Introduction
Heat stroke has a high incidence and fatality rate
(1), which will continue to increase
with global warming (2). Results of
epidemiological survey show that the average fatality rate of heat
stroke is between 10 and 15%. Once developing into severe heat
stroke, it is easy to be complicated by multiple organ dysfunction
syndrome (MODS), with a fatality rate >40%. Of the survivors,
over 30% of patients suffer from long-term nervous system diseases
(2,3). The cluster treatment strategy of ‘early
rapid cooling, early rapid expansion, early anticoagulation, and
active support of organ function’ can effectively prevent multiple
organ dysfunctions and shorten the length of ICU stay, so as to
improve prognosis (4). Heatstroke is
further divided into typical heatstroke and exertional heatstroke
according to whether there are fatigue factors in thermal exposure
process (5). Due to its high
fatality rate, possibly determining relevant factors for the
prognosis of patients reduce the long-term effects on their
outcomes.
Prealbumin (PA), synthesized by the liver, the level
of which is related to protein metabolism, can sensitively reflect
the body's nutrition and liver synthesis function, associated with
the inflammatory response in the body (6). Widely used in clinical practice, it has
been confirmed to predict the mortality and re-hospitalization rate
in patients with acute heart failure (7), mortality in patients with acute kidney
injury (8,9), mortality in patients with peritoneal
dialysis (10) and mortality in burn
patients (11). It can also predict
infectious complications after gastric operation (12) and the prognosis of patients in the
medical department ICU (13).
At present, the determination of prognostic risk
factors for heatstroke patients worldwide, mainly based on the
analysis of the relationship between multiple univariates and
outcomes in clinical data, ignores the interactive relationship
among multiple univariates. In addition, whether prealbumin can
predict the prognosis of heatstroke has not been studied.
Therefore, in this study, a multivariate analysis of the
relationship between clinical parameters and disease outcomes of
heatstroke patient at admission in the past 10 years was performed,
to determine whether the serum prealbumin level was associated with
short-term (28 days) mortality, so as to provide a theoretical
basis for guiding their rational treatment and improving the
prognosis.
Patients and methods
Subjects
A retrospective study of 118 heatstroke patients
admitted to the ICU in the Affiliated Changzhou No. 2 People's
Hospital of Nanjing Medical University (Changzhou, China), from
June 2010 to November 2017, was performed. Except for 1 case of age
<18 years, 2 cases of chronic hepatic insufficiency, 2 cases of
chronic renal insufficiency and 11 cases with incomplete data
caused by dying or discharging within 24 h of admission, 102
patients were eventually included. Among them, 61 male patients and
41 female patients, aged from 20 to 89 years, were divided into
normal serum prealbumin group (n=79) and low prealbumin group
(n=23) according to the difference in PA expression. In normal
serum prealbumin group, there were 50 male patients and 29 female
patients, aged from 20 to 73 years, and in low prealbumin group, 11
male patients and 12 female patients, aged from 25 to 89 years. All
patients were diagnosed in accordance with China's Diagnostic
Standards and Treatment Principles of Occupational Heatstroke
(GB11508-89). Exclusion criteria were: Patients aged <18 years,
knowing liver and kidney dysfunction and with incomplete data
caused by death or discharging within 24 h of admission, were
excluded.
This study was approved by the Ethics Committee of
the Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical
University. Patients and their family members were informed and
consented to all treatments and examinations.
Grouping scheme
A COBAS8000 analyzer (Roche Diagnostics,
Indianapolis, IN, USA) was used to determine serum prealbumin
content by immunoturbidimetry. Patients were divided into normal
serum prealbumin group (>15 mg/dl) and low prealbumin group (≤15
mg/dl) according to serum prealbumin level.
Observation indicators
Main observation indicators: i) General clinical
data and vital signs on admission, including age, sex, body mass
index (BMI), systolic blood pressure, heart rate and respiratory
rate; ii) major underlying diseases such as hypertension, diabetes
mellitus, chronic obstructive pulmonary disease, stroke and tumor
and iii) laboratory inspection data, including white blood cell
(WBC) count, hemoglobin, platelet (PLT) count, blood sodium, blood
potassium, alanine aminotransferase (ALT), aspartate
aminotransferase (AST), total bilirubin (TBIL), total cholesterol
(TCH), lactic dehydrogenase (LDH), phosphocreatine kinase (CPK),
forebrain natriuretic peptide (BNP), troponin I (TNI), serum
creatinine (Cr), albumin (ALB), prealbumin (PA), C-reactive protein
(CRP), procalcitonin (PCT), prothrombin time (PT), activated
partial thromboplastin time (APTT), fibrinogen (FBG) and D-dimer.
Blood samples from all patients were obtained within 24 h of
admission, used for the determination of the above indicators.
Secondary observation indicators: i) Invasive
positive pressure ventilation (IPPV), co-infection, shock, length
of ICU stay and outcome during the ICU were compared between two
groups of patients; ii) the study endpoints, deaths at admission,
were recorded, and the survival curve plotted; iii) Cox regression
analysis was performed based on the clinical data of patients and
iv) ROC curve was plotted based on Cox multivariate independent
prognostic indicators.
Statistical analysis
SPSS 20.0 (IBM Corp., Armonk, NY, USA) software was
used for data processing, Shapiro-Wilk for the normality test. The
measurement data in accordance with the normal distribution were
expressed as mean ± standard deviation (SD), and t-test was used
for the comparison between two groups. That not in accordance with
the normal distribution were expressed as median (quartile) [M
(QL-QU)], and Mann-Whitney U test was used for the comparison
between two groups. The χ2 test was used for comparison
among count data, Kaplan-Meier survival curves for the analysis of
survival rates of two groups and Mantel-Cox was the log-rank test.
Univariate Cox regression analysis was performed to determine the
association between each potential variable and the mortality in
patients, and multivariate Cox regression analysis to identify
independent variables predicting the mortality in patients. The
receiver operating characteristic (ROC) curve was plotted for
statistically significant indicators of multivariate regression
analysis. The area under the ROC curve (AUC) was calculated to
assess the ability of indicators to predict the mortality.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of clinical data of
patients
The clinical data of the groups were compared, and
it was found that there was no statistically significant difference
between them in age, sex, BMI, heart rate, respiratory rate,
history of hypertension, history of diabetes mellitus, COPD,
history of stroke, history of tumor and number of typical heat
stroke (P>0.05), and in NA, K, hemoglobin, WBC CPK, PCT and CRP
(P>0.05), but there were significant differences in clinical
variables PLT, ALT, AST, TBIL, ALB, TCH, LDH, TNI, BNP, creatinine,
PT, APTT, FBG and D-dimer (P<0.05) (Table I).
 | Table I.Characteristics of study cohort. |
Table I.
Characteristics of study cohort.
| Characteristics | All (n=102) | Normal serum
prealbumin group (n=79) | Low prealbumin group
(n=23) | P-value |
|---|
| PA (mg/dl), median
(IQR) | 19.56±6.21 | 22.05±4.51 | 10.99±2.38 | – |
| Age (years), median
(IQR) | 66 (50-77.25) | 66 (52.50–77.50) | 73 (49-77) | 0.916 |
| Male, n (%) | 61 (59.80) | 50 (63.29) | 11 (47.83) | 0.229 |
| BMI
(kg/m2), median (IQR) | 24.55
(20.58–26.93) | 24.60
(20.60–26.80) | 22.80
(20.50–26.35) | 0.819 |
| SBP (mmHg) | 120.39±22.93 | 123.86±21.67 | 108.47±23.60 | 0.004 |
| Heart rate (bpm),
median (IQR) | 102.5 (80-124.5) | 96 (79-117.5) | 113 (94.5-138) | 0.017 |
| Respiratory rate
(bpm), median (IQR) | 21 (18-27.25) | 21 (18-26.50) | 24 (18.50–28.50) | 0.572 |
| Previous disease |
|
Hypertension, n (%) | 29 (28.43) | 26 (32.91) | 3 (13.04) | 0.071 |
| Diabetes
mellitus, n (%) | 11 (10.78) | 9 (11.39) | 2 (8.69) | 0.714 |
| COPD, n
(%) | 5 (4.90) | 3 (3.79) | 2 (8.69) | 0.315 |
| Stroke,
n (%) | 8 (7.84) | 7 (8.86) | 1 (4.35) | 0.679 |
| Tumor,
n (%) | 4 (3.92) | 2 (2.53) | 2 (8.69) | 0.218 |
| NA (mmol/l), median
(IQR) | 137.20
(131.35–140.15) | 137.70
(131.00–140.20) | 136.90
(132.40–138.80) | 0.788 |
| K (mmol/l) | 3.56±0.67 | 3.60±0.62 | 3.41±0.78 | 0.267 |
| Hemoglobin
(g/l) | 124.22±17.72 | 124.97±16.21 | 121.60±22.34 | 0.425 |
| WBC
(109/l), median (IQR) | 12.84
(9.96–15.99) | 12.17
(9.02–15.26) | 13.88
(12.10–18.19) | 0.070 |
| PLT
(109/l), median (IQR) | 121.10
(80-172.25) | 132
(92-179.20) | 87 (51-117.50) | 0.006 |
| ALT (U/l), median
(IQR) | 100.50
(51.18–152.95) | 95 (50-139.50) | 127
(95.90-267) | 0.016 |
| AST (U/l), median
(IQR) | 140.55
(90.13–213.63) | 118
(83.30–180.70) | 185.30
(141.50-430) | <0.001 |
| TBIL (umol/l),
median (IQR) | 17
(10.35–24.10) | 15.60
(10-20.90) | 22.60
(17.60–27.55) | 0.004 |
| ALB (g/l) | 34.08±4.81 | 35.22±4.43 | 30.15±3.96 | <0.001 |
| TCH (mmol/l),
median (IQR) | 3.45
(2.79–4.11) | 3.59
(3.05–4.21) | 3.20
(2.60–3.66) | 0.006 |
| LDH (U/l), median
(IQR) | 416.80
(316.15–643.40) | 394
(308.45–566.60) | 503.40
(394.65–915.90) | 0.020 |
| CPK (U/l), median
(IQR) | 754.55
(290.90-2,059.78) | 688.80
(289.15–1831.45) | 1,109
(495.15-3,081.60) | 0.172 |
| TNI (ng/ml), median
(IQR) | 0.15
(0.04–0.58) | 0.10
(0.02–0.36) | 0.57
(0.16–0.84) | <0.001 |
| BNP (pg/ml), median
(IQR) | 488 (178-2633) | 394
(170-1,970) | 2480
(255-6505) | 0.037 |
| Creatinine
(umol/l), median (IQR) | 93.25
(73.83–127.15) | 87
(72.05–117.90) | 108.40
(88.30–138.10) | 0.036 |
| PCT (ng/ml), median
(IQR) | 3.42
(0.90–13.39) | 3.10
(0.81–10.66) | 5.85
(2.79–25.30) | 0.054 |
| CRP (mg/l), median
(IQR) | 4.45 (1.38-14) | 4.20
(1.62–12.95) | 8.50 (0.75-25) | 0.680 |
| PT(s), median
(IQR) | 15.10
(12.78–18.93) | 14.10
(12.40–16.75) | 24
(17.20–27.65) | <0.001 |
| APTT(s), median
(IQR) | 29.55
(25.35–36.58) | 27.80
(24.95–31.60) | 37.50
(34.50–43.85) | <0.001 |
| FBG (g/l), median
(IQR) | 2.16
(1.70–2.61) | 2.22
(1.81–2.78) | 1.80
(1.31–2.40) | 0.015 |
| D-dimer (mg/l),
median (IQR) | 1.87
(1.03–4.64) | 1.76
(0.76–4.37) | 3.11
(1.75–10.99) | 0.010 |
Comparison of adverse conditions in
the course of treatment, length of stay in the hospital, IPPV times
and length of ICU stay between two groups of patients
The adverse conditions, length of stay in the
hospital and IPPV times in two groups of patients were counted. It
was found that the incidence of infection, shock and IPPV was
significantly lower in normal serum prealbumin group of patients
than those in low prealbumin group, with a statistically
significant difference (P<0.05). There was no statistically
significant difference between two groups of patients in the length
of stay in the hospital and length of ICU stay (P>0.05)
(Table II).
 | Table II.Adverse conditions in the course of
treatment, length of stay in the hospital and length of ICU stay in
both groups. |
Table II.
Adverse conditions in the course of
treatment, length of stay in the hospital and length of ICU stay in
both groups.
| Index | Normal serum
prealbumin group (n=79) | Low prealbumin
group (n=23) | P-value |
|---|
| Typical heatstroke,
n (%) | 54 (68.36) | 15 (65.22) | 0.803 |
| Infection, n
(%) | 45 (56.96) | 17 (73.91) | 0.156 |
| Shock, n (%) | 18 (22.78) | 15 (65.22) | <0.001 |
| IPPV, n (%) | 19 (24.05) | 13 (56.52) | 0.005 |
| Days of ICU
(days) | 8 (6-12) | 7 (3-10) | 0.161 |
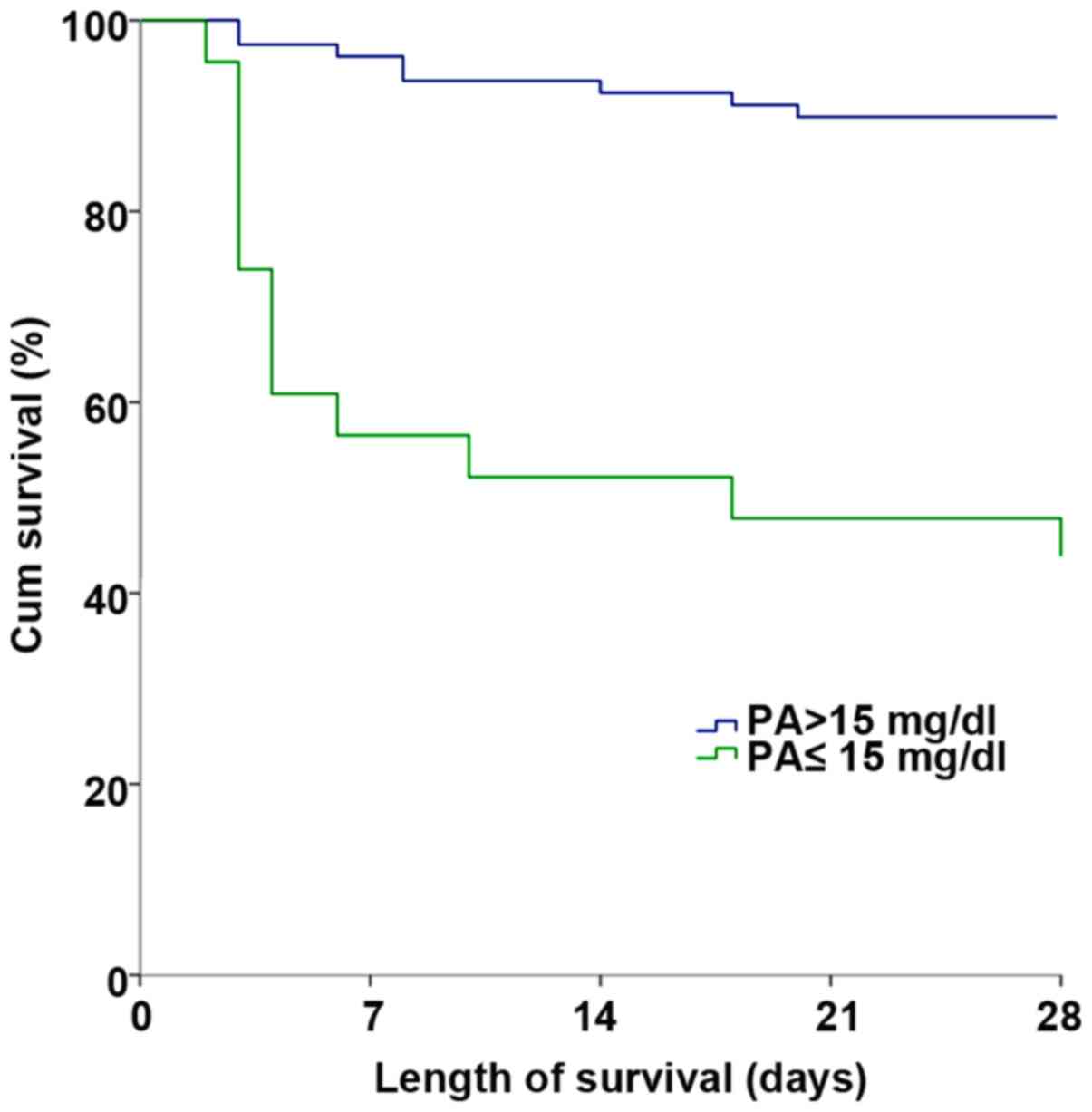
Length of survival of patients at
admission
The length of survival of two groups of patients at
admission was calculated. It was found that altogether 8 patients
died in normal serum prealbumin group of 79 patients, with the
length of survival of 26.177±0.649 days, and 13 patients died in
low prealbumin group of 23 patients, with the length of survival of
16.130±2.559 days. By comparison, it was found that there was a
statistically significant difference in short-term survival rate
between the groups of patients (χ2=29.101, P<0.001)
(Fig. 1).
Cox regression analysis
A univariate Cox regression analysis of the
collected data was performed. It was found that prognostic factors
for heatstroke patients were IPPV, heart rate, WBC count, PLT
count, ALB, PA, TBIL, LDH, CPK, Cr, PCT, PT, APTT, D-dimer and
co-infection and shock at admission. Then, a multivariate Cox
regression analysis of indicators with differences in univariates
was performed. It was found that independent prognostic-related
factors for heatstroke patients were IPPV (OR: 7.111, 95% CI:
1.977–25.574, P=0.003), PA level (OR: 1.204, 95% CI: 1.068–1.357,
P=0.002), PLT level (OR: 1.010, 95% CI: 1.002–1.019, P=0.017), ALB
level (OR: 1.159, 95% CI: 1.021–1.316, P=0.022), CPK level (per
1,000 U/l) (OR: 1.152, 95% CI: 1.052–1.262, P=0.002) and PT level
(OR: 1.096, 95% CI: 1.032–1.164, P=0.003) (Tables III and IV).
 | Table III.Predictors of mortality in heat
stroke patients, univariate Cox regression analysis. |
Table III.
Predictors of mortality in heat
stroke patients, univariate Cox regression analysis.
| Variables | β | SE | Wald | df | OR | 95% CI | P-value |
|---|
| Age | 0.008 | 0.014 | 0.335 | 1 | 1.008 | 0.981–1.035 | 0.563 |
| Sex | 0.126 | 0.449 | 0.078 | 1 | 1.134 | 0.470–2.736 | 0.780 |
| BMI | 0.026 | 0.059 | 0.196 | 1 | 1.027 | 0.914–1.153 | 0.658 |
| SBP | 0.013 | 0.010 | 1.689 | 1 | 1.013 | 0.993–1.033 | 0.194 |
| Heart rate | 0.023 | 0.008 | 8.555 | 1 | 1.024 | 1.008–1.040 | 0.003 |
| Respiratory
rate | 0.027 | 0.030 | 0.793 | 1 | 1.027 | 0.968–1.090 | 0.373 |
| Hypertension | −0.016 | 0.483 | 0.001 | 1 | 0.984 | 0.382–2.536 | 0.973 |
| Diabetes
mellitus | 0.240 | 0.744 | 0.104 | 1 | 1.271 | 0.296–5.459 | 0.747 |
| COPD | 3.084 | 4.285 | 0.518 | 1 | 21.854 |
0.005–96958.873 | 0.472 |
| Stroke | 0.563 | 1.025 | 0.302 | 1 | 1.757 | 0.236–13.091 | 0.582 |
| Tumor | 3.067 | 4.774 | 0.413 | 1 | 21.478 |
0.002–248485.152 | 0.521 |
| NA | 0.025 | 0.020 | 1.648 | 1 | 1.026 | 0.987–1.066 | 0.199 |
| K | −0.396 | 0.328 | 1.462 | 1 | 0.673 | 0.354–1.279 | 0.227 |
| WBC | 0.074 | 0.035 | 4.332 | 1 | 1.076 | 1.004–1.153 | 0.037 |
| Hemoglobin | 0.064 | 0.131 | 0.244 | 1 | 1.067 | 0.826–1.378 | 0.621 |
| per 10 g/l |
|
|
|
|
|
|
|
| PLT | 0.017 | 0.005 | 12.599 | 1 | 1.017 | 1.008–1.027 | <0.001 |
| ALT per 100
U/l | 0.091 | 0.062 | 2.163 | 1 | 1.095 | 0.97–1.236 | 0.141 |
| AST per 100
U/l | 0.050 | 0.038 | 1.674 | 1 | 1.051 | 0.975–1.133 | 0.196 |
| TBIL | 0.026 | 0.008 | 9.684 | 1 | 1.026 | 1.010–1.043 | 0.002 |
| ALB | 0.095 | 0.044 | 4.636 | 1 | 1.100 | 1.009–1.200 | 0.031 |
| TCH | −0.412 | 0.272 | 2.288 | 1 | 0.662 | 0.389–1.129 | 0.130 |
| LDH per 100
U/l | 0.129 | 0.043 | 8.920 | 1 | 1.138 | 1.045–1.239 | 0.003 |
| CPK per 1,000
U/l | 0.184 | 0.043 | 18.525 | 1 | 1.202 | 1.105–1.306 | <0.001 |
| TNI | 0.023 | 0.054 | 0.178 | 1 | 1.023 | 0.920–1.139 | 0.673 |
| BNP per 1,000
pg/ml | 0.033 | 0.024 | 1.837 | 1 | 1.033 | 0.986–1.083 | 0.175 |
| Creatinine per | 0.039 | 0.013 | 9.132 | 1 | 1.039 | 1.014–1.066 | 0.003 |
| 10 umol/l |
|
|
|
|
|
|
|
| PCT | 0.021 | 0.006 | 11.877 | 1 | 1.022 | 1.009–1.034 | 0.001 |
| CRP per 10
mg/l | 0.038 | 0.067 | 0.332 | 1 | 1.039 | 0.911–1.186 | 0.564 |
| PA | 0.189 | 0.041 | 21.536 | 1 | 1.208 | 1.115–1.308 | <0.001 |
| PT | 0.141 | 0.022 | 39.364 | 1 | 1.151 | 1.102–1.203 | <0.001 |
| APTT | 0.046 | 0.013 | 11.952 | 1 | 1.047 | 1.020–1.075 | 0.001 |
| FBG | −0.467 | 0.306 | 2.327 | 1 | 0.627 | 0.344–1.142 | 0.127 |
| D-dimer | 0.057 | 0.020 | 8.117 | 1 | 1.059 | 1.018–1.102 | 0.004 |
| Type | −0.067 | 0.463 | 0.021 | 1 | 0.936 | 0.378–2.318 | 0.886 |
| Infection | 2.705 | 1.025 | 6.966 | 1 | 14.960 | 2.007–111.533 | 0.008 |
| Shock | 1.838 | 0.484 | 14.401 | 1 | 6.285 | 2.432–16.242 | <0.001 |
| IPPV | 2.855 | 0.626 | 20.823 | 1 | 17.369 | 5.097–59.196 | <0.001 |
 | Table IV.Predictors of mortality in heat
stroke patients, multivariate Cox regression analysis. |
Table IV.
Predictors of mortality in heat
stroke patients, multivariate Cox regression analysis.
| Variables | β | SE | Wald | df | OR | 95.0% CI | P-value |
|---|
| IPPV | 1.962 | 0.653 | 9.022 | 1 | 7.111 | 1.977–25.574 | 0.003 |
| PLT | 0.010 | 0.004 | 5.646 | 1 | 1.010 | 1.002–1.019 | 0.017 |
| ALB | 0.148 | 0.065 | 5.221 | 1 | 1.159 | 1.021–1.316 | 0.022 |
| CPK | 0.142 | 0.046 | 9.319 | 1 | 1.152 | 1.052–1.262 | 0.002 |
| per 1,000 U/l |
|
|
|
|
|
|
|
| PA | 0.186 | 0.061 | 9.271 | 1 | 1.204 | 1.068–1.357 | 0.002 |
| PT | 0.091 | 0.031 | 8.811 | 1 | 1.096 | 1.032–1.164 | 0.003 |
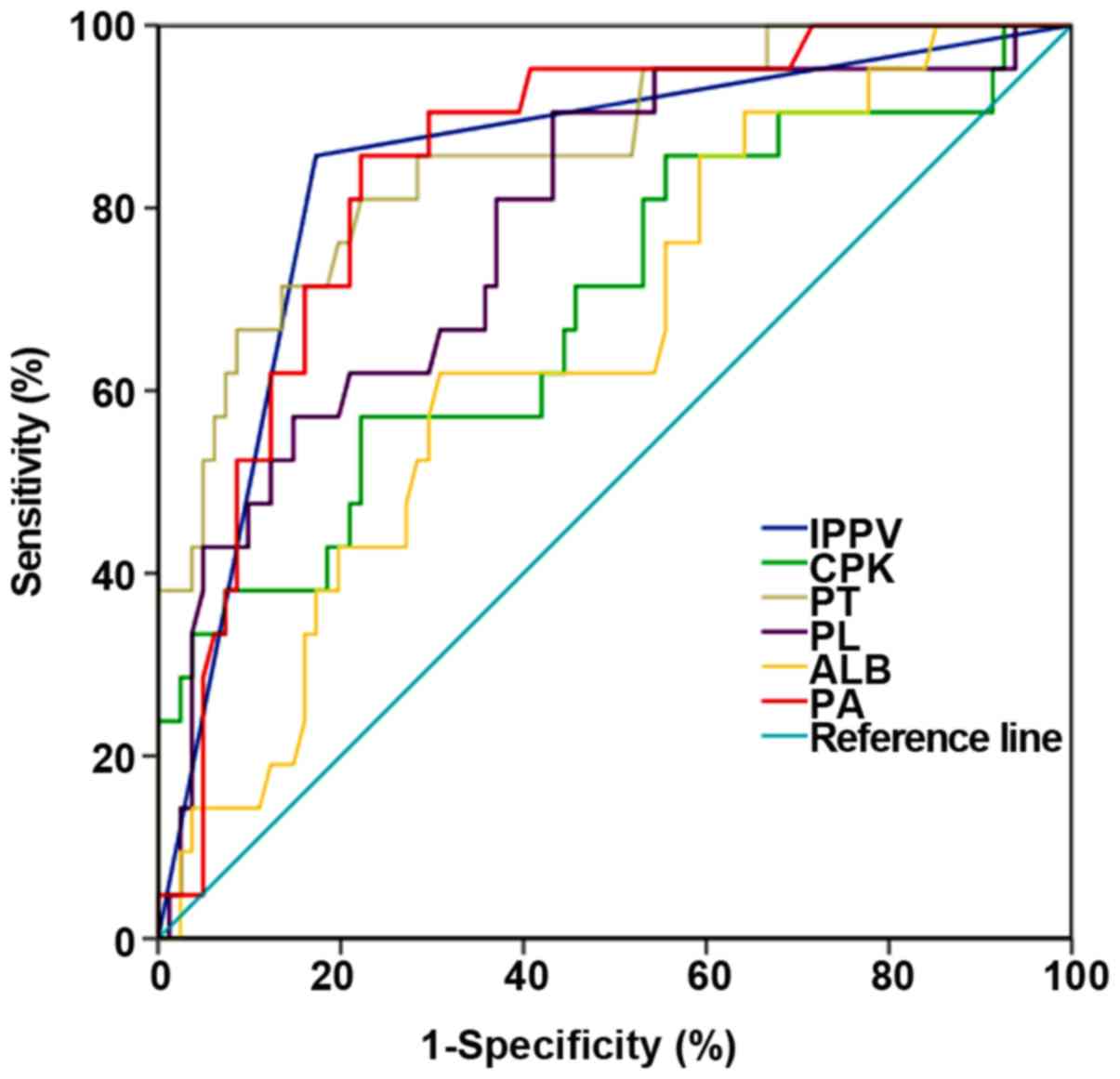
ROC curve analysis
ROC curve was plotted to further compare the role of
these six indicators in predicting patient mortality. It was found
that IPPVAUC=0.842 (95% CI: 0.743–0.941), PAAUC=0.845 (95% CI:
0.760–0.931), PLTAUC=0.780 (95% CI: 0.668–0.892), ALBAUC=0.652 (95%
CI: 0.527–0.777), CPKAUC=0.694 (95% CI: 0.558–0.829) and
PTAUC=0.862 (95% CI: 0.771–0.953). When prealbumin <17.95 mg/dl
was used as the death threshold for predicting at 28 days, the
sensitivity was 77.8%, and the specificity was 85.7% (Fig. 2 and Table
V).
 | Table V.Comparison of AUC among various
parameters for survival in heat stroke patients. |
Table V.
Comparison of AUC among various
parameters for survival in heat stroke patients.
| Variables | AUC | SE | P-value | 95% CI | Sensitivity | Specificity | Cut-off |
|---|
| IPPV | 0.842 | 0.051 | <0.001 | 0.743–0.941 | 0.857 | 0.827 |
|
| CPK | 0.694 | 0.069 | 0.006 | 0.558–0.829 | 0.571 | 0.778 | 1676.200 |
| PT | 0.862 | 0.046 | <0.001 | 0.771–0.953 | 0.810 | 0.778 | 17.650 |
| PLT | 0.780 | 0.057 | <0.001 | 0.668–0.892 | 0.905 | 0.568 | 125.500 |
| ALB | 0.652 | 0.064 | 0.032 | 0.527–0.777 | 0.619 | 0.691 | 32.450 |
| PA | 0.845 | 0.044 | <0.001 | 0.760–0.931 | 0.857 | 0.778 | 17.950 |
Discussion
In this study, the relationship between serum
prealbumin and mortality in heatstroke patients was shown. The
total in-hospital mortality was (20.6%) in this study cohort, lower
than that in other reports (14,15).
Possible reasons are: First, all heatstroke patients were admitted
to the ICU for standard treatment. Secondly, a certain degree of
clinical experience was accumulated in the early stage of this
group, that is, the cluster treatment strategy of ‘early rapid
cooling, early rapid expansion, early anticoagulation, and active
support of organ function’ was proposed, effectively reducing the
mortality. Finally, some critically ill patients were not included
due to their incomplete data, as a result of failure of admission
or admission to hospital in <24 h, which affected the
calculation of mortality. In this study, prealbumin has been shown
to be a predictor of mortality in heatstroke patients. The
cumulative survival rate of patients with prealbumin >15 mg/dl
is significantly better than that of patients with prealbumin ≤15
mg/dl. The results of multivariate Cox regression analysis have
confirmed that low serum prealbumin level on admission is an
independent risk factor for the poor prognosis of heatstroke
patients. ROC curve analysis has shown that the AUC of prealbumin
is greater than that of albumin, with the sensitivity and
specificity of it higher than those of albumin, indicating that it
is satisfactory for judging the prognosis of heatstroke patients,
and that prealbumin may be clinically useful. This is the first
clinical study at home and abroad of serum prealbumin level in
heatstroke patients.
Composed of the same four subunits, the main
function of serum prealbumin is to transport thyroxine and vitamin
A, which promotes lymphocyte maturation and immunity enhancement.
Due to its small base and shorter half-life (2 days), prealbumin
can effectively reflect the synthesis of protein in the body, which
has become an internationally recognized indicator of nutritional
status. Moreover, as a negative acute phase reaction protein, the
expression of serum prealbumin has a significant decrease in tumor,
cirrhosis and inflammatory response (16). According to the Nutritional Health
Consensus Group, serum prealbumin >15 mg/dl indicates a lower
risk of malnutrition (17).
Therefore, in this study, 15 mg/dl of serum prealbumin was selected
as the cut-off value, 23 patients had ≤15 mg/dl, accounting for
22.5%. In low prealbumin group of patients, the levels of PLT, ALB
and TCH were lower, PT and APTT significantly prolonged, and there
was no significant difference in PCT and CRP reflecting the
inflammatory state.
Cardiac dysfunction is the leading cause of death in
patients with heat-related diseases (18). Experimental studies have confirmed
that under heat stress conditions, severe damage to myocardial
cells is characterized by vacuolar changes and partial necrosis of
cells (19). Clinical studies have
found that myocardial markers are significantly elevated after heat
stress, the elevated levels of which can better predict the
severity of myocardial damage and heat stroke (20). The multivariate regression analysis
of this study has shown that CPK has certain value in judging the
condition and prognosis, consistent with the results of Ye et
al (21).
Coagulation dysfunction can occur in the early stage
of heatstroke patients, resulting in DIC whose characteristic
pathological injury is extensive thrombosis. On the one hand,
continuous consumption causes thrombocytopenia and clotting factor
deficiency leading to bleeding. On the other hand, extensive
thrombosis causes tissue perfusion disorder that is one of the
important mechanisms of the occurrence of severe heat stroke MODS
(22,23). The multivariate regression analysis
of Zhao et al (24) found
that DIC is an main risk factor affecting the prognosis of
exertional heatstroke patients, with the mortality of patients
coexisting of 70.83%. Therefore, coagulation dysfunction is the
most severe complication of heatstroke (24). In the blood specimen monitoring of
patients with severe heat stroke within 24 h of admission, Pan
et al (25) found that PT and
APTT significantly prolong, but PLT significantly decreases in
death group compared with survival group. The analysis of receiver
operating characteristic (ROC) curve has shown that PLT has a
predictive value for patients with severe heat stroke (25). Li et al (26) found that prothrombin time on
admission, international normalized ratio and fibrinogen can
effectively predict the clinical prognosis of heatstroke patients
(26). In this study, a multivariate
regression analysis was performed on the clinical data of 102
heatstroke patients. The results showed that PT and PLT are
independent risk factors affecting the prognosis of patients among
all the main clinical parameters collected at admission.
In this study, patients with low serum prealbumin
level are found to be correlated with invasive positive pressure
ventilation, with invasive mechanical ventilation being an
independent risk factor for death in heatstroke patients.
Nutritional supplementation in patients with low prealbumin level
may shorten extubation time.
Increasing evidence shows that not caused by simple
thermal exposure resulting in direct injury, the pathophysiological
response of severe heat stroke MODS is a systemic inflammatory
response syndrome secondary to thermal injury, which in turn
manifests as ‘sepsis’, further triggering MODS (27,28).
After heatstroke, the pro-inflammatory and anti-inflammatory
factors in the body are out of balance, and then tissue damage
caused by pro-inflammatory response and immunosuppression caused by
excessive anti-inflammatory response may occur in patients
(29). Experimental studies have
shown that when the inflammatory response in heatstroke rats is
reduced with different methods, the degree of organ damage is
reduced, with improved survival rate (30). At present, there are few studies on
the changing trends of PCT and CRP, generally considered to be
acute phase biomarkers of inflammatory response, in heatstroke
patients. Serum PCT monitoring was performed on 68 exertional
heatstroke patients within 2 h of admission. Tong et al
(31) found that the PCT of
non-survivors is higher than that of survivors. After confounding
factors adjusted, PCT concentration is also an independent risk
factor for mortality, but may not be a good indicator predicting
heat stroke accompanied by infection (31). The studies of Hausfater et al
show that serum PCT can be induced to release even in the absence
of bacterial infection in exertional heatstroke patients (14). Surprisingly, although the CRP level
is moderately elevated [4.45 (1.38-14) mg/l], there is no
statistically significant difference between survival group and
non-survival group [4.2 (1.4, 12.9) vs. 8.7 (1.4, 24.7), P=0.360].
CRP, also an acute phase reaction protein, is positively correlated
with the degree of inflammation and tissue damage (32), but often taking time to respond and
increase serum concentration after infection or inflammation
(33). Studies have also shown that
the kinetics of CRP is slower in sepsis and systemic inflammatory
response than that in inflammatory mediator (including PCT, leptin,
IL-6 and TNF-α) (34,35). Therefore, the decline in CRP
sensitivity in the early stage of heatstroke can be explained by
these facts. Used as a differential diagnosis in patients with
non-infectious high fever at the early stage of admission, CRP
saves patients' valuable time and avoids unnecessary diagnostic
checks.
In the univariate analysis used to determine main
risk factors affecting the prognosis in this group of studies, the
results obtained are similar to those worldwide, indicating that
these parameters are representative. Multivariate Cox regression
analysis is used to remove confounding factors and screen out
independent risk factors affecting the prognosis of heatstroke, so
as to analyze and evaluate the sensitivity and specificity of each
risk factor. Besides, it evaluates the effect of prealbumin on
prognosis for the first time, being the highlight of this group of
studies, with accurate and reliable results.
There are also some shortcomings in this study.
First, study design is observation, so the causal relationship
between prealbumin and other markers needs further confirmation.
Secondly, the number of cases included in the study, which is
relatively small, is derived from a single hospital, with limited
representativeness that may cause statistical bias. In addition,
most of the cases are mainly typical heat stroke, cannot fully
representing other types of heat stroke. Therefore, further
long-term multi-center clinical studies with large samples are
necessary for confirming factors affecting the prognosis of
heatstroke.
In summary, the measurement of prealbumin is a
useful tool evaluating the nutritional and inflammatory status of
heatstroke patients. Moreover, the lower level of serum prealbumin
is independently associated with the increase of death risk.
Acknowledgements
Not applicable.
Funding
This study was supported by Changzhou Medical and
Health Guiding Science and Technology Project (WZ201021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QY and WL collected and interpreted the general
information of patients. JY and JJ acquired and analyzed the
οbservation indicators. TX and YZ contributed to statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Affiliated Changzhou No. 2 People's Hospital of Nanjing Medical
University (Changzhou, China). Patients who participated in this
research had complete clinical data. The signed informed consents
were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glazer JL: Management of heatstroke and
heat exhaustion. Am Fam Physician. 71:2133–2140. 2005.PubMed/NCBI
|
|
2
|
Bouchama A and Knochel JP: Heat stroke. N
Engl J Med. 346:1978–1988. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hammami MM, Bouchama A, Al-Sedairy S,
Shail E, AlOhaly Y and Mohamed GE: Concentrations of soluble tumor
necrosis factor and interleukin-6 receptors in heatstroke and
heatstress. Crit Care Med. 25:1314–1319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee EJ, Lee SW, Park JS, Kim SJ and Hong
YS: Successful treatment of severe heat stroke with selective
therapeutic hypothermia using an automated surface cooling device.
Resuscitation. 84:e77–e78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leon LR: Heat stroke and cytokines. Prog
Brain Res. 162:481–524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vandevyver S, Dejager L, Vandenbroucke RE
and Libert C: An acute phase protein ready to go therapeutic for
sepsis. EMBO Mol Med. 6:2–3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Franco J, Formiga F, Trullas JC, Salamanca
Bautista P, Conde A, Manzano L, Quirós R, Franco ÁG, Ezquerro AM
and Montero-Pérez-Barquero M; RICA investigators group, : Impact of
prealbumin on mortality and hospital readmission in patients with
acute heart failure. Eur J Intern Med. 43:36–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie Q, Zhou Y, Xu Z, Yang Y, Kuang D, You
H, Ma S, Hao C, Gu Y, Lin S, et al: The ratio of CRP to prealbumin
levels predict mortality in patients with hospital-acquired acute
kidney injury. BMC Nephrol. 12:302011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berbel MN, Góes CR, Balbi AL and Ponce D:
Nutritional parameters are associated with mortality in acute
kidney injury. Clinics (São Paulo). 69:476–482. 2014. View Article : Google Scholar
|
|
10
|
Lee KH, Cho JH, Kwon O, Kim SU, Kim RH,
Cho YW, Jung HY, Choi JY, Kim CD, Kim YL, et al: Low prealbumin
levels are independently associated with higher mortality in
patients on peritoneal dialysis. Kidney Res Clin Pract. 35:169–175.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang HT, Yim H, Cho YS, Kim D, Hur J, Kim
JH, Lee BC, Seo DK, Kim HS and Chun W: Prediction of clinical
outcomes for massively-burned patients via serum transthyretin
levels in the early postburn period. J Trauma Acute Care Surg.
72:999–1005. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bae HJ, Lee HJ, Han DS, Suh YS, Lee YH,
Lee HS, Cho JJ, Kong SH and Yang HK: Prealbumin levels as a useful
marker for predicting infectious complications after gastric
surgery. J Gastrointest Surg. 15:2136–2144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Dai L, Wang X, Wang Y, Zhou L, Chen
M and Wang H: Predictive value of the C-reactive
protein-to-prealbumin ratio in medical ICU patients. Biomarkers
Med. 11:329–337. 2017. View Article : Google Scholar
|
|
14
|
Hausfater P, Hurtado M, Pease S, Juillien
G, Lvovschi VE, Salehabadi S, Lidove O, Wolff M, Bernard M,
Chollet-Martin S, et al: Is procalcitonin a marker of critical
illness in heatstroke? Intensive Care Med. 34:1377–1383. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bouchama A, Dehbi M, Mohamed G, Matthies
F, Shoukri M and Menne B: Prognostic factors in heat wave related
deaths: A meta-analysis. Arch Intern Med. 167:2170–2176. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davis CJ, Sowa D, Keim KS, Kinnare K and
Peterson S: The use of prealbumin and C-reactive protein for
monitoring nutrition support in adult patients receiving enteral
nutrition in an urban medical center. J Parenter Enteral Nutr.
36:197–204. 2012. View Article : Google Scholar
|
|
17
|
Prealbumin in Nutritional Care Consensus
Group, : Measurement of visceral protein status in assessing
protein and energy malnutrition: Standard of care. Nutrition.
11:169–171. 1995.PubMed/NCBI
|
|
18
|
Wang X, Yuan B, Dong W, Yang B, Yang Y,
Lin X and Gong G: Humid heat exposure induced oxidative stress and
apoptosis in cardiomyocytes through the angiotensin II signaling
pathway. Heart Vessels. 30:396–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quinn CM, Duran RM, Audet GN, Charkoudian
N and Leon LR: Cardiovascular and thermoregulatory biomarkers of
heat stroke severity in a conscious rat model. J Appl Physiol
(1985). 117:971–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hausfater P, Doumenc B, Chopin S, Le
Manach Y, Santin A, Dautheville S, Patzak A, Hericord P, Mégarbane
B, Andronikof M, et al: Elevation of cardiac troponin I during
non-exertional heat-related illnesses in the context of a heatwave.
Crit Care. 14:R992010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye J, Mo W, Chen Y and Yang A: An analysis
of laboratory results of parameters of organ function in patients
with heat stroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue.
27:658–661. 2015.(In Chinese). PubMed/NCBI
|
|
22
|
Jilma B and Derhaschnig U: Disseminated
intravascular coagulation in heat stroke: A hot topic. Crit Care
Med. 40:1370–1372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leon LR and Helwig BG: Heat stroke: Role
of the systemic inflammatory response. J Appl Physiol (1985).
109:1980–1988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao JJ, Zhou JJ, Hu J, Zhou FH, Kang HJ,
Liu H, Pan L and Song Q: Analysis of risk factors affecting
prognosis of exertional heat stroke. Zhonghua Wei Zhong Bing Ji Jiu
Yi Xue. 25:515–518. 2013.(In Chinese). PubMed/NCBI
|
|
25
|
Pan ZG, Shao Y, Liu YN, Gu ZT, Zhang XQ,
Xu YQ and Su L: Relationship between early coagulability parameters
at admission and outcome in patients with severe heatstroke.
Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 25:725–728. 2013.(In
Chinese). PubMed/NCBI
|
|
26
|
Li Z, Wang Z, Tu X, Lu W, Fang Y, Gao J,
Yu S, He R, Shi K and Chu H: Analysis of indicators for clinical
prognosis of the patients with heat stroke. Zhonghua Jizhen Yixue
Zazhi. 25:1058–1061. 2016.(In Chinese).
|
|
27
|
Liu Z, Sun X, Tang J, Tang Y, Tong H, Wen
Q, Liu Y and Su L: Intestinal inflammation and tissue injury in
response to heat stress and cooling treatment in mice. Mol Med Rep.
4:437–443. 2011.PubMed/NCBI
|
|
28
|
Leon LR, Blaha MD and DuBose DA: Time
course of cytokine, corticosterone, and tissue injury responses in
mice during heat strain recovery. J Appl Physiol (1985).
100:1400–1409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim CL and Mackinnon LT: The roles of
exercise-induced immune system disturbances in the pathology of
heat stroke: The dual pathway model of heat stroke. Sports Med.
36:39–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsu CC, Niu CS and Lin MT: Decrease of
heatstroke-induced multiorgan dysfunction by whole body cooling in
streptozotocin-induced diabetic rats. Chin J Physiol. 52:47–55.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tong HS, Liu YS, Wen Q, Tang YQ, Yuan FF
and Su L: Serum procalcitonin predicting mortality in exertional
heatstroke. Emerg Med J. 29:113–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Danesh J, Wheeler JG, Hirschfield GM, Eda
S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB and Gudnason V:
C-reactive protein and other circulating markers of inflammation in
the prediction of coronary heart disease. N Engl J Med.
350:1387–1397. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martin C, Boisson C, Haccoun M, Thomachot
L and Mege JL: Patterns of cytokine evolution (tumor necrosis
factor-alpha and interleukin-6) after septic shock, hemorrhagic
shock, and severe trauma. Crit Care Med. 25:1813–1819. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pourakbari B, Mamishi S, Zafari J,
Khairkhah H, Ashtiani MH, Abedini M, Afsharpaiman S and Rad SS:
Evaluation of procalcitonin and neopterin level in serum of
patients with acute bacterial infection. Braz J Infect Dis.
14:252–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yousef AA, Amr YM and Suliman GA: The
diagnostic value of serum leptin monitoring and its correlation
with tumor necrosis factor-alpha in critically ill patients: A
prospective observational study. Crit Care. 14:R332010. View Article : Google Scholar : PubMed/NCBI
|