Introduction
In addition to being the most prevalent mental
disorder, depression is one of the leading causes of disability
across the world (1). Major
depressive disorder is among the most severe and debilitating
psychiatric illnesses (2). Although
serotonin-norepinephrine reuptake inhibitors and selective
serotonin reuptake inhibitors are generally effective in the
treatment of depression, it can take several weeks for the patients
to experience the complete therapeutic benefits (3). In addition, ~1/3 of these patients fail
to respond to the current pharmacotherapy and there is a high rate
of relapse among those who do (4).
Therefore, the development of novel therapeutic agents capable of
inducing rapid and sustained antidepressant responses is urgently
required to treat patients with depression who are resistant to the
currently available pharmacological agents.
The pharmacological agent
(5S,10R)-5-methyl-10,11-dihydro-5H-dibenzo(A,D)cyclohepten-5,10-imine
hydrogen maleate (MK-801) is a high affinity, noncompetitive
antagonist of N-methyl-D-aspartate (NMDA) (5). Previous results have indicated the
antidepressant action of rapastinel (formerly GLYX-13) in
preclinical models of depression (6). However, the reports of the
antidepressant activity of MK-801 in rodents are inconsistent.
MK-801 at 0.1 mg/kg has been demonstrated to significantly decrease
the immobility time during the forced swimming test (FST) in mice
(7). Notably, MK-801 has been
indicated to have a short half-life, persisting for 3 h following
the administration of a single dose (7). Furthermore, MK-801 at 0.03 and 0.1
mg/kg exhibited antidepressant activity that lasted for 1 h in
mice, whereas the antidepressant effects were not evident at 24 h
in FST (8). MK-801 at 0.1 mg/kg
significantly enhances the decreased sucrose consumption 2 days
following single-dose administration in the sucrose preference test
(SPT) (8). However, no
anti-anhedonia effect was detected 4 or 7 days following the
administration of the single dose in a social defeat stress model
(9). These findings suggest that
MK-801 induces a rapid antidepressant effect in the social defeat
stress model, although the effect was not long lasting (9).
The US Food and Drug Administration granted
rapastinel the Fast Track designation in 2014 (10). Studies have demonstrated that
rapastinel, an NMDA receptor glycine-site functional partial
agonist, induces a rapid and long-lasting antidepressant-like
effect without psychotomimetic side effects in animal models
(11). In another study, it was
revealed that the antidepressant effect of rapastinel persisted for
5 days following treatment with a single dose in the social defeat
stress model of depression (12). A
recent placebo-controlled study suggested that rapastinel at 5 and
10 mg/kg produces a rapid and sustained antidepressant effect
following a single intravenous infusion in patients with depression
who had not responded to other antidepressants, without eliciting
any psychotomimetic or other notable side effects (13).
The aim of the present study was to demonstrate that
MK-801 elicits a sustained antidepressant effect in the established
chronic unpredictable mild stress (CUMS) mouse model. The
antidepressant effects of MK-801 and rapastinel were evaluated in
mice subjected to behavioral tests in the CUMS model of depression.
As brain-derived neurotrophic factor (BDNF), a subtype of
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
(GluA1) and phosphorylated mammalian target of rapamycin (p-mTOR)
signaling are implicated in the mechanisms underlying the
antidepressant action of the NMDA receptor antagonists (11,14,15),
western blot analysis was performed to examine the effects of
MK-801 and rapastinel on the protein expression of BDNF, GluA1 and
p-mTOR in the medial prefrontal cortex (mPFC), nucleus accumbens
(NAc), dentate gyrus (DG) and CA3 of the hippocampi regions
(12,16).
Materials and methods
Animals
A total of 40 young, healthy male CD1 (ICR) mice
(body weight, 22–25 g; age, 6–8 weeks) born and reared in the
animal facility of Renmin Hospital of Wuhan University (Wuhan,
China) were used in the present study. All animals were maintained
in a room at 22±2°C with 60±5% relative humidity and a 12-h
light/dark cycle (lights on between 07:00-19:00 h). Mice had ad
libitum access to water and food when the stressors were not
applied. The stressors were applied to mice outside their living
area in a separate procedure room.
The present study was conducted in strict accordance
with the recommendations of the Guide for the Care and Use of
Laboratory Animals and was approved by the Institutional Animal
Care and Use Committee of Wuhan University.
Drugs and drug administration
On the day of intraperitoneal injection a single
dose of vehicle (10 ml/kg; 0.9% saline), MK-801 (0.1 mg/kg,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and rapastinel (10
mg/kg, Tocris Bioscience, Bristol, UK) were administered to mice
(Fig. 1A). Previously reported doses
of MK-801 (0.1 mg/kg) and rapastinel (10 mg/kg) were used (7–9,11). If a drug exhibited antidepressant
activity that lasted for >24 h in FST or 2 days in SPT mice, it
was considered long-lasting (8,9).
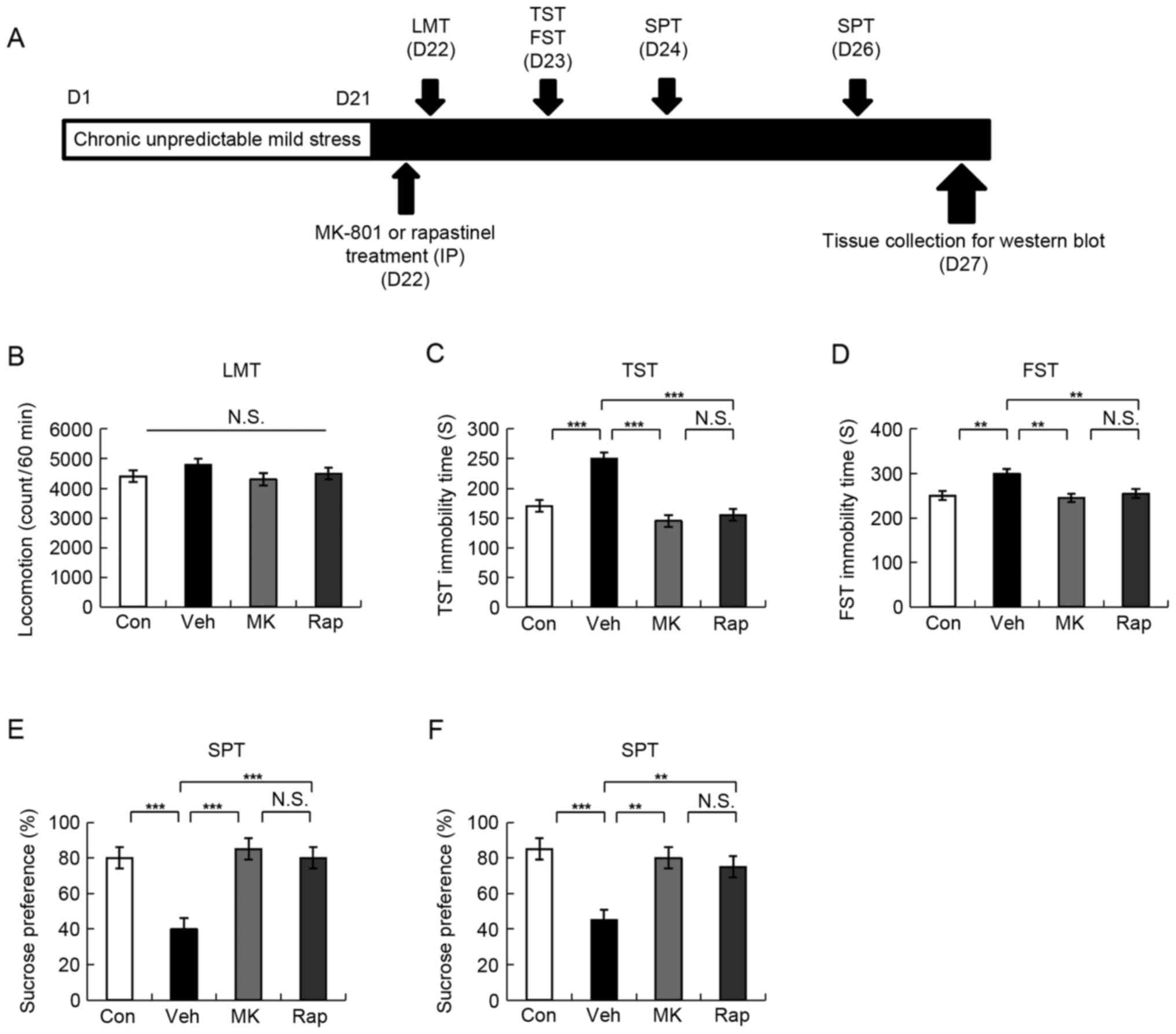 | Figure 1.Schedule of CUMS model, drug
administration, behavioral tests and brain sampling. (A) CUMS was
performed for 21 days. Stressed mice were used in the subsequent
experiments. Vehicle, MK-801 (0.1 mg/kg), or rapastinel (10 mg/kg)
was administered intraperitoneally (D22). LMT, TST and FST were
performed 2, 4 and 8 h after injection of a single dose,
respectively (D22-23). A 1% SPT test was performed 2 (D24) and 4
days (D26) after a single-dose injection. The collection of the
brain regions was performed at D27. (B) LMT, (C) TST, (D) FST, and
(E and F) 1% SPT results were determined. Values were presented as
the mean ± standard error of the mean (10 mice/group). **P<0.01
and ***P<0.001 as indicated. Con, control; Veh, vehicle; MK,
MK-801; Rap, rapastinel; CUMS, chronic unpredictable mild stress;
LMT, locomotion test; TST, tail suspension test; FST, forced swim
test; SPT, sucrose preference test; D, day; N.S., not
significant. |
CUMS mice model
The CUMS procedure was performed as previously
described (11). CUMS mice model
consisted of a range of unpredictable stressors, which alone are
insufficient to induce sustained effects. The animal model
consisted of random chronic exposure to a variety of unpredictable
stressors, which are listed in Table
I. To ensure the application of unpredictable stress and
prevent habituation, all stressors were randomly scheduled during a
7-day experimental period and repeated three times throughout the
21-day experimental period, the SPT was used to evaluate the
successful establishment of the CUMS model as previously described
(11). Control mice (the housing
conditions, age, sex and weight of the control mice were the same
as the study group) were bred in a separate room and did not come
in contact with the stressed groups. There were four treatment
groups as follows (n=10 mice/group): Control (10 ml/kg; distilled
water), vehicle (10 ml/kg; distilled water), MK-801 (0.1 mg/kg) and
rapastinel (10 mg/kg).
 | Table I.Schedule of stressor used in chronic
unpredictable mild stress model. |
Table I.
Schedule of stressor used in chronic
unpredictable mild stress model.
| Day | Duration | Stressor |
|---|
| 1, 8, 15 | 24 h | Water
deprivation |
| 2, 9, 16 | 24 h | Food
deprivation |
|
| 2 h | Physical
restraint |
| 3, 10, 17 | 24 h | Water
deprivation |
| 4, 11, 18 | 24 h | Soiled cage |
|
| 5 min | Forced swimming at
4°C |
| 5, 12, 19 | 24 h | Food
deprivation |
|
| 1 h | Exposure to an
empty bottle |
| 6, 13, 20 | 24 h | Soiled cage |
|
| Overnight | Overnight
illumination |
| 7, 14, 21 | 24 h | Exposure to a
foreign object |
|
| 5 min | Cage tilt
(45°C) |
Behavioral tests
All behavioral tests were performed as reported
previously (17–19). The tests were performed in a quiet
room. All experimental mice were placed in the behavioral test room
for ~30 min prior to the test and were returned to the housing room
soon after the tests.
The locomotion test (LMT) was performed using an
animal movement analysis system SCANETMV-40 (Melquest Co., Ltd.,
Toyama, Japan). Mice were placed in transparent Plexiglas cages
(560×560×330 mm). Under house lighting, cumulative exercise was
recorded for 60 min as previously described (18,19) and
the mice cages were cleaned between testing sessions. The LMT was
performed 2 h following the injection of a single dose (D22).
During the tail suspension test (TST), the
experimental mice were taken away from the living area and a piece
of adhesive tape was placed ~2 cm from the tip of the tail. The
tape was used to hang mice individually on a hook. The immobility
time of tail suspension for each mouse, which was determined by a
skilled observer, was video recorded for 10 min as previously
described (17,18). When mice hung passively and
completely motionless, they were considered immobile. TST was
performed 4 h following the injection of a single dose (D23).
The FST was performed to assess immobility time in
mice. Mice were placed individually in a cylinder (31×23 cm), which
contained ~15 cm of water (23±1°C). The experimental mice were
assessed using an automated forced-swim apparatus SCANETMV-40
(Melquest Co., Ltd.). When a mouse remained within the same
rectangle for 0.3 sec after the rectangle was set up, the mouse was
considered immobile. Immobility time was calculated from the
activity time using the apparatus analysis software (Melquest Co.,
Ltd., Toyama, Japan) as follows: Total time-active time. The
cumulative immobility time of the mice was recorded for 6 min
(17,19). FST was performed 8 h following the
injection of a single dose (D23).
To perform the SPT, mice were exposed to 1% sucrose
solution and water for ~48 h, deprived for 4 h and then exposed to
two identical bottles containing water and 1% sucrose solution for
1 h. The bottles containing sucrose and water were weighed prior to
and following 1 h of exposure. Subsequently, the sucrose preference
was evaluated as follows: The bottle containing sucrose was weighed
prior to 1 h of exposure (S1), the bottle containing water was
weighed prior to 1 h of exposure (W1), the bottle containing
sucrose was weighed following 1 h of exposure (S2), the bottle
containing water was weighed following 1 h of exposure (W2), the
sucrose preference %=(S1-S2)/[(W1-W2) + (S1-S2)] ×100%. A 1% SPT
test was performed 2 (D24) and 4 days (D26) following a single-dose
injection.
Western blot analysis of BDNF, GluA1,
and p-mTOR protein expression
Western blot analysis was performed as reported
previously (17). Mice were
sacrificed and their brains were immediately harvested. Coronal
sections ~1 mm thick were cut and bilateral tissue punches of mPFC,
NAc, DG and CA3 of the hippocampi were selected on ice using a
SZ-LED Kenis light microscope, and stored at −80°C. Tissue samples
were homogenized in Laemmli lysis buffer (Gold Biotechnology Inc.,
St. Louis, MO, USA). The samples were centrifuged at 3,000 × g at
4°C for 8 min to obtain the supernatants. Protein concentration was
determined using the DC protein assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), and samples were incubated for 5 min at
95°C with an equal volume of 4% SDS, 125 mM Tris/HCl, 0.1%
bromophenol blue (pH 6.8), 20% glycerol and 10% β-mercaptoethanol.
Aliquots (10 µg) were separated by 10% SDS-PAGE. Subsequently,
proteins were transferred onto polyvinylidene difluoride membranes
using a Mini Trans-Blot Cell. Membranes were blocked with 2% bovine
serum albumin (Bio-Rad Laboratories, Inc.) in Tris buffered saline
with 0.1% Tween-20 (TBST) for ~1 h at 22±2°C. Blots were incubated
with primary antibodies against BDNF (1:500 dilution; cat. no.
H-117, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), GluA1
(cat. no. ab31232; dilution 1.67 µl/ml, Abcam, Cambridge, MA, USA),
β-actin (1:10,000 dilution, Sigma-Aldrich; Merck KGaA) and p-mTOR
(1:1,000 dilution, Cell Signaling Technology, Inc., Danvers, MA,
USA) overnight at 4°C. The following day, blots were washed three
times in TBST and incubated with horseradish peroxidase conjugated
secondary anti-rabbit antibody (cat. no. sc-2357; 1:5,000 dilution;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 1
h at room temperature. Following a final three washes with TBST,
bands were detected using enhanced chemiluminescence (Bio-Rad
Laboratories, Inc.) and a western blotting detection system. The
images were captured and the optical density of the bands was
analyzed using a LAS3000 imaging and detection system (Fujifilm
Corporation, Tokyo, Japan).
Statistical analysis
Data were presented as the mean ± standard error of
the mean. PASW Statistics 19 (formerly SPSS Statistics; SPSS, Inc.,
Chicago, IL, USA) was used for analysis and the groups were
compared using one-way analysis of variance followed by Fishers
least significant difference post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of intraperitoneal
administration of MK-801 and rapastinel in the CUMS model
MK-801 provides a rapid antidepressant effect in the
social defeat stress model (9).
Notably, rapastinel has been demonstrated to produce a rapid and
long-lasting antidepressant effect in CUMS and social defeat stress
models (9,11,12). In
the present study, the effects of MK-801 and rapastinel were
assessed in the established CUMS model (Fig. 1A).
LMT indicated no statistically significant
difference among the four groups (Fig.
1B). In the TST and FST, 0.1 mg/kg MK-801 and 10 mg/kg
rapastinel significantly attenuated the increased immobility times
in stressed mice (P<0.001 and P<0.005; Fig. 1C and D, respectively). The SPT
results demonstrated that the sucrose preference of mice treated
with a single injection MK-801 or rapastinel was significantly
increased compared with that of the vehicle-treated group
(P<0.001 and P<0.01; Fig. 1E
(D24) and F (D26), respectively). These behavioral data suggest
that MK-801 elicited a rapid and long-lasting (5 days)
antidepressant effect in the CUMS model, which was consistent with
the antidepressant effect of rapastinel.
Protein expression of BDNF, GluA1 and
p-mTOR in the mPFC brain regions following intraperitoneal
administration of MK-801 and rapastinel
Western blot analysis was performed to detect the
protein expression of BDNF, synaptogenesis marker GluA1 (14), and p-mTOR in the mPFC 5 days after
the administration of a single dose of MK-801 and rapastinel
(Fig. 2A). Results indicated that
CUMS significantly reduced the protein expression of BDNF
(P<0.01), GluA1 (P<0.001) and p-mTOR (P<0.01) compared
with the control group in the mPFC (Fig.
2A). Notably, MK-801 and rapastinel significantly attenuated
the decreased protein expression of BDNF (P<0.01), GluA1
(P<0.001) and p-mTOR (P<0.05) in the mPFC (Fig. 2A).
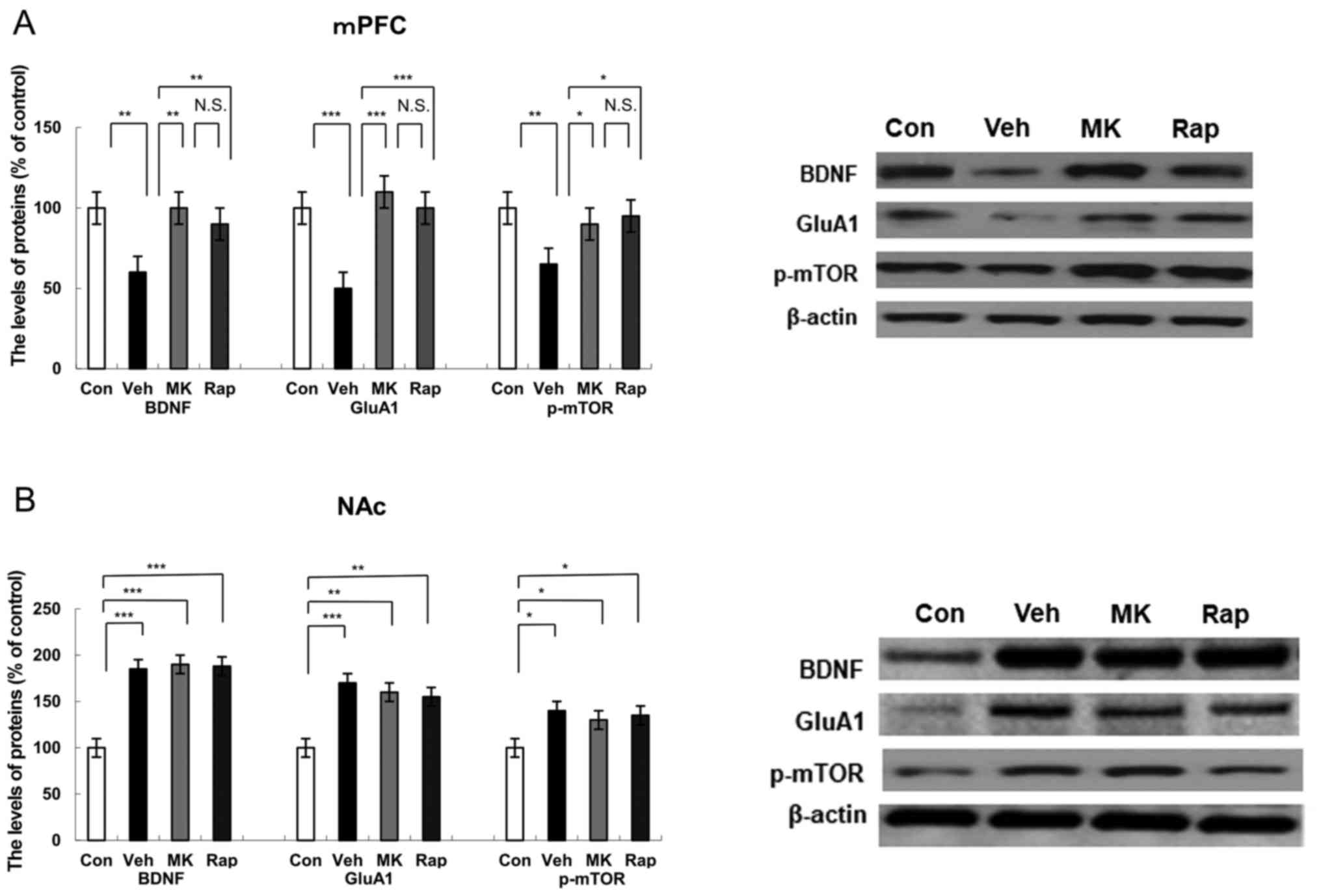 | Figure 2.Effects of MK-801 and rapastinel on
BDNF, GluA1 and p-mTOR protein expression in the mPFC and NAc.
Protein expression of BDNF, GluA1 and p-mTOR in the (A) mPFC and
(B) NAc was determined using western blot analysis. Values are
expressed as a percentage of those of the control mice and
presented as the mean ± standard error of the mean (n=6 or 7).
*P<0.05, **P<0.01, ***P<0.001 as indicated. Con, control;
Veh, vehicle; MK, MK-801; Rap, rapastinel; BDNF, brain-derived
neurotrophic factor; GluA1, Glutamate A1; p-mTOR, phosphorylated
mammalian target of rapamycin; mPFC, medial prefrontal cortex; NAc,
nucleus accumbens; N.S., not significant. |
Protein expression of BDNF, GluA1,
p-mTOR in the NAc following intraperitoneal administration of
MK-801 and rapastinel
Western blot analysis was used to assess protein
expression of BDNF, GluA1 and p-mTOR in the NAc (Fig. 2B). CUMS significantly increased the
protein expression of BDNF (P<0.001), GluA1 (P<0.001) and
p-mTOR (P<0.05) in the NAc group compared with the control
(Fig. 2B). Notably, MK-801 and
rapastinel had no significant effect on the increased protein
expression of BDNF, GluA1 and p-mTOR compared with the vehicle
group (Fig. 2B).
Protein expression of BDNF, GluA1 and
p-mTOR in the DG of the hippocampus following intraperitoneal
administration of MK-801 and rapastinel
Western blot analysis indicated the protein
expression of BDNF, GluA1 and p-mTOR in the DG of the hippocampus.
CUMS significantly decreased the protein expression of BDNF, GluA1
and p-mTOR compared with the control group in the DG of the
hippocampus (all P<0.01; Fig.
3A). Notably, MK-801 and rapastinel significantly attenuated
the decreased expression of BDNF, GluA1 and p-mTOR protein in the
DG of the hippocampus (P<0.05 and P<0.01; Fig. 3A).
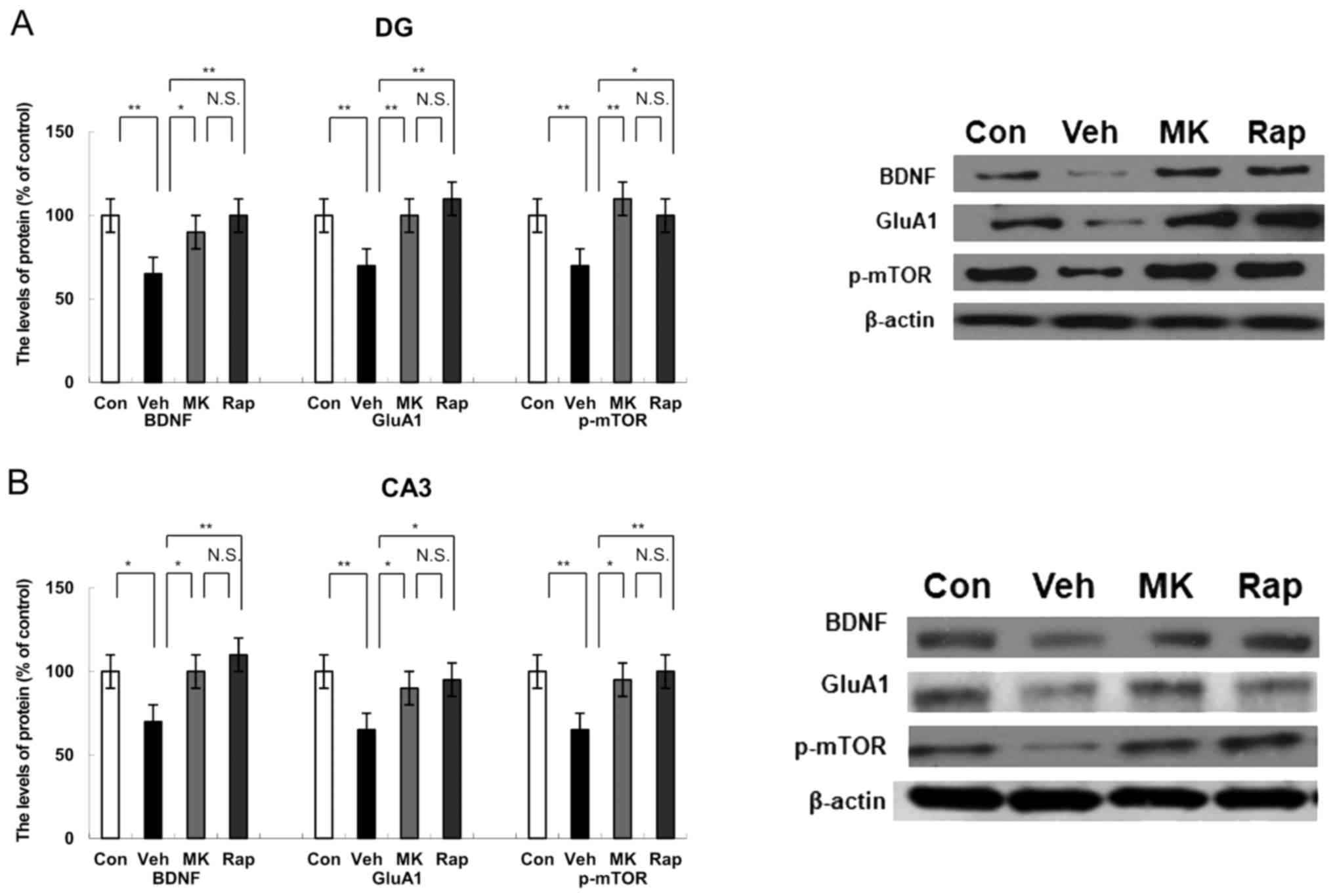 | Figure 3.Effects of MK-801 and rapastinel on
the BDNF, GluA1 and p-mTOR protein expression in the DG and CA3 of
the hippocampus regions. (A) Protein expression of BDNF, GluA1 and
p-mTOR in the (A) DG and (B) CA3 of the hippocampus regions was
determined using western blot analysis. Values were expressed as a
percentage of that of control mice and presented as the mean ±
standard error of the mean (n=6 or 7). *P<0.05 and **P<0.01,
as indicated. Con, control; Veh, vehicle; MK, MK-801; Rap,
rapastinel; BDNF, brain-derived neurotrophic factor; GluA1,
Glutamate A1; p-mTOR, phosphorylated mammalian target of rapamycin;
DG, dentate gyrus. |
Protein expression of BDNF, GluA1 and
p-mTOR in the CA3 region of hippocampus following intraperitoneal
administration of MK-801 and rapastinel
Western blot analysis was performed to measure the
protein expression of BDNF, GluA1 and p-mTOR in the CA3 region of
the hippocampus. CUMS significantly reduced the protein expression
of BDNF (P<0.05), GluA1 (P<0.01) and p-mTOR (P<0.01)
compared with the control group within the CA3 of the hippocampus
(Fig. 3B). However, MK-801 and
rapastinel significantly attenuated the decreased protein
expression of BDNF, GluA1 and p-mTOR compared with the vehicle
group (P<0.05 and P<0.01; Fig.
3B).
Discussion
The present study was performed to further elucidate
the mechanisms responsible for the long-lasting antidepressant
effects observed in the CUMS model following single-dose
administration of MK-801. In the established CUMS model, MK-801
significantly increased the protein expression of BDNF, GluA1 and
p-mTOR in the mPFC, DG and CA3 of the hippocampus, resulting in a
sustained antidepressant effect. Notably, the protein expression of
BDNF in the mPFC, DG and CA3 of the hippocampus were significantly
lower compared with the control mice. Furthermore, the
administration of a single dose of MK-801 increased the protein
expression level of BDNF in the mPFC, DG and CA3 of the hippocampus
compared with the vehicle control in CUMS model mice. The
expression of GluA1 and p-mTOR proteins in the CUMS model mice was
significantly lower compared with in the control mice; however,
MK-801 significantly increased the expression of GluA1 and p-mTOR
proteins in the mPFC, DG and CA3 of the hippocampus in the CUMS
model mice when measured 5 days following drug administration.
Notably, MK-801 evoked the same long-lasting antidepressant
response as rapastinel, which is currently being tested in a phase
II clinical development program as an adjunctive therapy for major
depressive disorder (clinicaltrials.gov identifier NCT01684163). On the
basis of these findings, it was suggested that BDNF-TrkB, GluA1
neurotransmitter and p-mTOR signaling in the PFC and hippocampus
may serve vital roles in the sustained antidepressant effect of
MK-801.
NMDA receptors are predominantly identified at
excitatory synapses and are ubiquitously expressed in the central
nervous system (20). These
receptors have become targets for drug development for the
treatment of depression (20,21).
Ketamine, an NMDA receptor antagonist, is one of the most appealing
antidepressants for treatment-resistant depression (22,23).
However, it is typically associated with psychotomimetic side
effects. Ketamine is a racemic mixture containing equal parts of
S-ketamine and R-ketamine (18). It
has been reported that R-ketamine is a potent, long-lasting and
safe antidepressant (18). In
vivo imaging studies have revealed reduced glutamate levels in
the PFC/anterior cortex of patients with depression (24–26).
Postmortem data suggested that NMDA receptor protein expression is
altered in the PFC of patients with depression (27). Similarly, CP-101,606 (an NR2B
subunit-selective NMDAR antagonist) was reported to reduce
depression scores (28). As MK-801
is an NMDA receptor noncompetitive antagonist, it is also
reasonable to assume that its long-lasting antidepressant-like
effects involve an NMDA receptor-mediated process akin. Although
MK-801 has been considered to increase locomotor activity in the
past (7), no significant effects at
doses of 0.1 mg/kg were indicated in the present study, which is
consistent with a recent report (9).
Consistent with the result of a previous report
(8), the present study indicated
that MK-801 had a rapid antidepressant effect (9). To the best of our knowledge, this is
the first study to report the long-lasting antidepressant effects
of MK-801 in the CUMS model. In the present study, a single-dose
intraperitoneal injection of MK-801 produced a long-lasting (5-day)
antidepressant effect in the CUMS model to a similar extent as
rapastinel. However, the precise mechanisms underlying this effect
remain unclear. A previous study revealed that the etiology of
depression is associated with the PFC and hippocampus (12). Notably, mice and humans exhibit
functional homology in these two regions of the brain (29). A recent study suggested changes in
the NAc may be associated with depression and its functional
abnormalities may be primarily concentrated in the shell rather
than the nucleus (30). Therefore,
to understand the antidepressant effect of MK-801, the mPFC, NAc,
DG and CA3 of the hippocampus were examined in the present
study.
BDNF is associated with the pathophysiology of
depression and the BDNF gene may be responsible for susceptibility
to depression (15). It has been
reported that the expression of BDNF is conspicuously reduced in
the PFC and hippocampi of patients with depression, whereas the
expression of BDNF is significantly increased following
antidepressant treatment (31–33). A
previous study suggested that depression is associated with changes
in brain neurotransmitters, including dopamine,
5-hydroxytryptamine, glutamate and γ-aminobutyric acid (34). Furthermore, changes in the steady
state concentration or imbalance of neurotransmitters may be
associated with depression (34).
The glutamate system represents a novel target for the treatment of
depression, inhibiting the release of neurotransmitters and
regulates postsynaptic responses (16). Previous studies have identified that
blocking the mTOR signaling pathway may reduce the antidepressant
effect of NMDA receptor antagonists and prevent neuronal
regeneration in animal depression models (11,20).
p-mTOR is the activated form of mTOR. In the present study, a
noticeable decrease in the protein expression of BDNF, GluA1 and
p-mTOR in the mPFC, DG and CA3 of the hippocampus were indicated,
while a significant increase in the expression of BDNF, GluA1 and
p-mTOR proteins in the NAc of CUMS mice was determined. These
findings suggest that the occurrence and development of depression
may be mediated by the BDNF-TrkB, glutamate neurotransmitter and
mTOR signaling pathways in these regions. These results are
consistent with those reported by Shirayama et al (35). According to a previous report,
synaptogenesis and neuronal regeneration serve a role in the
prolonged antidepressant effect associated with NMDA receptor
antagonists (14,16). The present results suggested that a
single dose of MK-801 or rapastinel significantly attenuated the
decrease in the expression of BDNF, GluA1 and p-mTOR proteins in
the mPFC, DG and CA3 of the hippocampus of the CUMS model. It is
likely that the sustained increase in the expression of BDNF, GluA1
and p-mTOR protein in the mPFC, DG and CA3 of the hippocampus is
involved in the long-lasting (5-day) effect of MK-801 and
rapastinel. However, further detailed studies are required to fully
understand the role of synaptogenesis and neuronal regeneration in
the long-lasting antidepressant response. The present study also
identified that the expression of BDNF, GluA1 and p-mTOR proteins
did not significantly change in the NAc following antidepressant
treatment with MK-801 or rapastinel, which suggests that MK-801 or
rapastinel cannot inhibit p-mTOR signaling pathway or block the
release of GluA1 in NAc. However, further research is required.
In conclusion, the present study demonstrated that a
single dose of MK-801 produced a sustained antidepressant effect in
the established CUMS model of depression, and that MK-801 elicits a
rapid and sustained antidepressant effect similar to rapastinel.
Furthermore, it is likely that increased synaptogenesis and
neuronal regeneration in the mPFC, DG and CA3 of the hippocampus
may be involved in the sustained antidepressant response.
Therefore, the present findings suggest that the NMDA antagonist
MK-801 may be considered as a rapid and long-lasting therapeutic
agent in patients with depression, once the safety and efficacy of
MK-801 are proven in a clinical setting.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 8157051331).
Availability of data and materials
The datasets during and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
J-CC conceived and designed the experiments. B-KY
performed the experiments. JQ and YN analyzed the data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Wuhan University.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Monteggia LM and Zarate CA Jr:
Antidepressant actions of ketamine: From molecular mechanisms to
clinical practice. Curr Opin Neurobiol. 30:139–143. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hashimoto K: Inflammatory biomarkers as
differential predictors of antidepressant response. Int J Mol Sci.
16:7796–7801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaki S and Fukumoto K: Potential of
glutamate-based drug discovery for next generation antidepressants.
Pharmaceuticals (Basel). 8:590–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schosser A, Serretti A, Souery D,
Mendlewicz J, Zohar J, Montgomery S and Kasper S: European group
for the study of resistant depression (GSRD)-where have we gone so
far: Review of clinical and genetic findings. Eur
Neuropsychopharmacol. 22:453–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong EH, Kemp JA, Priestley T, Knight AR,
Woodruff GN and Iversen LL: The anticonvulsant MK-801 is a potent
N-methyl-D-aspartate antagonist. Proc Natl Acad Sci USA.
83:7104–7108. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burgdorf J, Zhang XL, Nicholson KL,
Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA and Moskal
JR: GLYX-13, a NMDA receptor glycine-site functional partial
agonist, induces antidepressant-like effects without ketamine-like
side effects. Neuropsychopharmacology. 38:729–742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Autry AE, Adachi M, Nosyreva E, Na ES, Los
MF, Cheng PF, Kavalali ET and Monteggia LM: NMDA receptor blockade
at rest triggers rapid behavioural antidepressant responses.
Nature. 475:91–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zanos P, Moaddel R, Morris PJ, Georgiou P,
Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, et
al: NMDAR inhibition-independent antidepressant actions of ketamine
metabolites. Nature. 533:481–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang B, Ren Q, Ma M, Chen QX and Hashimoto
K: Antidepressant effects of (+)-MK-801 and (−)-MK-801 in the
social defeat stress model. Int J Neuropsychopharmacol. 19(pii):
pyw0802016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moskal JR, Burgdorf JS, Stanton PK, Kroes
RA, Disterhoft JF, Burch RM and Khan MA: The development of
rapastinel (Formerly GLYX-13); A rapid acting and long lasting
antidepressant. Curr Neuropharmacol. 15:47–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu Y, Wang C, Xue Z, Li C, Zhang J, Zhao
X, Liu A, Wang Q and Zhou W: PI3K/AKT/mTOR signaling-mediated
neuropeptide VGF in the hippocampus of mice is involved in the
rapid onset antidepressant-like effects of GLYX-13. Int J
Neuropsychopharmacol. 18(pii): pyu1102014.PubMed/NCBI
|
|
12
|
Yang B, Zhang JC, Han M, Yao W, Yang C,
Ren Q, Ma M, Chen QX and Hashimoto K: Comparison of R-ketamine and
rapastinel antidepressant effects in the social defeat stress model
of depression. Psychopharmacology (Berl). 233:3647–3657. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Preskorn S, Macaluso M, Mehra DO, Zammit
G, Moskal JR and Burch RM; GLYX-13 Clinical Study Group, .
Randomized proof of concept trial of GLYX-13, an
N-methyl-D-aspartate receptor glycine site partial agonist, in
major depressive disorder nonresponsive to a previous
antidepressant agent. J Psychiatr Pract. 21:140–149. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duman RS and Aghajanian GK: Synaptic
dysfunction in depression: Potential therapeutic targets. Science.
338:68–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lepack AE, Fuchikami M, Dwyer JM, Banasr M
and Duman RS: BDNF release is required for the behavioral actions
of ketamine. Int J Neuropsychopharmacol. 18:pyu0332014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohgi Y, Futamura T and Hashimoto K:
Glutamate signaling in synaptogenesis and NMDA receptors as
potential therapeutic targets for psychiatric disorders. Curr Mol
Med. 15:206–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren Q, Ma M, Ishima T, Morisseau C, Yang
J, Wagner KM, Zhang JC, Yang C, Yao W, Dong C, et al: Gene
deficiency and pharmacological inhibition of soluble epoxide
hydrolase confers resilience to repeated social defeat stress. Proc
Natl Acad Sci USA. 113:E1944–E1952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao
W, Ma M, Dong C and Hashimoto K: R-ketamine: A rapid-acting and
sustained antidepressant without psychotomimetic side effects.
Transl Psychiatry. 5:e6322015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma
M, Han M and Hashimoto K: Comparison of ketamine,
7,8-dihydroxyflavone and ANA-12 antidepressant effects in the
social defeat stress model of depression. Psychopharmacology
(Berl). 232:4325–4335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM,
Iwata M, Li XY, Aghajanian G and Duman RS: mTOR-dependent synapse
formation underlies the rapid antidepressant effects of NMDA
antagonists. Science. 329:959–964. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller OH, Yang L, Wang CC, Hargroder EA,
Zhang Y, Delpire E and Hall BJ: GluN2B-containing NMDA receptors
regulate depression-like behavior and are critical for the rapid
antidepressant actions of ketamine. Elife. 3:e035812014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krystal JH, Sanacora G and Duman RS:
Rapid-acting glutamergic antidepressants: The path to ketamine and
beyond. Biol Psychiatry. 73:1133–1141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lodge D and Mercier MS: Ketamine and
phencyclidine: The good, the bad and the unexpected. Br J
Pharmacol. 172:4254–4276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haj-Mirzaian A, Kordjazy N, Haj-Mirzaian
A, Ostadhadi S, Ghasemi M, Amiri S, Faizi M and Dehpour A: Evidence
for the involvement of NMDA receptors in the antidepressant-like
effect of nicotine in mouse forced swimming and tail suspension
tests. Psychopharmacology (Berl). 232:3551–3561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hasler G, Bonwetsch R, Giovacchini G,
Toczek MT, Bagic A, Luckenbaugh DA, Drevets WC and Theodore WH:
5-HT1A receptor binding in temporal lobe epilepsy patients with and
without major depression. Biol Psychiatry. 62:1258–1264. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luykx JJ, Laban KG, van den Heuvel MP,
Boks MP, Mandl RC, Kahn RS and Bakker SC: Region and state specific
glutamate downregulation in major depressive disorder: A
meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev.
36:198–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feyissa AM, Chandran A, Stockmeier CA and
Karolewicz B: Reduced levels of NR2A and NR2B subunits of NMDA
receptor and PSD-95 in the prefrontal cortex in major depression.
Prog Neuropsychopharmacol Biol Psychiatry. 33:70–75. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Preskorn SH, Baker B, Kolluri S, Menniti
FS, Krams M and Landen JW: An innovative design to establish proof
of concept of the antidepressant effects of the NR2B subunit
selective N-methyl-D-aspartate antagonist, CP-101,606, in patients
with treatment-refractory major depressive disorder. J Clin
Psychopharmacol. 28:631–637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duman RS, Aghajanian GK, Sanacora G and
Krystal JH: Synaptic plasticity and depression: New insights from
stress and rapid-acting antidepressants. Nat Med. 22:238–249. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren Q, Ma M, Yang C, Zhang JC, Yao W and
Hashimoto K: BDNF-TrkB signaling in the nucleus accumbens shell of
mice has key role in methamphetamine withdrawal symptoms. Transl
Psychiatry. 5:e6662015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang C, Shirayama Y, Zhang JC, Ren Q and
Hashimoto K: Regional differences in brain-derived neurotrophic
factor levels and dendritic spine density confer resilience to
inescapable stress. Int J Neuropsychopharmacol. 18:pyu1212015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hashimoto K: Sigma-1 receptor chaperone
and brain-derived neurotrophic factor: Emerging links between
cardiovascular disease and depression. Prog Neurobiol. 100:15–29.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang B, Yang C, Ren Q, Zhang JC, Chen QX,
Shirayama Y and Hashimoto K: Regional differences in the expression
of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF
and preproBDNF in the brain confer stress resilience. Eur Arch
Psychiatry Clin Neurosci. 266:765–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hashimoto K, Malchow B, Falkai P and
Schmitt A: Glutamate modulators as potential therapeutic drugs in
schizophrenia and affective disorders. Eur Arch Psychiatry
Neurosci. 263:367–377. 2013. View Article : Google Scholar
|
|
35
|
Shirayama Y, Yang C, Zhang JC, Ren Q, Yao
W and Hashimoto K: Alterations in brain-derived neurotrophic factor
(BDNF) and its precursor proBDNF in the brain regions of a learned
helplessness rat model and the antidepressant effects of a TrkB
agonist and antagonist. Eur Neuropsychopharmacol. 25:2449–2458.
2015. View Article : Google Scholar : PubMed/NCBI
|

















