Introduction
Diabetic foot ulcer (DFU) is a disorder observed
primarily in developing countries. The blood supply in the
extremities of patients with diabetes is poor, thus leading to the
development of DFUs (1). The wounds
require a long time to heal and may be incurable; dry gangrene can
also develop as a result of DFU, which may lead to amputation in
severe cases (1). Diabetes
associated with acquired coagulopathies, particularly those caused
by vitamin K deficiency or liver disease, may lead to excessive
bleeding from the DFU and delay the healing process (2). In these cases, the use of fibrin
adhesives and collagen matrices to the affected area are required
(3). Such wounds can be infected by
various pathogens that are difficult to treat, including fungi from
the genera Fusarium or Aspergillus (4,5). The
study of the pathogenesis of DFU has focused on poor wound healing
and delayed wound healing due to dry gangrene (6,7).
Numerous studies have attempted to elucidate the mechanisms
underlying refractory DFU and signal transduction pathways in DFU
lesions; however these studies have not been successful (8,9). A study
suggested that offloading, debridement and moist dressing are
currently used to treat DFUs with little success (9). Unsuccessful DFU treatment is caused by
a rage of factors, including the varying comorbidities observed for
each diabetic patient (10). Poor
wound healing is considered to be associated with poor glycemic
control, refractory infection, anti-inflammatory cytokines and a
relative or absolute deficiency of corresponding cytokine
receptors, and vascular lesion (11). Refractory DFU lesions primarily form
due to decreased expression levels of epidermal growth factor (EGF)
and its receptor in DFU lesions (12). It has been demonstrated that applying
active EGF to the DFU lesion may promote wound healing (13,14). EGF
may also promote the proliferation of gliocytes and fibroblasts,
neo-epidermal thickening, peripheral nerve regeneration, as well as
mediate the proliferation, migration and differentiation of
gliocytes and fibroblasts (15).
Previous studies have demonstrated that EGF stimulates protein
synthesis by modulating the replication and signal transduction of
epidermal cell DNA and RNA (16,17).
Previous studies investigated the repair function of the EGF
receptor in the colonic mucosa of patients with ulcerative colitis
(18–21). The authors demonstrated that EGF was
involved in repairing inflamed mucous membranes in these patients.
On the basis of these previous studies, and in order to aid the
development of novel pharmaceutical devices based on nanotechnology
for the treatment of refractory or non-healing DFU, the present
study attempted to elucidate the mechanism underlying the
therapeutic effect of EGF on DFU.
Materials and methods
Animals
A total of 65 healthy New Zealand white rabbits
(age, 2 years old; male to female, 1:1; weight, 2.5–3.0 kg) were
provided by the Jiaxiang Xian Yongwang Rabbit Sales Center (Jining,
China). All the rabbits were conventionally raised with natural
light, laminar circulation filter air, an indoor temperature of
25±1°C and the humidity at 40±5% (22–24). All
animals had free access to food and water. The present study was
approved by the Ethics Committee of The Affiliated Qingdao Hiser
Hospital of Qingdao University (Qingdao, China).
Experimental reagents
The following materials and reagents were used in
the present study: EGF solution (Wuhan Healthgen Biotechnology
Corp., Wuhan, China); Sumianxin (Jilin Huamu Animal Health Product
Co., Ltd., Changchun, China); alloxan monohydrate (Hefei Bomei
Biotechnology Co., Ltd., Hefei, China); glucometer and blood
glucose kit (Hua Xia Kangning Xuzhou Medical Technology Co., Ltd.,
Xuzhou, China); RNA PCR kit (AMV) Ver.3.0 (Takara Biotechnology
Co., Ltd., Dalian, China); and the RNAprep Pure Tissue kit [cat.
no. DP431; Tiangen Biotech (Beijing) Co., Ltd., Beijing,
China].
Experimental equipment
The following equipment were used in the present
study: NanoDrop ND-1000 Ultraviolet Spectrometry Photometer
(Shanghai Spectrum Instruments, Co., Ltd., Shanghai, China);
CO2 incubator (Shanghai SANTN Instruments Co., Ltd.,
Shanghai, China); a gel imaging system (UVP, Inc., Upland, CA,
USA); a −80°C freezer (Wuxi Guanya Refrigeration Technology Co.,
Ltd., Wuxi, China); an light microscope; a transmission electron
microscope; Nucleic Acid and Protein Analyzer (Suzhou Qile Electron
Technology Co., Ltd., Kunshan, China); and a gradient PCR
instrument (Beijing Bohui Innovation Technology Co., Ltd., Beijing,
China).
Induction of type II diabetes
New Zealand rabbits were fed with a
high-fat/high-sucrose diet for 2 months prior to the induction of
type II diabetes, as previously described (25,26).
Following this 2-month period, diabetes was induced by
administering rabbits with 50 mg/kg body weight alloxan monohydrate
(2.5%) via the ear of each rabbit every 3 days following 6 h of
fasting. On day 10, the rabbits were fasted overnight (8–10 h with
water) prior to the collection of blood samples. The rabbits that
exhibited a fasting blood glucose level >11.1 mmol/l were
admitted to the type II diabetes model. A total of 48 rabbits were
included in the present study as 17 rabbits did not meet the
experimental standard.
Experimental groups and therapeutic
measures
The remaining 48 type II diabetes rabbits were
randomly and equally divided into treatment and control groups. The
legs of all rabbits were disinfected with 100 ml iodophor solution
(Shandong Lierkang Medical Technology Co., Ltd., Dezhou, China) and
animals were anesthetized using 200 mg/kg Sumianxin administered to
the hind leg, were muscle was abundant. A 10×10 mm section of
full-thickness skin was excised, followed by a radial debridement
of the wound. For the treatment group, 100 mg/l EGF solution was
applied to the wound every day for 1 month; continuous contact
between the EGF solution and wound was maintained as described
previously (27). No treatment was
administered to the control group. During the experiment, the
feeding and sanitary conditions of the 2 groups were the same. A
digital camera was used to capture images of the DFU wound, and
record the wound healing rate and healing area in real time once
every five days, using Masson and hematoxylin and eosin (HE)
staining. The wound healing rate calculation formula was as
follows: Wound healing rate = (original wound area - non healing
area)/original wound area.
Histological analysis
Following 20 days of treatment, a 2-mm region of
newly produced granulation tissue from the DFU site was collected
from all rabbits in both groups. Prior to tissue collection, the
rabbits were injected with 200 mg/kg body weight Sumianxin
intramuscularly (22,23). The collected samples were irrigated
with distilled water and divided into four sections. These tissue
sections were then used for Masson staining, HE staining, electron
microscopy observation and reverse transcription-polymerase chain
reaction (RT-PCR) analysis of EGF mRNA levels.
Masson staining
Healing and granulation tissue specimens were
removed from the surface of the diabetic foot ulcer. The samples
were fixed in 10% formaldehyde solution, embedding in paraffin and
dewaxed (thickness, 6 µm) Staining was performed according to the
manufacturer's protocol connective tissue Masson staining kit
(Abcam, Cambridge, UK). Samples were incubated with Masson staining
solution at 37°C for 5 min. Following two washes with 0.2% acetic
acid for 5 min, the sections were stained with 1% aniline for 5 min
at room temperature. Following two more washing steps with 0.2%
acetic for 5 min, section were dehydrated 10 sec at room
temperature with absolute alcohol and rendered transparent 5 min at
room temperature using xylene. Sections were observed using a light
microscope (magnification, ×400).
HE staining
After 15 days, healing specimens were removed from
the surface of the diabetic foot ulcer of the experimental group
and fixed (10 min; 20°C) in 40 g/l paraformaldehyde solution.
Gradient ethanol dehydration (35°C, 1.5 h) and xylene (30 min;
35°C) was used in the sample preparation prior to embedding the
specimens in paraffin (60 min, 56°C). The specimens were sliced
into 5-µm-thick sections and xylene was used to dewax the samples
(37°C, 10 min). They were stained with HE for 5 min 35°C. The
growth of fibroblasts and blood vessels was observed using a light
microscope (magnification, ×1,600).
Molecular level detection
Total RNA was extracted using TRIzol reagents
(Invitrogen; Thermo Fisher Scientific, Inc., Inc., Waltham, MA,
USA) from the granulation tissue samples. This was performed
according to the manufacturer's instructions. The synthesis of cDNA
was carried out using a reverse transcription kit (RevertAid First
Strand cDNA Synthesis kit; Fermentas; Thermo Fisher Scientific,
Inc.). The reaction mixture was incubated at 37°C for 30 min
followed by inactivated at 85°C for 5 min. Polymerase chain
reaction was performed with cDNA as template and the reaction
volume was 20 µl. EGF mRNA expression was analyzed by
semi-quantitative RT-PCR using the Qiaquick PCR kit (Qiagen, Inc.,
Valencia, CA, USA). The thermocycling conditions were as follows:
42°C for 30 min, 94°C for 5 min, and 40 cycles of 94°C for 45 sec
and 60°C for 80 sec. β-actin was used as internal reference gene.
Amplification products were analyzed using the following protocol:
A total of 5 µl/lane RT-PCR reaction mixture was separated by 2%
agarose gel electrophoresis at a voltage of 80 V. Samples were
visualized using ethidium bromide. A gel imaging system was used to
observe the gel and capture images, and the gray values were
calculated using software (Shanghai Peiqing Science and Technology
Co., Ltd., Shanghai, China). Densitometry analysis was performed
using Bio1D (Vilber Lourmat, Marne la-Vallée, France). The primer
sequences for EGF and β-actin are shown in Table I.
 | Table I.Primer sequences used for reverse
transcription-polymerase chain reaction analysis. |
Table I.
Primer sequences used for reverse
transcription-polymerase chain reaction analysis.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) | Size (bp) |
|---|
| Epidermal growth
factor |
TTGCTGCTCTACCTCCACCAT |
CTGCATTCACATTTGTTGTGC | 354 |
| β-actin |
CAACACGCCGGCCATGTA |
TCCATGCCCAGGAAGGAG | 429 |
Capillary number
Fresh granulation tissue samples (1.0×1.0 mm) were
obtained from the wound surfaces of animals of the control and
treatment groups. Samples were fixed for 10 min at 20°C with 20%
formaldehyde, dehydrated at 35°C for 1.5 h and embedded in paraffin
for 2 min 22°C. Continuous sections (thickness, 5 µm) were stained
with HE solution as described. A light microscope was used to
analyze the sections (magnification, ×400). The number of
capillaries with a complete lumen in each visual field was
calculated and three visual fields per section were observed.
Statistical analysis
The data is presented as mean ± standard deviation.
Experiments were performed in duplicate. SPSS statistics software
(version 19.0; IBM Corp., Armonk, NY, USA) was used for data
analysis. A normal distribution detection and homogeneity of
variance test were conducted prior to comparison tests among
groups. Non-normal data were analyzed using Kruskal-Wallis test.
P<0.05 was used to indicate a statistically significant
difference.
Results
EGF increases wound healing rates
The wound healing of rabbits in the EGF treatment
group was faster than that of rabbits in the control group
(Fig. 1). The mean time for the
wound to become fully epithelized. In the experimental group, 78%
of the animals were healed following 12 days of treatment, while in
the control group 25% of animals exhibited wound closure following
25 days observation. The rabbits in the control group healed by day
25 and the healing rate was 35%.
EGF increases granulation tissue
production, neutrophil numbers, collagen uniformity and the
extracellular matrix
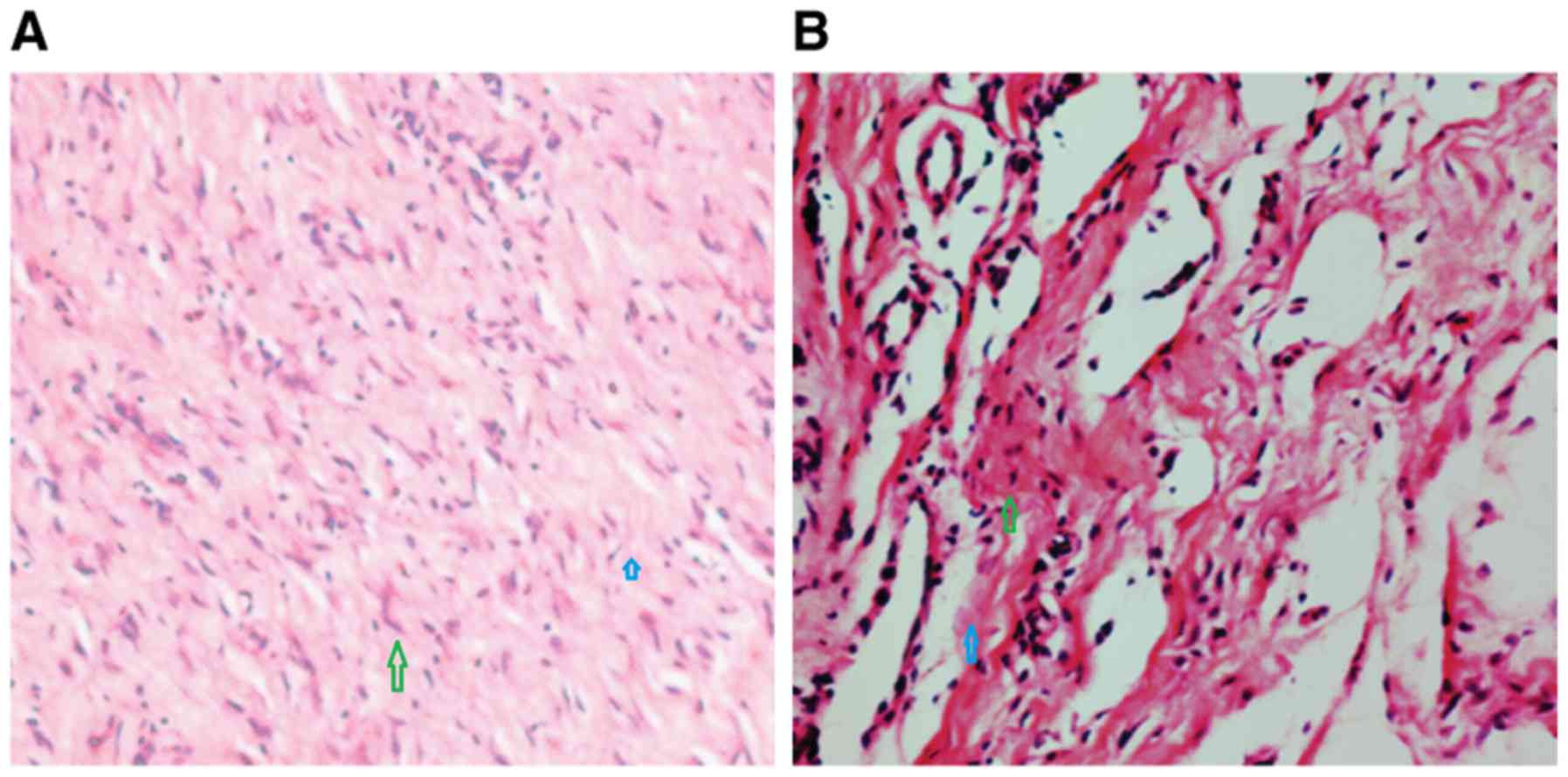
The number of neutrophils in the experimental group
was lower than that in the control group. Histological sections
from the treatment and control groups were collected at 20 days
following treatment. Granulation tissue production and the number
of clustered fibroblasts were increased in the treatment group when
compared with the control group (Fig.
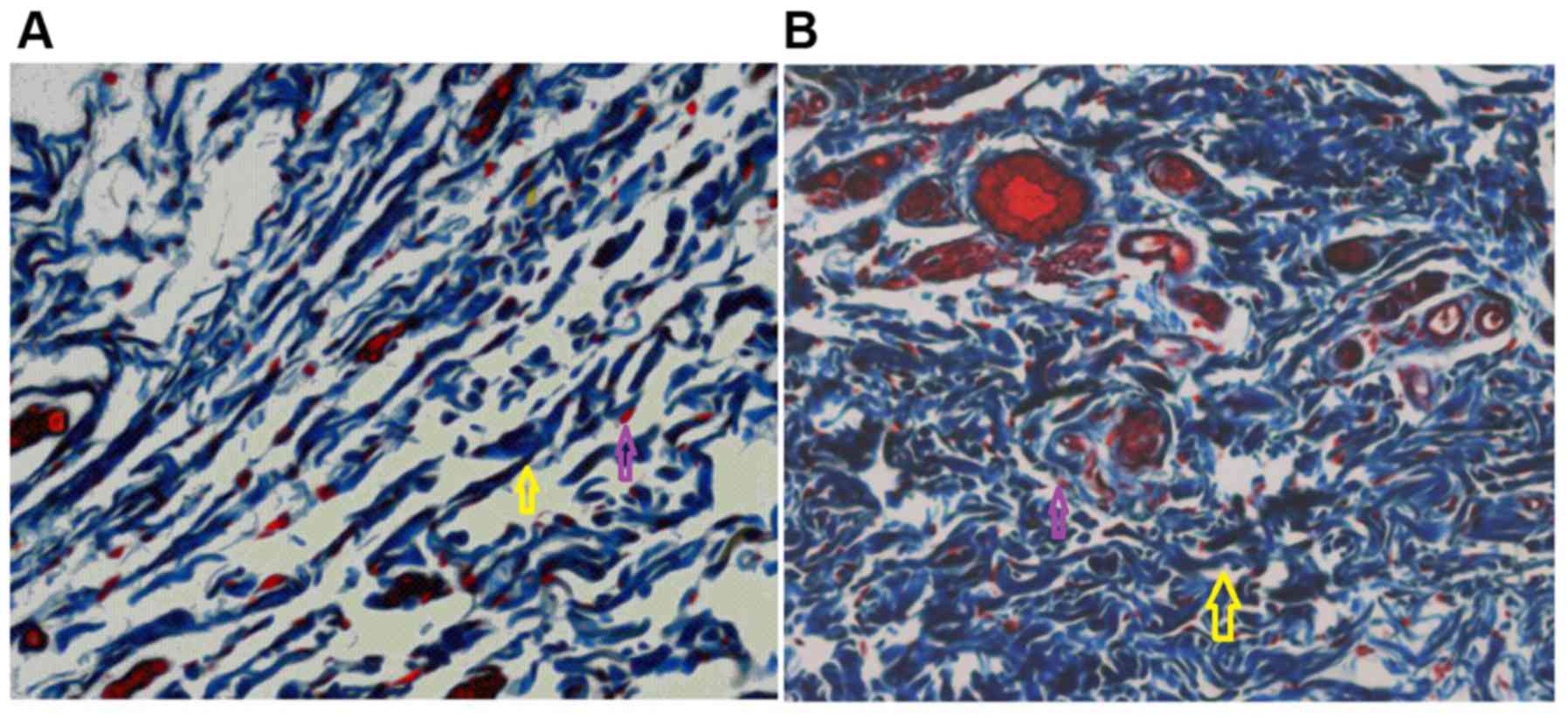
1). The collagen bundles in the treatment group were dense and
ordered, and the extracellular matrix was more abundant when
compared with that of the control group (Fig. 2). In the control group, wound healing
was relatively slow, histological structures were not evident and
the collagen fibers were relatively sparse (Fig. 2).
EGF increases the number of
capillaries and fibroblasts, and improves fibroblast
morphology
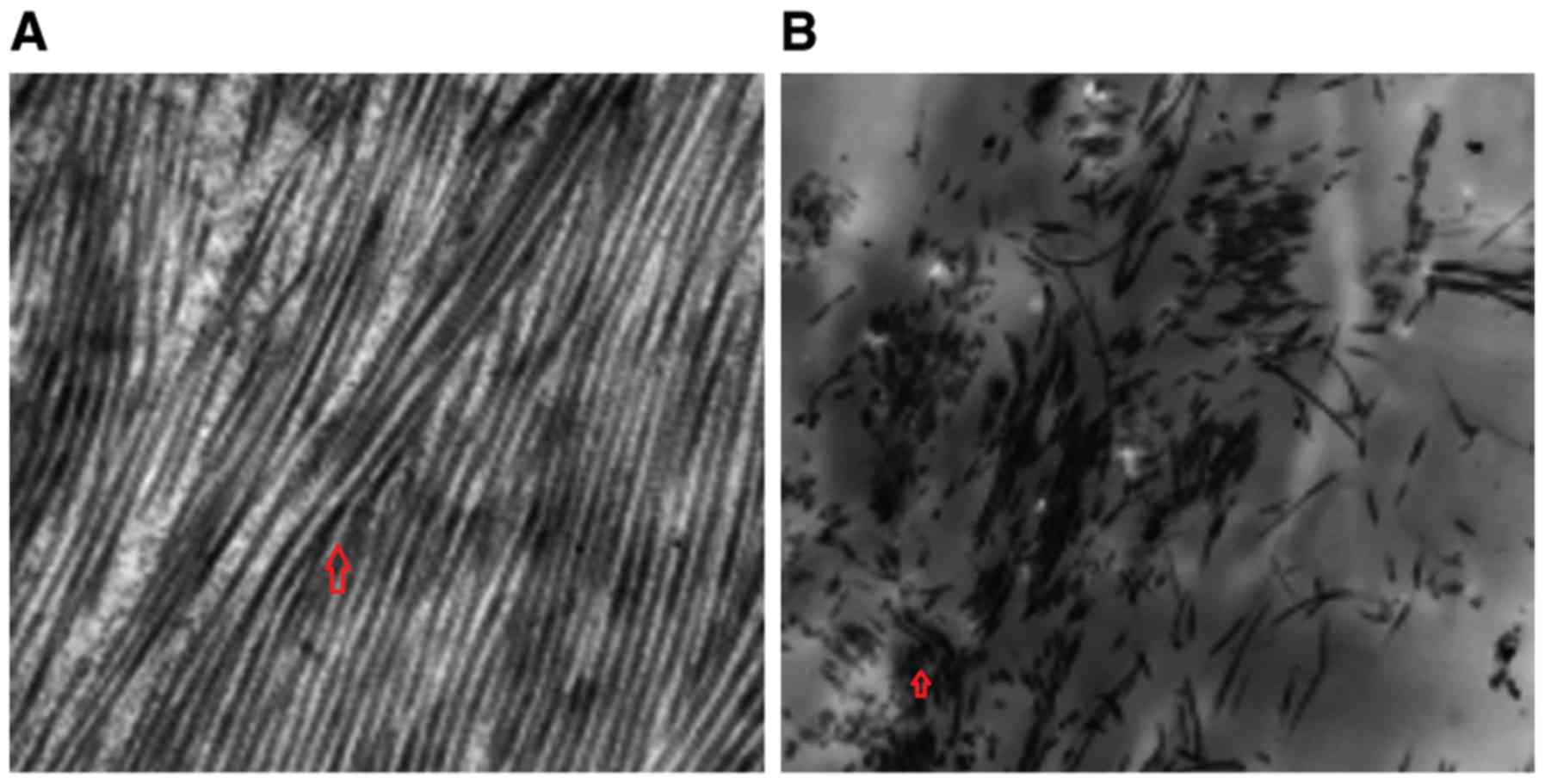
The number of fibroblasts and capillaries were
increased in the treatment group compared with the control group
(Fig. 3). The fibroblasts in the
treatment group exhibited a uniform morphology and were abundant in
organelles when compared with the fibroblasts in the control
group.
Exogenous EGF treatment increases EGF
mRNA expression
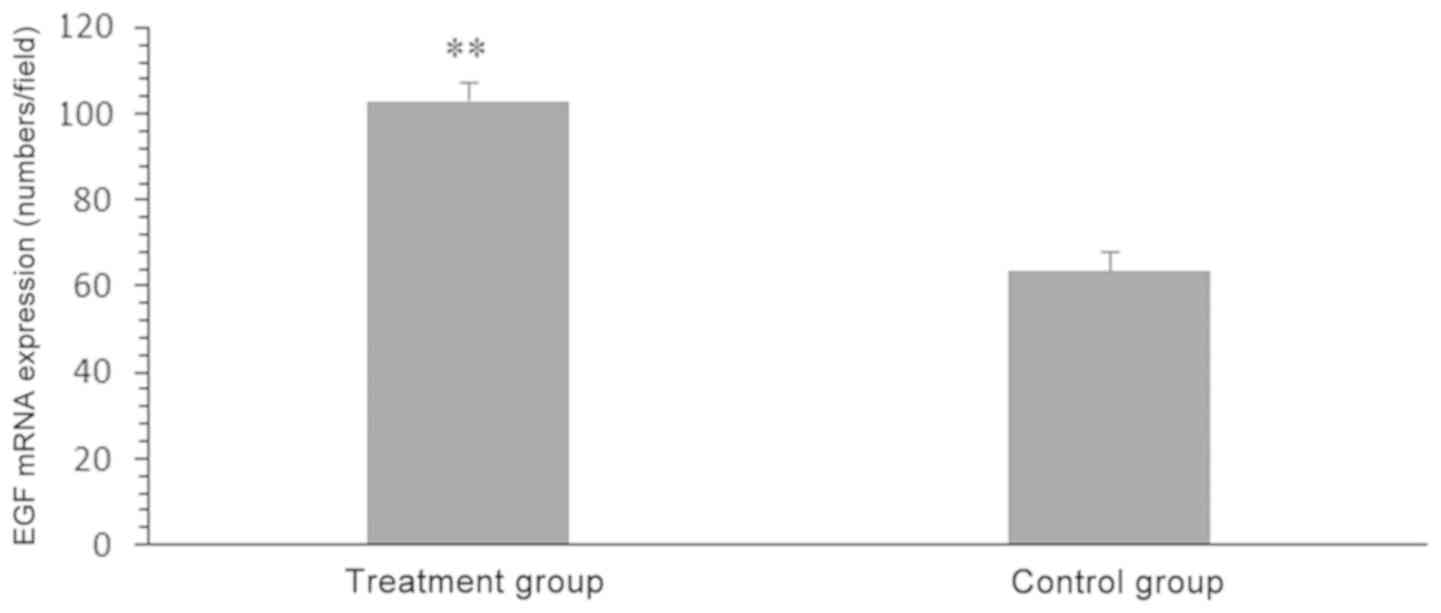
The relative gray value of EGF mRNA in the treatment
group was higher than that of the control group was (103.27±4.27
vs. 63.88±4.36; P<0.05; Fig.
4).
Discussion
When normal tissue is acutely injured, epithelial
cells, fibrocytes and fibroblasts are damaged and the normal
physiological process of wound healing is initiated (28). In DFU, the wound healing process is
hindered by the accumulation of advanced glycation end products
(AGEs), which is due to the high blood glucose levels of patients
with diabetes (29,30). AGEs competitively bind to the EGF
receptor, thus preventing the binding of EGF and perpetuating the
initial injury to the vascular endothelial cells and fibroblasts
(31,32). This leads to an increase in apoptosis
of injured vascular endothelial cells and fibroblasts, and a
decrease in cytokines and collagen production; collectively these
phenomena decrease angiogenesis (33). The levels of inflammatory mediators
also increase in the extracellular matrix of DFU lesions (34,35). The
wound healing process is inhibited by decreased neutrophil
granulocyte numbers, inflammation, inflammatory cell chemotaxis and
granulation tissue production (36).
This type of wound takes a long time to heal and may never heal
completely. However, topical treatments consisting of antibacterial
nanoparticles (such as silver nanoparticle between 1 and 100 nm)
have been used against different pathogens, particularly
Staphylococcus aureus and it was observed that these
treatments can encourage wound healing (37,38).
Previous studies have demonstrated that the
degradation of growth factors, such as EGF, and their cell surface
receptors are the main cause of refractory DFU wounds (39,40). EGF
was the first growth factor used to study wound healing (41,42). A
previous in vitro study demonstrated that EGF may promote
mitosis and glycolysis (43). EGF
receptors exist on the majority of cell surface membranes (44,45). EGF
induces the migration of inflammatory cells away from the wound,
leading to an improvement of the wound microenvironment and the
nutritional status of the tissue (46). Also, EGF can promote epidermal
proliferation increasing fibroblast numbers in partial thickness
wounds (47–49). Cellulose promotes cell proliferation
and wound repair (50). The
aforementioned studies support the results of the present study,
which indicates that EGF may serve a positive role in the DFU wound
healing process.
EGF is secreted by circulating platelets,
macrophages and mononuclear cells (51). The results of the present study
demonstrated that EGF mRNA expression in the group treated with
exogenous EGF was higher than that of the control group. This may
demonstrate that exogenous EGF promotes the expression of
endogenous EGF, which upregulates partial EGF gene expression
(52,53). Experiments with single and multiple
linear regression models should be designed and regression analyses
performed to support the notion that exogenous EGF promotes
endogenous EGF protein expression.
In conclusion, the results of the present study
suggest that EGF binds to its corresponding receptor on epidermal
cell and fibroblast cell surface membranes in order to build
collagenous tissue, and accelerate the generation of wound
granulation and epithelial tissues, which accelerate the wound
healing process. The treatment of diabetic foot ulcer is long and
expensive, and causes great pain to patients and families.
Therefore, it is of great importance to find a novel treatment
method DFUs. EGF demonstrated positive effects in promoting wound
healing.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and ZX designed the study. WH, QD and ZW were
responsible for the collection and analysis of the data. JM and XC
performed data analysis and interpretation. JZ and ZX prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Affiliated Qingdao Hiser Hospital of Qingdao
University (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iraj B, Khorvash F, Ebneshahidi A and
Askari G: Prevention of diabetic foot ulcer (DFU). Int J Prev Med.
4:373–376. 2013.PubMed/NCBI
|
|
2
|
Wojciechowski VV, Calina D, Tsarouhas K,
Pivnik AV, Sergievich AA, Kodintsev VV, Filatova EA, Ozcagli E,
Docea AO, Arsene AL, et al: A guide to acquired vitamin K
coagulophathy diagnosis and treatment: The Russian perspective.
Daru. 25:102017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asadi M, Alamdari DH, Rahimi HR,
Aliakbarian M, Jangjoo A, Abdollahi A, Bahar MM, Azadmand A,
Forghani N, Sadegh MN, et al: Treatment of life threatening wounds
with a combination of allogenic platelet rich plasma, fibrin glue
and collagen matrix, and a literature review. Exp Ther Med.
8:423–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Călina D, Docea AO, Roşu L, Zlatian O,
Roşu AF, Anghelina F, Rogoveanu O, Arsene AL, Nicolae AC, Drăgoi
CM, et al: Antimicrobial resistance development following surgical
site infections. Mol Med Rep. 15:681–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tănase A, Colita A, Ianosi G, Neagoe D,
Branisteanu DE, Calina D, Docea AO, Tsatsakis A and Ianosi SL: Rare
case of disseminated fusariosis in a young patient with graft vs.
host disease following an allogeneic transplant. Exp Ther Med.
12:2078–2082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blumberg SN, Berger A, Hwang L, Pastar I,
Warren SM and Chen W: The role of stem cells in the treatment of
diabetic foot ulcers. Diabetes Res Clin Pract. 96:1–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mcardle C, Lagan K, Spence S and McDowell
D: Diabetic foot ulcer wound fluid: The effects of pH on DFU
bacteria and infection. J Foot Ankle Res. 8 Suppl 1:A82015.
View Article : Google Scholar
|
|
8
|
Buchberger B, Follmann M, Huppertz H and
Wasem J: Health technology assessment (HTA) on the importance of
growth factors for the treatment of diabetic foot ulcers (DFU). Das
Gesundheitswesen. 72:491–501. 2010. View Article : Google Scholar
|
|
9
|
Sheehan P, Jones P, Caselli A, Giurini JM
and Veves A: Percent change in wound area ofdiabetic foot ulcers
over a 4-week period is a robust predictor of complete healing in a
12-week prospective trial. Diabetes Care. 26:1879–1882. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gilligan AM, Waycaster CR and Landsman AL:
Wound closure in patients with DFU: A cost-effectiveness analysis
of two cellular/tissue-derived products. J Wound Care. 24:149–156.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh K, Singh VK, Agrawal NK, Gupta SK
and Singh K: Association of toll-like receptor 4 polymorphisms with
diabetic foot ulcers and application of artificial neural network
in DFU risk assessment in type 2 diabetes patients. Biomed Res Int.
2013:3186862013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mcdonnell J, Redekop K, Verboom P, Lovas K
and Kalo Z: PDG15: The cost of treating diabetic foot ulcers (dfu)
with apligraf in the Netherlands. Value Health. 4:5082001.
View Article : Google Scholar
|
|
13
|
Hong JP, Jung HD and Kim YW: Recombinant
human epidermal growth factor (EGF) to enhance healing for diabetic
foot ulcers. Ann Plast Surg. 56:394–398; discussion 399–400. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robson MC: Invited discussion: Recombinant
human epidermal growth factor (EGF) to enhance healing for diabetic
foot ulcers. Ann Plast Surg. 56:399–400. 2006. View Article : Google Scholar
|
|
15
|
Choi JS, Leong KW and Yoo HS: In vivo
wound healing of diabetic ulcers using electrospun nanofibers
immobilized with human epidermal growth factor (EGF). Biomaterials.
29:587–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohan VK: Recombinant human epidermal
growth factor (REGEN-D 150): Effect on healing of diabetic foot
ulcers. Diabetes Res Clin Pract. 78:405–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aktaş Ş, Baktıroğlu S, Demir L, Kılıçoğlu
Ö, Topalan M, Güven E, Mirasoğlu B and Yanar F: Intralesional
application of epidermal growth factor in limb-threatening ischemic
diabetic foot ulcers. Acta Orthop Traumatol Turc. 50:277–283.
2016.PubMed/NCBI
|
|
18
|
Dumantepe M, Fazliogullari O, Seren M,
Uyar I and Basar F: Efficacy of intralesional recombinant human
epidermal growth factor in chronic diabetic foot ulcers. Growth
Factors. 33:128–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen F, Lin L, Zhang HJ, Ye XX, Zhao WZ,
Wang T and Yang XN: Roles of hepatocyte growth factor, c-Met and
epidermal growth factor receptor in repair of colonic mucosa from
patients with ulcerative colitis. Shiji Hua Ren Xiao Hua Za Zhi.
14:594–599. 2006.(In Chinese).
|
|
20
|
Chen F, Lin L, Zhang HJ and Wang T: Effect
of epidermal growth factor receptor in the repair of colonic mucosa
in patients with ulcerative colitis. Zhongguo Lin Chuang Kang Fu Za
Zhi. 9:104–106. 2005.(In Chinese).
|
|
21
|
Sun L, Kwok E, Gopaluni B and Vahidi O:
Pharmacokinetic-pharmacodynamic modeling of metformin for the
treatment of type II diabetes mellitus. Open Biomed Eng J. 5:1–7.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu YH, Yun SF, Zhou SM and Tian XY:
Influence of Sumianxin Anesthesia on Physiology in Rabbits.
Zhongguo Bi Jiao Yi Xue Za Zhi. 16:475–478. 2006.(In Chinese).
|
|
23
|
Cao CY, Kang N, Yan L, Hu ZY, Shen ZH and
Wang Q: Anesthetic effect of sumianxin II combined with chloral
hydrate on rabbit. Zhongguo Bi Jiao Yi Xue Za Zhi. 24:25–18.
2014.(In Chinese).
|
|
24
|
Huang J: Scientific breeding management of
experimental rabbits. Animal Husbandry Veterinarian of Zhejiang.
3:471995.
|
|
25
|
Li X, Liu W, Kou H, Zhou W, Li T, Dong B
and Liang P: Experimental study of image-guided percutaneous
microwave ablation in rabbit lung VX2 tumor model. Int J Clin Exp
Pathol. 7:905–913. 2014.PubMed/NCBI
|
|
26
|
Yuan ZW, Pei LY, Qiu JL, et al: Anesthesia
of commonly used experimental animals. Chinese J Comparative
Medicine. 14:245–247. 2004.(In Chinese).
|
|
27
|
Pixin R and Shengfu D: Effects of
epidermal growth factor (EGF) on circumflex function of isolated
pulmonary artery in rats. Chinese J Pathophysiol. 2:173–176.
1994.(In Chinese).
|
|
28
|
Liu Y, Pei G and Jiang S: New porous
beta-tricalcium phosphate as scaffold for bone tissue engineering.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 21:1123–1127. 2007.(In
Chinese). PubMed/NCBI
|
|
29
|
Ertugrul BM, Buke C, Ersoy OS, Ay B,
Demirez DS and Savk O: Intralesional epidermal growth factor for
diabetic foot wounds: The first cases in Turkey. Diabetic Foot
Ankle. 6:284192015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan HJ, Yu JH, Cui GM, Zhang WY, Yang X
and Dong QJ: Insulin pump for the treatment of diabetes in
combination with ulcerative foot infections. J Biol Regul Homeost
Agents. 30:465–470. 2016.PubMed/NCBI
|
|
31
|
Thomson SE, McLennan SV and Twigg SM:
Growth factors in diabetic complications. Expet Rev Clin Immunol.
2:403–418. 2006. View Article : Google Scholar
|
|
32
|
Han HM, Guo L, Jiang LJ, Jiang XY, Lv YL,
Pang JK, Bai ZM, Che WJ, Xu RH, Yu P and Li Q: A microarray study
on the molecular mechanism for the therapeutic effect of Antidotal
and Myogenic Ointment on the foot ulcer in diabetic rats. Zhongguo
Wei Zhong Bing Ji Jiu Yi Xue. 23:621–624. 2011.(In Chinese).
PubMed/NCBI
|
|
33
|
Wan Y, Yang YJ, Li YS, Li XJ, Zhang W, Liu
M and Tang HB: Effects of San-huang-sheng-fu oil on peripheral
circulatory disorders and foot ulcers in diabetic rats and the
mechanisms. Zhonghua Shao Shang Za Zhi. 32:168–175. 2016.(In
Chinese). PubMed/NCBI
|
|
34
|
Demidova-Rice TN, Hamblin MR and Herman
IM: Acute and impaired wound healing: Pathophysiology and current
methods for drug delivery, part 2: Role of growth factors in normal
and pathological wound healing: Therapeutic potential and methods
of delivery. Adv Skin Wound Care. 25:349–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jennings JA, Crews RM, Robinson J,
Richelsoph K, Cole JA, Bumgardner JD, Yang Y and Haggard WO: Effect
of growth factors in combination with injectable silicone resin
particles on the biological activity of dermal fibroblasts: A
preliminary in vitro, study. J Biomed Mater Res B Appl Biomater.
92:255–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan N, Wang C, Wang Y, Yu T, Long Y,
Zhang X and Ran X: Preparation of autologous platelet-rich gel for
diabetic refractory dermal ulcer and growth factors analysis from
it. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 22:468–471. 2008.(In
Chinese). PubMed/NCBI
|
|
37
|
Zhou LS, Liao ZJ, Zhang Q, Luo M, Lu G and
Zhang W: Bio-inductive effects of inorganic elements on skin wound
healing. Zhonghua Shao Shang Za Zhi. 21:363–366. 2005.(In Chinese).
PubMed/NCBI
|
|
38
|
Jiang XH, Zhou WM, He YZ, Wang Y, Lv B and
Wang XM: Effects of lipopeptide carboxymethyl chitosan
nanoparticles on Staphylococcus aureus biofilm. J Biol Regul
Homeost Agents. 31:737–743. 2017.PubMed/NCBI
|
|
39
|
Buteică AS, Mihăescu DE, Grumezescu AM,
Vasile BS, Popescu A, Călina D and Mihăiescu OM: The citotoxicity
of (non)magnetic nanoparticles tested on Escherichia coli
and Staphylococcus aureus. Dig J Nanomater Biostruct.
5:651–655. 2010.
|
|
40
|
Acosta JB, Savigne W, Valdez C, Franco N,
Alba JS, del Rio A, López-Saura P, Guillén G, Lopez E, Herrera L
and Férnandez-Montequín J: Epidermal growth factor intralesional
infiltrations can prevent amputation in patients with advanced
diabetic foot wounds. Int Wound J. 3:232–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Galkowska H, Wojewodzka U and Olszewski
WL: Chemokines, cytokines, and growth factors in keratinocytes and
dermal endothelial cells in the margin of chronic diabetic foot
ulcers. Wound Repair Regen. 14:558–565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pedro A, López Saura, Jorge Berlanga
Acosta, José I, Fernández Montequín, Carmen Valenzuelá Silva,
Odalys González Díaz, William Savigne, Lourdes Morejoń Vega,
Amauryś del Río Martín, Luis Herrera Martínez, et al:
Intralesional human recombinant epidermal growth factor for the
treatment of advanced diabetic foot ulcer: From proof of concept to
confirmation of the efficacy and safety of the procedure. Zhur.
Eksptl'. i Teoret. Fiz. 21:406–409. 2011.
|
|
43
|
Bennett SP, Griffiths GD, Schor AM, Leese
GP and Schor SL: Growth factors in the treatment of diabetic foot
ulcers. Br J Surg. 90:133–146. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Margolis DJ, Bartus C, Hoffstad O, Malay S
and Berlin JA: Effectiveness of recombinant human platelet-derived
growth factor for the treatment of diabetic neuropathic foot
ulcers. Wound Repair Regen. 13:531–536. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Buchberger B, Follmann M, Freyer D,
Huppertz H, Ehm A and Wasem J: The evidence for the use of growth
factors and active skin substitutes for the treatment of
non-infected diabetic foot ulcers (DFU): A health technology
assessment (HTA). Exp Clin Endocrinol Diabetes. 119:472–479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Galstyan GR, Ignatieva VI, Avksentieva MV
and Dedov II: Pharmacoeconomic analysis of epidermal growth factor
(HeberprotP®) for the treatment of diabetic foot ulcers.
Endocrine Surgery. 7:4–15. 2013. View Article : Google Scholar
|
|
47
|
Tuyet HL, Nguyen Quynh TT, Vo Hoang Minh
H, Thi Bich DN, Do Dinh T, Le Tan D, Van HL, Le Huy T, Doan Huu H
and Tran Trong TN: The efficacy and safety of epidermal growth
factor in treatment of diabetic foot ulcers: The preliminary
results. Int Wound J. 6:159–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tiaka EK, Papanas N, Manolakis AC and
Georgiadis GS: Epidermal growth factor in the treatment of diabetic
foot ulcers: An update. Perspect Vasc Surg Endovasc Ther. 24:37–44.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Afshari M, Larijani B, Fadayee M, Farzaneh
Darvishzadeh, Ghahary A, Pajouhi M, Bastanhagh MH, Baradar-Jalili R
and Vassigh AR: Efficacy of topical epidermal growth factor in
healing diabetic foot ulcers. Therapy. 2:759–765. 2016. View Article : Google Scholar
|
|
50
|
Lihuan D: Regenerated cellulose skin
repair materials: preparation and performance. Zhongguo Zu Zhi Gong
Cheng Yan Jiu. 19:6098–6103. 2015.(In Chinese).
|
|
51
|
Singla S, Singla S, Kumar A and Singla M:
Role of epidermal growth factor in healing of diabetic foot ulcers.
Indian J Surg. 74:451–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gałkowska H and Olszewski W: Cellular and
molecular basis of impaired healing of diabetic foot ulcers. Polish
J Surg. 79:720–727. 2007. View Article : Google Scholar
|
|
53
|
Li Q, Wang Y and Yang L: Curative effect
observation of recombinant human epidermal growth factor in
treatment of oral ulcer in children with hand-foot-mouth disease.
Erke Yao Xue Za Zhi. 23:533–547. 2015.(In Chinese).
|