Introduction
Autoimmune thyroid disease is a common chronic
lymphocytic inflammatory disease, which does not exhibit any of the
typical symptoms of systemic infection (1), in which thyroid function is
progressively reduced, which severely affects the quality of life
of patients (2). Hashimoto's
thyroiditis (HT) is a common tissue-specific autoimmune thyroid
disease. This can be induced by the termination of immune tolerance
due to abnormal immune system regulation, as well as an
inappropriate autoimmune response against the thyroid (3).
Autophagy is a highly conserved physiological
process that involves lysosome-mediated self-digestion and the
recycling of intracellular components, including the cell membrane
and organelles, particularly mitochondria, cytoskeletal components
and protein complexes (4). Autophagy
can remove injured or aging biological macromolecules and
organelles in cytoplasm, thus maintaining protein turnover and
intracellular homeostasis, as well as regulating cell survival,
differentiation and development (5).
Autophagy is a regulatory stress response that participates in
multiple physiological and pathological cell processes, including
embryonic development, aging and cancer (5). It is maintained at a basic level in
resting cells for the elimination of defective organelles and
misfolded proteins, the prevention of excessive protein
accumulation and to cope with endoplasmic reticulum stress
(6). Additionally, autophagy is an
important inflammatory regulator that is associated with
inflammasome and cytokine elimination (6). Autophagy maintains cell homeostasis,
protecting cells from injury and death (4). Therefore, autophagy is a necessary
physiological process for normal cell function and survival.
A study has demonstrated that the phosphoinositide
3-kinase/protein kinase B/mechanistic target of rapamycin
(PI3K/Akt/mTOR) signalling pathway can regulate autophagy as well
as apoptosis (7). PI3K/Akt controls
cell function by regulating the expression of numerous downstream
molecules, including Mtor (7). mTOR
is a serine/threonine kinase in the phosphatidyl inositol
3′kinase-related kinase family that is associated with the
proliferation and growth of non-neuronal cells (8). Neurons are non-proliferative cells, but
previous research has indicated that neuron size is associated with
mTOR regulation (9). It has also
been demonstrated that mTOR activation is required for neuron
extension direction, dendritic development and spine shape
(8). PI3K/Akt signalling pathway
activation regulates mTOR protein expression to result in the
inhibition of neuronal apoptosis (9). Phosphorylated Akt can activate mTOR and
mTOR inhibits downstream molecule ULK1 complex to negatively
regulate autophagy (10).
miRNAs have the potential to regulate the expression
of the majority of human genes (11). miRNAs participate in biological
processes of autoimmune disease by regulating the development,
differentiation, maturation and signal transduction of immune cells
(11). Furthermore, they have an
important role in maintaining immune cell homeostasis and normal
cell function regulation and therefore have the potential to be
used in novel disease therapies (12). It has been identified that >100
miRNAs may contribute to autoimmune disease. These miRNAs have the
potential to influence the development and function of innate and
acquired immune cells (12). Li and
Ding (13) recently demonstrated
that miR-125a also promoted serum pro-inflammatory cytokine
expression in patients with lupus nephritis. In the present study,
the function and underlying mechanisms of miR-125a in thyroiditis
were examined.
Materials and methods
Mice and cell culture
Female 6-week-old C57BL/6 mice (n=12; 18–20 g) were
purchased from Chongqing Medical University (Chongqing, China),
housed in a clean-grade animal breeding centre at 20–24°C, humidity
of 55–60% and were given free access to food and water in a 12 h
alternating dark/light cycle. Mice were divided into the control
(n=6) and thyroiditis model (n=6) groups. In the thyroiditis model
group, mice were subcutaneously injected with 100 µg/ml porcine
thyroglobulin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
weekly for 2 weeks. The peripheral blood of mice (100 µl) was
subsequently collected into centrifuge tubes containing heparin
(Sigma-Aldrich; Merck KGaA) under anaesthesia, and then the mice
were sacrificed by decapitation. In control group, mice were
subcutaneously injected with normal saline once a week. The use of
animals in the present study was reviewed and approved by the
Ethics Committee of The Zhongshan District of Chongqing General
Hospital (Chongqing, China).
The mouse macrophage cell line RAW264.7 cell was
obtained from The Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China) and cultured in Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 U/ml streptomycin at
37°C and 5% CO2.
Hematoxylin and eosin (H&E)
staining
Following the induction of thyroiditis, thyroid
tissue was fixed with 4% paraformaldehyde for 24 h at room
temperature. Thyroid tissue was sectioned into 10-µm-thick sections
and H&E staining was performed at room temperature for 10 min.
Thyroid tissue was examined using a confocal microscope (Nikon
Eclipse Ti; Nikon Corporation, Tokyo, Japan) at a magnification of
×100 to confirm the establishment of the model.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the mouse peripheral
blood using TRIzol reagent (Life Technologies; Thermo Fisher
Scientific, Inc.). cDNA was reverse transcribed using the miScript
II RT kit (Qiagen China Co., Ltd., Shanghai, China). RT-qPCR was
performed in triplicate using the miScript SYBR Green PCR kit
(Qiagen China Co., Ltd.) with the StepOnePlus PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Initial denaturation
was performed at 95°C for 30 sec, followed by 40 cycles at 95°C for
30 sec, 60°C for 30 sec and 72°C for 30 sec. The primer sequences
used were as follows: miRNA-125a: Forward,
5′-CTGAACTAGTGTGTGCGGAAACACTCAGGG-3′ and reverse,
5′-CTGAAAGCTTGGAGTGACAGATCTGTGTCCTC-3′; U6: Forward,
5′-CCGCCCGCCGCCAGGCCCC-3′ and reverse, 5′-ATATGGAACGCTTCACGAATT-3′.
The expression of miRNA-125a was quantified using the
2−ΔΔCq method (14,15).
Cell transfection and establishment of
an in vitro model of thyroiditis
Cells (5×105) were seeded into 6-well
plates, and transfected with 100 ng miR-125a
(5′-ACAGGUGAGGUUCUUGGGAGCC-3′) or a combination of miR-125a and a
PI3K inhibitor (20 nM LY294002) for 36 h at 37°C using
Lipofectamine® 2000 (both Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. In
control group, cells (5×105) were transfected with 100
ng negative control miRNA (5′-CCCCCCCCCCCCCCCCCCCCC-3′) using
Lipofectamine® 2000 according to the manufacturer's
protocol. Cells were subsequently seeded into 6 (1×106
cell/well) or 96 (1×103 cell/well) well plates in DMEM
and stimulated with human interferon-γ (IFN-γ; 10 ng/ml;
Sigma-Aldrich; Merck KGaA) for 12 h at 37°C. RAW264.7 cells were
stimulated with IFN-γ to establish the in vitro model of
experimental autoimmune inflammation (16).
MTT assay
Cells (1×103/well) were plated in 96-well
plates and MTT (5 mg/ml; 20 µl; Thermo Fisher Scientific, Inc.) was
added into each well for 4 h at 37°C. Dimethyl sulfoxide (150 µl;
Thermo Fisher Scientific, Inc.) was subsequently added into the
wells for 20 min at 37°C. Optical density values were measured with
an automatic microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA) at 492 nm.
Flow cytometry
After 4 h of transfection, cells were plated in
6-well plates (1×106/well) in DMEM with 10% FBS for 48 h
at 37°C and subsequently washed with PBS. Cells were resuspended in
binding buffer (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
and stained with annexin-V fluorescein isothiocyanate (5 µl) and
propidium iodide (5 µl; KeyGen Biotech Co., Ltd.) for 15 min in the
dark at room temperature. Samples were analysed using a Beckman
Coulter flow cytometer (Beckman Coulter, Inc., Fullerton, CA, USA)
and FlowJo software (version 7.6.1; FlowJo LLC, Ashland, OR,
USA).
Immunocytochemical analysis of
autophagy
Cells were washed with PBS, fixed with 4%
paraformaldehyde for 15 min at room temperature and permeabilized
with 0.1% Triton X-100 for 15 min at room temperature. Cells were
subsequently incubated with anti-microtubule-associated protein
1α/1β-light chain 3 (LC3) antibody (cat. no. 3868; 1:1,000; CST
Biological Reagents Co., Ltd., Shanghai, China) for 1 h at room
temperature, followed by incubation with Alexa Fluor®
488-conjugated goat anti-rabbit antibody (cat. no. 4412; 1:2,000,
CST Biological Reagents Co., Ltd.) for 1 h at room temperature.
Fluorescence was visualized with a confocal laser-scanning
microscope (Zeiss GmbH, Jena, Germany) at a magnification of
×20.
Cytokine assay
Tumor necrosis factor-α (TNF-α; cat. no. EM008-96),
interleukin (IL)-1β (cat. no. EM001-96), IL-6 (cat. no. EM004-96)
and IL-18 (cat. no. EH047-96) levels were measured in cell
supernatant using sandwich ELISA kits (ExCell; Genetimes
Technology, Inc., Shanghai, China) according to the manufacturer's
protocol.
Western blot analysis
Cells were washed with PBS, harvested and lysed with
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China). Protein content was measured with a
bicinchoninic assay following centrifugation at 1,000 × g for 10
min at 4°C. Protein (50 µg) was loaded into each lane, separated
using 10% SDS-PAGE and transferred onto polyvinylidene fluoride
membranes. The membranes were blocked with 5% skim milk powder for
2 h at 37°C and subsequently incubated overnight at 4°C with
primary antibodies against LC3 (cat. no. 4108; 1:1,000), autophagy
protein 5 (Atg 5; cat. no. 12994; 1:2,000), PI3K (cat. no. 4249;
1:2,000), phosphorylated (p)-Akt (cat. no. 4060; 1:1,000), p-mTOR
(cat. no. 5536; 1:1,000) and GAPDH (cat. no. 5174; 1:5,000; all CST
Biological Reagents Co., Ltd.). The membrane was washed in
tris-buffered saline with Tween-20 and incubated with horseradish
peroxidase-conjugated goat anti-rabbit antibody (cat. no. 7074;
1:5,000; CST Biological Reagents Co., Ltd.). Bands were visualized
using an enhanced chemiluminescence plus blotting reagent and
Quantity One 1-D 3.0 software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation of independent experiments (n=3). Statistical analysis
was performed using one-way analysis of variance followed by
Tukey's post-hoc test using SPSS software (version 17.0; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
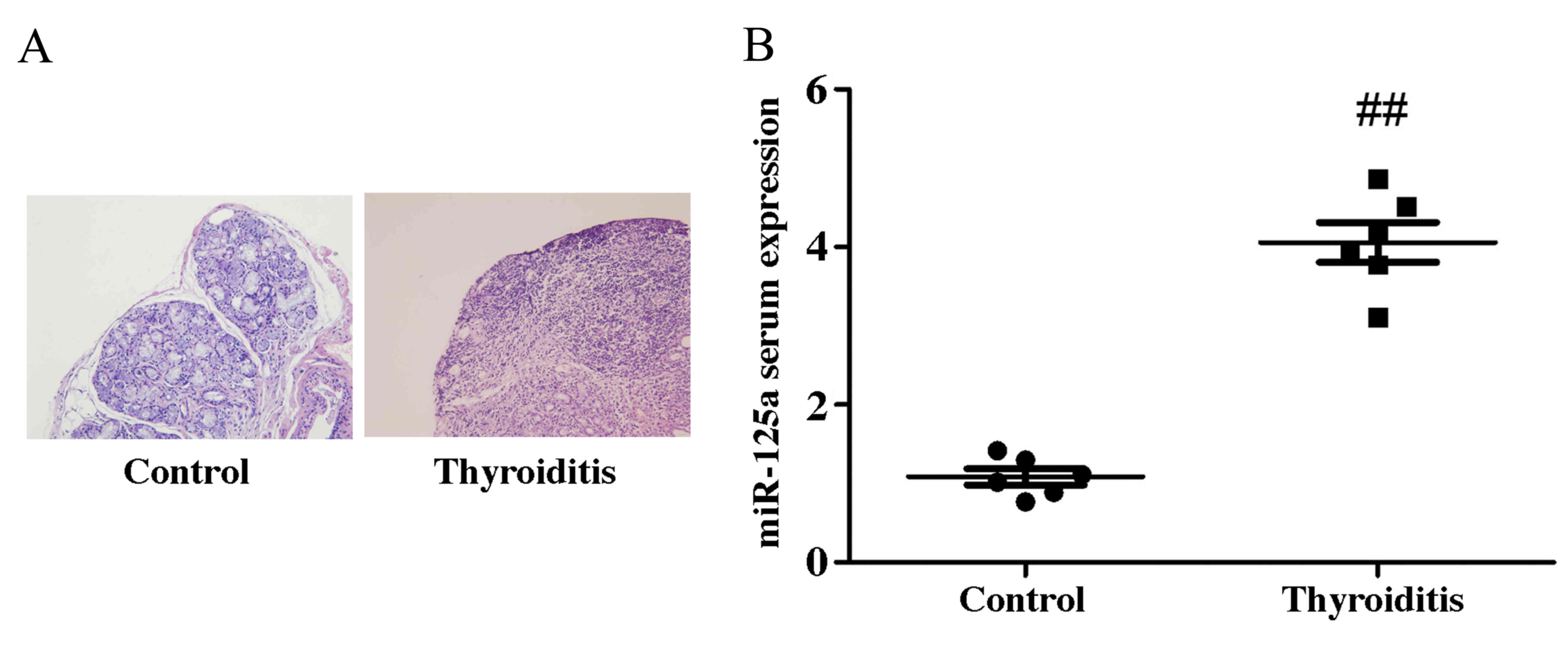
miR-125a serum expression increases in
a mouse model of thyroiditis
RT-qPCR was performed to detect miR-125a serum
expression. miR-125a expression was significantly upregulated in
thyroiditis mice, compared with the control group (Fig. 1). This suggests that miR-125a may be
associated with the pathophysiology of thyroiditis.
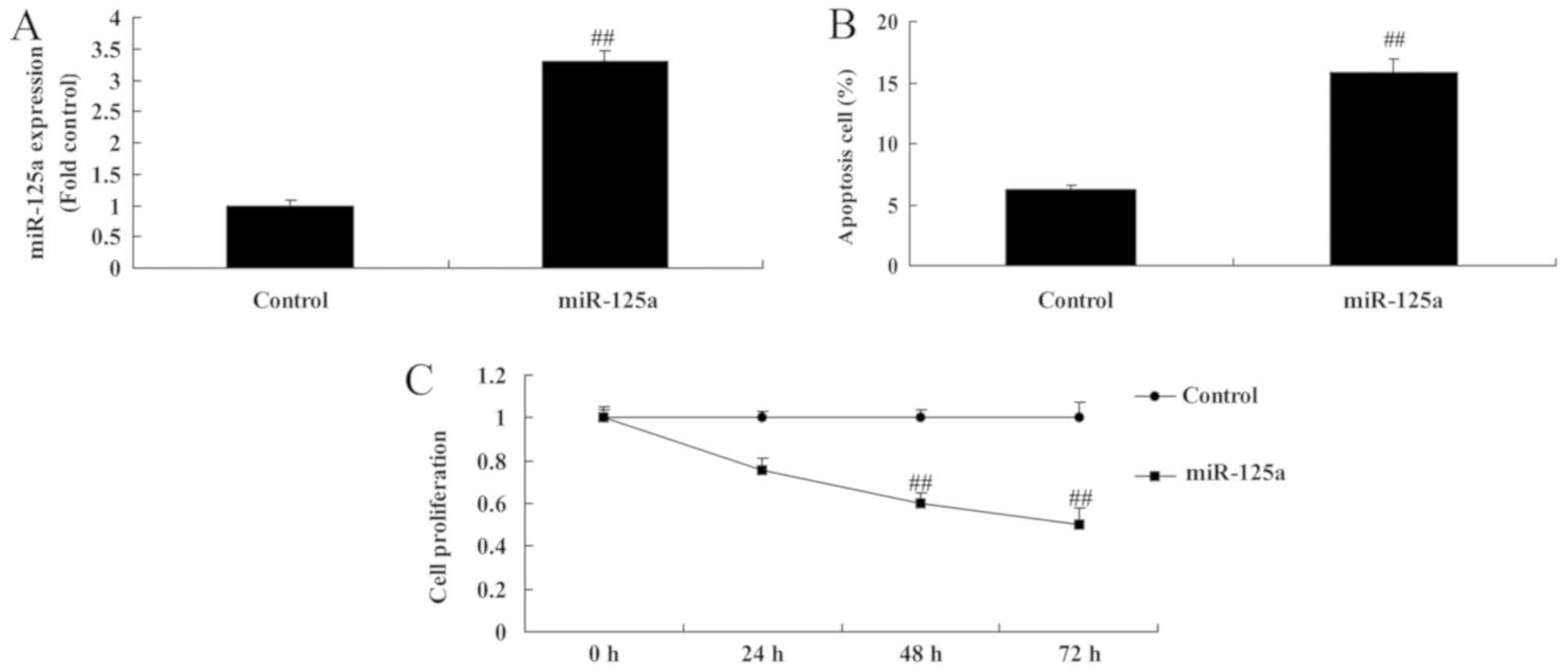
miR-125a upregulation induces
apoptosis and reduces cell proliferation in vitro
The effect of miR-125a on cell growth in an in
vitro thyroiditis model was examined. miR-125a was
significantly upregulated in miRNA-125a mimic transfected cells
(Fig. 2A). The upregulation of
miRNA-125a significantly increased the apoptotic rate (Fig. 2B) and reduced cell proliferation
(Fig. 2C) compared with the control
group.
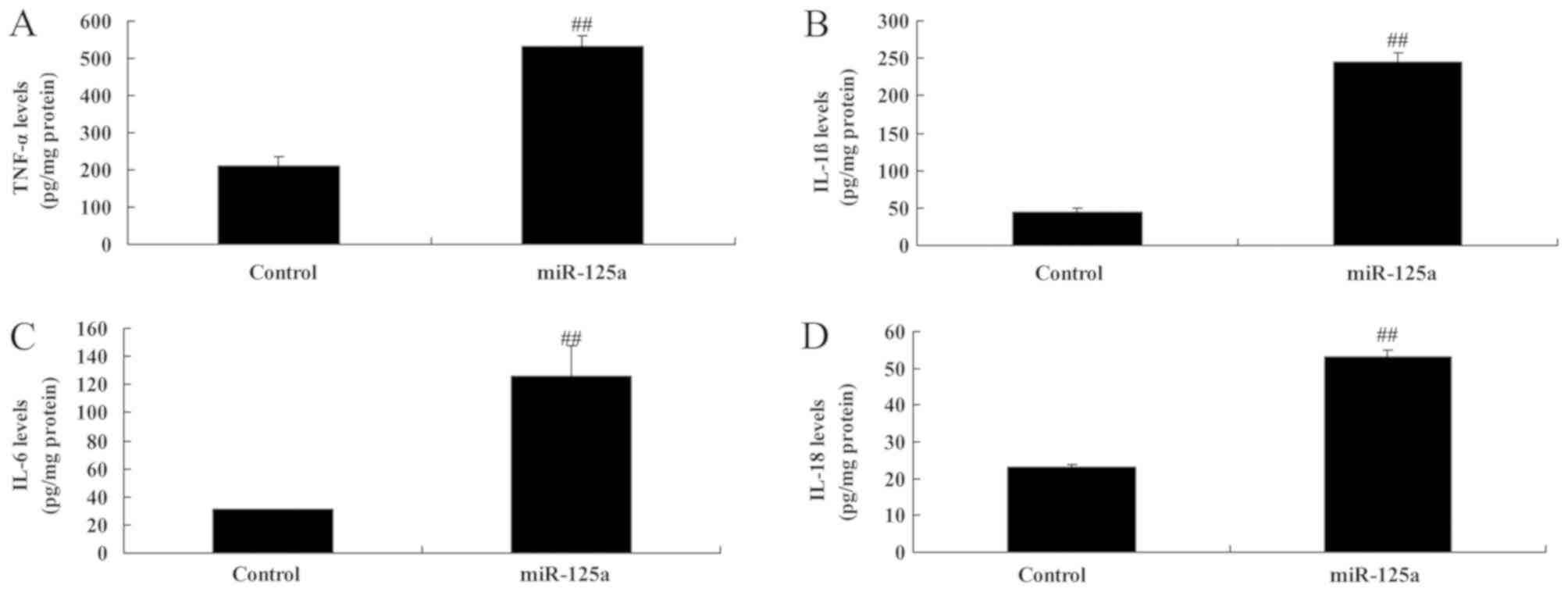
miR-125a upregulation induces the
expression of pro-inflammatory factors in vitro
Levels of TNF-α, IL-1β, IL-6 and IL-18 were
significantly increased in the in vitro model of thyroiditis
when transfected with miR-125a mimics, compared with the control
group (Fig. 3), indicating that
miR-125a may regulate pro-inflammatory factor expression in
thyroiditis.
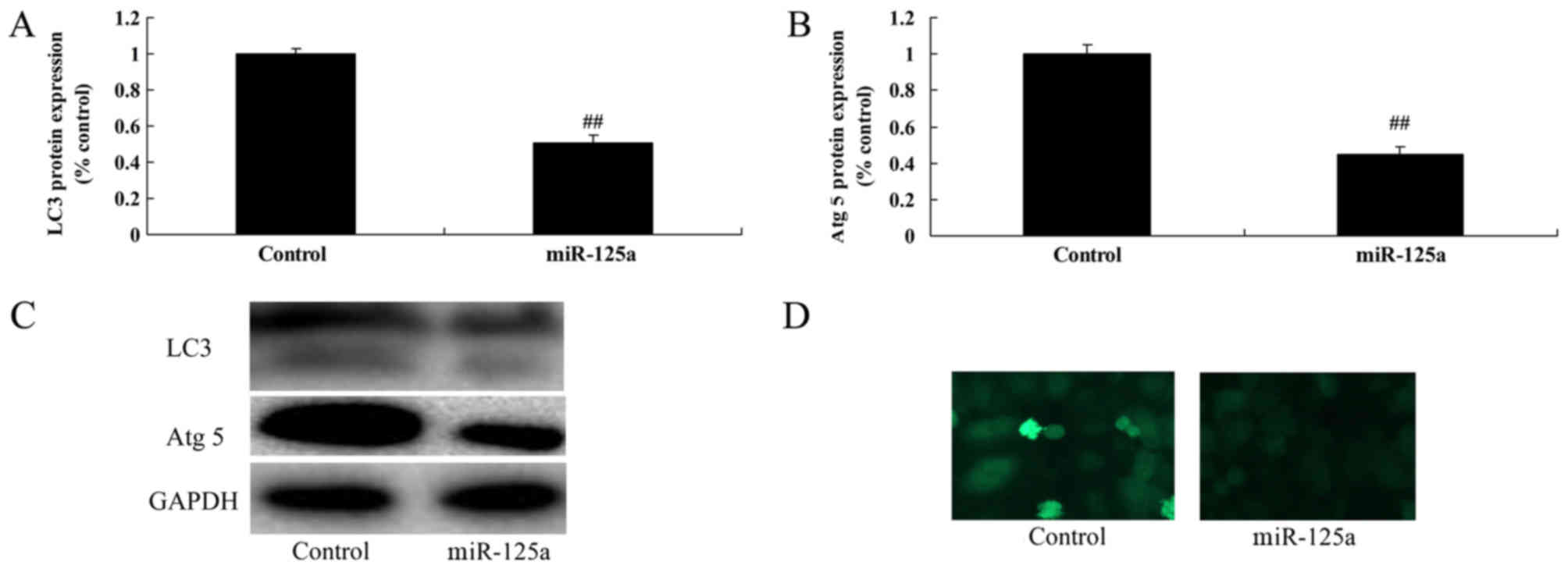
miR-125a upregulation reduces
autophagy in vitro
The effect of miR-125a on the expression of
autophagy-associated proteins was investigated. The expression of
autophagy proteins LC3 and Atg 5 was significantly decreased in the
miR-125a mimic transfected group compared with the control
(Fig. 4), suggesting that miR-125
may reduce autophagy in thyroiditis.
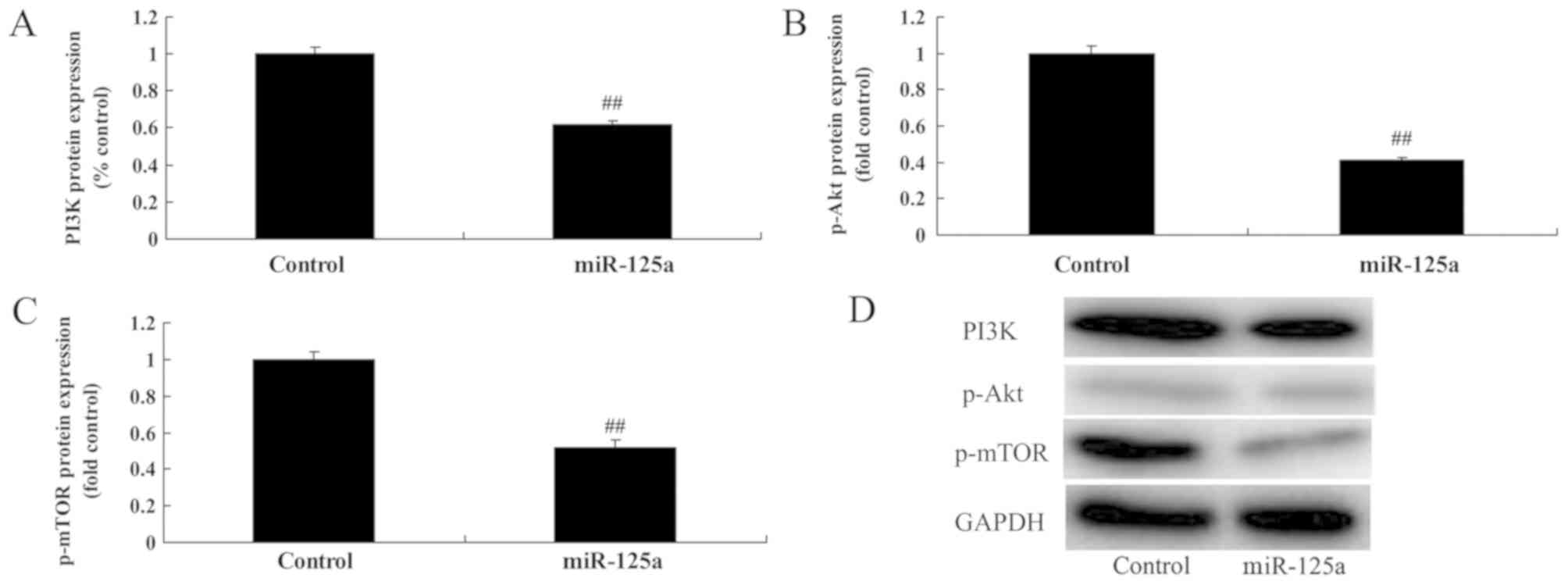
miR-125a suppresses PI3K/Akt/mTOR
signalling pathway activation in vitro
miR-125a upregulation significantly suppressed PI3K,
p-Akt and p-mTOR levels, compared with the control group (Fig. 5), suggesting that miR-125a suppressed
autophagy through inhibition of the PI3K/Akt/mTOR signalling
pathway.
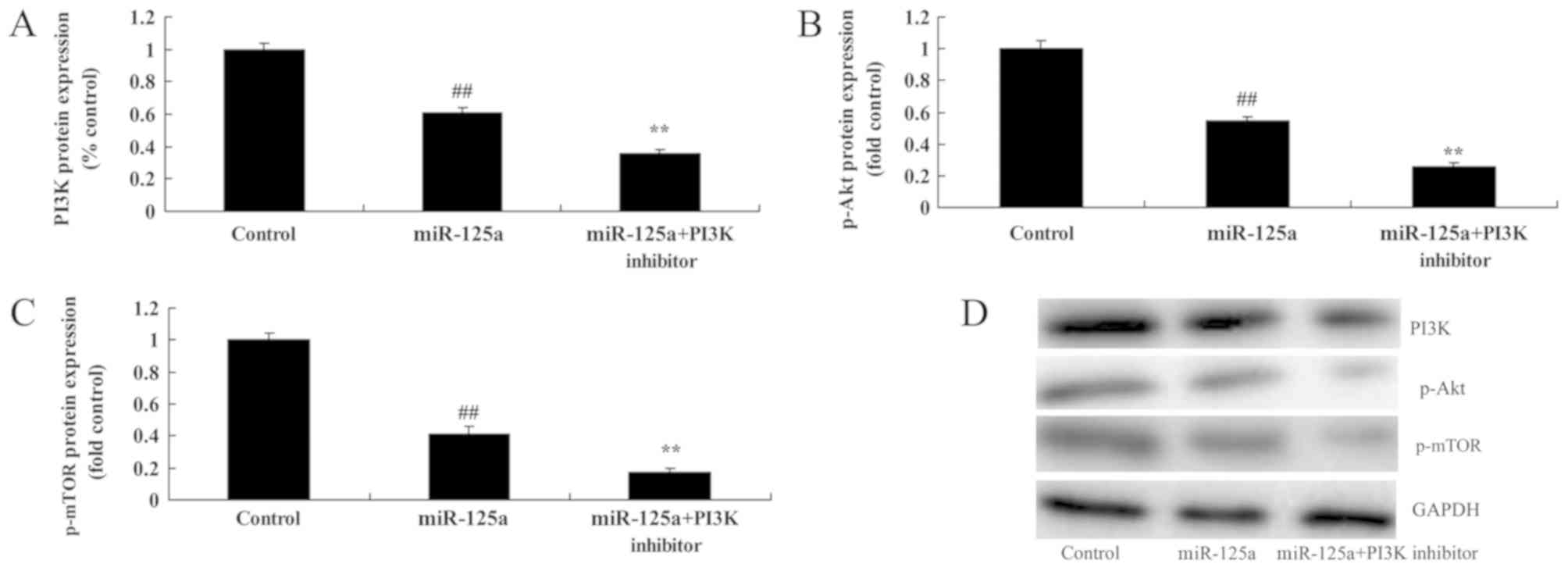
PI3K inhibition enhances the
miRNA-125a-mediated suppression of the PI3K/Akt/mTOR signalling
pathway, induction of apoptosis and inhibition of cell
proliferation in vitro
To examine the role of PI3K in the function of
miRNA-125a in thyroiditis, a PI3K inhibitor, 20 nM of LY294002 was
used to inhibit the PI3K/Akt/mTOR signalling pathway in the in
vitro thyroiditis model. PI3K inhibitor and miRNA-125a mimics
significantly reduced PI3K, p-Akt and p-mTOR levels by miRNA-125a,
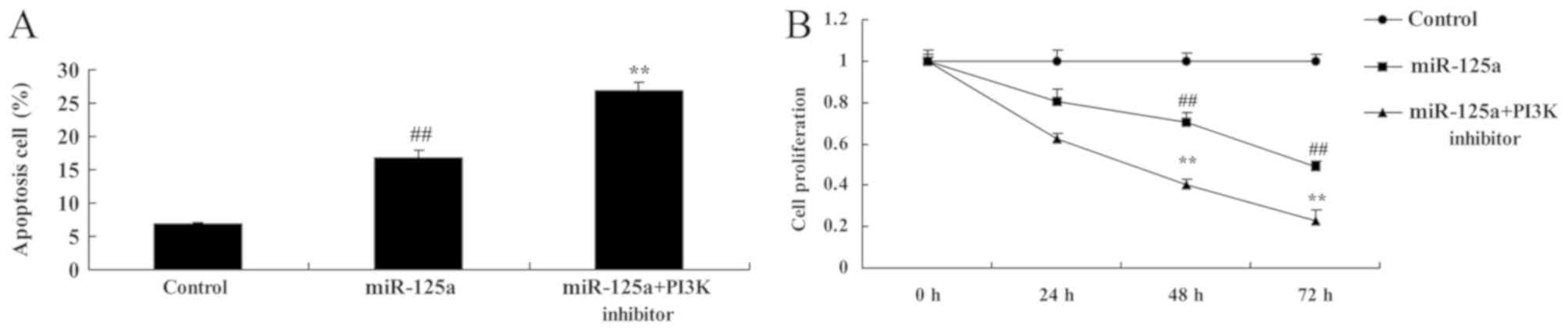
compared with the miRNA-125a mimic alone (Fig. 6). Apoptotic rate was also
significantly increased and cell proliferation was further
decreased by the PI3K inhibitor and miRNA-125a mimic, compared with
the miRNA-125a mimic alone (Fig.
7).
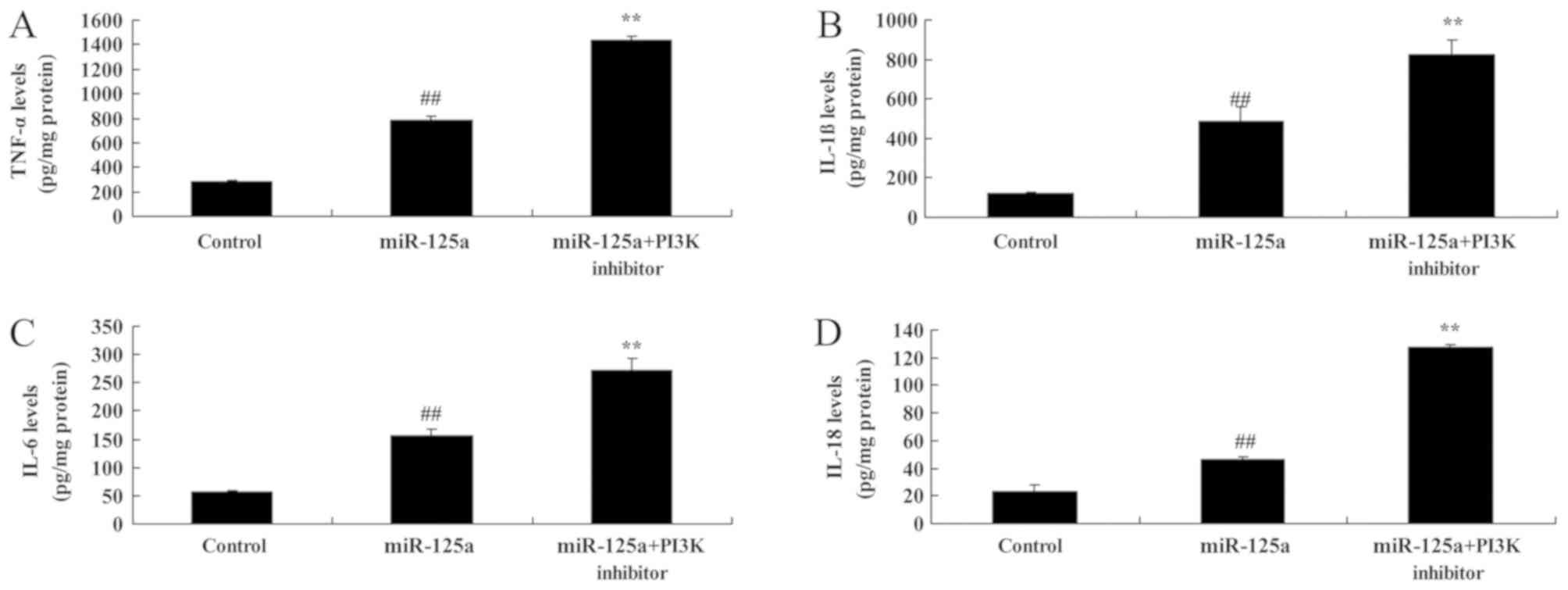
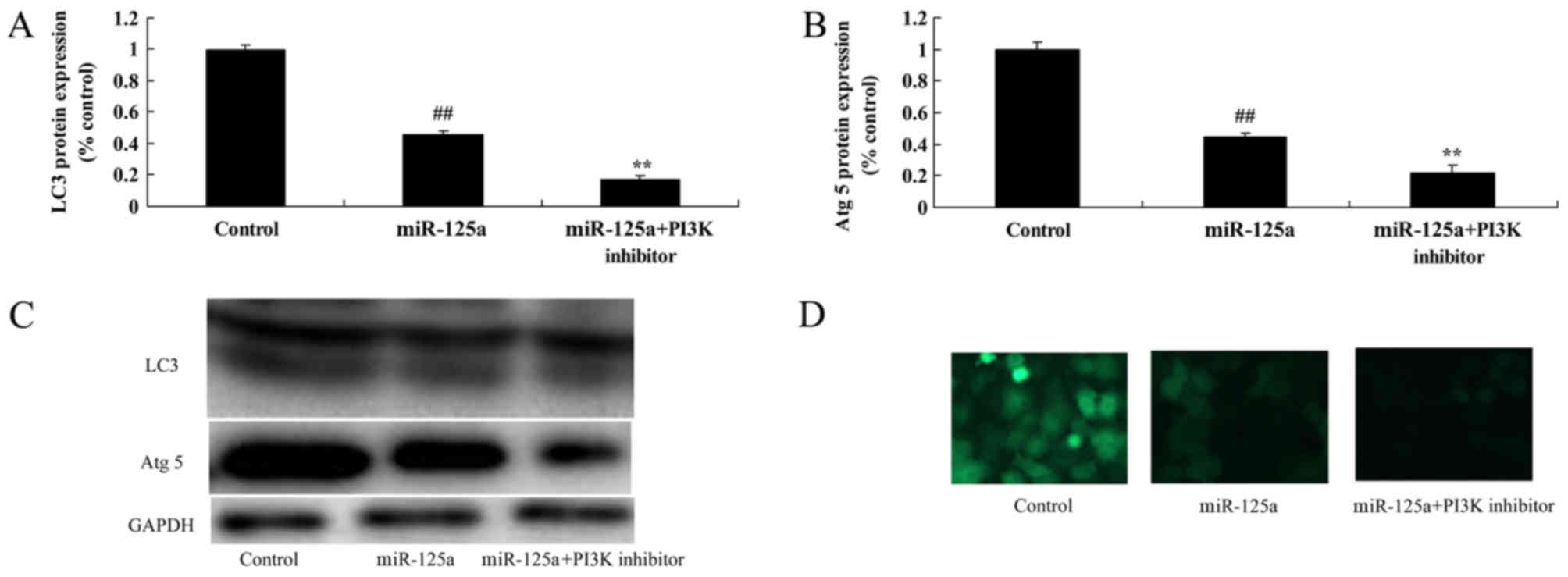
PI3K inhibition enhances the
miRNA-125a-mediated increase in pro-inflammatory mediators and
decrease in autophagy in vitro
The inhibition of PI3K in combination with the
miRNA-125a mimics significantly increased TNF-α, IL-1β, IL-6 and
IL-18 expression compared with miRNA-125a mimic alone (Fig. 8). PI3K inhibition also resulted in a
further decrease in LC3 and Atg 5 protein expression thyroiditis,
compared with miRNA-125a mimic alone (Fig. 9).
Discussion
HT, also referred to as chronic lymphocytic
thyroiditis, is a T-cell-mediated organ specific autoimmune disease
(17). It is the most common type of
thyroiditis and typically develops in middle-aged women, with the
population morbidity at 2% (18).
The present study revealed that miR-125a serum expression was
upregulated in a mouse model of thyroiditis compared with the
control group. However, only 12 mice were used in the current study
and larger cohorts should be used in future research to validate
this result. In the current study, RAW264.7 cells stimulated with
IFN-γ were used to establish the in vitro model of
experimental autoimmune inflammation, however they were not uses as
a model of experimental autoimmune thyroiditis as that hypothesis
was not adequately supported by previous studies. Other experiment
models will be used in a further study.
It has previously been indicated that lymphocytic
inflammatory disorder is a major source of ROS (17). Excessive ROS production is associated
with allergic dermatitis, continuous inflammation, lupus
erythematosus and multiple dermatitis (19). Autophagy defects have been identified
in many diseases and are associated with an increased
susceptibility to autoimmune and inflammatory disease development
(20). HT is an autoimmune
inflammatory disease and autophagy has an important role in its
pathogenesis (20). The present
study demonstrated that miR-125a upregulation reduced the
expression of autophagy proteins LC3 and Atg in vitro.
Kim et al (21) recently reported that miR-125a
inhibits autophagy activation during mycobacterial infection. The
development of HT is closely associated with a signalling network
of cytokines, neurotransmitters and other endocrine hormones in the
thyroid (22). This signalling
network can regulate the differentiation and growth function of
thyroid cells in an autocrine and paracrine manner. Inflammatory
cytokines associated with this disease include monocyte
chemoattractant protein 1 and TNF-α (23). The present study identified that the
upregulation of miR-125a induced the expression of inflammatory
factors IL-18, IL-6, IL-1β and TNF-α in vitro. Li et
al (13) recently demonstrated
that miR-125a also promotes serum pro-inflammatory cytokine
expression in patients with lupus nephritis.
Akt is a serine/threonine kinase that regulates cell
function by phosphorylating various enzymes, kinases and
transcription factors following its activation (24). For example, activated Akt can
phosphorylate B cell lymphoma-1 and caspases to inhibit apoptosis
and act on the class O forkhead box transcription factors member
and nuclear factor-κB to regulate cell survival (25). The PI3K/Akt signalling pathway is a
molecular pathway that is required for cell survival and has
important roles in apoptosis and tumor genesis (25). Its regulatory effect on downstream
autophagy-related molecules, including mTOR, has been extensively
recognized (9). The results of the
present study revealed that miR-125a upregulation suppressed
PI3K/Akt/mTOR signalling pathway activation in vitro.
It is well established that necrosis can induce the
inflammatory response, the release of toxic cytokines and
peripheral cell injury (7). By
contrast, apoptosis is a process of programmed cell death that does
not induce an inflammatory response and is therefore
neuroprotective (10). Research has
confirmed that autophagy is regulated by the PI3K/Akt signalling
pathway following neuronal mechanical injury. The PI3K/Akt/mTOR
pathway has a critical role in the growth, proliferation and
survival of cells (10). However,
some research has demonstrated that this pathway can induce cell
death, particularly necrosis. PI3K/Akt/mTOR pathway activation can
inhibit autophagy, which may lead to an increase in necrotic cells
and a decrease in neuronal apoptosis and overall cellular activity
(9). The present study indicated
that the inhibition of PI3K enhanced the ability of miRNA-125a to
increase the inflammatory response in macrophages via the
PI3K/Akt/mTOR signalling pathway. Tang et al (26) identified that miR-125a also inhibits
the invasive ability of hepatocellular carcinoma cells via
regulation of the PI3K/Akt/mTOR signalling pathway.
In conclusion, miR-125a increased inflammation and
inhibited autophagy in an in vitro model of thyroiditis
through regulation of the PI3K/Akt/mTOR signalling pathway. Further
investigation is required to fully elucidate the mechanisms
involved in autoimmune thyroiditis. The present study provides
evidence for a potential mechanism involved in autoimmune
thyroiditis pathophysiology.
Acknowledgements
This study was supported by Medical Science and
Technology Innovation Project of Chongqing General Hospital (grant
no. 2017MSXM02).
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
DC, XH, SL, HD, HG, RH and BZ performed the
experiments and wrote the manuscript. DC designed the experiment
and performed the data analysis.
Ethics approval and consent to
participate
The use of animals in the present study was reviewed
and approved by the Ethics Committee of The Zhongshan District of
Chongqing General Hospital (Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Byun JI, Lee ST, Jung KH, Sunwoo JS, Moon
J, Lim JA, Lee DY, Shin YW, Kim TJ, Lee KJ, et al: Effect of
immunotherapy on seizure outcome in patients with autoimmune
encephalitis: A prospective observational registry study. PLoS One.
11:e01464552016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Winther KH, Watt T, Bjørner JB, Cramon P,
Feldt-Rasmussen U, Gluud C, Gram J, Groenvold M, Hegedüs L, Knudsen
N, et al: The chronic autoimmune thyroiditis quality of life
selenium trial (CATALYST): Study protocol for a randomized
controlled trial. Trials. 15:1152014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nazarpour S, Ramezani Tehrani F, Simbar M,
Tohidi M, Alavi Majd H and Azizi F: Effects of levothyroxine
treatment on pregnancy outcomes in pregnant women with autoimmune
thyroid disease. Eur J Endocrinol. 176:253–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HM, Kim ES and Koo JS: Expression of
autophagy-related proteins in different types of thyroid cancer.
Int J Mol Sci. 18:E5402017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh BK, Sinha RA, Ohba K and Yen PM:
Role of thyroid hormone in hepatic gene regulation, chromatin
remodeling, and autophagy. Mol Cell Endocrinol. 458:160–168. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin CI, Whang EE, Donner DB, Du J, Lorch
J, He F, Jiang X, Price BD, Moore FD Jr and Ruan DT: Autophagy
induction with RAD001 enhances chemosensitivity and
radiosensitivity through Met inhibition in papillary thyroid
cancer. Mol Cancer Res. 8:1217–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG,
Yoo HJ and Lee SJ: Synergistic cytotoxicity of BIIB021 with
triptolide through suppression of PI3K/Akt/mTOR and NF-κB signal
pathways in thyroid carcinoma cells. Biomed Pharmacother. 83:22–32.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pringle DR, Vasko VV, Yu L, Manchanda PK,
Lee AA, Zhang X, Kirschner JM, Parlow AF, Saji M, Jarjoura D, et
al: Follicular thyroid cancers demonstrate dual activation of PKA
and mTOR as modeled by thyroid-specific deletion of Prkarla and
Pten in mice. J Clin Endocrinol Metab. 99:E804–E812. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Souza EC, Ferreira AC and Carvalho DP: The
mTOR protein as a target in thyroid cancer. Expert Opin Ther
Targets. 15:1099–1112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petrulea MS, Plantinga TS, Smit JW,
Georgescu CE and Netea-Maier RT: PI3K/Akt/mTOR: A promising
therapeutic target for non-medullary thyroid carcinoma. Cancer
Treat Rev. 41:707–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prabahar A and Natarajan J: MicroRNA
mediated network motifs in autoimmune diseases and its crosstalk
between genes, functions and pathways. J Immunol Methods.
440:19–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Otsu H, Watanabe M, Inoue N, Masutani R
and Iwatani Y: Intraindividual variation of microRNA expression
levels in plasma and peripheral blood mononuclear cells and the
associations of these levels with the pathogenesis of autoimmune
thyroid diseases. Clin Chem Lab Med. 55:626–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H and Ding G: Elevated serum
inflammatory cytokines in lupus nephritis patients, in association
with promoted hsa-miR-125a. Clin Lab. 62:631–638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu H, Hu S, Xu S, Gao Y, Zeng F and Shui
H: miR-29b regulates Ang II-induced EMT of rat renal tubular
epithelial cells via targeting PI3K/AKT signaling pathway. Int J
Mol Med. 42:453–460. 2018.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia N, Chen G, Liu M, Ye X, Pan Y, Ge J,
Mao Y, Wang H, Wang J and Xie S: Anti-inflammatory effects of
luteolin on experimental autoimmune thyroiditis in mice. Exp Ther
Med. 12:4049–4054. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buschur E, Sarma AV, Pietropaolo M, Dunn
RL, Braffett BH, Cleary PA, Cowie C, Larkin ME, Wessells H, Nathan
DM, et al: Self-reported autoimmune disease by sex in the diabetes
control and complications trial/epidemiology of diabetes
interventions and complications (DCCT/EDIC) study. Diabetes Care.
37:e28–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Höfling DB, Chavantes MC, Juliano AG,
Cerri GG, Knobel M, Yoshimura EM and Chammas MC: Low-level laser in
the treatment of patients with hypothyroidism induced by chronic
autoimmune thyroiditis: A randomized, placebo-controlled clinical
trial. Lasers Med Sci. 28:743–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee J, Yi S, Kang YE, Chang JY, Kim JT,
Sul HJ, Kim JO, Kim JM, Kim J, Porcelli AM, et al: Defective
ciliogenesis in thyroid hürthle cell tumors is associated with
increased autophagy. Oncotarget. 7:79117–79130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu C, Wu F, Mao C, Wang X, Zheng T, Bu L,
Mou X, Zhou Y, Yuan G, Wang S and Xiao Y: Excess iodine promotes
apoptosis of thyroid follicular epithelial cells by inducing
autophagy suppression and is associated with Hashimoto thyroiditis
disease. J Autoimmun. 75:50–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JK, Yuk JM, Kim SY, Kim TS, Jin HS,
Yang CS and Jo EK: MicroRNA-125a inhibits autophagy activation and
antimicrobial responses during mycobacterial infection. J Immunol.
194:5355–5365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raad H, Eskalli Z, Corvilain B, Miot F and
De Deken X: Thyroid hydrogen peroxide production is enhanced by the
Th2 cytokines, IL-4 and IL-13, through increased expression of the
dual oxidase 2 and its maturation factor DUOXA2. Free Radic Biol
Med. 56:216–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koc A, Batar B, Celik O, Onaran I, Tasan E
and Sultuybek GK: Polymorphism of the NFKB1 affects the serum
inflammatory levels of IL-6 in Hashimoto thyroiditis in a Turkish
population. Immunobiology. 219:531–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xian H, Wang F, Teng W, Yang D and Zhang
M: Thyroid hormone induce a p53-dependent DNA damage through
PI3K/Akt activation in sperm. Gene. 615:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu P, Liao LY, Zhao TT, Mo XM, Chen GG
and Liu ZM: GPER/ERK&AKT/NF-κB pathway is involved in
cadmium-induced proliferation, invasion and migration of
GPER-positive thyroid cancer cells. Mol Cell Endocrinol. 442:68–80.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang H, Li RP, Liang P, Zhou YL and Wang
GW: miR-125a inhibits the migration and invasion of liver cancer
cells via suppression of the PI3K/AKT/mTOR signaling pathway. Oncol
Lett. 10:681–686. 2015. View Article : Google Scholar : PubMed/NCBI
|