Introduction
Ovarian cancer is one of the three most common
malignant tumor types of the female reproductive system; it poses a
serious threat to women's health and is associated with high
mortality (1). The symptoms of the
disease are frequently non-specific, which hampers early detection,
so that the majority of patients present with advanced-stage
disease at the time-point of diagnosis (2). Therefore, the treatment of advanced
ovarian cancer is particularly important in the clinic.
Studies have confirmed that tumor growth is highly
dependent on blood vessels, and tumor metastasis and prognosis are
also closely linked to angiogenesis (3–6).
Although anti-angiogenic agents, e.g. bevacizumab, are used for
almost all patients with ovarian cancer, the cure rate is not
increased (7,8). Furthermore, predictive biomarkers are
currently insufficient and are urgently required. Basic fibroblast
growth factor (bFGF), belonging to the family of FGFs, is one of
the strongest vascular growth factors involved in the processes of
proliferation and differentiation of a wide variety of cell types.
Previous studies have indicated that the P7 high-affinity
bFGF-binding peptide is able to inhibit the proliferation and
invasion of various cell types induced by bFGF (9–12).
Angiogenesis has a fundamental role in normal ovarian physiology as
well as in the pathogenesis of ovarian cancer, promoting tumor
growth and progression through ascites formation and metastatic
spread (7). However, whether P7 also
inhibits bFGF-induced proliferation and angiogenesis of human
epithelial ovarian cancer cells has not been previously reported,
to the best of our knowledge. In the present study, the effect of
P7 on bFGF-induced proliferation and invasion of SKOV3 ovarian
cancer cells was assessed in vitro, providing an
experimental basis for the targeted treatment of epithelial ovarian
cancer.
Materials and methods
Materials
The SKOV3 human ovarian cancer cell line was
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). P7 peptides (PLLQATLGGGS) with a purity of
>98% was synthesized by Beijing SBS Genetech Corp. (Beijing,
China) and recombinant human bFGF was obtained from Peprotech Inc.
(Rocky Hill, NJ, USA). RPMI-1640 medium and fetal bovine serum
(FBS) were from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Morphological examination
SKOV3 cells in the exponential growth phase were
seeded into 96-well culture plates in 100 µl RPMI-1640 medium
containing 0.4% FBS at a density of 1×104 cells/well at
37°C in a humidified atmosphere containing 5% CO2. After
addition of 100 µl medium containing P7 at different final
concentrations (0.25, 1, 4 and 16 µM) and culture for 48 h, the
cell morphology was observed with an inverted microscope (Olympus
IX70; Olympus, Tokyo, Japan). SKOV3 cells with addition of 100 µl
complete medium were used as a control group.
MTT cell proliferation assay
Cell viability was determined using an MTT assay.
SKOV3 cells in the exponential growth phase were seeded into
96-well culture plates in 100 µl medium at a density of
1×104 cells/well. The cells were serum-starved for 24 h.
In the P7-treated group, 100 µl medium containing different
concentrations of P7 was added to each well, followed by incubation
for 48 h. In the bFGF-treated group, 100 µl medium containing
different concentrations of bFGF (0.1, 1, 10 and 100 ng/ml) was
added. Furthermore, in the P7+bFGF group, 100 µl medium containing
different concentrations of P7 mixed with 10 ng/ml bFGF
(IC50) was added to the wells in quadruplicate. SKOV3
cells with addition of RPMI-1640 medium containing 0.4% FBS were
used as the control group, while 100 µl bFGF (10 ng/ml) was added
for the positive control group. After 48 h of incubation, the MTT
assay was performed by adding 20 µl MTT solution (5 mg/ml in PBS;
Beyotime Institute of Biotechnology, Haimen, China) to each well,
followed by further incubation for 4 h. Subsequently, 150 µl
dimethyl sulfoxide (Beijing Chemical Industry Co. Ltd., Beijing,
China) was added. After shaking for 10 min, the optical density
(OD) value was measured at a wavelength of 570 nm using a
microplate reader (BIO-TEK800; Biotech Instruments, Winooski, VT,
USA). Each treatment was performed in triplicate and the results
were expressed as a percentage of the control: Cell proliferation
rate
(%)=(ODbFGF-ODControl)/ODControl
×100%. The inhibition rate of P7 on bFGF-induced SKOV3 cell
proliferation was calculated as follows: Inhibition rate
(%)=[(ODbFGF-ODControl)-(ODP7-ODControl)]/(ODbFGF-ODControl)
×100%.
Scratch wound cell migration
assay
Exponentially growing SKOV3 cells were seeded into
6-well culture plates at a density of 1×104 cells/well,
followed by starved incubation for 24 h. In the middle of each
well, a 0.5 cm-wide linear scratch was generated with a 20-µl
filter tip. The detached cells were rinsed with PBS 3 times so as
to prevent their re-attachment in the scratched area. Subsequently,
100 µl DMEM containing 4 µM P7 for the P7 group, 10 ng/ml bFGF for
the bFGF group or 50 µl 8 µM P7 + 50 µl 20 ng/ml bFGF for the
P7+bFGF group was added to each well separately, while 100 µl DMEM
was added to the blank control. Following incubation for 0, 6, 12
and 24 h, an inverted microscope was used to observe wound closure
and capture images of the cells. The distance the cells had
migrated into the scratched area was measured with ImagePro Express
software 6.0 (Media Cybernetics, Rockville, MD, USA). Cell
migration was quantified using the following formula: Cell
migration (%)=(scratch edge distance at 0 h-migration distance at
6, 12 or 24 h)/scratch edge distance at 0 h ×100%.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The mRNA levels of urokinase-type plasminogen
activator (uPA), matrix metallopeptidase (MMP)2 and MMP9 were
assessed as factors associated with cell invasion. SKOV3 cells were
incubated in 100 µl RPMI-1640 medium containing 0.4% FBS with 4 µM
P7 for the P7 group or 10 ng/ml bFGF for the bFGF group, or 50 µl 8
µM P7+50 µl 20 ng/ml bFGF for the P7+bFGF group for 24 h. Cells
incubated with 100 µl RPMI-1640 medium containing 0.4% FBS were
used as the blank control. Total RNA was isolated with a Qiagen
RNeasy Micro kit (cat. no. 74004; Qiagen, Hilden, Germany). RT was
performed by using a Takara PrimeScript™ RT reagent kit (cat. no.
RR037A, Takara Bio Inc., Otsu, Japan). The first-strand
complementary DNA synthesis was performed using 500 ng isolated
RNA. PCR primers of the genes are displayed in Table I. qPCR was performed using
SYBR® Premix Ex Taq™ (cat. no. DRR041A; Takara Bio Inc.)
with the following thermocycling conditions: Initial denaturation,
95°C for 5 min; amplification, 95°C for 10 sec, 60°C for 10 sec,
72°C for 10 sec, gathering fluorescence signal at 72°C for 40
cycles; dissolve, 95°C for 5 sec, 65°C for 1 min, 97°C termination
for 1 cycle; cooled at 40°C for 30 sec. Each sample was anlysed 3
times and the average value was recorded. The relative expression
levels of uPA, MMP2 and MMP9 were normalized to β-actin levels. The
2−ΔΔCq value was used to represent the relative gene
expression (13).
 | Table I.Sequences and amplicon sizes of the
oligonucleotide primers used for quantitative polymerase chain
reaction. |
Table I.
Sequences and amplicon sizes of the
oligonucleotide primers used for quantitative polymerase chain
reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Amplicon size
(bp) |
|---|
| UPA |
TGTGAGATCACTCTGGCTTTGGAA |
CCTTGGAGGGAACAGACGAG | 223 |
| MMP2 |
AATGCCATCCCCGATAACC |
GCTCAGCAGCCTAGCCAGTC | 155 |
| MMP9 |
GGGGGAAGATGCTGCTGTT |
GCCGGTCCTGGCAGAAATAG | 172 |
| β-actin |
CATTGCCGACAGGATGCAG |
CTCGTCATACTCCTGCTTGCTG | 169 |
Statistical analysis
Each experiment was performed at least three times
and values are expressed as the mean ± standard deviation. The
statistical data analysis was performed using one-way analysis of
variance between groups, and Tukey's multiple-comparisons test was
used to compare between pairs of groups by using SPSS 19.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
P7 does not affect the morphology of
SKOV3 cells
In the presence of P7, the cells grew well and their
morphology observed under the inverted microscope was normal
compared with that in the control group. P7 had no significant
effect on SKOV3 cell morphology within the range of concentrations
tested (0.25-16 µM; Fig. 1). These
results suggested that, at the concentrations assessed, P7 had no
cytotoxic effect on SKOV3 cells.
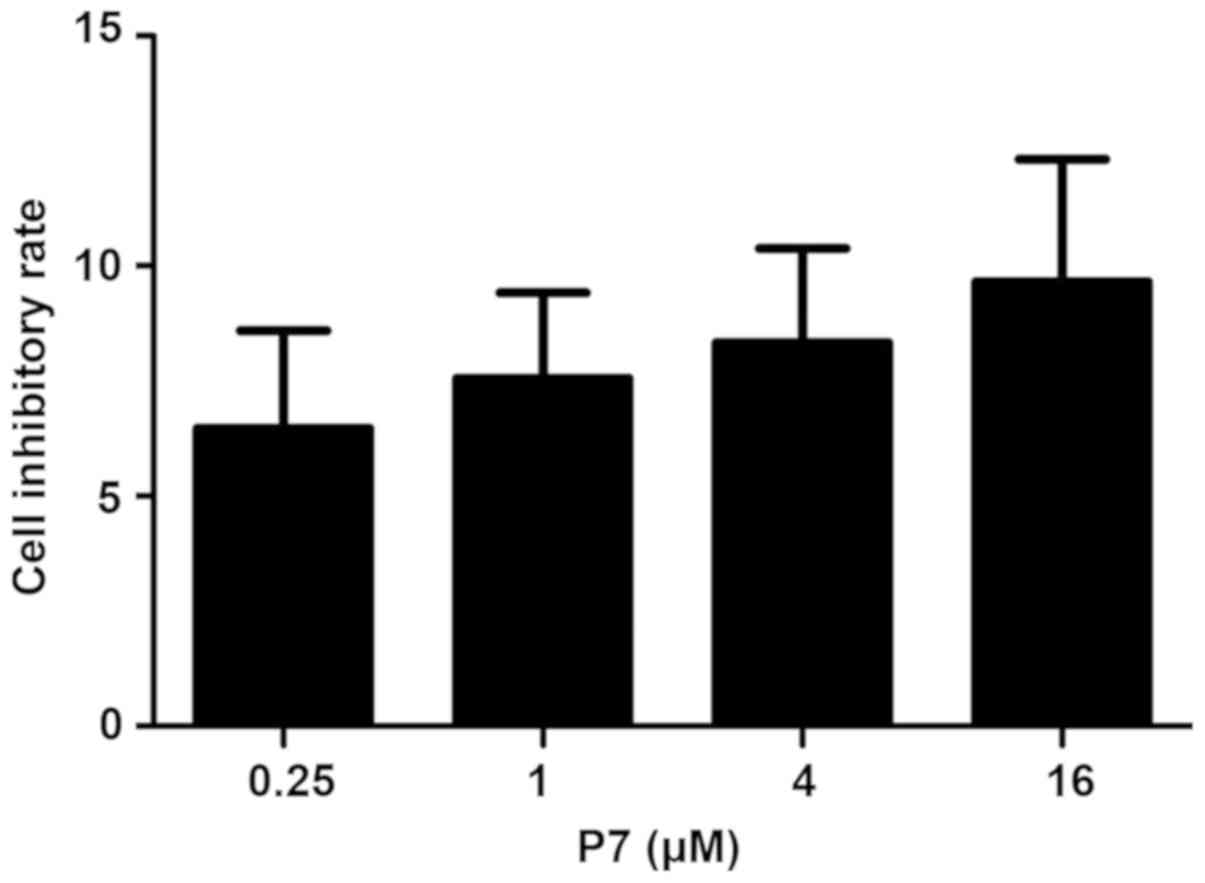
Effects of P7, bFGF and P7+bFGF on the
proliferation of SKOV3 cells
The cell proliferation was expressed as a percentage
of the control. Among the P7-treated groups, the inhibition rate of
0.25 µM P7 on SKOV3 cells was 6.47±2.12% and this rate was enhanced
with increasing concentrations of P7 (Fig. 2). However, the inhibitory rate
between the different groups exhibited no statistically significant
difference (P>0.05). Overall, the results of the MTT assay
indicated that P7 slightly inhibited SKOV3 cell proliferation, but
this was not significant (Fig.
2).
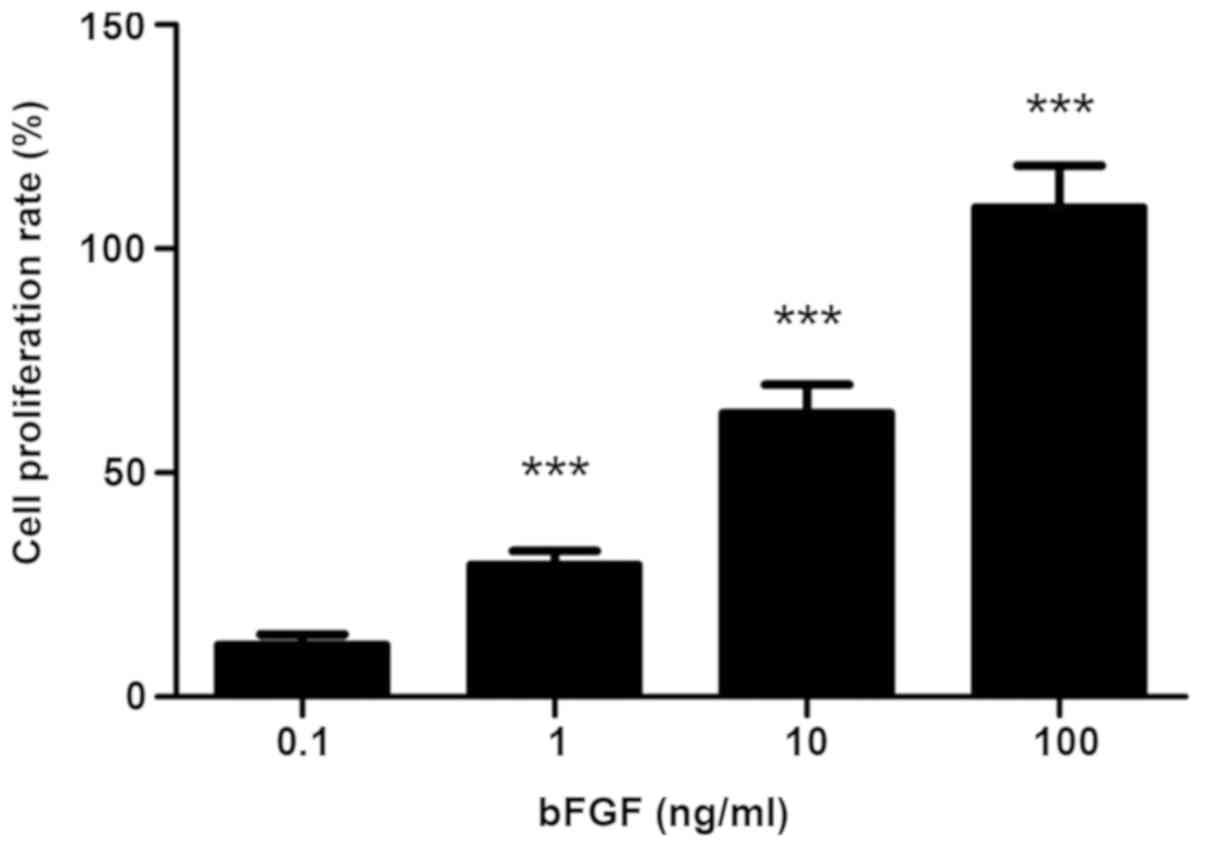
In the bFGF-treated groups, the viability of the
SKOV3 cells was significantly enhanced in a dose-dependent manner
(P<0.001; Fig. 3). When SKOV3
cells were treated with 10 ng/ml bFGF, the proliferation rate
reached >60%.
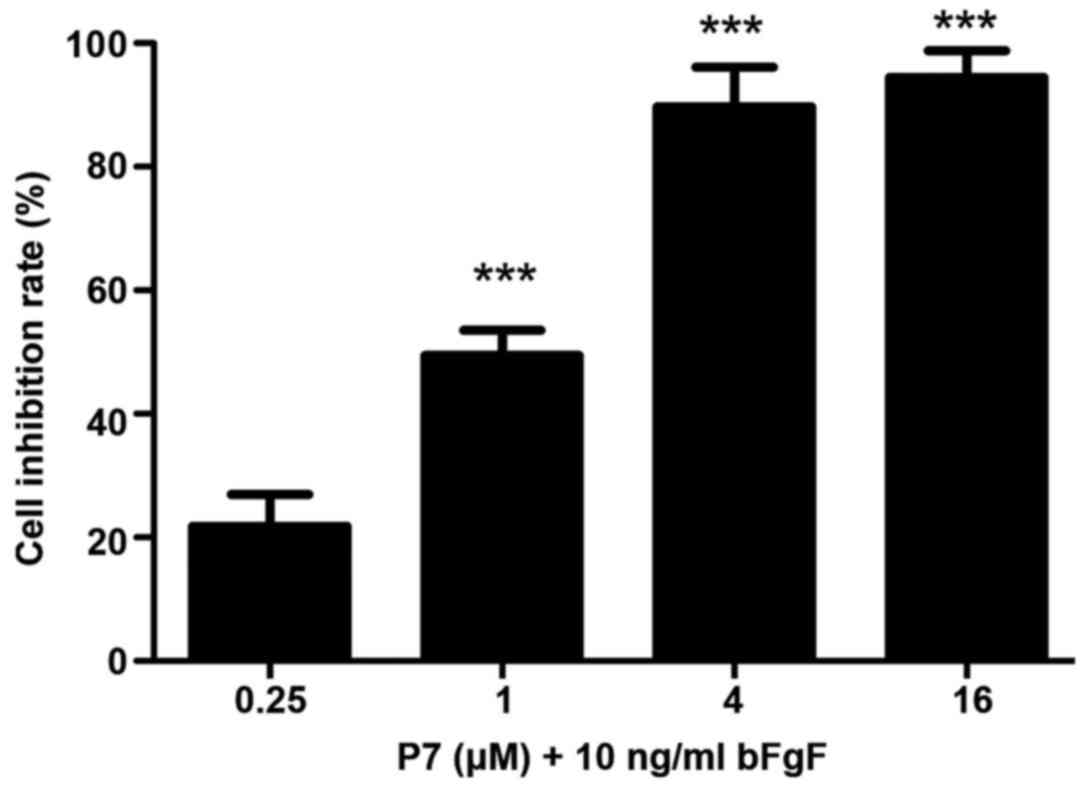
In the P7+bFGF group, P7 significantly reduced the
bFGF-stimulated proliferation of SKOV3 cells at concentrations from
0.25 to 4 µM (P<0.001; Fig. 3).
In the group treated with 4 µM P7, the mean inhibitory rate of
SKOV3 cells was nearly 90% (Fig.
4).
Effects of P7, bFGF and P7+bFGF on the
migration of SKOV3 cells
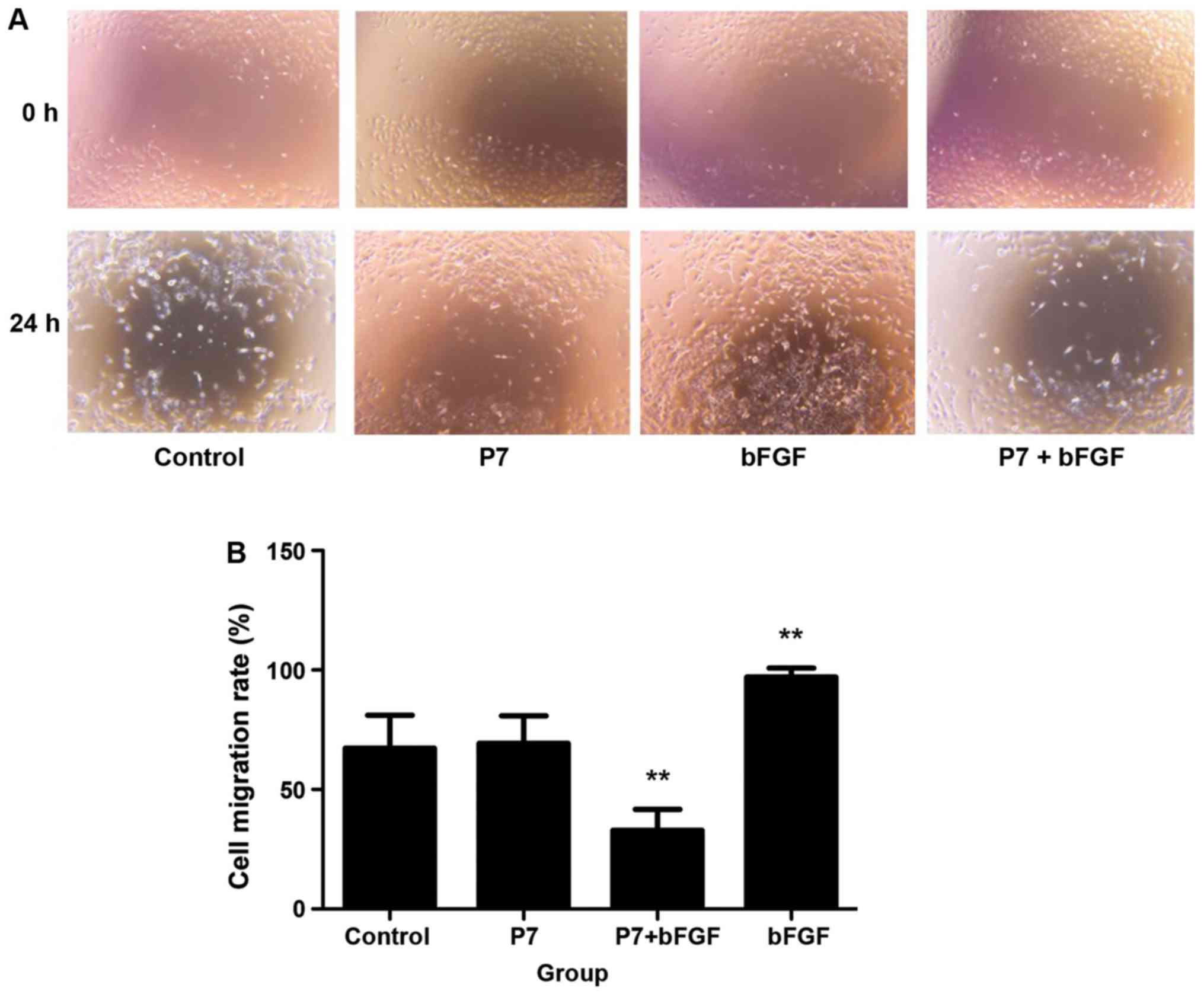
The results of the cell scratch assay indicated that
after 24 h, the SKOV3 cells in the bFGF group had almost migrated
into the total scratched area, while an obvious gap was still
present in the P7+bFGF group (Fig.
5A). Quantitative analysis of cell migration indicated a
statistically significant difference between the P7+bFGF group and
the bFGF group (P<0.01; Fig.
5B).
Effects of P7, bFGF and P7+bFGF on the
expression of uPA, MMP2 and MMP9
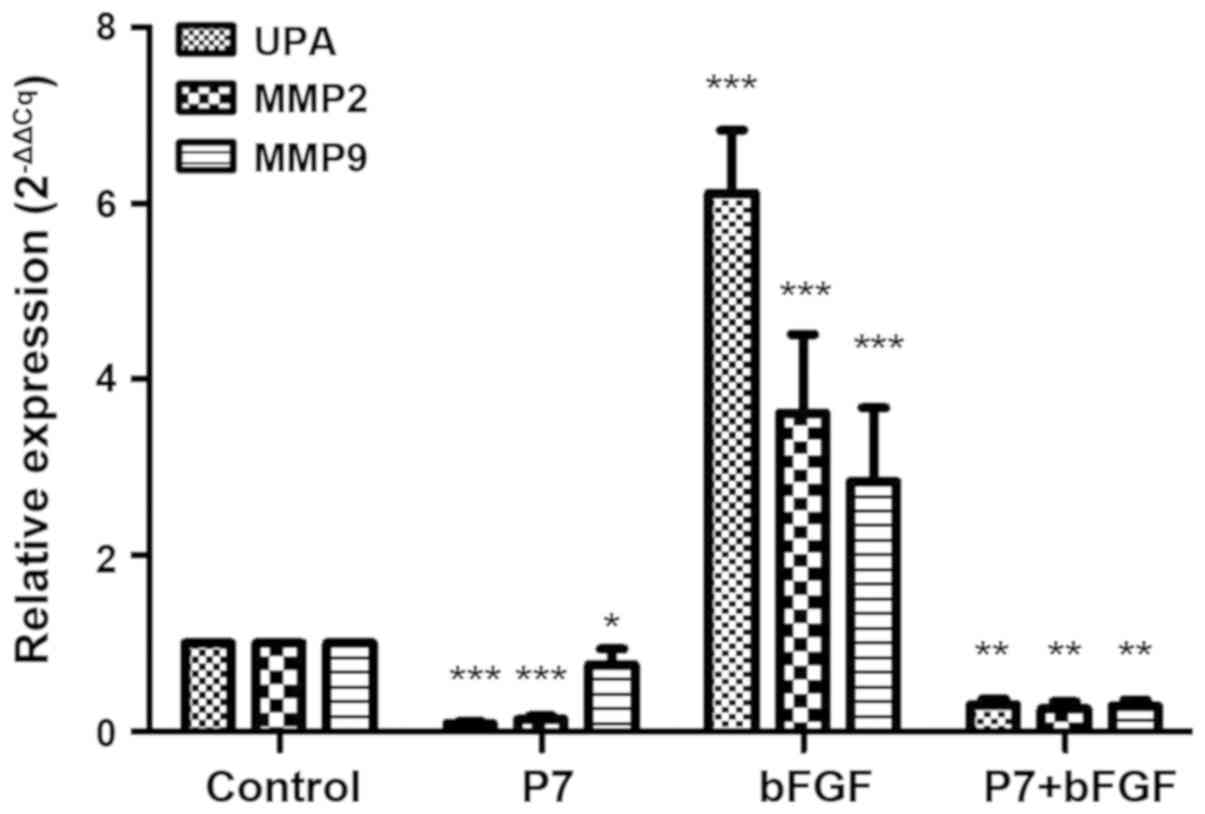
RT-qPCR analysis confirmed that uPA, MMP2 and MMP9
were all expressed in SKOV3 cells. After treatment with P7, the
expression of these genes was significantly inhibited. In the bFGF
group, the expression of the genes was significantly increased. The
expression levels in the P7+bFGF group were low (Fig. 6). In the P7 group, it was
demonstrated that P7 had the strongest inhibitory effect on uPA,
followed by MMP2, and the weakest inhibitory effect on MMP9. The
bFGF group demonstrated that bFGF had the strongest effect to
enhance uPA expression, followed by MMP2, and the weakest effect on
MMP9 expression.
Discussion
Epithelial ovarian cancer has been reported to be
the leading cause of mortality among gynecologic malignancies
worldwide during the last twenty years. Ovarian cancer is
characterized by a high degree of malignancy, insidious onset, high
invasive capacity and fast growth. Screening based on the detection
of cancer antigen (CA)125 and transvaginal sonography did not
markedly reduce the mortality rate associated with ovarian cancer
(14). Due to the insufficient
diagnostic methods for early-onset ovarian cancer, most patients
are diagnosed at a late stage. Therefore, chemotherapy is important
in the treatment of ovarian cancer, but the adverse effects of
drugs and multi-drug resistance affect the therapeutic efficacy of
ovarian carcinoma (15,16).
Angiogenesis is required for invasive tumor growth
and metastasis. Inhibition of angiogenesis is considered to be a
promising approach for antitumor therapy. Bevacizumab is a
monoclonal antibody against vascular endothelial growth factor and
was approved by the US Food and Drug Administration in February
2004 for the first-line treatment of advanced colorectal cancer; it
has been used to successfully treat colorectal cancer and advanced
non-small cell lung cancer (7,17).
Bevacizumab also has efficacy against recurrent or
treatment-resistant ovarian cancer. Combined with paclitaxel, it
rapidly reduces serum CA125 levels of patients with advanced
chemoresistant ovarian cancer and significantly reduces
cancer-associated symptoms (18).
bFGF is one of the most potent vascular growth factors, which
regulates the expression of various invasion-associated factors,
including uPA and MMPs, to promote the degradation of the
extracellular matrix and the destruction of the basal layer to
inhibit tumor invasion, metastasis and spread (6). Overexpression of bFGF was reported in
the A90 and A121 ovarian cancer cell lines, and cell proliferation
was significantly enhanced under exogenous bFGF stimulation
(19). The present study also
confirmed that bFGF promotes the proliferation of SKOV3 cells, and
it significantly increased the amount of viable SKOV3 cells in a
dose-dependent manner. This supports the hypothesis that inhibiting
tumor cell growth and tumor angiogenesis via a targeting inhibition
of bFGF to impair its biological activity may provide a novel
approach for the development of drugs (20).
Peptides have become a focus of drug research due to
their high activity and specificity, while having relatively few
side effects. Phage display technology was used to screen the novel
lead peptide P7 from a random peptide library, based on its ability
to specifically bind to bFGF (21).
The present study indicated that P7 had almost no effect on the
morphology, proliferation and migration of SKOV3 cells, but had a
significant inhibitory effect on cell proliferation and migration
induced by bFGF. It is therefore indicated that P7 has a slight
cytotoxic effect on SKOV3 cells, but inhibits SKOV3 cell
proliferation and migration by specifically binding to bFGF. This
supports the hypothesis that P7 may be utilized as a specific
treatment for ovarian cancer.
uPA is a type of serine proteolytic enzyme, and it
may activate MMPs and proteolytic enzymes from a variety of
precursors in tumor tissue, and degrade extracellular matrix and
basement membrane components of the tumor and vessel wall,
resulting in an increase of the tumor vessel wall permeability.
Fibrinolytic enzymes may stimulate angiogenesis by increasing the
activity of vascular endothelial growth factor to promote the
growth and spread of tumors (22).
Studies have confirmed that the content of uPA is higher in the
center of the tumor than that in the normal adjacent tissue, and is
correlated with the depth of invasion and metastasis. MMPs are
zinc-dependent endopeptidases, and have a high expression and
activity in most types of tumor tissue, which promotes cell
invasion and migration, and induces angiogenesis via numerous ways,
to enhance metastasis of tumor cells. The expression levels of
MMP-2 and MMP-9 are associated with the degree of malignancy and
angiogenesis of tumors (23).
Studies have indicated that MMPs are expressed in ovarian cancer
tissues, and their expression level is associated with the clinical
stage and prognosis of ovarian cancer (24). The present study indicated that bFGF
significantly increases the expression of uPA, MMP-2 and MMP-9 in
SKOV3 cells, confirming that bFGF is associated with the invasive
ability of ovarian cancer cells. bFGF may promote the degradation
of extracellular matrix by upregulating the expression of uPA,
MMP-2 and MMP-9, and increase vascular permeability to promote
tumor cell proliferation. P7 significantly reduced the expression
of these three genes in bFGF-induced SKOV3 cells, which indicates
that P7 may specifically inhibit bFGF-induced tumor cell invasion
and proliferation by sequestering the increasing expression effect
of bFGF on invasion-associated factors. In the present study, it
was revealed that P7 had the greatest inhibitory effect on uPA,
followed by MMP2 and MMP9. However, the exact mechanisms remain
elusive and further studies are required to investigate them.
In conclusion, the present study indicated that P7
inhibits the proliferation and migration of ovarian cancer cells
induced by bFGF, and downregulates the expression of
invasion-associated factors, including uPA, MMP-2 and MMP-9. It was
revealed that treatment with P7 inhibits the proliferation,
migration and invasion of bFGF-induced human ovarian cancer SKOV3
cells in vitro. Due to its prominent effects, it is
recommended that the use of P7 as an anticancer agent for ovarian
tumors should be pursued.
Acknowledgements
Not applicable.
Funding
This work was supported by the Wenzhou science and
technology bureau foreign cooperation project (grant no.
H20110015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WQ initiated and guided the study, and revised the
manuscript. QC and ZY performed experiments, and wrote the
manuscript. XC and LS contributed to data collection and
processing. All authors have read and approved the final version of
the manuscript.
Ethical approval and informed consent
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koshiyama M, Matsumura N and Konishi I:
Subtypes of ovarian cancer and ovarian cancer screening.
Diagnostics. 7:E122017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaya AM and Rustin GJ: Vascular disrupting
agents: A new class of drug in cancer therapy. Clin Oncol.
17:277–290. 2005. View Article : Google Scholar
|
|
4
|
Al-Abd AM, Alamoudi AJ, Abdel-Naim AB,
Neamatallah TA and Ashour OM: Anti-angiogenic agents for the
treatment of solid tumors: Potential pathways, therapy and current
strategies-A review. J Adv Res. 8:591–605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bielenberg DR and Zetter BR: The
contribution of angiogenesis to the process of metastasis. Cancer
J. 21:267–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burger RA: Overview of anti-angiogenic
agents in development for ovarian cancer. Gynecol Oncol.
121:230–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Monk BJ, Minion LE and Coleman RL:
Anti-angiogenic agents in ovarian cancer: Past, present, and
future. Ann Oncol. 27 (Suppl):i33–i39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chase DM, Chaplin DJ and Monk BJ: The
development and use of vascular targeted therapy in ovarian cancer.
Gynecol Oncol. 145:393–406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan L, Li W, Ying S, Shi L, Wang Z, Chen
G, Ye H, Wu X, Wu J, Liang G and Li X: A peptide derivative serves
as a fibroblast growth factor 2 antagonist in human gastric cancer.
Tumour Biol. 36:7233–7241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo W, Yu Y, Wang R, He D, Wang C, Zeng X,
Chen X, Tan X, Huang T and Wu X: P7 peptides targeting bFGF
sensitize colorectal cancer cells to CPT-11. Int J Mol Med.
33:194–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Y, Gao S, Li Q, Wang C, Lai X, Chen X,
Wang R, Di J, Li T, Wang W and Wu X: The FGF2-binding peptide P7
inhibits melanoma growth in vitro and in vivo. J Cancer Res Clin
Oncol. 138:1321–1328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q, Gao S, Yu Y, Wang W, Chen X, Wang R,
Li T, Wang C, Li X and Wu X: A novel bFGF antagonist peptide
inhibits breast cancer cell growth. Mol Med Rep. 6:210–214.
2012.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buys SS, Partridge E, Black A, Johnson CC,
Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B,
et al: Effect of screening on ovarian cancer mortality: The
prostate, lung, colorectal and ovarian (PLCO) cancer screening
randomized controlled trial. Jama. 305:2295–2303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Collins T, Gray K, Bista M, Skinner M,
Hardy C, Wang H, Mettetal JT and Harmer AR: Quantifying the
relationship between inhibition of VEGF receptor 2, drug-induced
blood pressure elevation and hypertension. Br J Pharmacol.
175:618–630. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayman SR, Leung N, Grande JP and Garovic
VD: VEGF inhibition, hypertension, and renal toxicity. Curr Oncol
Rep. 14:285–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arkenau HT, Brunetto AT, Barriuso J, Olmos
D, Eaton D, de Bono J, Judson I and Kaye S: Clinical benefit of new
targeted agents in phase I trials in patients with advanced
colorectal cancer. Oncology. 76:151–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cohn DE, Valmadre S, Resnick KE, Eaton LA,
Copeland LJ and Fowler JM: Bevacizumab and weekly taxane
chemotherapy demonstrates activity in refractory ovarian cancer.
Gynecol Oncol. 102:134–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crickard K, Gross JL, Crickard U, Yoonessi
M, Lele S, Herblin WF and Eidsvoog K: Basic fibroblast growth
factor and receptor expression in human ovarian cancer. Gynecol
Oncol. 55:277–284. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hill DS, Martin S, Armstrong JL, Flockhart
R, Tonison JJ, Simpson DG, Birch-Machin MA, Redfern CP and Lovat
PE: Combining the endoplasmic reticulum stress-inducing agents
bortezomib and fenretinide as a novel therapeutic strategy for
metastatic melanoma. Clin Cancer Res. 15:1192–1198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Yan Q, Huang Y, Huang H, Su Z, Xiao
J, Zeng Y, Wang Y, Nie C, Yang Y and Li X: Isolation of a novel
basic FGF-binding peptide with potent antiangiogenetic activity. J
Cell Mol Med. 14:351–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Binder BR, Mihaly J and Prager GW:
uPAR-uPA-PAI-1 interactions and signaling: A vascular biologist's
view. Thromb Haemost. 97:336–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Torng PL, Mao TL, Chan WY, Huang SC and
Lin CT: Prognostic significance of stromal metalloproteinase-2 in
ovarian adenocarcinoma and its relation to carcinoma progression.
Gynecol Oncol. 92:559–567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmalfeldt B, Prechtel D, Harting K,
Spathe K, Rutke S, Konik E, Fridman R, Berger U, Schmitt M, Kuhn W
and Lengyel E: Increased expression of matrix metalloproteinases
(MMP)-2, MMP-9, and the urokinase-type plasminogen activator is
associated with progression from benign to advanced ovarian cancer.
Clin Cancer Res. 7:2396–2404. 2001.PubMed/NCBI
|