Introduction
Neonatal purulent meningitis has been given closer
attention because of its high mortality rate in neonatal period and
high disability rate in childhood. Moreover, the incidence rate of
neonatal purulent meningitis is increased year by year due to the
inappropriate use of antibiotics and increase of drug-resistance
bacteria. Its incidence rate is 0.02–0.1% in live births and 0.3%
in premature infants. In addition, its mortality rate is as high as
40–58%, and 40–50% of survivors suffer from neurological sequelae
(1). Furthermore, the symptoms of
neonatal purulent meningitis are not typical, and the
indiscriminate use of antibiotics can lead to atypical changes in
cerebrospinal fluid (CSF), thus making the early diagnosis of
neonatal purulent meningitis difficult. Therefore, exploring an
effective detection method for early diagnosis of neonatal purulent
meningitis is of important clinical significance. Currently, it is
considered that TNF-α is an important cytokine participating in the
inflammatory response, its level is elevated in CSF of neonates
with purulent meningitis (2). With
the improvement in technology, the fluid-attenuated inversion
recovery (FLAIR) sequence has shown a good value in the
differential diagnosis of lesions in the meninges and brain
parenchyma, which can selectively inhibit CSF and obtain
high-resolution T2 contrast images at the same time (3). The application of CSF TNF-α detection
alone or magnetic resonance imaging (MRI) enhanced FLAIR sequence
scanning alone in purulent meningitis has been reported (4,5). In this
study, the diagnostic value of MRI enhanced FLAIR sequence scanning
combined with CSF TNF-α detection in neonatal purulent meningitis
was investigated, so as to improve the accuracy rate of diagnosis
of neonatal purulent meningitis, providing a basis for early
treatment in clinical practice.
Patients and methods
General data
Neonates with fever and sepsis admitted to the
neonatal intensive care unit (NICU) of Linyi Women and Children's
Hospital (Linyi, China) from April 2015 to May 2018 received CSF
routine and biochemical examinations. Fifty neonates with purulent
meningitis were randomly selected as the purulency group, and 50
neonates with viral meningitis (VM) were selected as the virus
group. Fifty neonates without purulent meningitis were selected as
the no meningitis group. The neonates were 0–28 days old. There
were 37 boys and 13 girls in the purulency group, 33 boys and 17
girls in the virus group, and 35 boys and 15 girls in the no
meningitis group. No significant differences were found in age and
sex among the three groups (P>0.05), and they were comparable.
Diagnostic criteria: i) Neonates conforming to the diagnostic
criteria for purulent meningitis (referred to the fourth edition of
Practical Neonatology) (6), with
common clinical symptoms including fever (sustainable or
transient), vomiting, irritability and sleepiness accompanied by
disturbance of consciousness, ii) neonates with bacteria found in
the culture of CSF or positive CSF in smear test, and iii) those
with no bacteria detected in the culture of CSF or negative smear
test result, but with typical CSF changes, turbid appearance,
significantly increased white blood and obviously elevated protein.
Diagnostic criteria for VM (referred to the seventh edition of
Textbook of Pediatrics) (7): i)
Neonates diagnosed with VM according to the characteristics of
neonates' electroencephalogram (EEG) and MRI images and serological
examination, and ii) those with no bacteria detected in the culture
of CSF or negative smear test result, no overt increase in cell
count, and slight increase in protein level. Exclusion criteria: i)
Premature infants and low birth weight infants, ii) neonates with
intracranial hemorrhage complicated with purulent infection, iii)
neonates with congenital nervous system malformations, or iv) those
with congenital genetic metabolic diseases. Signed informed
consents were obtained from the parents of neonates. The
examinations of CSF routine, biochemistry and culture, TNF-α
detection and head MRI FLAIR sequence examination were carried out
at the early stage of the disease. This study was approved by the
Ethics Committee of Linyi Women and Children's Hospital.
Examination methods
Specimen collection
CSF was taken from neonates via lumbar puncture,
followed by routine examination (biochemistry and cytology).
Peripheral venous blood (5 ml) was collected and centrifuged at
1,500 × g at room temperature for 15 min. Then the supernatant was
collected and stored at −80°C.
Measurement of TNF-α in CSF
Double-antibody enzyme-linked immunosorbent assay
(ELISA) was applied. After one freeze-thaw cycle, specimens were
measured by a specially-assigned individual according to the
manufacturer's instructions provided by the Academy of Military
Medical Sciences.
Scanning parameters and scanning
method of MRI FLAIR sequence scanning
A Philips Achieva 1.5T superconducting magnetic
resonance imager was used. Scanning parameters are as follows:
field of view (FOV) = 24 cm × 24 cm, layer thickness = 5 mm,
interval = 1.7 mm, TR8500ms, TE92ms, TI2400ms, and the acquisition
time was 150 sec. An 8-channel Sense head coil was adopted. The
scanning should be done with neonates in a quiet state. If
necessary, 6% chloral hydrate was taken orally (at a dose of 1.5
ml/kg) half an hour before the examination. Both MRI plain scan and
enhanced MRI scan were required. The plain scan included the spin
echo sequence FLAIR of the cross section and the lateral axis. For
the enhanced scan, gadopentetate dimeglumine (Gd-DTPA) was injected
first via the cubital veins at a dose of 0.1 mmol/kg. The
cross-sectional fast FLAIR sequence scanning was immediately
performed to ensure that the scanning conditions of the FLAIR
sequence were consistent.
Evaluation and analysis of scanned
images
The MRI images of neonates were independently
evaluated by two magnetic resonance physicians with rich clinical
experience using the ‘double-blind method’. If the two physicians
had different evaluations, a third physician would be selected for
evaluation. The FLAIR and enhanced FLAIR of the head of neonates
were analyzed and evaluated based on the location and number of
purulent meningitis. The enhancement, extent and quantity of
cranial nerve, meningeal blood vessel, CSF and adjacent brain
parenchyma were evaluated. (−), (+) and (++) indicated negative,
positive and strongly positive, respectively, which were used to
describe the signal intensity of purulent meningitis. The total
positive rate = (number of positive cases + number of strongly
positive cases) / total number of cases. Apparent diffusion
coefficient (ADC) images and ADC values were generated
automatically by computer software. The ADC value was the average
of 3 measurements.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
17.0 software (SPSS, Inc., Chicago, IL, USA) was used for data
analyses. Measurement data were expressed as mean ± standard
deviation (mean ± SD), and t-test was employed. χ2 test
was applied for enumeration data. Enumeration data not conforming
to normal distribution could be converted into measurement data
based on reality. Factors with reference value in the univariate
analysis were subjected to further logistic regression analysis, so
as to determine the risk factors for purulent meningitis. ANOVA was
used for multiple comparisons and Least Significant Difference was
the post-hoc test. P<0.05 suggested that the difference was
statistically significant.
Results
Comparisons of clinical data and
biochemical indicators of neonates in the three groups
As to clinical symptoms of neonates, there were no
statistically significant differences in fever [100% (50/50), 100%
(50/50), 100% (50/50)], convulsive seizure [33 (66.00%), 30
(60.00%), 15 (30.00%)], sleepiness with low spirits [45 (90.00%),
41 (82.00%), 36 (72.00%)], jaundice [38 (76%), 12 (24.00%), 26
(52.00%)], irritability [33, (66.00%), 25 (50.00%), 19 (38.00%)]
and dyspnea [18 (20.00%), 13 (26.00%), 12 (24.00%)] among the
purulency, virus and no meningitis groups (P>0.05). However, the
increase of TNF-α (P=0.005), white blood cell count (P=0.032),
erythrocyte sedimentation rate (ESR) (P=0.028), rate of cerebral
edema alone (P=0.042) and rate of cerebral edema combined with
abnormal EEG (P=0.030) in the purulency group were overtly higher
than those in the virus and no meningitis groups (Table I).
 | Table I.Comparisons of biochemical indexes of
children in the three groups. |
Table I.
Comparisons of biochemical indexes of
children in the three groups.
|
|
| Pandy test [n
(%)] |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Groups | The increase of TNF-α
(pg/ml) | Positive | Negative | ESR (mm/h) | Positive CSF culture
[n (%)] | White blood cell
count >5×108/l [n (%)] | Cerebral edema alone
[n (%)] | Cerebral edema
combined with abnormal EEG [n (%)] |
|---|
| Purulency group | 132.58±31.72 | 60 | 40 |
50.95±9.93 | 35 (70.00) | 40 (80.00) | 37 (74.00) | 23 (46.00) |
| Virus group | 21.65±0.31 | 72 | 28 | 17.05±10.38 | 20 (40.00) | 5
(10.00) | 12 (40.00) | 6
(20.00) |
| No meningitis
group | 0 | 0 | 100 |
12.35±6.29 | 0
(0.00) | 0
(0.00) | 0
(0.00) | 0
(0.00) |
| P-value | 0.006 | 0.074 |
| 0.028 | 1.583 | 0.032 | 0.042 | 0.030 |
Results of MRI enhanced FLAIR sequence
scanning in the three groups
The results showed that there were 45 neonates with
positive (+, ++) high signal intensity and 5 neonates with negative
high signal intensity in MRI enhanced FLAIR sequence scanning in
the purulency group, while in the virus group, there were 35 cases
of positive (+, ++) high signal intensity and 15 cases of negative
high signal intensity. All 50 neonates in the no meningitis group
had negative high signal intensity. Based on the χ2
test, the differences were statistically significant (P=0.042). The
comparison of ADC value showed that there was a statistically
significant difference between the purulency, virus and no
meningitis groups (P=0.006) (Table
II).
 | Table II.Comparisons of MRI enhanced FLAIR
sequence scan results between the two groups. |
Table II.
Comparisons of MRI enhanced FLAIR
sequence scan results between the two groups.
|
| HVS signal intensity
[n (%)] |
|
|---|
|
|
|
|
|---|
| Groups | (−) | (+) | (++) | Total positive rate
(%) | ADC value
(×10−5 mm2/sec) |
|---|
| Purulency group | 4 | 19 | 27 | 92.00 | 31.19±8.01 |
| Virus group | 15 | 20 | 15 | 70.00 | 63.48±15.42 |
| No meningitis
group | 50 | 0 | 0 | 0.00 |
|
| P-value |
|
|
| 0.042 | 0.058 |
Analyses on risk factors for purulent
meningitis
Logistic regression analysis was carried out, and it
was found that the risk factors for purulent meningitis included
increased white blood cell count, enhanced TNF-α value, cerebral
edema alone, cerebral edema complicated with abnormal EEG, elevated
ESR and decreased ADC value (Table
III).
 | Table III.Logistic regression analysis of risk
factors for neonatal purulent meningitis. |
Table III.
Logistic regression analysis of risk
factors for neonatal purulent meningitis.
| Category | β | SE | Wald | Odds ratio | 95% confidence
interval | P-value |
|---|
| White blood cell
count |
0.481 | 0.668 |
8.845 | 2.033 | 1.617–4.875 | 0.001 |
| TNF-α |
0.805 | 0.654 | 16.531 | 3.213 | 1.768–9.969 | 0.001 |
| Cerebral edema
alone |
0.563 | 0.491 |
4.610 | 1.602 | 1.191–3.679 | 0.041 |
| Cerebral edema
complicated with abnormal EEG |
0.429 | 0.391 |
5.789 | 1.964 | 1.430–5.285 | 0.040 |
| ESR |
1.336 | 0.018 |
7.587 | 1.973 | 1.235–6.031 | 0.018 |
| ADC value | −1.229 | 0.019 | 14.148 | 2.326 | 1.467–7.115 | 0.001 |
Value of CSF TNF-α detection combined
with MRI enhanced FLAIR sequence scanning
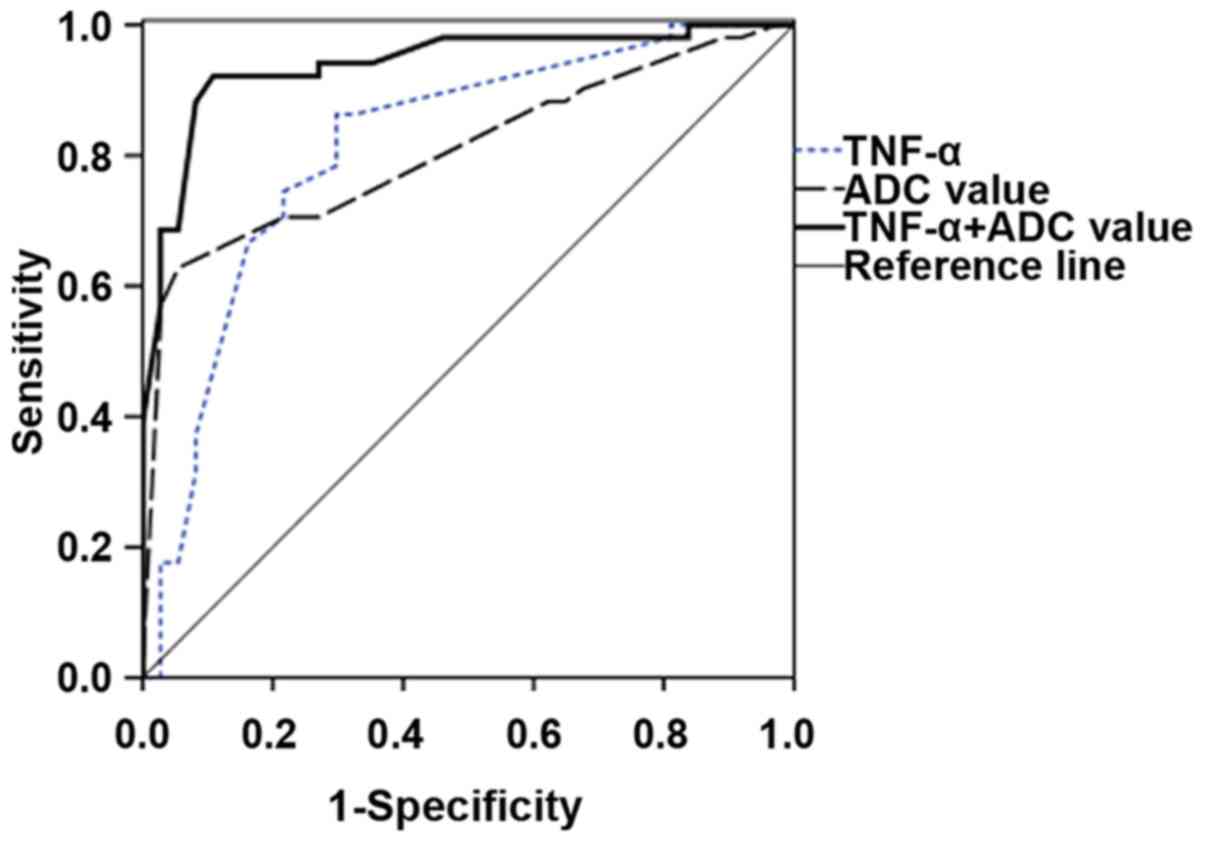
According to the receiver operating characteristic
(ROC) curves, when the Youden index was at its maximum, the
critical values of ADC in TNF-α detection and MRI enhanced FLAIR
sequence examination were 74.21 pg/ml and 45.66×10−5
mm2/sec, respectively. In the measurement of ADC value,
TNF-α detection combined with MRI enhanced FLAIR had better
sensitivity, specificity, positive predictive value, negative
predictive value, diagnostic accuracy and area under curve (AUC)
than TNF-α detection alone and MRI enhanced FLAIR alone. The AUC of
ROC curve showed that the the AUC of ADC value in the TNF-α
combined detection with MRI enhanced FLAIR sequence examination was
0.925, which was higher than those in MRI enhanced FLAIR sequence
examination (0.845) and TNF-α detection (0.730) (P<0.05),
showing statistically significant differences (Fig. 1 and Table
IV).
 | Table IV.Comparison of TNF-α and MRI enhanced
FLAIR sequence ADC values in the diagnosis of neonatal purulent
meningitis. |
Table IV.
Comparison of TNF-α and MRI enhanced
FLAIR sequence ADC values in the diagnosis of neonatal purulent
meningitis.
|
|
|
|
|
|
|
| AUC statistics |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Detection index | Sensitivity (%) | Specificity (%) | Positive predictive
value (%) | Negative predictive
value (%) | Diagnostic accuracy
(%) | Youden index | AUC | P-value | 95% CI | Diagnostic
cutoff |
|---|
| TNF-α | 75.7 | 85.2 | 81 | 80.1 | 82.3 | 0.611 | 0.730 | 0.004 | 0.657–0.811 | 74.21 pg/ml |
| ADC value | 78.8 | 91.1 | 80 | 77.9 | 84.4 | 0.701 | 0.845 | 0.001 | 0.754–0.887 | 45.66×10−5
mm2/sec |
| TNF-α+ADC | 86.6 | 93.8 | 96.8 | 85.5 | 87.8 | 0.806 | 0.925 | 0.001 | 0.831–0.927 |
|
Discussion
Significance and importance of TNF-α in CSF.
Neonatal purulent meningitis is a central nervous system infection,
of which the incidence rate is 0.02–0.1% in live births and up to
0.3% in premature infants. Besides, its mortality rate is 40–58%,
and 40–50% of survivors suffer from sequelae, such as epilepsy,
hydrocephalus and mental retardation (1). For the diagnosis of purulent
meningitis, routine and biochemical examinations of CSF are
important means. TNF in CSF has a comprehensive biological
activity, which is able to kill tumor cells, regulate T and B
lymphocytes and has antisuppurative and antiviral effects (8). TNF is composed of TNF-α and TNF-β, in
which TNF-α is secreted by activated monocytes and macrophages
(9). Under physiological conditions,
TNF in the body has a low concentration and participates in various
physiological immune processes, activating neutrophils and K cells,
promoting the growth and differentiation of B cells and stimulating
the proliferation of T cells (10).
However, the concentration of TNF is evidently increased, and its
proportional relationships with other cytokines are disordered
under pathological conditions, resulting in disorder of immune
regulation and participating in the development of related
diseases. Therefore, TNF is a potential fibrotic factor or
inflammatory mediator, which plays an important role in the acute
phase and inflammatory sclerosis (11). A study has manifested that in
purulent meningitis, TNF-α is significantly elevated in CSF of
neonates and involved in the inflammatory response (4). Mediators produced by viral RNA
infection and endotoxin in septic lipopolysaccharide (LPS) will
stimulate immune cells and infected cells to produce TNF and
interleukin-6 (12). TNF-α can
induce the production of a variety of cytokines. When neonates
suffer from acute central nervous system infection, TNF-α is
significantly increased, resulting in fever, shock and increased
vascular permeability in neonates (13). This is also consistent with the
results of our present study.
MRI enhanced FLAIR sequence examination and its
contribution. The images were obtained via the MRI enhanced FLAIR
sequence examination. This method can eliminate the artifacts of
the blood vessels, showing flow voids and fully displaying the
subtle lesions (14). The FLAIR
sequence can suppress the CSF signal and obtain images with higher
T2-weighted degree, showing lesions not visible on enhanced T1WI.
In particular, the small lesions in the early stage of purulent
meningitis can be overtly diagnosed using the enhanced FLAIR
technique, to better and more timely assess the disease. Moreover,
a wider range of effective information with high accuracy can be
obtained through such a technique, which cannot only reflect the
enhanced form and characteristics of lesions in the meninges, but
also better reflect the grade and extent of lesions. In this study,
the MRI enhanced FLAIR sequence scanning combined with CSF TNF-α
detection was applied, and the results showed that its sensitivity,
specificity, positive predictive value, negative predictive value,
diagnostic accuracy and AUC were superior to those of TNF-α
detection alone and MRI enhanced FLAIR sequence ADC value
measurement alone.
In conclusion, our study demonstrated that the MRI
enhanced FLAIR technique combined with CSF TNF-α detection has the
highest sensitivity and specificity in the diagnosis of purulent
meningitis. Therefore, it is proposed to use the MRI enhanced FLAIR
technique combined with CSF TNF-α detection in the diagnosis of
purulent meningitis, helping to improve the clinical diagnosis rate
of neonatal purulent meningitis.
Acknowledgements
Not applicable.
Funding
This study was supported by Linyi Key Research and
Development Project (no. 2016ZK003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ conceived the study and drafted the manuscript.
YZ and XS performed and analyzed MRI FLAIR sequence scanning. JW
analyzed the general data of patients and revised the manuscript.
AS helped with ELISA. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Linyi Women and Children's Hospital (Linyi, China). Patients who
participated in this study had complete clinical data. Signed
informed consents were obtained from the parents of the
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
The Collaborative Group For Neonatal
Meningitis Study TC, ; Liu CQ: Epidemiology of neonatal purulent
meningitis in Hebei Province, China: A multicenter study. Zhongguo
Dang Dai Er Ke Za Zhi. 17:419–424. 2015.(In Chinese). PubMed/NCBI
|
|
2
|
Prasad R, Kapoor R, Srivastava R, Mishra
OP and Singh TB: Cerebrospinal fluid TNF-α, IL-6, and IL-8 in
children with bacterial meningitis. Pediatr Neurol. 50:60–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeevanandham B, Kalyanpur T, Gupta P and
Cherian M: Comparison of post-contrast 3D-T1-MPRAGE, 3D-T1-SPACE
and 3D-T2-FLAIR MR images in evaluation of meningeal abnormalities
at 3-T MRI. Br J Radiol. 90:201608342017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pfausler B, Grubwieser G, Bösch S, Vollert
H, Herald M and Schmutzhard E: Cerebrospinal fluid-filtration
reduces TNF alpha in bacterial meningitis-CSF. Eur J Neurol.
2:570–572. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abe M, Takayama Y, Yamashita H, Noguchi M
and Sagoh T: Purulent meningitis with unusual diffusion-weighted
MRI findings. Eur J Radiol. 44:1–4. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sohn CH, Sevick RJ, Frayne R, Chang HW,
Kim SP and Kim DK: Fluid attenuated inversion recovery (FLAIR)
imaging of the normal brain: Comparisons between under the
conditions of 3.0 Tesla and 1.5 Tesla. Korean J Radiol. 11:19–24.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nigrovic LE, Fine AM, Monuteaux MC, Shah
SS and Neuman MI: Trends in the management of viral meningitis at
United States children's hospitals. Pediatrics. 131:670–676. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bociąga-Jasik M, Garlicki A, Cieśla A,
Kalinowska-Nowak A, Sobczyk-Krupiarz I and Mach T: The diagnostic
value of cytokine and nitric oxide concentrations in cerebrospinal
fluid for the differential diagnosis of meningitis. Adv Med Sci.
57:142–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Panato AP, Tomasi LT, Simon CS, Madeira K,
Simoes LR, Medeiros LR, Barichello T and Rosa MI: Meta-analysis
identifies tumor necrosis factor-alpha and interleukin-1 beta as
diagnostic biomarkers for bacterial and aseptic meningitis. Curr
Neurovasc Res. 11:340–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Splendiani A, Puglielli E, De Amicis R,
Necozione S, Masciocchi C and Gallucci M: Contrast-enhanced FLAIR
in the early diagnosis of infectious meningitis. Neuroradiology.
47:591–598. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Letiembre M, Echchannaoui H, Ferracin F,
Rivest S and Landmann R: Toll-like receptor-2 deficiency is
associated with enhanced brain TNF gene expression during
pneumococcal meningitis. J Neuroimmunol. 168:21–33. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HJ, Shim KW, Lee MK, Park MS, Kim SH,
Kim EY, Park S and Kim TS: Tuberculous encephalopathy without
meningitis: Pathology and brain MRI findings. Eur Neurol.
65:156–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Šumanović-Glamuzina D, Čulo F, Čulo MI,
Konjevoda P and Jerković-Raguž M: A comparison of blood and
cerebrospinal fluid cytokines (IL-1β, IL-6, IL-18, TNF-α) in
neonates with perinatal hypoxia. Bosn J Basic Med Sci. 17:203–210.
2017.PubMed/NCBI
|
|
14
|
Vaswani AK, Nizamani WM, Ali M, Aneel G,
Shahani BK and Hussain S: Diagnostic accuracy of contrast-enhanced
FLAIR magnetic resonance imaging in diagnosis of meningitis
correlated with CSF analysis. ISRN Radiol. 2014:5789862014.
View Article : Google Scholar : PubMed/NCBI
|