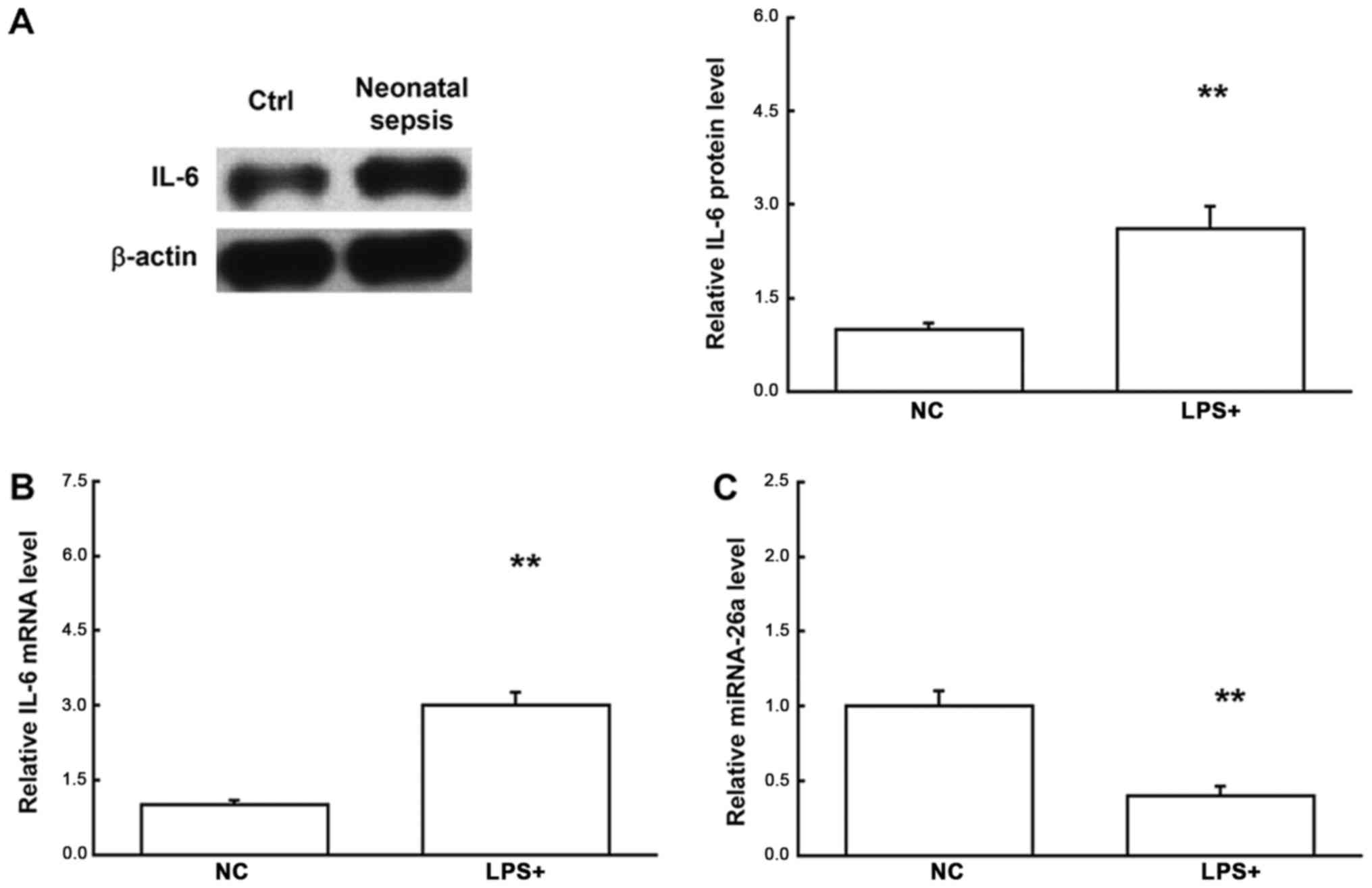
To investigate the mRNA and protein expression
levels of IL-6 in the blood mononuclear cells and serum,
quantitative real-time PCR, western blot analysis, and ELISA were
performed, respectively. Our results from the quantitative
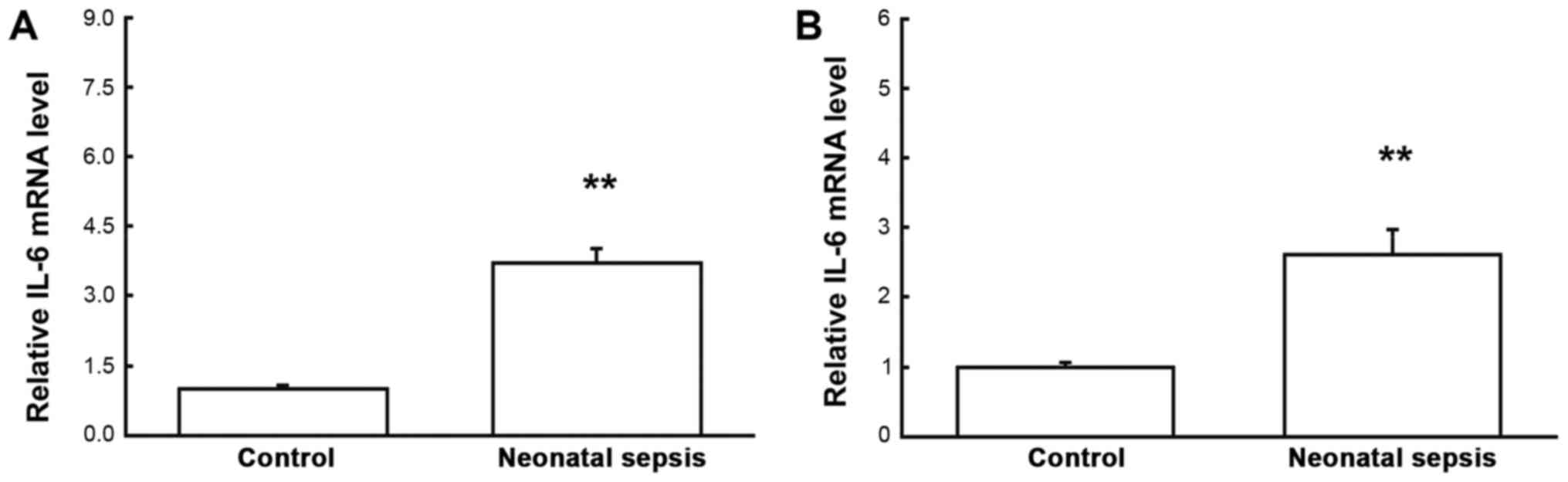
real-time PCR showed that, compared with the control group, the
mRNA expression levels of IL-6 in both the blood mononuclear cells
and serum samples were significantly elevated for neonatal sepsis
(both P<0.05) (Fig. 1). Similar
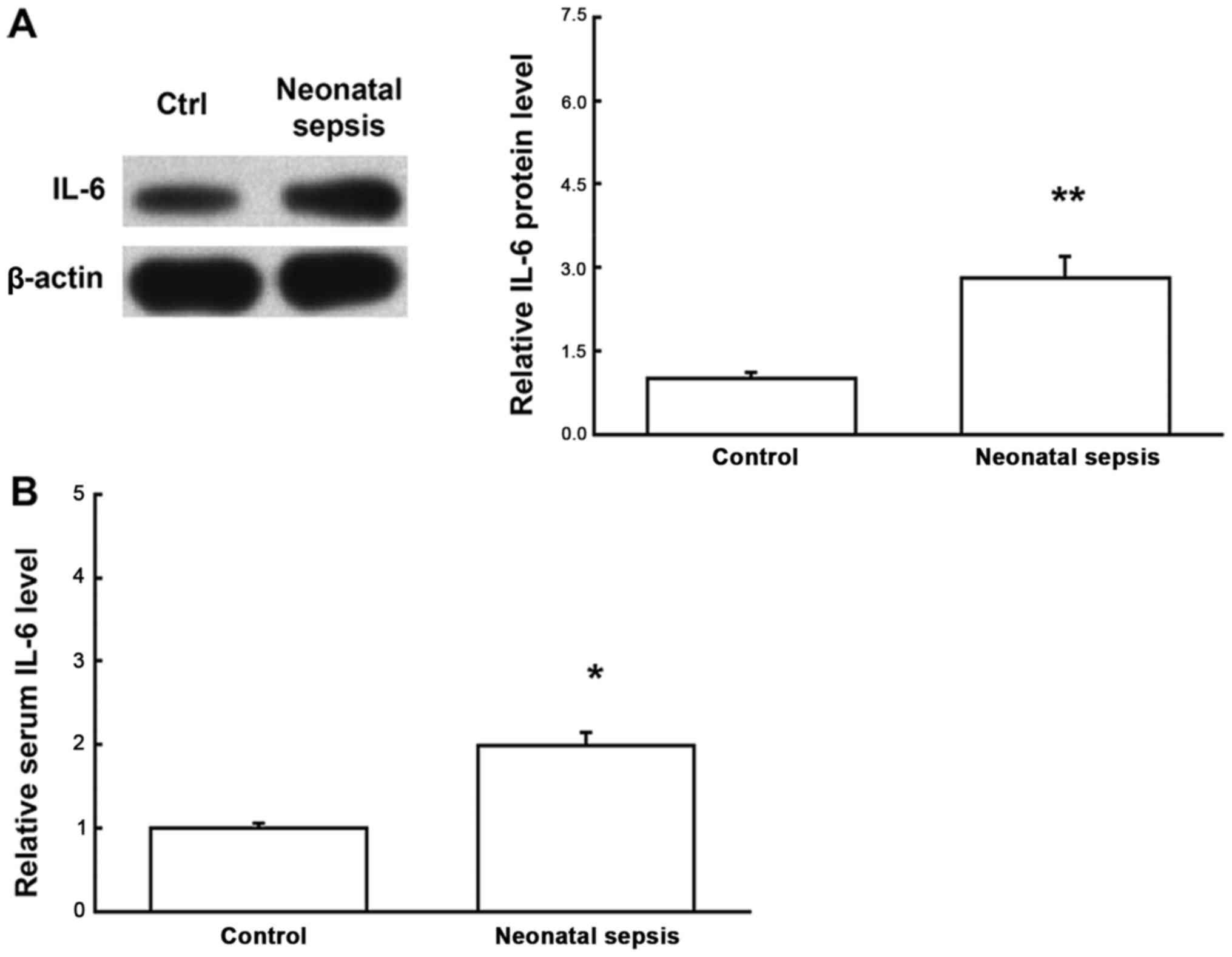
results were obtained for the detection of protein expression
levels of IL-6. Western blot analysis showed that, compared with
the control group, the protein expression levels of IL-6 in the
blood mononuclear cells for the neonatal sepsis were significantly
increased (P<0.05). Moreover, ELISA showed that, compared with
the control group, the serum IL-6 contents were significantly
increased for the neonatal sepsis (P<0.05) (Fig. 2). Taken together, these results
suggest that both the mRNA and protein expression levels of IL-6 in
the blood mononuclear cells are elevated in neonatal sepsis,
leading to elevated serum IL-6 contents, which might be involved in
regulating the pathogenesis of neonatal sepsis.
Changed expression levels of miRNA-26a
in neonatal sepsis
Based on the bioinformatics prediction analysis,
miRNA-26a was recognized as the up-stream regulator for IL-6
(Fig. 3). Therefore, the expression
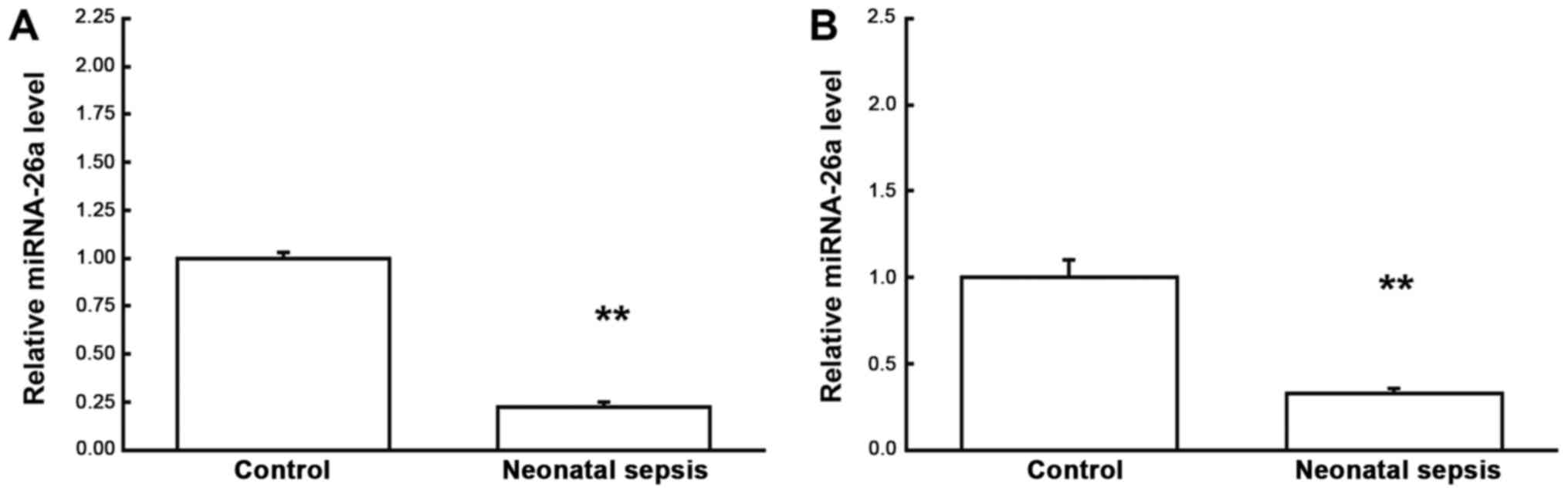
levels of miRNA-26a in neonatal septic samples were analyzed by the
quantitative real-time PCR. Our results showed that compared with
the control group, the expression levels of miRNA-26a were
significantly declined in the neonatal septic samples (P<0.05)
(Fig. 4), indicating that miRNA-26
might contribute to the pathogenic process of neonatal sepsis,
probably via regulating the expression of target gene IL-6 on the
transcription level.
Changed expression levels of miRNA-26a
and IL-6 in in vitro septic model
The in vitro septic model was established by
LPS induction in the THP-1 cells, and the expression levels of
miRNA-26a and IL-6 were analyzed. Our results showed that, compared
with the control group, the miRNA-26a level was significantly
declined, while the IL-6 expression level was significantly
elevated, in the LPS-induced THP-1 cells (Fig. 5). These results confirm the
expression patterns of and the regulatory relationship between
miRNA-26a and IL-6 in the neonates with sepsis.
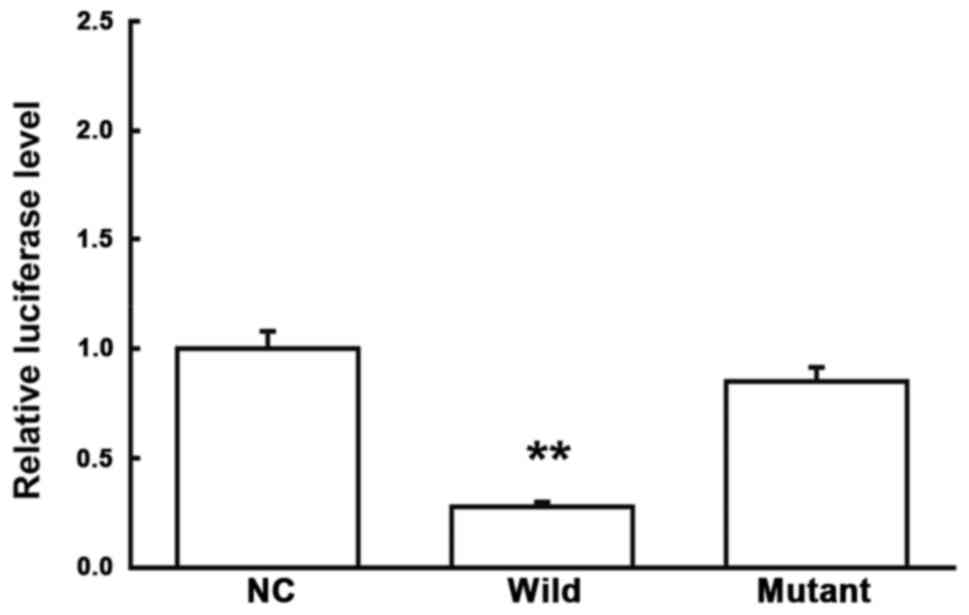
Direct interaction between miRNA-26a
and IL-6
To confirm the direct interaction between miRNA-26a
and IL-6, dual-luciferase reporter assay was performed. Our results
showed that, compared with the NC group, the co-transfection of
agomiRNA-26a and pMIR-REPORT plasmids significantly declined the
luciferase in the cells (P<0.05), while no significant changes
were observed in the cells transfected with the plasmid containing
mutant 3′-UTR (P>0.05) (Fig. 6).
These results suggest that miRNA-26a could directly bind with the
3′-UTR of IL-6 to regulate the gene expression.
Discussion
In the present study, the expression levels of
miRNA-26a in the blood mononuclear cells and serum samples from the
neonatal sepsis cases were analyzed, as well as its down-stream
target gene IL-6 (mRNA and protein expression levels). Moreover,
in vitro models were established by the LPS induction in
THP-1 cells, and the expression levels of miRNA-26a and IL-6 were
detected. The mechanism through which miRNA-26a targeted on its
down-stream IL-6 to contribute to the pathogenesis of neonatal
sepsis was primarily investigated.
Neonatal infectious disease is still one of the
commonly seen diseases in the neonatal period, and severe cases
could induce sepsis, multiple organ failure, and even death. With
the development of economy and medical technologies, although the
survival rate of neonates has been rising over the past decades,
more than 1 million newborns still die of serious infection each
year, according to the World Health Organization (WHO), in which
the death cases associated with neonatal sepsis or pneumonia might
be up to 1 million (13). It is
widely accepted that the activation of neutrophils, lymphocytes,
and mononuclear macrophages, and the release of endogenous
mediators, might play key roles in the pathogenesis and development
of neonatal sepsis. Recently, evidence suggests that the role of
cytokines cannot be ignored either. It has been shown that the
elevated levels of CXCR4, CXCL12, TNF-α, IL-6, and IL-8 are
associated with the neonatal sepsis infection (10,14–16).
IL-6 is a potent cytokine synthesized by mononuclear
cells, phagocytes, T cells, B cells, vascular endothelial cells,
fibroblasts, and other cells in response to IL-1 and small amount
of TNF-α (17). Under normal
conditions, the IL-6 content is minimal, with, however, high
biological activity, exerting functions mainly through the
paracrine and autocrine effects (18). In the inflammation, IL-6 can induce
the production of C-reactive protein and fibrinogen in the body,
which can also promote the formation of thrombosis (19). Elevated IL-6 level in the body can
induce the pathogenesis of inflammatory diseases by binding to the
IL-6 receptor, such as rheumatoid arthritis and Crohn's disease
(20). In the rheumatoid arthritis,
IL-6 can stimulate the T lymphocytes and B lymphocytes to secret
inflammatory mediators, promoting the maturation of B lymphocytes
and enhancing the effects of IL-1β and TNF-α. In the inflammatory
responses, IL-6 exerts chemotaxis-inducing effects on other
inflammatory cells, such as lymphocytes and mononuclear macrophages
(21).
To further explore the specific role of IL-6 in the
pathogenesis of trauma and infection, Riedemann et al
(22) have shown that IL-6 could
significantly promote the expression of C5a on the mRNA level,
suggesting that IL-6 enhances the secondary inflammatory mediator
C5a to exert its pro-inflammatory effects. Moreover, Pritts et
al (23) have shown that nuclear
NF-kb and activated protein 1 (AP-1) are involved in the
stimulating signal transition process in the synthesis of IL-6
within effector cells. Although great progress has been made
concerning the investigation of the role of IL-6 in the neonatal
sepsis pathogenesis, the detailed cellular and molecular mechanisms
remain to be further explored. In this study, elevated expression
levels of IL-6 (mRNA and protein levels) were detected in both the
blood mononuclear cells and serum samples from the neonates with
sepsis. These results suggest that inflammatory infection might
activate the mononuclear cells and lymphocytes, which could secret
large amounts of IL-6 to induce large-scale antigen immune
response. These findings were in line with the responses of the
somatic cell damage.
miRNAs are important gene regulatory factors widely
involved in a variety of pathophysiological processes, such as
tumor cell proliferation, invasion and metastasis, hypertension,
diabetes, and atherosclerosis (24,25). The
dysregulation of miRNA-26a contributes to various biological
processes, including the natural immune responses against the
invasion of pathogenic microorganisms, the development and
differentiation of organs and/or tissues, and the pathogenesis of
various solid tumors and hematopoietic malignancy. miRNA-26a has
been shown to be able to activate the innate immune responses by
controlling the secretion of various inflammatory chemokines
(26,27). Meanwhile, miRNA-26a also plays an
important role in the regulation of stem cell differentiation. For
example, miRNA-26a has been shown to be involved in the
differentiation of hepatic stem cells into mature hepatocytes and
biliary tract cells, as well as the differentiation of
adipose-derived stem cells into osteoblasts, by regulating the
transcription factor Smad family (28,29).
miRNA-26a is expressed in a variety of cancer cells and cancerous
tissues, which can inhibit the proliferation of nasopharyngeal
carcinoma, breast cancer, and HCC cells (30–34).
Based on the bioinformatics prediction, our results showed that
miRNA-26a was closely related to IL-6, which was likely to be an
up-stream miRNA regulating IL-6 expression. Previous literature has
shown that miRNA-26a negatively regulates the expression levels of
IL-6 (35). In this study, our
results showed that the miRNA-26a expression level was
significantly declined, while the expression level of IL-6 was
significantly elevated, in the mononuclear cells from the neonatal
sepsis cases. Moreover, similar results were observed in the
expression levels of miRNA-26a and IL-6 in the serum samples from
the neonatal sepsis. These results suggest that the downregulated
miRNA-26a in the mononuclear cells could increase the expression
level of IL-6, as well as its secretion into the serum in neonatal
sepsis. Moreover, the serum expression levels of miRNA-26a and IL-6
might, to some extent, reflect the inflammatory responses and
tissue injuries. In addition, THP-1 cells were induced by LPS to
simulate the in vitro septic environment, and the results
further confirmed the expression patterns of miRNA-26a and IL-6,
and their interaction. Furthermore, the dual-luciferase reporter
assay showed that IL-6 was the direct target of miRNA-26a.
In conclusion, our results showed that, in the
neonatal sepsis, the serum miRNA-26a content was significantly
declined, which regulated the expression levels of the target gene
IL-6, to further changing the expression levels of related
proteins. These findings might contribute to the understanding of
the roles of miRNA-26a and IL-6 in the pathogenesis of neonatal
sepsis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QC conceived and designed the study, read and
analyzed the documents and collected and analyzed data. LT read and
analyzed the documents and collected and analyzed the data. YW
conceived and designed the study, read and analyzed the documents,
drafted and revised the manuscript and gave the final approval of
the version to be published. All authors take responsibility for
the content of the paper.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Hospital of Shandong University and written
informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Satar M and Ozlü F: Neonatal sepsis: A
continuing disease burden. Turk J Pediatr. 54:449–457.
2012.PubMed/NCBI
|
|
2
|
Liu L, Johnson HL, Cousens S, Perin J,
Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al:
Global, regional and national causes of child mortality: An updated
systematic analysis for 2010 with time trends since. Lancet.
379:2151–2161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raymond SL, Stortz JA, Mira JC, Larson SD,
Wynn JL and Moldawer LL: Immunological defects in neonatal sepsis
and potential therapeutic approaches. Front Pediatr. 5:142017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu YQ, Shen J, Zhou QL, Zhao HW, Liu LR
and Liu X: Interleukin-6 and interleukin-8 in diagnosing neonatal
septicemia. J Biol Regul Homeost Agents. 30:1107–1113.
2016.PubMed/NCBI
|
|
5
|
Delanghe JR and Speeckaert MM:
Translational research and biomarkers in neonatal sepsis. Clin Chim
Acta. 451:46–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anderson AE, Pratt AG, Sedhom MA, Doran
JP, Routledge C, Hargreaves B, Brown PM, Cao Lê KA, Isaacs JD and
Thomas R: IL-6-driven STAT signalling in circulating CD4+
lymphocytes is a marker for early anticitrullinated peptide
antibody-negative rheumatoid arthritis. Ann Rheum Dis. 75:466–473.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tezono K, Sarker KP, Kikuchi H, Nasu M,
Kitajima I and Maruyama I: Bioactivity of the vascular endothelial
growth factor trapped in fibrin clots: Production of IL-6 and IL-8
in monocytes by fibrin clots. Haemostasis. 31:71–79.
2001.PubMed/NCBI
|
|
8
|
Vila N, Reverter JC, Yague J and Chamorro
A: Interaction between interleukin-6 and the natural anticoagulant
system in acute stroke. J Interferon Cytokine Res. 20:325–329.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Celik HT, Portakal O, Yigit S, Hascelik G,
Korkmaz A and Yurdakok M: Efficacy of new leukocyte parameters
versus serum C-reactive protein, procalcitonin and interleukin-6 in
the diagnosis of neonatal sepsis. Pediatr Int. 58:119–125. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steinberger E, Hofer N and Resch B: Cord
blood procalcitonin and Interleukin-6 are highly sensitive and
specific in the prediction of early-onset sepsis in preterm
infants. Scand J Clin Lab Invest. 74:432–436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Z, Xiao SB, Xu P, Xie Q, Cao L, Wang D,
Luo R, Zhong Y, Chen HC and Fang LR: miR-365, a novel negative
regulator of interleukin-6 gene expression, is cooperatively
regulated by Sp1 and NF-kappaB. J Biol Chem. 286:21401–21412. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Subspecialty Group of Neonatology
Pediatric Society Chinese Medical Association, . Editorial Board
Chinese Journal of Pediatrics. Protocol for diagnosis and treatment
of neonatal septicemia. Zhonghua Er Ke Za Zhi. 41:897–899. 2003.(In
Chinese). PubMed/NCBI
|
|
13
|
Qazi SA and Stoll BJ: Neonatal sepsis: A
major global public health challenge. Pediatr Infect Dis J. 28 1
Suppl:S1–S2. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou M, Cheng S, Yu J and Lu Q:
Interleukin-8 for diagnosis of neonatal sepsis: A meta-analysis.
PLoS One. 10:e01271702015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tunc T, Cekmez F, Cetinkaya M, Kalayci T,
Fidanci K, Saldir M, Babacan O, Sari E, Erdem G, Cayci T, et al:
Diagnostic value of elevated CXCR4 and CXCL12 in neonatal sepsis. J
Matern Fetal Neonatal Med. 28:356–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv B, Huang J, Yuan H, Yan W, Hu G and
Wang J: Tumor necrosis factor-α as a diagnostic marker for neonatal
sepsis: A meta-analysis. ScientificWorldJournal. 2014:4714632014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DeLong WG Jr and Born CT: Cytokines in
patients with polytrauma. Clin Orthop Relat Res. 1–65.
2004.PubMed/NCBI
|
|
18
|
Stover JF, Sakowitz OW, Schoning B,
Rupprecht S, Kroppenstedt SN, Thomale UW, Woiciechowsky C and
Unterberg AW: Norepinephrine infusion increases interleukin-6 in
plasma and cerebrospinal fluid of brain-injured rats. Med Sci
Monit. 9:Br382–Br388. 2003.PubMed/NCBI
|
|
19
|
Baeuerle PA and Henkel T: Function and
activation of NF-kappa B in the immune system. Annu Rev Immunol.
12:141–179. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tone M, Powell MJ, Tone Y, Thompson SA and
Waldmann H: IL-10 gene expression is controlled by the
transcription factors Sp1 and Sp3. J Immunol. 165:286–291. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo Q, Ma X, Wahl SM, Bieker JJ, Crossley
M and Montaner LJ: Activation and repression of interleukin-12 p40
transcription by erythroid Kruppel-like factor in macrophages. J
Biol Chem. 279:18451–18456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Riedemann NC, Neff TA, Guo RF, Bernacki
KD, Laudes IJ, Sarma JV, Lambris JD and Ward PA: Protective effects
of IL-6 blockade in sepsis are linked to reduced C5a receptor
expression. J Immunol. 170:503–507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pritts T, Hungness E, Wang Q, Robb B,
Hershko D and Hasselgren PO: Mucosal and enterocyte IL-6 production
during sepsis and endotoxemia-role of transcription factors and
regulation by the stress response. Am J Surg. 183:372–383. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Varshney J and Subramanian S: MicroRNAs as
potential target in human bone and soft tissue sarcoma
therapeutics. Front Mol Biosci. 2:312015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Witwer KW, Sisk JM, Gama L and Clements
JE: MicroRNA regulation of IFN-beta protein expression: Rapid and
sensitive modulation of the innate immune response. J Immunol.
184:2369–2376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones MR, Quinton LJ, Blahna MT, Neilson
JR, Fu S, Ivanov AR, Wolf DA and Mizgerd JP: Zcchc11-dependent
uridylation of microRNA directs cytokine expression. Nat Cell Biol.
11:1157–1163. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rogler CE, Levoci L, Ader T, Massimi A,
Tchaikovskaya T, Norel R and Rogler LE: MicroRNA-23b cluster
microRNAs regulate transforming growth factor-beta/bone
morphogenetic protein signaling and liver stem cell differentiation
by targeting Smads. Hepatology. 50:575–584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luzi E, Marini F, Sala SC, Tognarini I,
Galli G and Brandi ML: Osteogenic differentiation of human adipose
tissue-derived stem cells is modulated by the miR-26a targeting of
the SMAD1 transcription factor. J Bone Miner Res. 23:287–295. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen L, Zheng J, Zhang Y, Yang L, Wang J,
Ni J, Cui D, Yu C and Cai Z: Tumor-specific expression of
microRNA-26a suppresses human hepatocellular carcinoma growth via
cyclin-dependent and -independent pathways. Mol Ther. 19:1521–1528.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu J, He ML, Wang L, Chen Y, Liu X, Dong
Q, Chen YC, Peng Y, Yao KT, Kung HF and Li XP: MiR-26a inhibits
cell growth and tumorigenesis of nasopharyngeal carcinoma through
repression of EZH2. Cancer Res. 71:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang B, Liu XX, He JR, Zhou CX, Guo M, He
M, Li MF, Chen GQ and Zhao Q: Pathologically decreased miR-26a
antagonizes apoptosis and facilitates carcinogenesis by targeting
MTDH and EZH2 in breast cancer. Carcinogenesis. 32:2–9. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kota J, Chivukula RR, O'Donnell KA,
Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P,
Torbenson M, Clark KR, et al: Therapeutic microRNA delivery
suppresses tumorigenesis in a murine liver cancer model. Cell.
137:1005–1017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ciarapica R, Russo G, Verginelli F,
Raimondi L, Donfrancesco A, Rota R and Giordano A: Deregulated
expression of miR-26a and Ezh2 in rhabdomyosarcoma. Cell Cycle.
8:172–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang X, Liang L, Zhang XF, Jia HL, Qin Y,
Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, et al: MicroRNA-26a
suppresses tumor growth and metastasis of human hepatocellular
carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology.
58:158–170. 2013. View Article : Google Scholar : PubMed/NCBI
|