Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
disease characterized by persistent synovial inflammation of the
joint and invasion of the cartilage and bone. The etiology of RA
remains unclear; however, it is thought that dysregulation of the
internal environment may induce the pathophysiological process of
RA, which is closely associated with immune, genetic and
environmental factors (1,2). Tumor necrosis factor-α (TNF-α) serves a
key role in RA development and progression, and it is the earliest
effective inflammatory factor expressed during the process of
inflammation (3). Therefore,
suppression of TNF-α activation may inhibit disease progression and
further alleviate or cure the disease (4). As a cutting edge treatment strategy,
gene therapy facilitates the transfection of genes into tissues,
which is different from traditional drug injection, and allows for
the continuous and efficient delivery of therapeutic proteins to
the target site, thereby achieving the goal of sustained treatment
for the disease (5). Generally, gene
transfection is performed using biological, chemical and physical
methods, and each has its own advantages and disadvantages
(6).
Ultrasound-targeted microbubble destruction (UTMD)
is a novel method of gene transfection. It utilizes ultrasonic
cavitation to increase the permeability of the cell membrane and
promote the uptake of plasmids, and is considered to be a highly
efficient and safe method (7,8). A
previous study confirmed that UTMD effectively delivers insulin
like growth factor-1 (IGF-1) plasmid DNA into the injured Achilles
tendons of rats, effectively promoting tendon regeneration and
inhibiting adhesion (9). An
additional study confirmed the successful transfection of the EGFP
gene into the inflammatory synovial membrane of rabbits via UTMD
(10). Since muscle cells are
abundant in the body, transfected muscle cells may constantly
express and release therapeutic proteins. In addition,
intramuscular injection is a simple procedure, therefore, muscle
cells are an ideal target for gene therapy. The pathological basis
of RA is characterized by the secretion of a variety of
inflammatory factors by the synovium, which has a rich blood
supply. Therefore, it may also be a useful target for gene therapy.
In the present study, UTMD was performed to transfect the TNF-α
receptor (TNFR) gene into muscle and synovial cells in the inflamed
joints of rats with collagen-induced arthritis (CIA). The
transfection efficiency and therapeutic outcomes were then compared
to identify an optimal strategy for treating RA using gene therapy.
To the best of the author's knowledge, there have only been few
studies on this topic.
Materials and methods
Establishment of a rat model of
CIA
A total of 108 healthy female Sprague-Dawley rats
(age, 6–7 weeks; weight, ~200 g) were provided by Chengdu Dashuo
Experimental Animal Co., Ltd., Chengdu, China, and were housed in a
temperature-controlled room (20–26°C), at 50% humidity, with
standard and sufficient food and water and 12/12 h light/dark
cycles. All procedures (including rat anesthesia and euthanasia
procedures) involving animals were approved by the Sichuan
University Research Committee for Animal Research (Sichuan, China;
approval no. 2016052A). Bovine type II collagen acetic acid
solution (Chondrex, Redmond, WA, USA) was emulsified in an equal
volume of incomplete Freund's adjuvant (Chondrex). Each rat was
immunized intradermally at the base of the tail with 0.2 ml
emulsion (200 µg collagen/rat). A booster injection was
administered (0.1 ml emulsion; 100 µg collagen/rat) intradermally
in the tail at 7 days following the initial immunization. Arthritic
progression was monitored daily.
Amplification, extraction and
purification of plasmid DNA
The present study used the pEGFP plasmid (Roche
Diagnostics, Basel, Switzerland) that expresses enhanced green
fluorescent protein (EGFP), and a TNFR plasmid (GeneCopoeia, Inc.,
Rockville, MD, USA) that expresses TNFR. The manufacturer provided
these plasmids transfected into Escherichia coli and labeled
with EGFP. Plasmid extraction and purification were performed
according to the instructions provided with the plasmid extraction
kit (Qiagen GmbH, Hilden, Germany), and an ultraviolet
spectrophotometer was used to determine plasmid concentration by
measuring the optical density (OD) at wavelengths of 260 and 280
nm. If the OD 260/280 nm ratio was between 1.8 and 2.1, then the
sample was considered to be pure and uncontaminated. The plasmid
was diluted to 30 µg/100 µl and stored at −20°C prior to use.
Preparation of the SonoVue-DNA
complexes
The SonoVue microbubble suspensions (Bracco Imaging
S.p.A, Milano, Italy) were prepared at a density of
2–5×108/ml and a concentration of 5 mg/ml using 0.9%
saline solution. SonoVue (100 µl) was then mixed with 100 µl (30
µg) of the plasmid DNA solution and stored at 4°C prior to each
experiment.
Experimental grouping
CIA rats were randomly assigned to the following 6
groups: Group 1, plasmid + microbubble + ultrasound (muscle group);
group 2, plasmid + microbubble + ultrasound (joint group); group 3,
plasmid + ultrasound; group 4, plasmid + microbubble; group 5,
plasmid only and; group 6, untreated controls. Aside from group 2,
which was injected at the ankle joint, the other groups were
injected in the tibialis anterior muscle. Groups 1, 2 and 4 were
injected with the SonoVue/TNFR mixture (300 µg plasmid/rat), groups
3 and 5 were injected with the same dosage of TNFR plasmid alone,
and group 6 received no treatment. An ultrasound therapy device
(Sonic Master ES-2; OG Giken, Co., Ltd., Okayama, Japan) associated
with a probe frequency of 1 MHz, transducer area of 10
cm2 and a pulse repetition frequency of 100 Hz, was used
in this study. The output power (2 W/cm2), duty cycle
(20%) and time (5 min) were selected according to the parameters
used in our previous study (10).
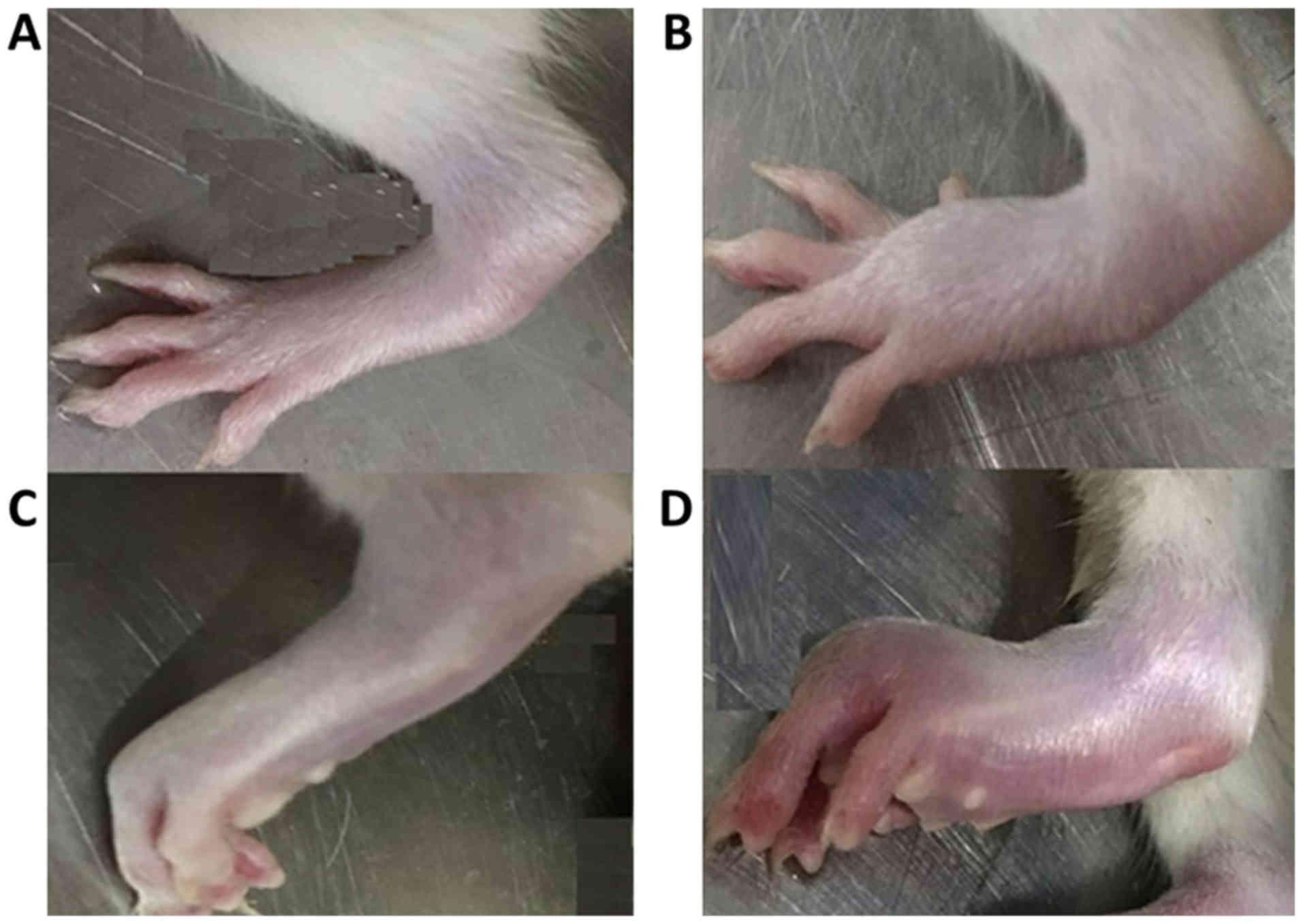
Arthritis scoring
After 2, 4 and 8 weeks of treatment, swelling of the
joints in the CIA rats was observed and recorded for arthritis
scoring. For each limb, arthritis was graded on a scale of 0 to 4
(Fig. 1): 0 represents a normal
joint; 1 represents redness or swelling of one type of joint (the
interphalangeal joint, metatarsophalangeal joint or the ankle
joint); 2 represents redness or swelling of two types of joints; 3
represents redness or swelling of all types of joints and; 4
represents severe swelling below the knee joint, with loss of
anatomical landmarks and the rat failing to bear weight. The scores
of all four limbs were added together to give a total score for
each rat; the highest possible score was 16.
Sample collection
Blood samples were collected in each group prior to
treatment, and the serum level of TNF-α was detected by ELISA. At
2, 4 and 8 weeks following treatment, rats were anesthetized by
isoflurane inhalation, placed in a supine position and blood
samples from both tibialis anterior muscles and ankle joints were
obtained. Blood samples were incubated at 4°C for 24 h, before they
were centrifuged at 4°C at 1,500 × g for 20 min. The serum was
subsequently collected and stored at −80°C. Muscles were fixed with
10% formalin for 48 h at room temperature. Joints were fixed in 10%
formalin for 72 h, and then decalcified in 10% EDTA for 60 days at
room temperature. Samples were embedded in paraffin and sectioned
to 4-µm thickness for hematoxylin and eosin (H&E) and
immunohistochemical (IHC) staining as described below.
Serological and pathological
analysis
The serum levels of TNF-α were determined using a
commercial ELISA kit (cat. no. RTA00; R&D Systems, Inc.,
Minneapolis, MN, USA), according to the manufacturer's protocol.
Differences in TNF-α levels were calculated by subtracting the
serum levels of TNF-α at different time points (2, 4, and 8 weeks)
from the serum levels prior to treatment.
H&E staining
Sections stained with H&E for 5 min and 5% eosin
staining for 5 sec at room temperature were observed using a light
microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan), and 5
typical fields for each sample were selected to observe joint
arthritis. Samples were scored from 0 to 3 individually depending
on the infiltration of inflammatory cells, synovial thickening,
cartilage degradation and bone destruction according to a previous
study (11).
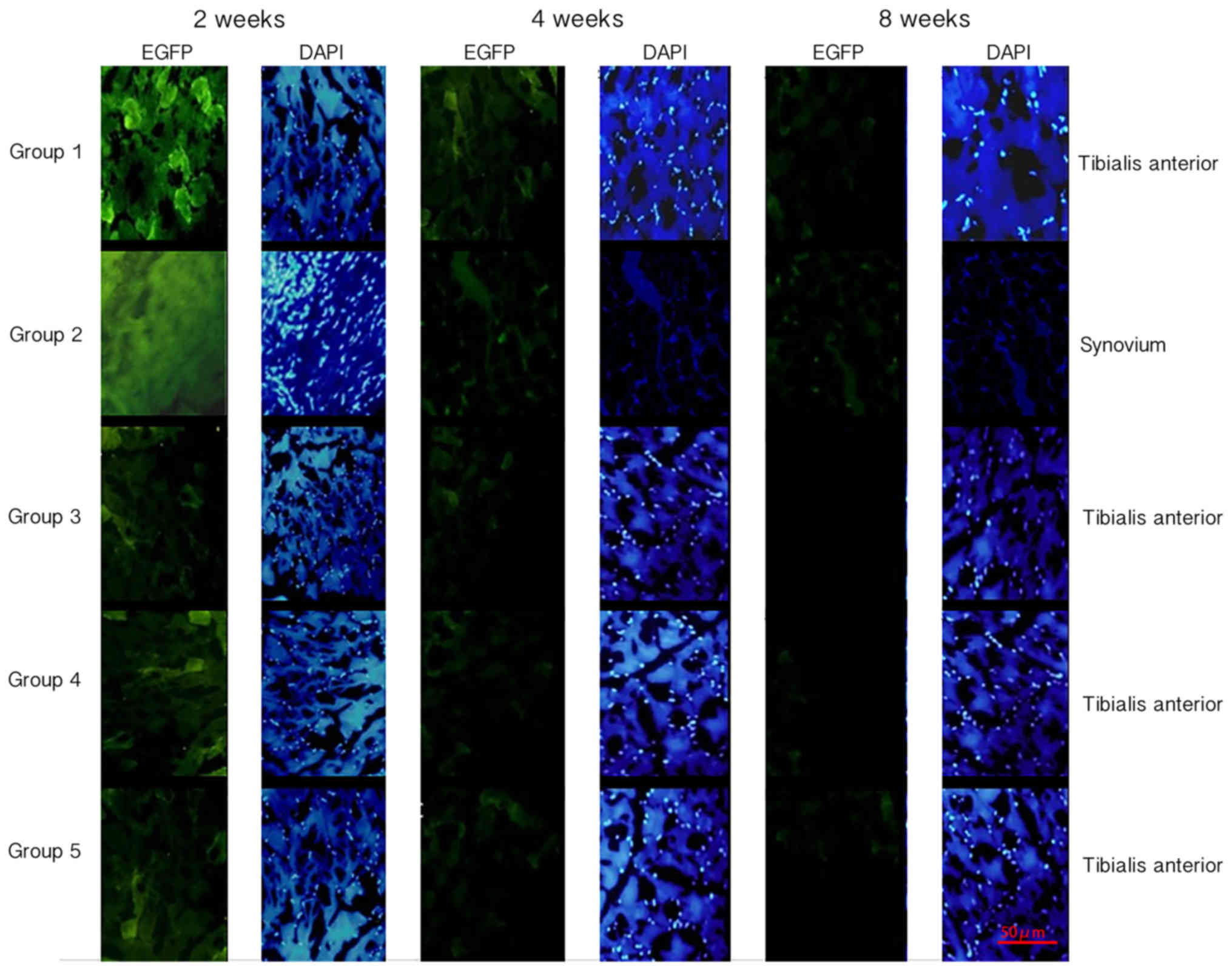
Fluorescence staining
In order to determine the plasmid transfection
efficiency, the muscle and synovial samples of rats in each group
were stained with DAPI (1 µg/ml) for 5 min at room temperature and
the expression of EGFP was evaluated. A total of ten fields of view
for each section were selected at random and observed using a
fluorescence microscope (Nikon ECLIPSE TE2000-U; Nikon Corporation,
Tokyo, Japan). The Image-Pro Plus 6.0 system (Media Cybernetics,
Inc., Rockville, MD, USA) was used to measure the mean fluorescence
intensity. The mean value of three measurements was used for
statistical analysis.
IHC staining
Tissue sections were deparaffinized and rehydrated
prior to IHC analysis. A total of 100 µl 10% goat serum (cat. no.
SP-KIT-B3; Fuzhou Maxim Biotechnology Development Co., Ltd.,
Fuzhou, China) was added to each section for blocking for 15 min at
room temperature. Samples were incubated with anti-TNF-α antibody
(1:100; cat. no. ab220210; Abcam, Cambridge, UK) at 4°C overnight.
The horseradish peroxidase conjugated goat secondary antibody (100
µl Bottle A from the ChemMate™ Envision; cat. no. K5007;
Dako Agilent Technologies, Inc., Santa Clara, CA, USA) was added
and incubated at 37°C for 45 min. DAB working liquid was then added
and staining was observed under a light microscope (magnification,
×100). The sections were then observed using a light microscope
(Olympus BX51; Olympus Corporation) and five random fields were
selected. The Image-Pro Plus 6.0 system (Media Cybernetics) was
used to measure the mean density of TNF-α staining, and the mean
values were calculated and used for statistical analysis.
Western blotting
The expression of TNF-α in the synovium was examined
by western blotting. Total protein was extracted using a EpiQuik
Whole Cell Extraction kit (EpiGentek Group, Inc., Farmingdale, NY,
USA) according to the manufacturer's protocol. The bicinchoninic
acid protein determination method (cat. no. P0012; Beyotime
Institute of Biotechnology, Haimen, China) was utilized and 40 µg
protein was subsequently loaded per lane. The samples were
separated by 5% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and then transferred to polyvinylidene fluoride
membranes. Membranes were subsequently blocked with 50 ml/l non-fat
milk for 2 h at room temperature. The rabbit anti-TNF-α polyclonal
antibody (1:500; cat. no. ab6671; Abcam) was added and incubated
for 2 h at room temperature. The membranes were then incubated with
horseradish peroxidase conjugated goat anti-rabbit IgG secondary
antibody (1:2,000; cat. no. ab6721; Abcam) for 1 h at 37°C. The
protein bands were normalized to GAPDH (1:500; cat. no. ab6671;
Abcam) was incubated for 2 h at room temperature. A Pierce™ ECL
Western Blotting Substrate (cat. no. 32106; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was utilized as the
visualization reagent. A BioSpectrum Imaging System (SmartChemi
500; Sagecreation, Beijing, China) was used to capture the
luminescent signals and optical density of the protein bands, which
were subsequently quantified using analysis software provided by
the manufacturer (Lane 1D™ 2011 edition; Beijing Sage
Creation Science Co., Ltd., Beijing, China).
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp., Armonk, NY, USA). The data are presented as
the mean ± standard deviation. Comparisons between two groups were
performed using the Wilcoxon rank sum test. Multigroup comparisons
were performed using the Kruskal-Wallis H rank sum test. Pairwise
comparisons were made using the Nemenyi test. P<0.05 was
considered to indicate a statistically significant difference.
Results
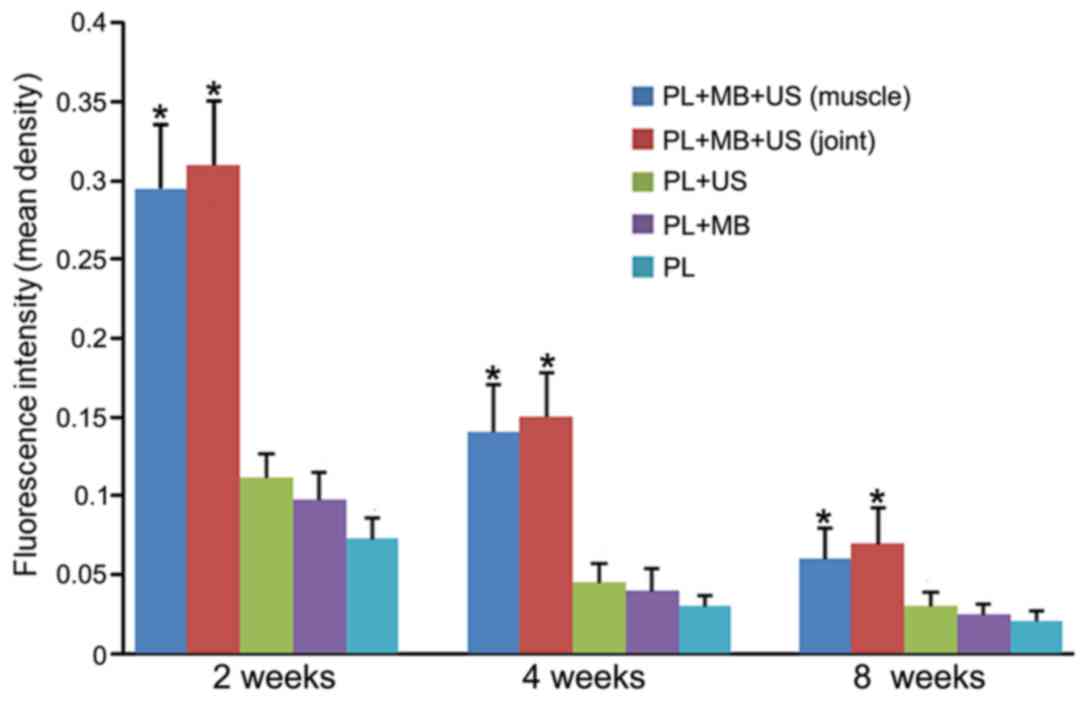
Transfection efficiency at different
time points
The plasmids employed in the present study were
labeled with EGFP; therefore, the level of green fluorescence was
observed under a fluorescence microscope following transfection. In
addition, DAPI was used to label double-stranded DNA in the nucleus
and displayed blue fluorescence under the microscope. Quantitative
analysis of green fluorescence was performed using Image-Pro Plus
software. Green fluorescence was observed in the muscle tissues of
groups 1, 3, 4 and 5 at each time point, and the fluorescence
intensity at 2 weeks was greater than that at 4 and 8 weeks for all
groups (Figs. 2 and 3). The fluorescence intensity in group 1
was greater than that of the other groups at all time points, and
the difference was statistically significant (P<0.05; Figs. 2 and 3). In group 2, green fluorescence was
observed in the synovial tissue, and the intensity showed no
significant difference when compared with group 1 (Figs. 2 and 3). The control group exhibited no
fluorescence in the muscle or joint tissues (data not shown).
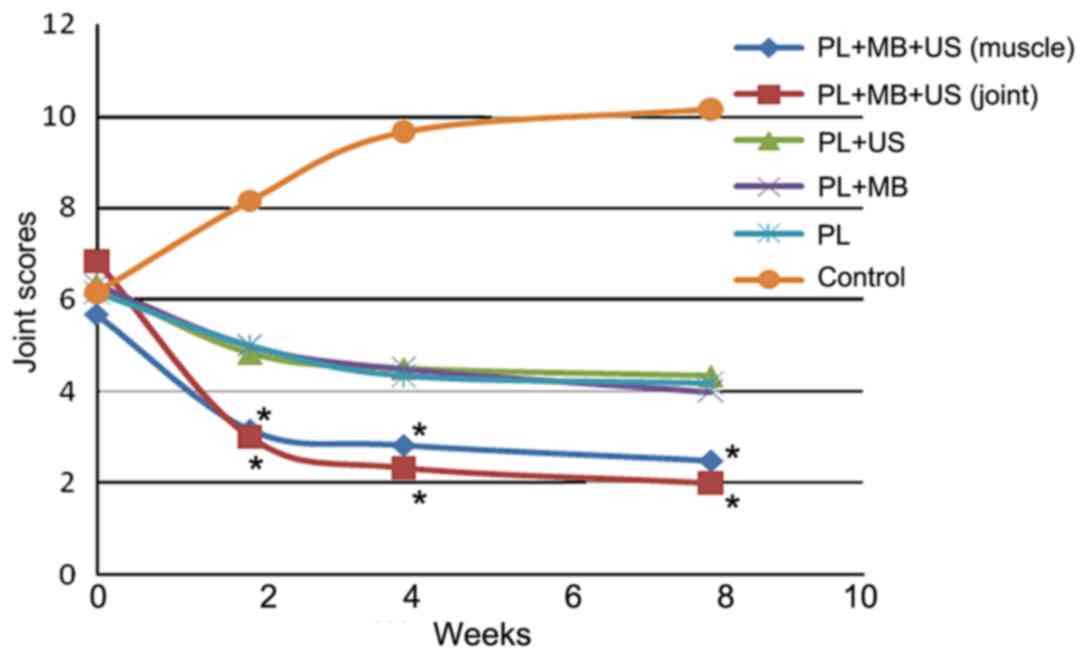
Arthritis scoring
Arthritis was scored at 2, 4 and 8 weeks following
treatment. According to the results, the treatment groups exhibited
decreasing arthritis scores over time (Fig. 4). The most marked reduction occurred
at 4 weeks, after which point, a gradual decline was observed until
the rate eventually stabilized. The score reduction in groups 1 and
2 was significantly greater than that in the other groups at 2, 4
and 8 weeks (P<0.05), regardless of whether the injection site
was the muscle or joint. The score reductions among groups 3, 4 and
5 were not significantly different (P>0.05; Fig. 4). The scores in the control group
exhibited an initial increase and then stabilized over time;
however, these scores remained higher than those of the other
experimental groups at 2, 4 and 8 weeks following treatment
(Fig. 4).
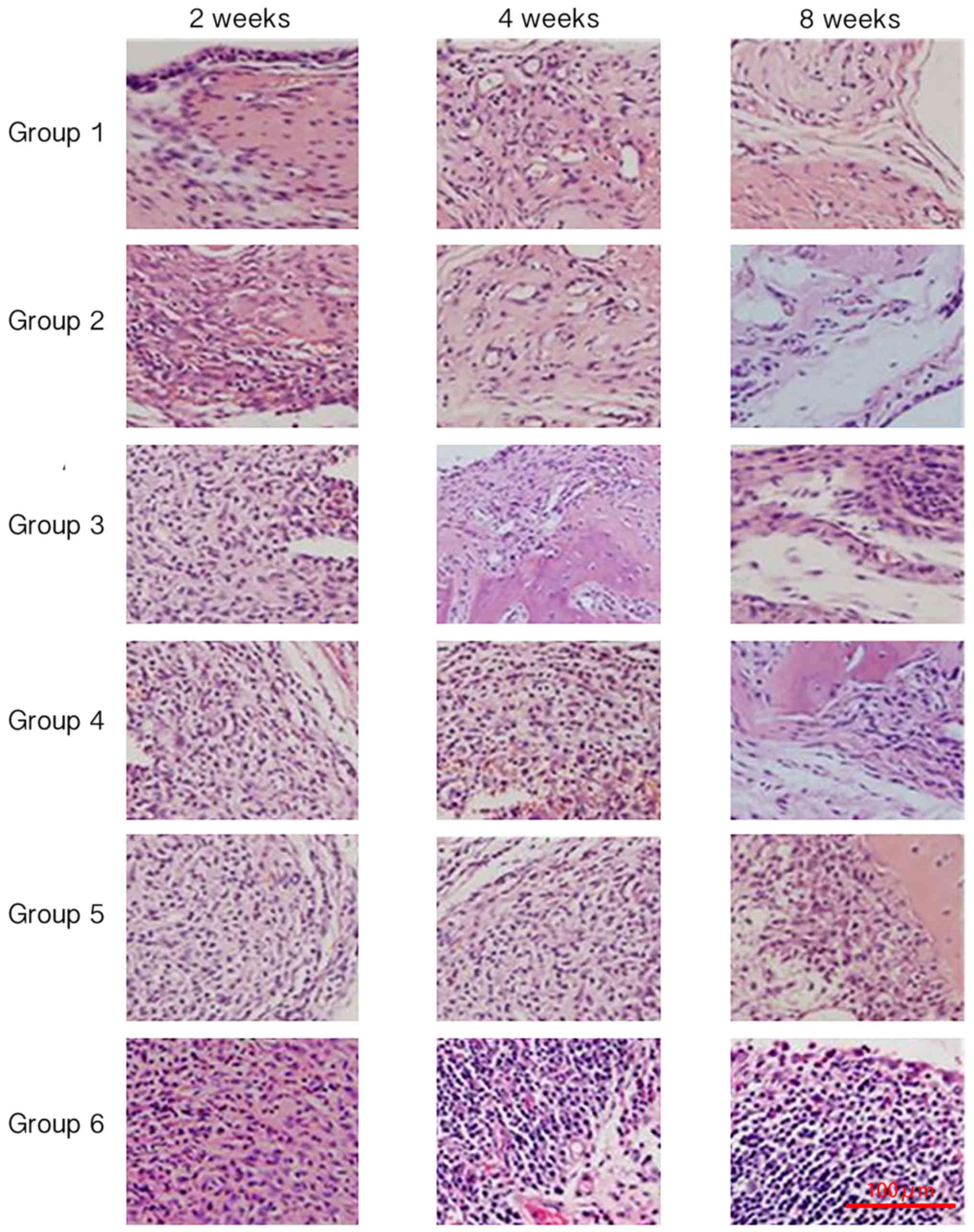
Pathological analysis using H&E
staining
Inflammatory cell infiltration and synovial
hyperplasia were observed to evaluate synovial inflammation at
different time points among the different groups. At 2 weeks of
treatment, significant cellular infiltration was observed in all
groups; most notably in the control group (Fig. 5). At 2, 4 and 8 weeks of treatment,
the inflammatory reaction was reduced in all treatment groups.
Groups 1 and 2 exhibited the lowest number of inflammatory cells
and the least synovial inflammation, and there was no marked
difference between groups 1 and 2 (Fig.
5). Groups 3, 4 and 5 exhibited more severe inflammation when
compared with groups 1 and 2, and group 6 demonstrated the most
severe inflammatory reaction (Fig.
5).
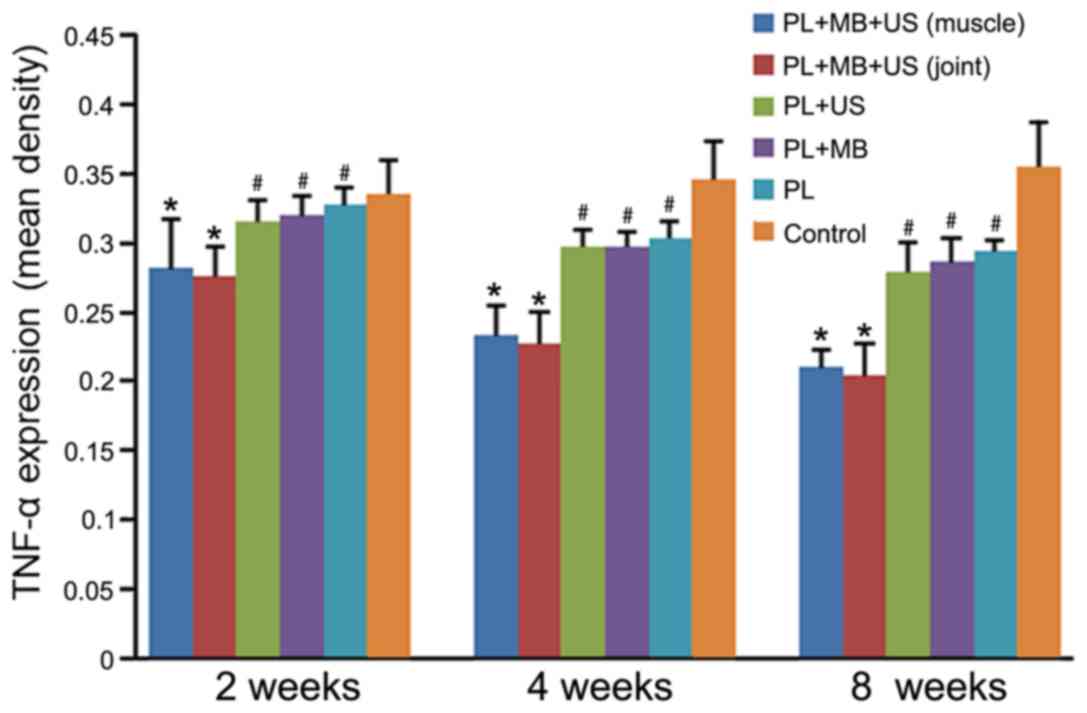
Pathological analysis by IHC
staining
IHC staining was performed in tissue sections
obtained at different time points and from different groups, and
the expression of TNF-α was detected. The results demonstrated that
the staining intensity in groups 1–5 decreased gradually with
increasing treatment time and reached their lowest point at 8 weeks
following treatment (Fig. 6).
However, group 6 exhibited a gradual increase in staining
intensity, reaching a peak at 8 weeks following treatment, and was
higher than that of the other groups (P<0.05; Fig. 6). The staining intensity in groups 1
and 2 was significantly lower than the other four groups, and no
significant difference between groups 1 and 2 was observed
(P>0.05). The staining intensity of groups 3, 4 and 5 groups
remained lower than that of group 6 at all the corresponding time
points (P<0.05), but no significant difference among these
groups was identified (P>0.05; Fig.
6).
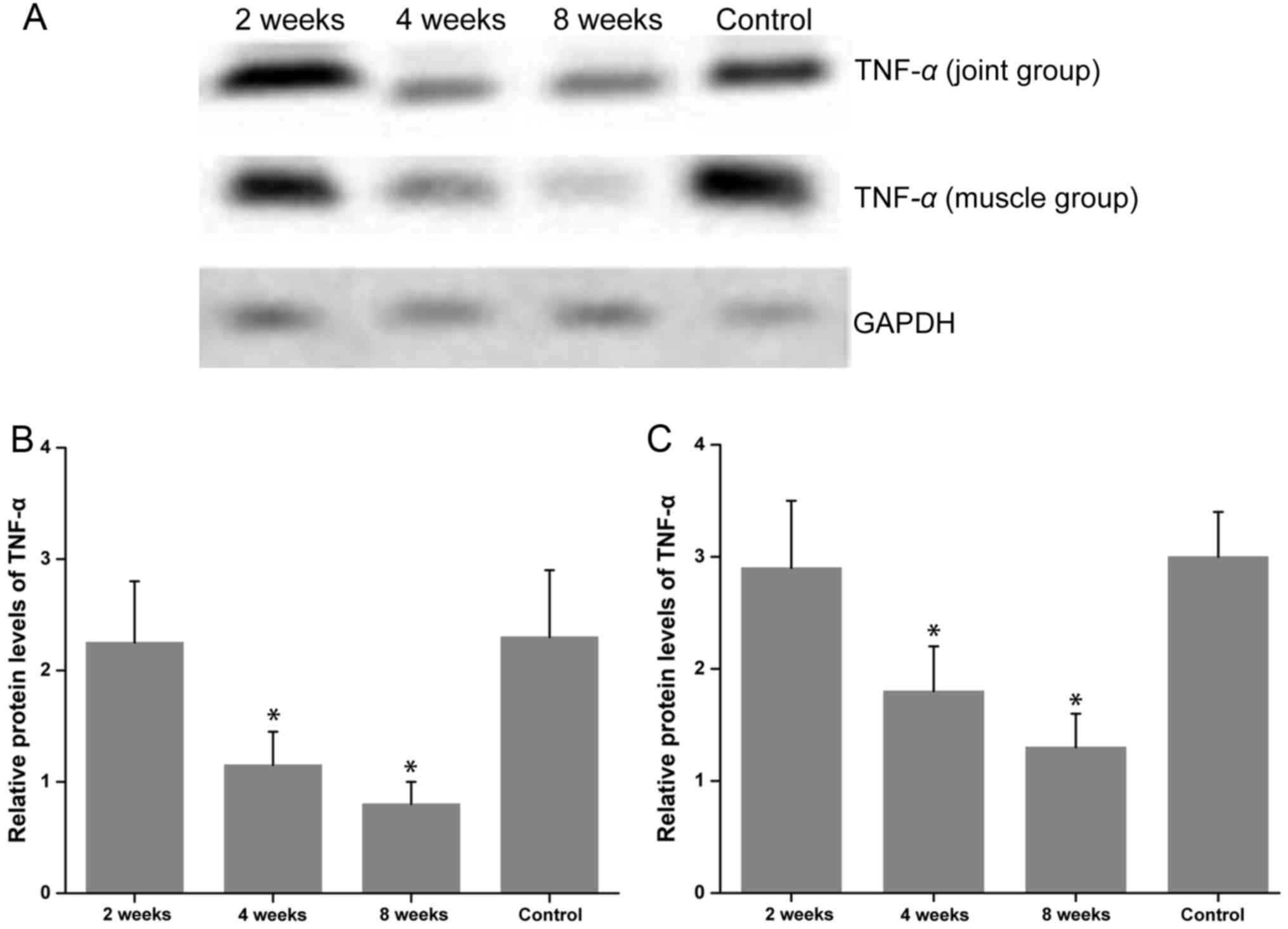
Western blotting analysis
The western blotting results demonstrated that the
expression of TNF-α in groups 1 and 2 were markedly decreased at 4
and 8 weeks post-treatment when compared with group 6 (P<0.05;
Fig. 7). In addition, TNF-α levels
gradually decreased over time and reached their lowest point at 8
weeks following treatment. In group 1, TNF-α levels were slightly
higher than in group 2 at all time points, but no significant
difference was identified between them (P>0.05; Fig. 7).
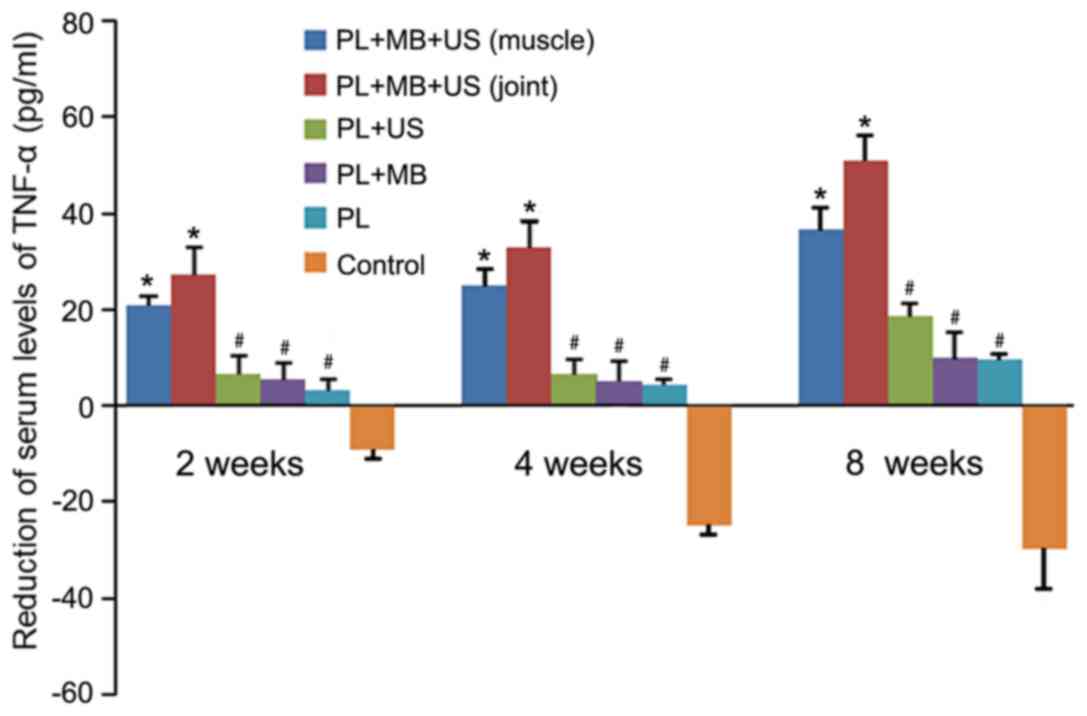
Serological analysis
Inflammation was evaluated by measuring the serum
levels of TNF-α at different time points among the experimental
groups. The results of the ELISA demonstrated that serum levels of
TNF-α were negatively correlated with treatment duration (Fig. 8). The concentration was at its
highest at the beginning of the treatment, and then gradually
decreased to its lowest point at 8 weeks following treatment. At 2,
4 and 8 weeks following treatment, a significant reduction in TNF-α
in groups 1 and 2 was observed when compared with the remaining
groups (P<0.05; Fig. 8). Group 1
exhibited a greater reduction in serum TNF-α levels when compared
with group 2; however, this did not reach statistical significance
(P>0.05). When compared with group 6, TNF-α levels in groups 3,
4 and 5 were significantly lower (P<0.05); however, no
significant difference was observed among groups 3, 4 and 5
(P>0.05; Fig. 8). The original
level of serum TNF- α was ~50 pg/ml (data not shown), as the
duration of treatment increased, the serum level of TNF-α in group
6 gradually increased by ~40 pg/ml, which indicated a plateaued at
~90 pg/ml (Fig. 8).
Discussion
RA is a common inflammatory joint disease. It is an
autoimmune disease with a complex pathogenesis and is thought to be
associated with abnormal regulation of the internal environment
(1). The main clinical symptoms of
RA include joint pain and stiffness accompanied by movement
disorders. Severe symptoms may affect a patients' quality of life,
and may even lead to disability and dysfunction, especially among
the young and middle-aged individuals. Therefore, it is of great
clinical significance to investigate novel treatment strategies for
RA. Although the etiology of RA is currently unclear, immune,
genetic, environmental and lifestyle factors (such as smoking) are
thought to be closely associated to the development of RA. At
present, it is generally considered that RA development involves an
imbalance between autoimmune induction and
pro-inflammatory/anti-inflammatory factors, which leads to chronic
inflammation (3,12). According to their mechanisms of
action, clinical medicines for RA treatment are classified into
nonsteroidal anti-inflammatory drugs, glucocorticoids,
disease-modifying anti-rheumatic drugs and TNF-α inhibitors.
However, despite these various treatment options for RA, their
curative effects are variable and far from satisfactory.
As a cutting-edge treatment, gene therapy delivers
cDNA encoding therapeutic proteins to cells in the target tissue
and facilitates continuous and efficient local secretion of gene
products. This negates the disadvantages of direct injection and
provides sustained treatment for the disease (5). There are currently a number of gene
therapy strategies; cDNA transfection can be administered
physically, chemically, or biologically. However, these methods
differ from each other in terms of efficiency, operation
feasibility, as well as therapeutic safety. For instance, although
chemical transfection is cheaper, easier to operate and requires no
special experimental conditions, the efficiency is low and
liposomes may be toxic to cells (5).
Physical transfection includes electroporation, cell microinjection
and gene gun techniques. These techniques provide high transfection
efficiencies; however, they also require more advanced equipment
and more complex surgical tools. Furthermore, they use high
pressure pulses that may cause significant cell death (13,14).
Biological methods primarily involve viral vector-mediated
transfection. This technique is highly efficient, easy to operate
and involves continuous and stable expression of exogenous genes
either in vitro or in vivo. Since viral vectors only
exert their function following integration with the nuclear genome
of host cells, this technique introduces a risk of carcinogenesis
and teratogenicity (9,10). Therefore, non-viral vectors, such as
plasmids, are considered to be safer and have lower immunogenicity
when used to carry therapeutic genes; however, their transfection
efficiency, duration of action, and expression levels are far lower
than those of viral vectors.
UTMD is a new combined transfection method that uses
microbubbles as the vector. Following intravenous injection or
local administration of plasmids encoding the genes of interest,
the cavitation effects of ultrasound are utilized to increase
membrane permeability, promote gene uptake and optimize vector
delivery, which in turn enhances the efficiency and safety of
transfection (7,15,16). In
a previous study, IGF-1 cDNA was successfully transfected into rat
Achilles tendons using UTMD, which promoted tendon regeneration and
provided novel insights into the clinical treatment of tendon
injury (9). An additional study also
confirmed that UTMD mediated the successful transfection of the
EGFP gene into the inflammatory synovium and muscle of
antigen-induced arthritis rabbits, which resulted in the continuous
production of fluorescent proteins (10). The optimal conditions were determined
to be a 1-MHz ultrasound pulse applied for 5 min with a power
output of 2 W/cm2 and a 20% duty cycle. Under these
conditions, EGFP was expressed at the highest levels and no normal
tissue was damaged (10).
As RA is a systemic disease, the introduction of
UTMD-based gene therapy for RA may confer a more favorable
treatment outcome, and also provide novel insights for the clinical
treatment of RA. In the present study, UTMD was utilized to
transfect the TNFR gene into muscle cells or the synovium of the
inflammatory joints of CIA rats. The transfection efficiency of
different methods at different sites was compared, and inflammatory
cytokines and the severity of arthritis were evaluated to identify
an optimal strategy for using UTMD in the treatment of RA. The CIA
model was established based on the principle of immune inflammation
induced by alloantigens, and its clinical symptoms primarily
include the development of multi-terminal arthritis. The model was
first established by Trentham et al in 1977 (17), and it has now become the most
commonly used arthritis model (16).
Yovandich et al (18) injected plasmids into the knee joints
of rabbits and rats. The results revealed that the reporter gene
was expressed in the synovial tissues; however, the transfection
efficiency was relatively low and expression lasted for only 2–5
days, after which point the protein was absorbed and degraded
rapidly by the synovial tissues. Lawrie et al (19) investigated ultrasonic
irradiation-combined naked DNA transfection and observed that gene
expression in vascular endothelial cells was elevated 10-fold. In a
subsequent study, Lawrie et al (20) combined microbubbles and plasmids
together with ultrasonic irradiation and obtained an increased
expression level, as well as a plasmid transfection efficiency of
300-fold higher than that of naked DNA transfection. In a previous
study performed by our research group, microbubbles and plasmids
were injected simultaneously into the knee joints of rabbits to
optimize the transfection conditions. Further experiments involving
the combined administration of ultrasonic irradiation with
microbubbles and plasmids, demonstrated a much higher transfection
efficiency of EGFP when compared to ultrasonic irradiation and
plasmids, plasmids and microbubbles, or plasmid only methods
(3).
The present study used an EGFP-labeled TNFR plasmid.
The two genes are simultaneously replicated and expressed, and the
transfection efficiency of the TNFR gene was therefore evaluated by
fluorescence intensity analysis and DAPI staining. According to the
results, fluorescence was detected in the muscles and synovium,
indicating successful transfection of the gene. The PL+MB+US muscle
group exhibited significantly higher fluorescence and staining
intensities when compared with the other groups at the
corresponding time points; however, no significant difference with
the PL+MB+US joint group was observed. The PL+US, PL+MB and PL
groups exhibited lower transfection efficiencies; however, no
significant difference among these three groups was observed.
Therefore, UTMD significantly improved gene transfection efficiency
in the muscles and inflammatory synovium.
Previous studies have evaluated the level of
inflammation in CIA rat models using an arthritis scoring system.
It is a simple and common method to determine relief or aggravation
of joint inflammation, by assessing the degree of joint redness or
swelling (21,22). In the present study, the arthritis
scores in the two PL+MB+US groups were the lowest at all time
points with the most significant reductions compared with other
treatment groups and the control. This indicated that UTMD-mediated
transfection was associated with the most significant remission of
inflammation and the most positive treatment outcome. The PL+US,
PL+MB and PL groups were not significantly different in terms of
the reduction in arthritis scores. As the control group was
untreated, its score increased gradually and plateaued at a certain
level.
In the normal synovial tissues of rats, there is a
low number of synovial cells and no inflammatory cell infiltration
or significant neovascularization is apparent. Synovitis is caused
by the activation and infiltration of systemic or local monocytes,
macrophages and mast cells, and by the formation of new vessels. In
the inflammatory synovium, massive inflammatory cell infiltration
and synovial thickening, as well as neovascularization were
observed (23). Using H&E
staining, the degree of inflammation can be determined by observing
the percentage of inflammatory cells and the thickness of the
synovium. In the present study, the PL+MB+US groups exhibited the
fewest inflammatory cells, no obvious synovial hyperplasia and the
mildest inflammation. No significant difference was identified
between the two PL+MB+US groups with different injection sites. The
control group presented with the most severe pathology with the
highest percentage of inflammatory cells, the thickest synovium and
the most significant inflammation.
Under inflammatory conditions, synoviocytes secrete
factors such as TNF-α, and RA patients show high levels of TNF-α in
their serum and synovium of inflammatory joints (24). By stimulating osteoclast formation,
TNF-α induces bone invasion and joint injury, which subsequently
aggravates inflammation (3).
Therefore, TNF-α is considered to be a pathogenic factor and a
marker. In clinical practice, inflammatory remission or aggravation
could be evaluated by measuring the levels of TNF-α in the synovium
or blood. Therefore, Lee et al (23) evaluated the effects of TNF-α
silencing in RA treatment and observed that the experimental group
exhibited a significant reduction in TNF-α levels when compared to
the control, and also confirmed that the serum levels of TNF-α were
positively associated with the level of inflammation. In the
current study, inflammatory remission was evaluated and the
treatment efficacies were compared among the different treatment
groups by measuring the levels of TNF-α. According to the results,
the PL+MB+US groups exhibited the greatest decrease in TNF-α levels
when compared with the remaining groups, indicating that UTMD
performed better than all other transfection modalities. Although
the muscle injection group demonstrated a smaller reduction
compared with the joint injection group, the difference was not
statistically significant. The authors hypothesize that this be
associated with the enriched blood supply in the inflammatory joint
synovium, which may promote the absorption of transfected genes.
However, this remains to be verified in future studies with larger
sample sizes.
Olkkonen et al (25) identified the presence of TNF-α in the
synovium of RA patients, and the degree of arthritis or synovitis
was evaluated by measuring the TNF-α concentration using IHC or
additional methods. The results of the present study demonstrated
that TNF-α was detected at all time points, and the staining
intensity in the treatment groups was decreased with increasing
treatment duration, which suggests that treatment resulted in
varying degrees of remission. Furthermore, the staining intensity
of the PL+MB+US groups was significantly lower than that of the
remaining groups, and the joint injection group exhibited a greater
reduction when compared with the muscle injection group; however,
the difference was not significant. The staining intensity of TNF-α
in the control rats increased gradually with increasing treatment
duration, no significant variation was observed following 8 weeks
of treatment (and 12 weeks after the model was established). This
suggests that, inflammation will progress to a chronic phase at 3
months without treatment. The results of IHC analysis were
consistent with those of the serum ELISA analysis, which confirmed
that UTMD-mediated transfection improved synovial and systemic
inflammation and was associated with an improved disease outcome
when compared with other transfection methods for the treatment of
arthritis.
However, the present study has some limitations.
Namely, evaluation of H&E staining was based on the observation
of inflammatory infiltration and synovial hyperplasia, which is
relatively subjective, thus objective criteria may be required for
future studies. In addition, the observation period was relatively
short and will be extended in future studies.
In conclusion, the results of the present study
demonstrated that UTMD-mediated gene transfection could
successfully introduce the TNFR gene into muscle cells and the
inflammatory synovium, and the transfection efficiency was
significantly higher when compared with other transfection
modalities. The transfected TNFR gene was successfully expressed at
a continuous level in CIA rats, and improved the symptoms of
arthritis, as well as reduced TNF-α expression in the synovial
tissues and peripheral blood. No significant differences in
treatment outcome were observed between the two sites of injection
(muscle or joint).
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (81671696).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW conducted the animal experiments, analyzed and
interpreted the experimental data and was a major contributor in
writing the manuscript. XT performed the animal experiments and
analyzed the pathological data. XX performed the animal experiments
and revised the manuscript. YT supervised the study design and
performed the animal experiments. LQ contributed to the conception
and design of the study, interpretated the data and critically
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in strict
accordance with institutional regulations and the study was
approved by the Sichuan University Research Committee for Animal
Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UTMD
|
ultrasound-mediated microbubble
destruction
|
|
TNFR
|
TNF-α receptor
|
|
CIA
|
collagen-induced arthritis
|
|
EGFP
|
enhanced green fluorescent protein
|
|
RA
|
rheumatoid arthritis
|
|
MB
|
microbubble
|
|
US
|
ultrasound
|
References
|
1
|
Boissier MC: Cell and cytokine imbalances
in rheumatoid synovitis. Joint Bone Spine. 78:230–234. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boissier MC, Semerano L, Challal S,
Saidenberg-Kermanac'h N and Falgarone G: Rheumatoid arthritis: From
autoimmunity to synovitis and joint destruction. J Autoimmun.
39:222–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moelants EA, Mortier A, Van Damme J and
Proost P: Regulation of TNF-α with a focus on rheumatoid arthritis.
Immunol Cell Biol. 91:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohan AK, Coté TR, Siegel JN and Braun MM:
Infectious complications of biologic treatments of rheumatoid
arthritis. Curr Opin Rheumatol. 15:179–184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghivizzani SC, Oligino TJ, Glorioso JC,
Robbins PD and Evans CH: Direct gene delivery strategies for the
treatment of rheumatoid arthritis. Drug Discov Today. 6:259–267.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Wang J, Satterle A, Wu Q, Wang J and
Liu F: Gene transfer to skeletal muscle by site-specific delivery
of electroporation and ultrasound. Biochem Biophys Res Commun.
424:203–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walton CB, Anderson CD, Boulay R and
Shohet RV: Introduction to the ultrasound targeted microbubble
destruction technique. J Vis Exp. (pii): 2963–2011. PubMed/NCBI
|
|
8
|
Wan C, Qian J, Li F and Li H:
Ultrasound-targeted microbubble destruction enhances
polyethylenimine-mediated gene transfection in vitro in human
retinal pigment epithelial cells and in vivo in rat retina. Mol Med
Rep. 12:2835–2841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang Y, Leng Q, Xiang X, Zhang L, Yang Y
and Qiu L: Use of ultrasound-targeted microbubble destruction to
transfect IGF-1 cDNA to enhance the regeneration of rat wounded
Achilles tendon in vivo. Gene Ther. 22:610–618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiang X, Tang Y, Leng Q, Zhang L and Qiu
L: Targeted gene delivery to the synovial pannus in antigen-induced
arthritis by ultrasound-targeted microbubble destruction in vivo.
Ultrasonics. 65:304–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu W, Sun X, Zhu L, Gan Y, Baiwu R, Wei
J, Li Z, Li R and Sun J: A novel BLyS peptibody down-regulates B
cell and T helper cell subsets in vivo and ameliorates
collagen-induced arthritis. Inflammation. 39:839–848. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Tian F and Wang F: Rheumatoid
arthritis-associated microRNA-155 targets SOCS1 and upregulates
TNF-α and IL-1β in PBMCs. Int J Mol Sci. 14:23910–23921. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Newman CM and Bettinger T: Gene therapy
progress and prospects: Ultrasound for gene transfer. Gene Ther.
14:465–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan K, Cheang P, Ho IA, Lam PY and Hui KM:
Nanosized bioceramic particles could function as efficient gene
delivery vehicles with target specificity for the spleen. Gene
Ther. 14:828–835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anderson CD, Urschitz J, Khemmani M, Owens
JB, Moisyadi S, Shohet RV and Walton CB: Ultrasound directs a
transposase system for durable hepatic gene delivery in mice.
Ultrasound Med Biol. 39:2351–2361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Myers LK, Rosloniec EF, Cremer MA and Kang
AH: Collagen-induced arthritis, an animal model of autoimmunity.
Life Sci. 61:1861–1878. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trentham DE, Townes AS and Kang AH:
Autoimmunity to type II collagen: An experimental model of
arthritis. J Exp Med. 146:857–868. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yovandich J, O'Malley B Jr, Sikes M and
Ledley FD: Gene transfer to synovial cells by intra-articular
administration of plasmid DNA. Hum Gene Ther. 6:603–610. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lawrie A, Brisken AF, Francis SE, Tayler
DI, Chamberlain J, Crossman DC, Cumberland DC and Newman CM:
Ultrasound enhances reporter gene expression after transfection of
vascular cells in vitro. Circulation. 99:2617–2620. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lawrie A, Brisken AF, Francis SE,
Cumberland DC, Crossman DC and Newman CM: Microbubble-enhanced
ultrasound for vascular gene delivery. Gene Ther. 7:2023–2027.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang G, Xu Z, Huang Y, Duan X, Gong W,
Zhang Y, Fan J and He F: Curcumin protects against collagen-induced
arthritis via suppression of BAFF production. J Clin Immunol.
33:550–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choy E: Understanding the dynamics:
Pathways involved in the pathogenesis of rheumatoid arthritis.
Rheumatology (Oxford). 51 Suppl 5:v3–v11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SJ, Lee A, Hwang SR, Park JS, Jang J,
Huh MS, Jo DG, Yoon SY, Byun Y, Kim SH, et al: TNF-α gene silencing
using polymerized siRNA/thiolated glycol chitosan nanoparticles for
rheumatoid arthritis. Mol Ther. 22:397–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lipsky PE, Davis LS, Cush JJ and
Oppenheimer-Marks N: The role of cytokines in the pathogenesis of
rheumatoid arthritis. Springer Semin Immunopathol. 11:123–162.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olkkonen J, Kouri VP, Hynninen J,
Konttinen YT and Mandelin J: Differentially expressed in
chondrocytes 2 (DEC2) increases the expression of IL-1β and is
abundantly present in synovial membrane in rheumatoid arthritis.
PLoS One. 10:e01452792015. View Article : Google Scholar : PubMed/NCBI
|