Introduction
Spinal cord injury (SCI) is associated with severe
morbidity in terms of neurological disability and has major impact
on quality of life, due to impaired bladder and bowel control
(1–3). The most prevalent causes of SCI are
traffic accidents and falls, and they generally occur as blunt
trauma (4,5).
Although grafting of tissue from peripheral nerves
to CNS lesions has unlocked some regenerative potential in mammals,
the human organism is incapable of restoring neural tissue
following SCI (6–8). On the contrary, the Mexican axolotl
(Ambystoma mexicanum), a widely used model in regenerative
biology, has the ability to regenerate from transection of the
spinal cord (9–12). Literature has reported different
durations of successful regeneration, ranging between 7 days and 23
months (9–11).
However, SCI models in the axolotl are traditionally
performed as spinal cord transection (or direct tail amputation),
thus the novelty in this study is in the application of a more
clinically relevant type of injury, namely a blunt contusion
injury. To our knowledge, no studies on blunt SCI in the axolotl
have been performed though this type of injury reflects a more
clinically relevant situation than transection (9–12).
In this proof of concept study, we demonstrate
anatomically and functionally that the axolotl is capable of
regenerating a clinically relevant SCI. In line with the scientific
tradition, it will then be the purpose of subsequent studies to
reveal the molecular mechanisms behind this phenomenon.
In conclusion, the purpose of the present study was
to investigate the axolotl's ability to regenerate from a blunt
contusion trauma.
Materials and methods
Animals and anesthesia
Animals used in the present study were Mexican
axolotls (Ambystoma mexicanum) (mean body mass ± STD: 12.12
g ± 1.25 g) obtained from a commercial breeder (Exoterra GmbH,
Holzheim, Germany). Animals were housed individually in plastic
containers with a 10 cm water depth and a 930 cm2
surface area with regular water change and a 12 h:12 h light:dark
cycle. They were fed every other day with protein enriched trout
pellets. Anesthesia was obtained using 200 mg/l
ethyl-4-aminobenzoate.
Overview
A randomized blinded controlled trial study was
performed, involving 6 animals in both a surgery and sham group.
The model was designed as a surgical intervention, with the aim of
inducing a blunt trauma to the spinal cord. Furthermore, all data
were blinded with respect to time.
All animals were examined using high-field magnetic
resonance imaging (MRI), including diffusion tensor imaging (DTI).
Furthermore behavioral modalities such as neurological
examinations, and swimming tests were performed as well. All
animals were sacrificed for histology at 9 weeks post injury (WPI).
Three additional animals (2 SCI, and 1 sham) were sacrificed
immediately after surgery for histology.
All modalities were applied at baseline
(pre-surgery). MRI protocols were applied immediately after
surgery. For follow-up, MRI and all other modalities were applied
every third week for 9 weeks.
Surgery
The animals were randomized to the SCI and sham
groups though ensuring similar group size, with the project
investigators being blinded. To induce a controlled spinal cord
contusion trauma, the animals underwent laminectomy of two adjacent
vertebral levels, caudal to the hind limbs. Under microscopic
magnification and with the animal in prone position, transection
through the keel to the spinal process midline was performed and
extended into bilateral horizontal sections. The spinal processes
and laminae were exposed. The vertebrae in the axolotl are of the
opisthocoelous type. A laminectomy was performed with a bilateral
micro-scissor cut, and the laminae elevated using forceps, leaving
the underlying dura and spinal cord exposed (Fig. 1).
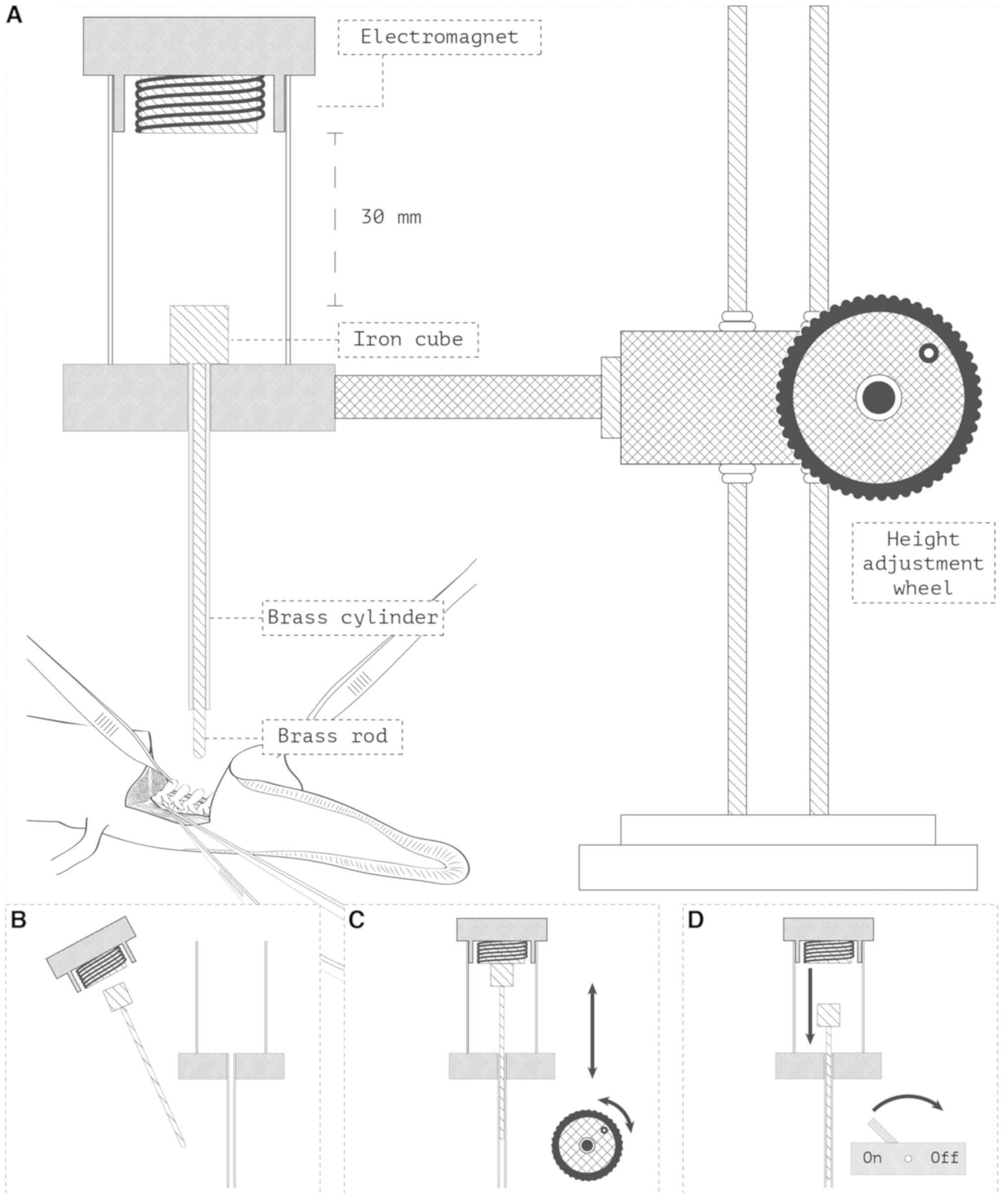
For the intervention group, a standardized trauma
unit was designed (Fig. 1). The
equipment allowed the rod of a 25 g weight to fall exactly 30 mm
onto the exposed spinal cord, transferring a theoretical 7.4 mJ of
energy directly to the spinal cord. These values were decided upon
from extensive piloting. During piloting we titrated to the lowest
falling height and weight which produced disruption of the dorsal
artery and hence macroscopical bleeding. Literature has reported no
previous standards for this given trauma. For the sham group, all
above-mentioned steps were performed, with exception of the spinal
cord trauma.
Observations during the first 3
weeks
To ensure wound closure and hinder additional
injury, the animals were only observed passively for the first 3
weeks. Animals were not disturbed, and water was changed gently
using a pump.
Histology
Tissue samples were fixed in 4% neutral buffered
formaldehyde solution, and decalcified using 2 M HCl for 1 h before
being processed in a Citadel Shandon 2000 tissue processor (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and embedded in
paraffin. The tissue blocks were sectioned into 20 µm sections to
yield longitudinal sections of the spinal cord. Sections were
deparaffined in xylene and rehydrated in decreasing alcohol
concentrations and then water. Staining was performed using Mayers
hematoxoxylin for 10 min followed by a 10 min rinse in tap water,
and alcoholic eosin (0.2% eosin in 80% ethanol) for 2 min. Sections
were dehydrated through increasing alcohol concentrations, cleared
in xylene and closed with Depex.
Anatomical MRI
Anatomical images of the injury were obtained using
a gradient echo 3D protocol using an Agilent 9.4 T MRI system and
following parameters: TE: 2.24 ms, TR: 4.44 ms, FOV: 50 ×50×50
mm3, matrix: 256×256×256 yielding isotropic voxels of
0.2 mm, and averages: 4. One scan was made at baseline (−0 WPI),
one immediately after surgery (+0 WPI), and then at 3 weeks
intervals, for 9 weeks (3, 6 and 9 WPI).
MRI images were described in a blinded manner using
simple dichotomous outcomes (‘yes’ or ‘no’) for each parameter.
Continuity of the spinal cord was assessed. Edema of the spinal
cord was defined as a lack of visual cerebrospinal fluid (CSF)
around the spinal cord, due to displacement of the CSF by the
edematous spinal cord.
As a quality assessment, the images were analysed
for complete transections, penetrating bone fragments and/or
dislocation of the spinal cord as well as post-surgical sequelae
incorporating artefacts such as hemosiderin depositions leading to
conditions that would make evaluation of the spinal cord
impossible.
Neurological examination
The animals were examined neurologically every third
week post-surgery. The tail caudal to the injury was stimulated
with both tactile (gentle touch with forceps) and nociceptive
stimuli (forceps pinching). For both stimuli, 3 attempts were
conducted, with the maximum response defining the score of the
test: 0 point: No response. 1 point: Local tail movement. 2 points:
Truncal movement. 3 points: Coordinated movement of limbs and/or
head alongside with truncal movement. 4 points: Animals with
immediate coordinated fast movement.
Swimming capacity test
At baseline and every third week from surgery,
animals were tested for swimming capacity in a swimming
respirometer using a gradual increase of 50 rpm/5 s, starting at
200 rpm. The maximum swimming capacity was then defined as the
moment when the animal would not produce sufficient force to
withhold against the water current. All data points were normalized
to the baseline mean for the given group yielding a relative
measure for water current.
Statistics
Statistical test of the swimming capacity was
performed as student's t-test. P-values below 5% were considered
significant. For the neurological score a non-parametric one-tailed
Mann-Whitney U test with a critical value of 5 was performed.
Ethical
We certify that all applicable institutional and
governmental regulations concerning the ethical use of animals were
followed during the course of the present study. The study was
conducted under the Approval ID: 2015-15-0201-0061 by the Danish
Animal Experiment Inspectorate.
Results
General observations
One sham group animal was sacrificed 7 days after
surgery due to insufficient wound closure. For the remaining
animals the wound was closed 2–3 WPI.
Histology
The Hemotoxylin-eosin stained tissue morphology
analyzed directly post-surgery (+0 WPI) showed that the
intervention group had severe injuries on their spinal cord i.e.,
the central canal was discontinued (Fig.
2A-1). The tissue surrounding the injury site was edematous and
thickened. The sham animals had intact spinal cords, suggesting
that surgery did not inflict damage. We observed no signs of edema
or infiltration (Fig. 2C-1).
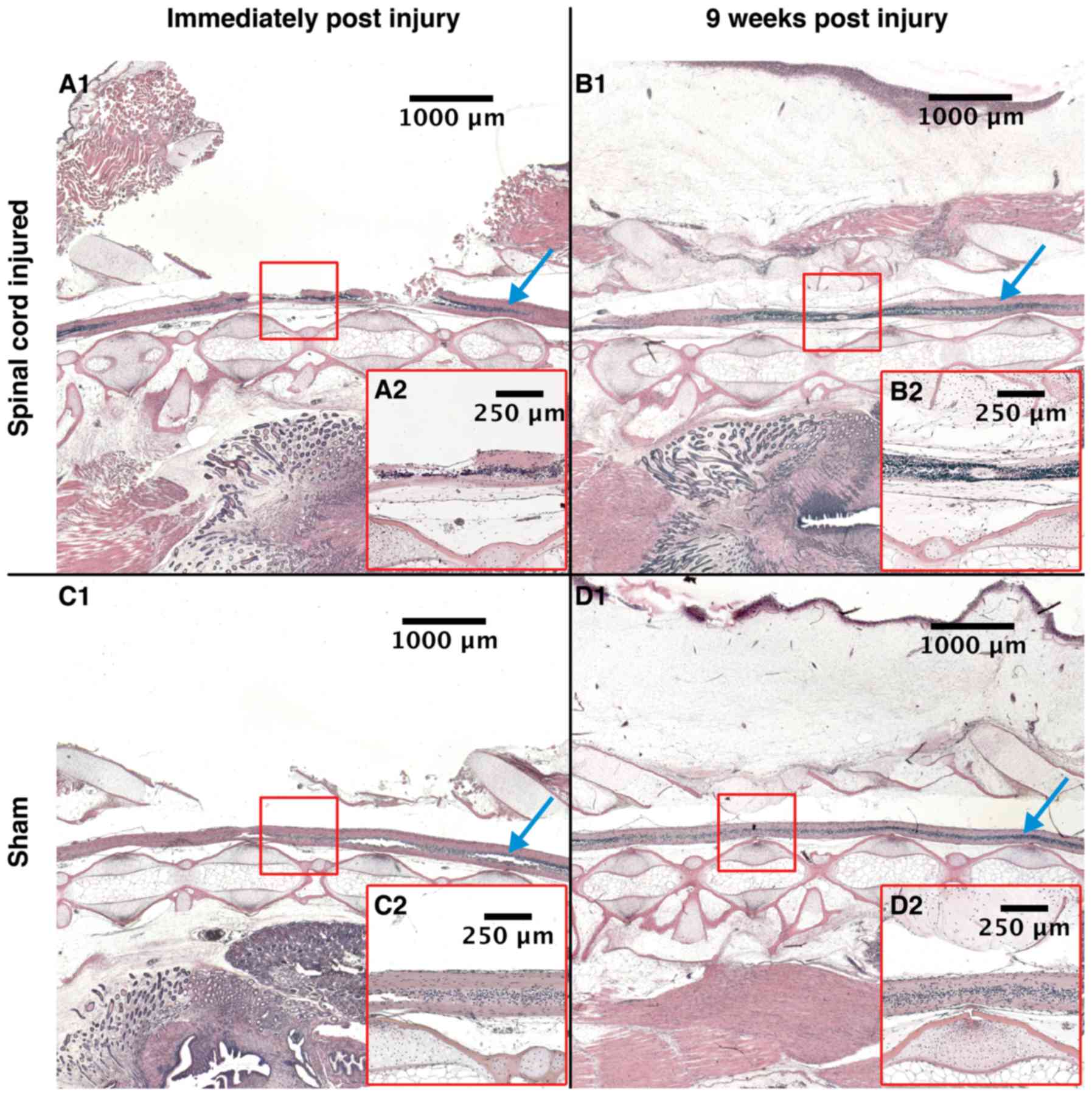 | Figure 2.Histology. Histological, sagittal
sections of spinal cords. (A-1) SCI animal at +0 WPI, (B-1) SCI
animal at 9 WPI, (C-1) Sham animal at +0 WPI and (D-1) Sham animal
at 9 WPI. Red square: The area of injury for the SCI animals, and
the area of laminectomy for the sham animals. Montage of
photographs taken using ×1.25 objective, scale bars of larger
images 1,000 µm and of smaller images 250 µm. (A-2, B-2, C-2 and
D-2) All show the marked area at magnification, ×5. Blue arrow:
Uninjured spinal cord. SCI, spinal cord injury; WPI, weeks post
injury. |
At 9 WPI, some differences in spinal cord morphology
between sham and intervention animals were observed. The spinal
cords of the sham animals were comparable to the samples acquired
post-surgery (+0 WPI), i.e. not showing any signs of discontinuity,
thickening, edema or infiltration (Fig.
2D-1). In contrast, the intervention animals showed ependymal
proliferation and a compressed spinal cord. The spinal cord and
central canal were both found continuous. Organization of the cells
was different in the regenerating spinal cord where cells with
small nuclei and cell bodies dominate, possibly
microglial/macrophages cells (Fig.
2B-1).
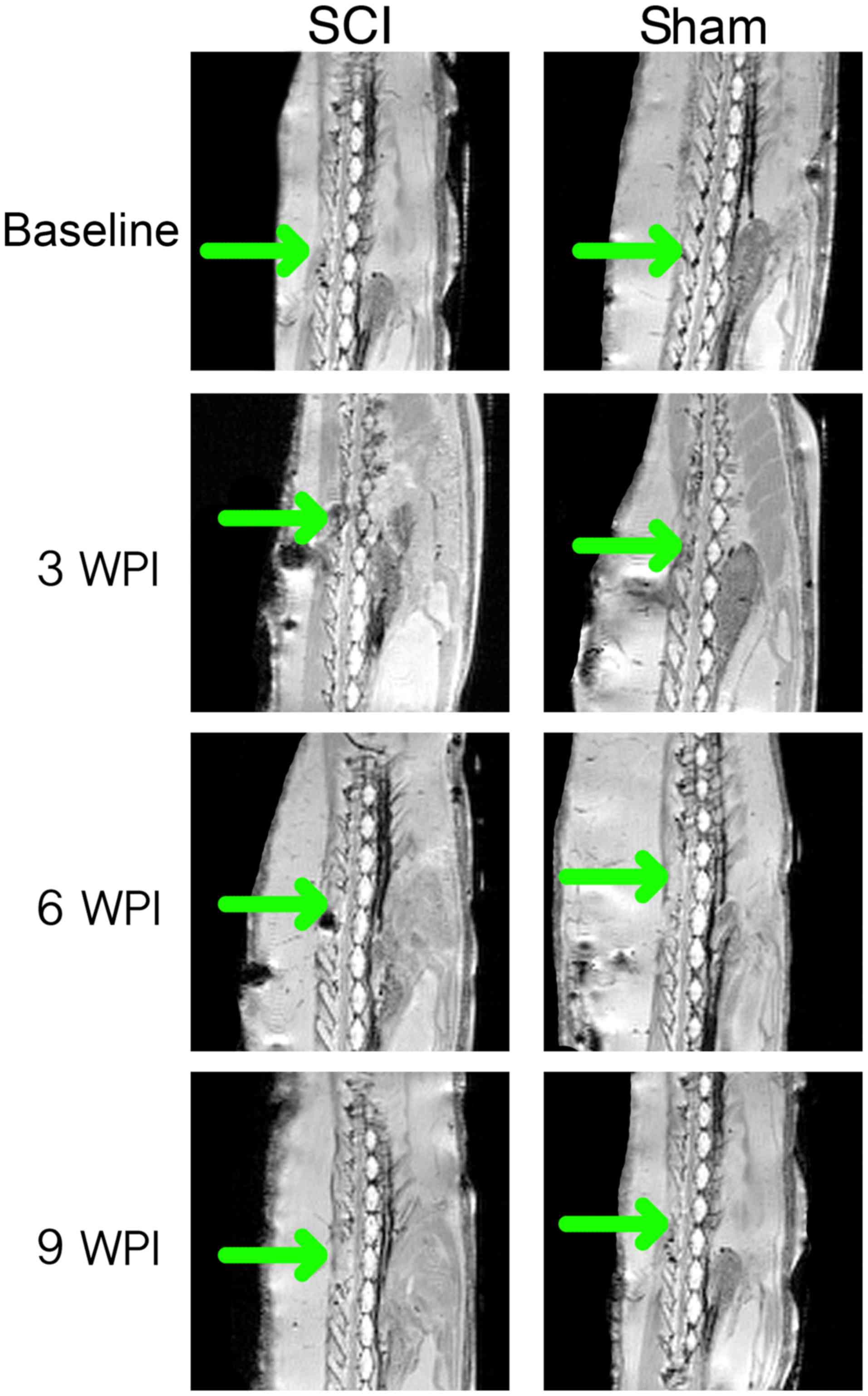
Anatomical MRI
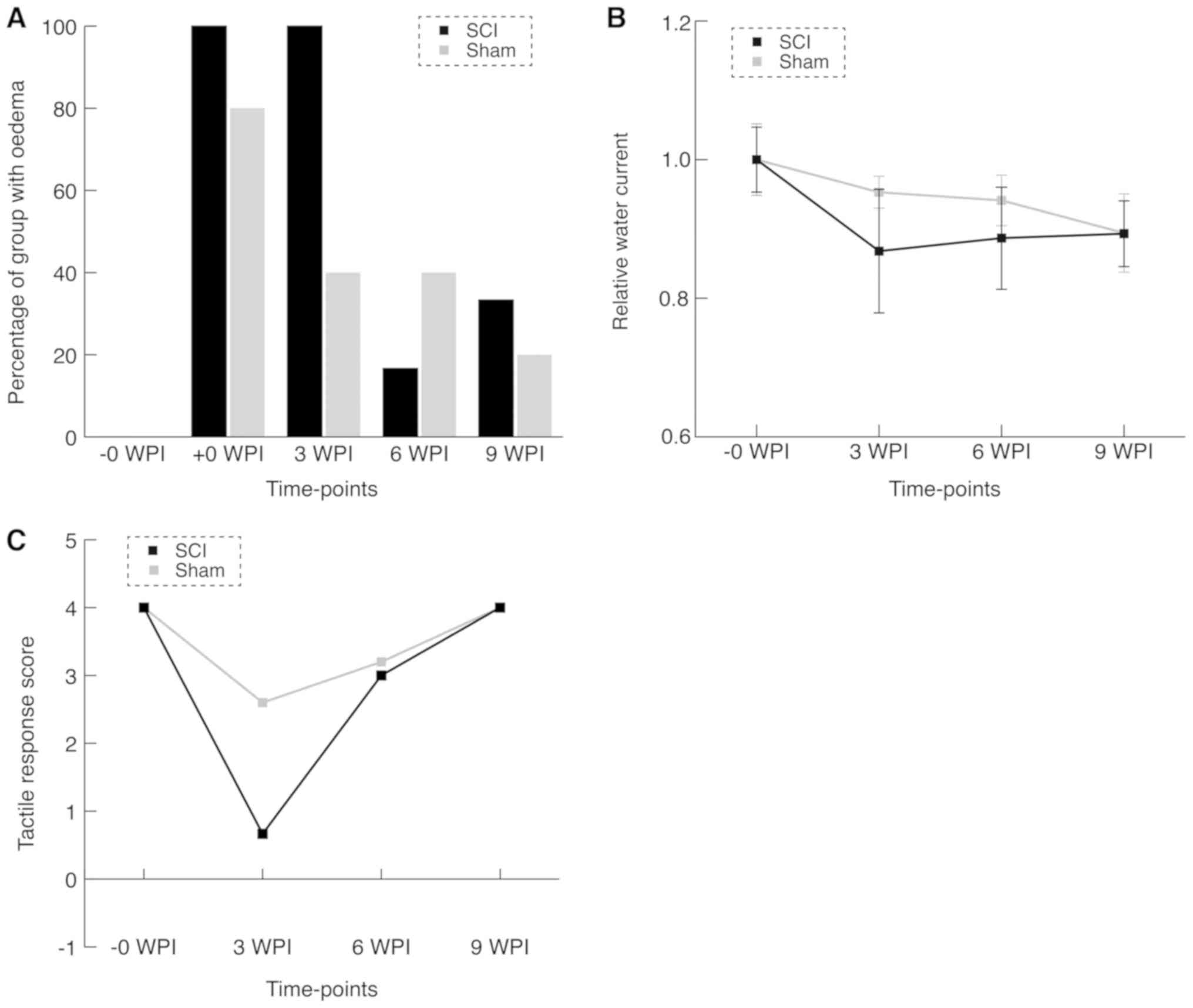
At baseline, all animals were blind-scored for
continuity and potential signs of injury (Figs. 3A and 4). Eight of the immediate post-surgical (+0
WPI) scans were impossible to describe in a satisfactory manner due
to artefacts and hemosiderin deposits. In the remaining three +0
WPI scans, edema was observed in the SCI group and not in the sham
group. At 3 WPI, all SCI animals were described and showed full
continuity, but still spinal cord edema was observed. MRI from two
animals in the sham group suggested edema as well, with the
remaining being comparable to baseline. At 6 WPI, MRI of the SCI
group was comparable to baseline, except for one animal. In the
sham group two animals had findings suggesting edema. Only one of
these animals was consistent with findings at 3 WPI. At 9 WPI, the
edema was observed in two animals in the SCI group. Edema that was
not identified in earlier MRI scans was found in one of the sham
group animals.
Swimming capacity test
The swimming capacity test did not at any time point
show significant differences between the sham and intervention
groups (Fig. 3B). However, we
observed that mean swimming capacity was lower in the SCI group at
3 WPI (P=0.34). Data revealed a significant difference (lower)
swimming capacity at baseline compared to the last follow up for
all animals (P=0.0008).
Neurological examination
When pinching the tail immediately after surgery in
pilot experiments we saw subtle clonus-like tail movements. Tactile
examination at 3 WPI yielded a non-significant difference of the
mean score between the groups (mean difference=2 points,
U-statistic: SCI = 6.5, Sham=12.5). This difference was diminished
at the later time points (Fig. 3C).
Considering the nociceptive part of the examination, we found no
difference at any time point. All animals except one SCI animal at
3 WPI (score: 2) scored 4 points throughout the entire
experiment.
Discussion
The present study demonstrates that the axolotl is
capable of morphologically regenerating the spinal cord following a
contusion trauma. However, we found that regeneration was
incomplete after 9 weeks. Bone regeneration seemed to be limited in
our model, which might be a consequence of the critical size defect
phenomenon; a phenomenon where bone injury size exceeds a given
threshold and the regenerative mechanism is not sufficiently
stimulated (13).
The axolotl regenerates by formation of terminal
vesicles derived from ependymal cells that proliferate and migrate
to the injury site and re-establish contact in the spinal cord
(9,14). Axons sprout and grow across the
injury, innervating the caudal segments (10). Hence, the axolotl spinal cord is
permissive of both axonal regrowth but also of ependymal cell
proliferation and glial tissue regeneration. In a study of larvae
of a related species, A. maculatum, applying 2 mm ablations,
histology showed regeneration 40 days post injury. All animals had
regained gait using their hind limbs, but only half of the animals
their ability to use their tail. This finding suggests a functional
cranio-caudal regrowth, supporting the view of glial tissue
regeneration from both stumps, allowing for axonal regrowth from
the cranial stump (9).
The human inability to regenerate the central
nervous system is multi-factorial. Among them is Chondroitin
sulphate, which mechanically restricts regrowth (15). Blood-derived monocytes and macrophage
polarization also inhibit regeneration (16). Myelin associated factors like Nogo-A
inhibit axonal sprouting (17,18).
Paradoxically, Nogo-A and its receptor have been shown to be part
of the axolotl genome (19). All
these steps are somehow overcome in the axolotl.
Krogh's principle states that: ‘For such a large
number of problems there will be some animal of choice or a few
such animals on which it can be most conveniently studied’. In
accordance with Krogh´s principle, this model can facilitate
research in spinal cord regeneration mechanisms itself, but also
work to test potential inhibitors of regeneration. Increasing
levels of potential mammalian inhibitory factors should be able to
terminate regeneration, negatively mirroring mammalian studies.
This kind of translational approach has been conducted for miRNA
125b. In the axolotl miRNA 125b was found to regulate regeneration
of the spinal cord (10). Increasing
the levels of miRNA 125b in a rodent SCI model increased
behavioural scores post-surgery (10). Application of our model in such
experiments will enhance the translational value due to a more
clinical relevant trauma mechanism. Intervention studies should
address the effects of modulating levels of myelin associated
inhibitory factors. Additionally, manipulation of chondroitin
sulphate, astrocytes and macrophages would be of interest for
understanding of the regulatory mechanisms, since these serve in
inhibiting SCI progression and regeneration in mammals (15,16,20).
The presence of increased number of ependymal cells
in the SCI group as well as the compressed morphology of the spinal
cord indicate that regeneration was not complete at 9 weeks (9
WPI). Furthermore, the presence of possible microglial cells needs
further investigation.
We did not produce immunohistochemistry sections,
since the study aimed to describe complete morphological
regeneration of the spinal cord (including the central-canal,
ependymal layers and vasculature), and not solely axonal
regeneration. Future intervention studies could implement axonal
staining for beta-III-tubulin, to visualize axonal regeneration in
detail.
There seems to be a discrepancy between neurological
and morphological outcomes, as all animals at final follow-up seems
to be neurological intact. Perhaps our methods were not refined
enough to identify small remaining differences. We may as well have
had benefitted from a longer timeframe to final follow-up. This
finding may reflect that the contusion trauma leaves a less perfect
environment for regeneration than surgical transection.
Though non-significant, the neurological tactile
examination showed a difference at 3 weeks, which diminished at
later time points, which was in coherence with our
expectations.
The swimming capacity and nociceptive tests did not
show differences between groups. The results support the conclusion
that substantial regeneration of neuronal connections happens
before 3 WPI.
Interestingly, neither group restored full swimming
capacity, suggesting that regeneration of other tissue (e.g. bone
and muscle) might be the limiting factor after 3 WPI. The keel of
the animals did not regenerate perfectly, maybe due to damages
being below critical size defect. This certainly changed the
hydrodynamics of the tail and probably the swimming capacity.
Since some of our results yielded insignificant
differences between groups, a type II error cannot be excluded;
hence a more fine-tuned scale or more replicates might have picked
up smaller differences.
The tactile and nociceptive neurological
examinations are observer dependent modalities. Both modalities
will be dependent on the force applied for both stimuli, which in
turn will increase variance on the estimate. However, the study was
designed as a randomized and fully blinded study, which would make
type-I-errors very unlikely to arise from these potential
inaccuracies. Secondly the examinations were performed by the same
person throughout the study, eliminating inter-observer
variance.
The results of the scans seem prone to noise in the
analysis. Some animals, that did not have edema, showed edema at
later time points. The chosen protocol does not itself visualize
edema, but rather visualizes the lack of CSF fluid. This makes the
protocol a surrogate marker edema.
The clonus-like movements observed have been
reported in other SCI studies on salamanders (9,21). We
interpret these as enhanced spinal reflexes similar to those known
from human SCI.
The trauma mechanism and force was chosen based on
our extensive pilots. During piloting we found a dose-response
between force and neurological impairment. This was to be expected,
from our knowledge on SCI in general. However, this study aimed to
establish the model and proof-of-concept. Spinal cord contusions
are a force dependent condition, and future model development
describing the dose-response systematically could be of great
interest. Unfortunately, no validated or conventional scale of SCI
severity exist for axolotls, which leaves a need for development of
such a tool, before commencing the work on a dose-response
study.
The axolotl is capable of regenerating a contusion
SCI in a randomized, investigator blinded study. The duration of
complete regeneration needs to be further investigated.
Acknowledgements
The authors would like to thank Associate Professor
Peter Agger, post.doc, Aarhus University: For his expertise and
time on developing the MRI protocols and Associate Professor
Steffen Ringgard, post.doc, Aarhus University: For his expertise
and time on developing the MRI protocols.
Funding
The present study was funded by the Riisfort
foundation (Grant no. 01-09-2015).
Availability of data and materials
Data are available on from the corresponding author
on reasonable request.
Authors' contributions
MMT, undertook project initiation, running and
piloting the experiments, data analyses, and was responsible for
the study and drafting of the manuscript. HL was responsible for
supervising and performing the experiments, statistical analyses,
and reviewing the manuscript. MP supervised the experiments
performed the calculations and reviewed the manuscript. DO
undertook the piloting and development of the histological
analyses, analyses of histological sections and reviewing the
manuscript. TWM made the histological sections, performed the
experiments and reviewed the manuscript. MMR was the main
supervisor, iniated the project, piloted and ran the experiments,
and performed the final review of the manuscript.
Ethics approval and consent to
participate
The present study was conducted under the approval
id: 2015-15-0201-0061 by the Danish Animal Experiment Inspectorate.
We certify that all applicable institutional and governmental
regulations concerning the ethical use of animals were followed
during the course of the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shavelle RM, DeVivo MJ, Brooks JC, Strauss
DJ and Paculdo DR: Improvements in long-term survival after spinal
cord injury? Arch Phys Med Rehabil. 96:645–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hicken BL, Putzke JD and Richards JS:
Bladder management and quality of life after spinal cord injury. Am
J Phys Med Rehabil. 80:916–922. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levi R, Hultling C, Nash MS and Seiger A:
The Stockholm spinal cord injury study: 1. Medical problems in a
regional SCI population. Paraplegia. 33:308–315. 1995.PubMed/NCBI
|
|
4
|
Bjørnshave Noe B, Mikkelsen EM, Hansen RM,
Thygesen M and Hagen EM: Incidence of traumatic spinal cord injury
in Denmark, 1990–2012: A hospital-based study. Spinal Cord.
53:436–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh A, Tetreault L, Kalsi-Ryan S, Nouri
A and Fehlings MG: Global prevalence and incidence of traumatic
spinal cord injury. Clin Epidemiol. 6:309–331. 2014.PubMed/NCBI
|
|
6
|
Aguayo AJ, Rasminsky M, Bray GM,
Carbonetto S, McKerracher L, Villegas-Pérez MP, Vidal-Sanz M and
Carter DA: Degenerative and regenerative responses of injured
neurons in the central nervous system of adult mammals. Philos
Trans R Soc Lond B Biol Sci. 331:337–343. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aguayo AJ, Björklund A, Stenevi U and
Carlstedt T: Fetal mesencephalic neurons survive and extend long
axons across peripheral nervous system grafts inserted into the
adult rat striatum. Neurosci Lett. 45:53–58. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richardson PM, Issa VM and Aguayo AJ:
Regeneration of long spinal axons in the rat. J Neurocytol.
13:165–182. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Butler EG and Ward MB: Reconstitution of
the spinal cord following ablation in urodele larvae. J Exp Zool.
160:47–65. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diaz Quiroz JF, Tsai E, Coyle M, Sehm T
and Echeverri K: Precise control of miR-125b levels is required to
create a regeneration-permissive environment after spinal cord
injury: A cross-species comparison between salamander and rat. Dis
Model Mech. 7:601–611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clarke JD, Alexander R and Holder N:
Regeneration of descending axons in the spinal cord of the axolotl.
Neurosci Lett. 89:1–6. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McHedlishvili L, Mazurov V and Tanaka EM:
Reconstitution of the central nervous system during salamander tail
regeneration from the implanted neurospheres. Methods Mol Biol.
916:197–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hutchison C, Pilote M and Roy S: The
axolotl limb: A model for bone development, regeneration and
fracture healing. Bone. 40:45–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lacroix S, Hamilton LK, Vaugeois A,
Beaudoin S, Breault-Dugas C, Pineau I, Lévesque SA, Grégoire CA and
Fernandes KJ: Central canal ependymal cells proliferate extensively
in response to traumatic spinal cord injury but not demyelinating
lesions. PLoS One. 9:e859162014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
James ND, Shea J, Muir EM, Verhaagen J,
Schneider BL and Bradbury EJ: Chondroitinase gene therapy improves
upper limb function following cervical contusion injury. Exp
Neurol. 271:131–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Silver J, Schwab ME and Popovich PG:
Central nervous system regenerative failure: Role of
oligodendrocytes, astrocytes and microglia. Cold Spring Harb
Perspect Biol. 7:a0206022014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Freund P, Wannier T, Schmidlin E, Bloch J,
Mir A, Schwab ME and Rouiller EM: Anti-Nogo-A antibody treatment
enhances sprouting of corticospinal axons rostral to a unilateral
cervical spinal cord lesion in adult macaque monkey. J Comp Neurol.
502:644–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zorner B and Schwab ME: Anti-Nogo on the
go: From animal models to a clinical trial. Ann N Y Acad Sci. 1198
Suppl 1:E22–E34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hui SP, Monaghan JR, Voss SR and Ghosh S:
Expression pattern of Nogo-A, MAG, and NgR in regenerating urodele
spinal cord. Dev Dyn. 242:847–860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wanner IB, Anderson MA, Song B, Levine J,
Fernandez A, Gray-Thompson Z, Ao Y and Sofroniew MV: Glial scar
borders are formed by newly proliferated, elongated astrocytes that
interact to corral inflammatory and fibrotic cells via
STAT3-dependent mechanisms after spinal cord injury. J Neurosci.
33:12870–12886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chevallier S, Landry M, Nagy F and
Cabelguen JM: Recovery of bimodal locomotion in the
spinal-transected salamander, Pleurodeles waltlii. Eur J Neurosci.
20:1995–2007. 2004. View Article : Google Scholar : PubMed/NCBI
|