Introduction
Severe hypokalemia is a fatal emergent clinical
condition in which the serum potassium concentration is >2.5
mmol/l (1); it presents as general
muscle weakness, cardiac arrhythmia, and respiratory muscle
paralysis. Even though this condition is fatal, a swift reversion
of the low serum potassium concentration in clinical patients is
not allowed due to the strict regulations regarding the
supplementation of potassium to prevent pseudohyperkalemia. This
approach is followed in most cases; however, it does not work well
in cases of severe hypokalemia, particularly when the condition is
fatal, e.g., after long-term fasting or insufficient food
consumption with severe diarrhea and vomiting (2), poisoning (3,4), after
cardiothoracic surgery (5,6), in subjects with hypokalemic periodic
paralysis (7) and severe
malnutrition. Furthermore, severe hypokalemia often concurs with
various critical conditions, including multiple organ failure,
severe infection, as well as fluid, electrolyte and acid-base
imbalances. These conditions are accompanied with various
underlying diseases and complications that require restricted speed
and volume of venous infusion. This presents a contradiction:
According to the established regulations, to avoid hyperkalemia,
the concentration and speed of intravenous potassium
supplementation should not exceed 40 mmol/l (0.3%) or 20 mmol/h
(1.5 g/h) in adults and 0.4 mmol/kg/h in children; furthermore,
direct intravenous injection of potassium is strictly prohibited
(1,8); however, to correct severe hypokalemia
in the presence or absence of critical diseases, rapid infusion of
a small volume of potassium solution may be beneficial.
Of note, the American Heart Association (AHA)
recommends an empirical intravenous injection of 2 mEq of potassium
in the first 1 min, followed by an intravenous infusion of 10 mEq
of potassium over 5–10 min in cases where hypokalemia led to
malignant ventricular arrhythmia or inevitable cardiac arrest
(9). Although several case reports
and randomized clinical trial investigations have suggested the
success of this novel recommendation (2,10,11),
more persuasive, evidence-based proof is required. Slow potassium
supplementation may not lead to treatment failure, increase the
severity of the disease and increase the risk of patient death;
however, empirical rapid potassium supplementation is potentially
dangerous. In the present study, an tailored rapid potassium
supplementation strategy was developed, which was based on the
hemodynamic principle, as well as the mechanism of potassium
distribution and balance, for calculating the proper concentration,
infusion rate and dose of potassium supplement for individuals with
bedside monitoring via electrocardiogram (ECG) and the serum
potassium concentration. In the era of precise medicine, with
exceptional cases and AHA recommendations (2,8,10,11),
this tailored rapid potassium supplementation strategy has the
potential to become the optimum treatment option in the future. On
the basis of an integrated approach, it was hypothesized that this
strategy may restore extremely low serum potassium concentrations
in patients admitted to emergency departments and intensive care
units.
Barium (Ba) is a silver-white alkaline metal that is
widely distributed in nature. Ba is almost non-toxic, but the
toxicity of Ba salt is determined by its solubility; Ba chloride
(BaCl2) is the most toxic soluble Ba salt (12). Ba may induce a low serum potassium
concentration via the following two synergistic mechanisms: i) Ba
ions activate Na+/K+-ATPase in the cell
membrane and block the potassium channels; this causes potassium
ions to be redistributed inside and outside of the cell, and
extracellular potassium reverses the chemical gradient into the
cell; ii) the potassium channel blockade makes it difficult for the
cell to excrete potassium, resulting in high intracellular and low
extracellular potassium, which causes membrane current inhibition
and produces a series of clinical manifestations associated with
low potassium, including muscle paralysis, respiratory failure and
fatal arrhythmia (13). This is also
the major cause of death due to acute BaCl2 poisoning
(14–16).
The animal model established in the present study is
based on the characteristics of BaCl2 poisoning, which
not only rapidly causes severe hypokalemia, but also easily induces
arrhythmia. The present study first established an animal model of
acute fatal severe hypokalemia with BaCl2 and then
performed a potassium supplementation experiment using this model.
The purpose of the present study was to compare the conventional
potassium supplementation method with a newly developed the
tailored rapid potassium supplementation strategy in order to
evaluate its efficiency and safety, and to provide an experimental
basis for the development of an effective, safe and scientific
strategy for the clinical rescue of fatal severe hypokalemia.
Materials and methods
Experimental animals
The Animal Ethics Committee of Sichuan University
(Chengdu, China) approved all of the procedures performed in the
present study. A total of 30 adult healthy male and female Japanese
white rabbits (weight, 2.5 kg; age, 12–14 weeks) were purchased
from the Animal Center of Sichuan Province (Chengdu, China), and
kept for 3–5 days in single cages. Animals were housed at a
temperature of 22–24°C and a relative humidity of 30–40% with free
access to food and water and a 12 h light/dark cycle prior to the
start of the experiment.
Establishment of animal disease
model
The conditions of the current study are based on
preliminary experimental results from acute toxicity experiments
assessing barium chloride in rabbits (17). Barium chloride gavage and continuous
potassium supplementation monitoring were performed under
anaesthesia. All rabbits were 12 h prior to the beginning of the
experiment and ate immediately following potassium supplementation.
After weighing, a 22-gauge catheter was inserted and placed in the
central ear artery of each rabbit for blood pressure monitoring and
blood sampling by connecting a T-branch tube. The rabbits were
anesthetized by an intraperitoneal injection of urethane (1,000
mg/5 ml/kg). After successful anesthesia, the rabbit was fixed on
the operation bench and a urinary catheter was inserted into the
empty bladder. A needle electrode was then inserted and connected
to the BL-420E+ Biological Function Experimental System
(Chengdu TME Technology Co., Ltd., Chengdu, China) for real-time
recording of ECG, blood pressure, respiratory rate and oxygen
saturation. BaCl2 [dose at which 50% of animal
mortalities occurred in 14 days (LD50), 168 mg/5 ml/kg]
was delivered by oral gavage. Every 30 min or whenever arrhythmia
occurred, blood samples of 0.5 ml were extracted from the central
ear artery of the rabbits to determine the serum potassium
concentration. When the serum potassium concentration reached
<2.5 mmol/l and was accompanied with arrhythmia, the fatal
severe hypokalemia model was considered established. The
experimental rabbits which did not meet the above criteria
regarding the serum potassium levels and ECG were ruled out.
Randomized grouping principle
The model animals were randomized using the
‘repeated fair coin-tossing method’. A total of 20 rabbits with
severe hypokalemia were randomly divided into two treatment groups
(n=10 per group): Conventional potassium supplementation group
(conventional treatment group) and tailored rapid potassium
supplementation group (tailored rapid treatment group).
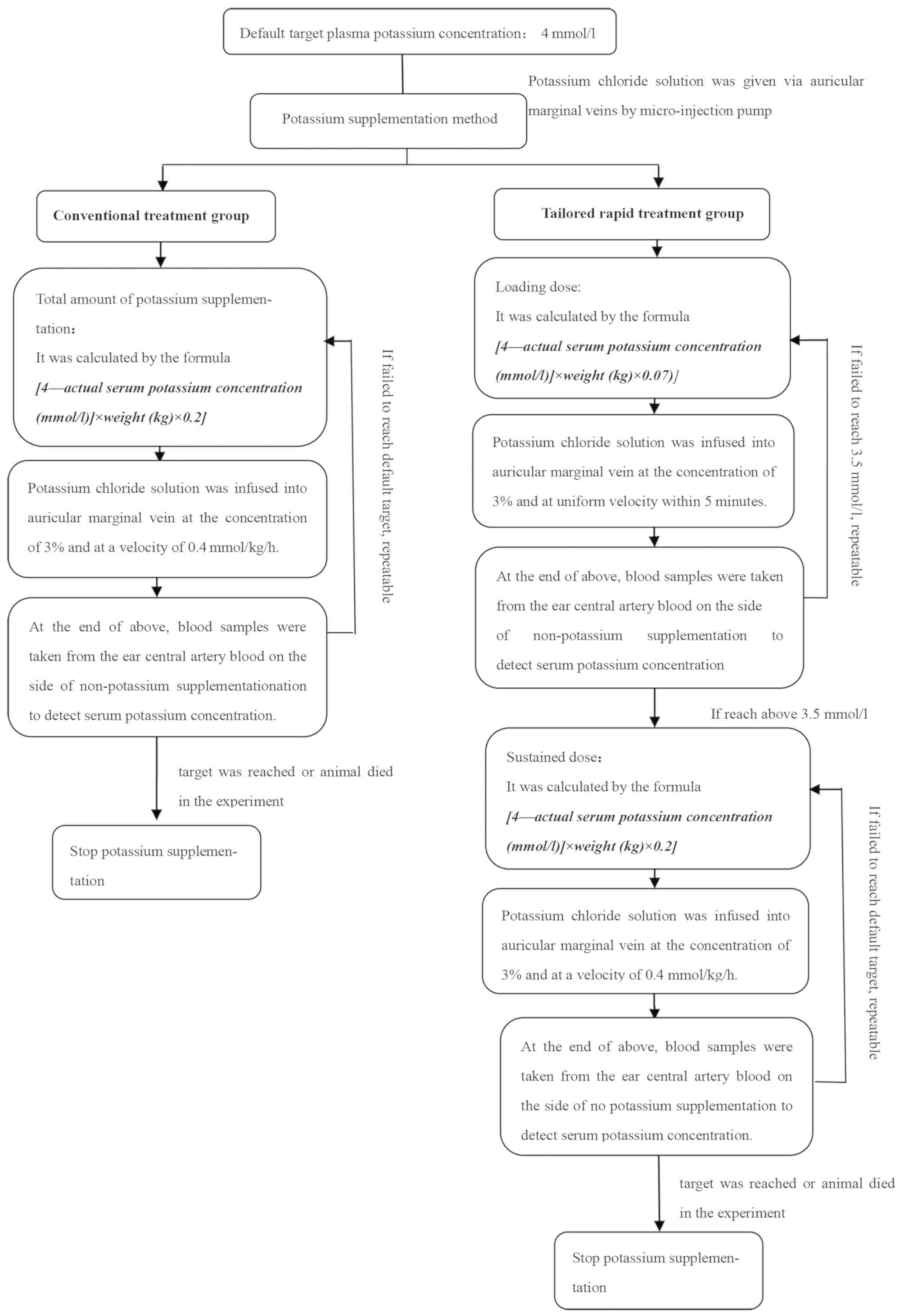
Potassium supplementation methods
Potassium chloride infusion was performed through
the auricular marginal vein by a micro-injection pump, and the
target serum potassium concentration was 4 mmol/l.
For the conventional treatment group, the total
amount of potassium supplement was calculated by the formula
[4-actual serum potassium concentration (mmol/l)] × weight (kg)
×0.2; this was infused into the auricular marginal vein at the
concentration of 3% and at the infusion rate of 0.4 mmol/kg/h
(18,19). If necessary, the potassium deficiency
was recalculated and potassium solution was infused to obtain the
present concentration. Potassium supplementation was stopped if the
target serum potassium concentration was reached or if the animal
died in the experiment.
The tailored rapid treatment group was administered
a loading dose and a maintenance dose. The loading dose, calculated
by the formula [4-actual serum potassium concentration (mmol/l)] ×
weight (kg) ×0.07, was infused into the auricular marginal vein at
a uniform infusion rate within 5 min; this dose was repeated until
the serum potassium concentration increased to 3.5 mmol/l.
Subsequently, a maintenance dose, calculated by the formula
[4-actual serum potassium concentration (mmol/l)] × weight (kg)
×0.2, was injected with a micro-pump at the infusion rate of 0.4
mmol/kg/h. All the potassium solutions were prepared and infused at
a concentration of 3%. In the case where the serum potassium
concentration did not increase to the target concentration, the
respective steps were repeated for a maintenance dose. Potassium
supplementation was stopped if the target serum potassium
concentration was reached or if the animal died in the experiment
(Fig. 1).
Observation parameters
In the experiment, the serum potassium concentration
was measured prior to and at the end of each potassium
supplementation cycle until the serum potassium concentration
reached the target level. All urine excreted during the potassium
supplementation procedure was collected for the measurement of
volume and the potassium concentration. The duration of potassium
infusion, the amount of potassium infused, mean arterial pressure
(MAP), respiratory rate (RR), ECG and adverse effects were
recorded. From then on, serum potassium concentrations were
measured once per day in the 14-day observation period. In
addition, the respiratory rate of rabbits was observed without
anaesthesia and ECGs were monitored via electrodes. The death of
rabbits was continuously observed following potassium
supplementation and no rescue measures were taken prior to
mortality, and the serum potassium concentration was determined
again before death.
Calculation method for observation
parameters
The serum potassium concentration prior to potassium
supplementation (mmol/l) was measured after successfully
establishing the model, and was defined as the serum potassium
concentration before the beginning of potassium supplementation
treatment.
The serum potassium concentration after potassium
supplementation (mmol/l) was defined as the serum potassium
concentration after stopping the treatment or at death of the
animal.
The duration of potassium infusion (h) was the time
interval of potassium supplementation treatment.
The actual total amount of potassium supplement
(mmol) was the total amount of serum potassium ions in the body
from the beginning to the end of the potassium supplementation.
The net amount of potassium supplement (mmol) was
the actual total amount of potassium supplement- the amount of
potassium excreted through the kidneys.
The total urinary volume (ml) was the total urinary
volume from the beginning to the end of the potassium
supplementation.
The amount of potassium excreted through the kidneys
(mmol) was the urine potassium concentration × the total urinary
volume.
The amount of extracellular fluid (ECF) potassium
(mmol) was the serum potassium concentration × the body weight (kg)
×0.2.
The ECF potassium alteration [post- vs.
pre-potassium supplementation (mmol)] was the ECF potassium
concentration after potassium supplementation-the ECF potassium
concentration prior to potassium supplementation.
The amount of the transcellular potassium shift
(mmol) was determined as the net amount of potassium
supplementation-ECF potassium alteration (post- vs. pre-potassium
supplementation).
The criteria for arrhythmias associated with
hypokalemia were as follows: Ventricular arrhythmias, including
various types of ventricular pre-mature beats, ventricular
tachycardia and ventricular fibrillation.
The duration of cardiac arrhythmia was the time of
beginning and disappearance of each arrhythmia.
Sample testing method
The serum potassium concentration was determined
using the following method: Blood samples were collected from the
central artery of rabbit ears and placed into the ABL800FLEX
automatic biochemical-blood gas analyser (Radiometer, Copenhagen,
Denmark). The serum potassium concentration was measured using the
ion selective electrode method.
The urine potassium concentration was determined as
follows: Urine samples were analyzed using the DxcUnicel 800
Synchron automatic biochemical analyser (Beckmann Coulter, Brea,
CA, USA) and the samples were measured via the indirect potential
method.
Statistical methods
SPSS.16 software was utilized to perform statistical
analysis (SPSS, Inc., Chicago, IL, USA). The results for all
measurement variables were first tested for normal distribution.
Variables with a normal distribution were expressed by arithmetic
means with standard deviations, while variables with an abnormal
distribution were expressed by the median. Student's t-tests or
analysis of variance F-tests (followed by an LSD or SNK post-hoc
test) were used to evaluate differences in the mean values of
variables with a normal distribution, and the rank-sum test was
used to evaluate differences in variables with an abnormal
distribution. Categorical variables were summarized by frequency
counts with percentages, while the chi-square test or Fisher's
exact test were used to evaluate differences between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Establishment of the rabbit model of
hypokalemia
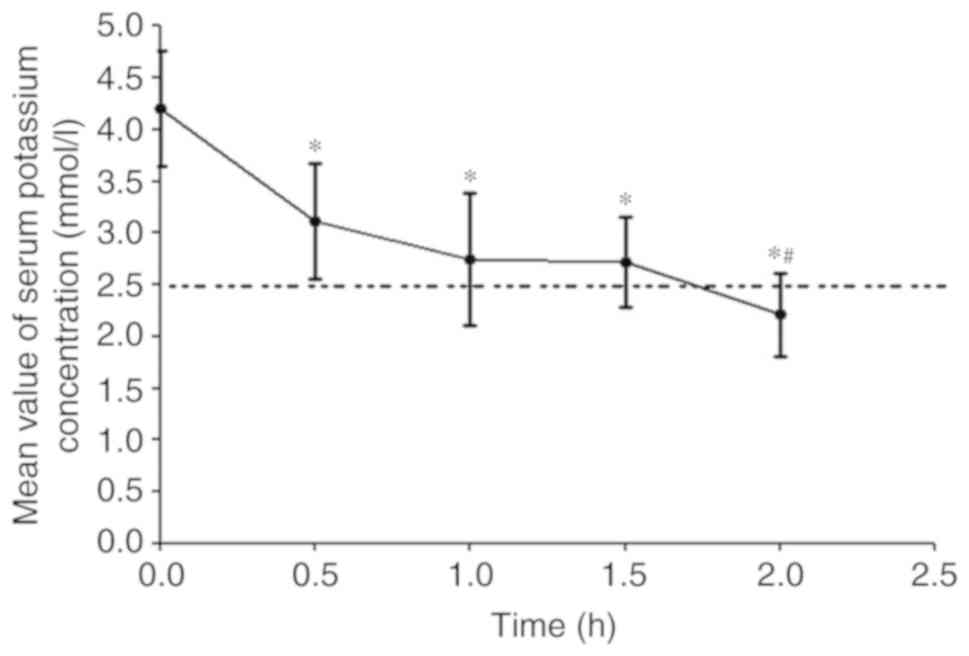
Following the administration of BaCl2,
only 20 in 30 experimental rabbits reached the experimental
requirements. The serum potassium concentration of these 20
experimental rabbits had significantly declined at 0.5 h
(P<0.05), maintained a stable decrease at 1–1.5 h (P<0.05)
and exhibited a further gradual decrease until 2 h, when it reached
<2.5 mmol/l (P<0.05), and arrhythmia occurred (Fig. 2). At this time-point, the
establishment of the fatal severe hypokalemia model in the 20
experimental rabbits was considered to be successful, and the
potassium supplementation experiments were started.
No significant differences between the two groups in
body weight, serum concentration of K+, Na+
and Cl−, blood pH, arterial partial pressure of oxygen
and PCO2, MAP, heart rate and RR were observed
(P>0.05; Table I).
 | Table I.Comparison of the baseline state of
the two groups prior to creating the model. |
Table I.
Comparison of the baseline state of
the two groups prior to creating the model.
| Variable | Conventional group
(n=10) | Tailored rapid
group (n=10) | P-value |
|---|
| Weight (kg) |
2.4±0.2 |
2.4±0.1 | 0.990 |
| Sex
(female/male) | 5/5 | 5/5 | 1.000 |
| K+
(mmol/l) |
4.0±0.4 |
4.0±0.7 | 0.908 |
| Na+
(mmol/l) | 137.2±4.5 | 140.2±6.1 | 0.226 |
| Cl−
(mmol/l) | 108.7±3.6 | 106.5±5.7 | 0.314 |
| Blood pH |
7.4±0.1 |
7.4±0.1 | 0.977 |
| PO2 (mm
Hg) |
91.1±7.9 |
89.1±16.4 | 0.724 |
| PCO2 (mm
Hg) |
27.2±4.2 |
28.9±0.1 | 0.338 |
| MAP (mm Hg) |
84.2±6.5 |
81.8±7.1 | 0.376 |
| Hr (beats/min) |
332.1±24.2 |
334.7±17.9 | 0.185 |
| RR
(breaths/min) |
59.4±9.9 |
61.1±7.4 | 0.399 |
Comparison of serum potassium
concentration at pre- and post-potassium supplementation, as well
as duration and dose of potassium supplementation between the two
groups
The serum potassium concentration prior to the
experiment, the actual amount of potassium supplement and the net
amount of potassium supplement were not significantly different
between the two groups (P>0.05). However, the serum potassium
concentration after potassium supplementation and the potassium
increment post- vs. pre-potassium supplementation in the tailored
rapid treatment group were significantly higher than those in the
conventional treatment group (P<0.05); Furthermore, the duration
of potassium supplementation in the tailored rapid group was
significantly shorter than that in the conventional group
(P<0.05), and the actual amount and the net amount of the
potassium supplement per hour in the tailored rapid treatment group
were significantly higher than those in the conventional treatment
group (P<0.05; Table II).
 | Table II.Comparison of observation parameters
pre- and post-potassium supplementation between the two groups. |
Table II.
Comparison of observation parameters
pre- and post-potassium supplementation between the two groups.
| Variable | Conventional group
(n=10) | Tailored rapid
group (n=10) | P-value |
|---|
| Serum potassium
concentration prior to potassium supplementation (mmol/l) | 2.1±0.3 | 2.2±0.2 | 0.601 |
| Serum potassium
concentration after potassium supplementation (mmol/l) | 3.6±0.6 | 4.6±0.4 | 0.001 |
| Potassium increase
post- vs. pre-potassium supplementation (mmol/l) | 1.5±0.8 | 2.4±0.3 | 0.003 |
| Duration of
potassium infusion (h) | 4.7±1.4 | 2.1±0.7 | <0.001 |
| Actual total amount
of potassium supplement (mmol) | 4.0±0.9 | 3.6±0.4 | 0.174 |
| Actual infusion
rate of potassium supplement (mmol/h) | 0.9±0.1 | 1.9±0.5 | <0.001 |
| Net amount of
potassium supplement (mmol) | 2.6±0.4 | 2.6±0.2 | 0.927 |
| Net infusion rate
of potassium supplement (mmol/h) | 0.6±0.1 | 1.4±0.4 | <0.001 |
| Total urine volume
(ml)a | 13.6±4.7 | 6.4±1.8 | <0.001 |
| Rate of urine
output (ml/h) | 2.9±0.6 | 3.2±0.3 | 0.177 |
| Urine potassium
concentration (mmol/l) | 108.4±19.7 | 164.9±18.1 | <0.001 |
| Amount of renal
potassium excretion (mmol) | 1.5±0.5 | 1.0±0.3 | 0.037 |
| Rate of renal
potassium excretion (mmol/h) | 0.3±0.1 | 0.5±0.1 | <0.001 |
| Amount of ECF
potassium before potassium supplementation (mmol) | 1.0±0.2 | 1.1±0.1 | 0.899 |
| Amount of ECF
potassium after potassium supplementation (mmol) | 1.8±0.3 | 2.2±0.2 | 0.001 |
| ECF potassium post-
vs. pre-potassium supplementation (mmol) | 0.7±0.4 | 1.2±0.2 | 0.002 |
| Amount of
transcellular potassium shift (mmol) | 1.8±0.6 | 1.4±0.2 | 0.043 |
| Rate of the
transcellular potassium shift (mmol/h) | 0.4±0.2 | 0.8±0.3 | 0.003 |
| Serum potassium
concentration at the occurrence of arrhythmia (mmol/l) | 2.1±0.3 | 2.1±0.4 | 0.769 |
| Duration of cardiac
arrhythmia (min) | 71.8±9.8 | 19.6±8.9 | <0.001 |
| Animals with
occurrence of hyperkalemia (n) | 0 | 0 | 1.000 |
| Surviving animals
(n) | 6 | 10 | 0.043 |
| Total number of
bleeds in 14-day period (n) | 18.4±7.2 | 25.7±3.7 | 0.010 |
| Total blood volume
withdrawn from the animals in 14-day period (ml) | 9.3±3.6 | 13.1±1.9 | 0.010 |
Comparison of renal potassium
excretion during the potassium supplement treatment between the two
groups
When the potassium supplementation was completed,
because of the short time of potassium supplementation in the
tailored rapid treatment group, the urine volume in this group was
less than that in the conventional treatment group (P<0.05), but
the average urine volume per hour in the two groups exhibited no
significant difference (P>0.05). Although the urine potassium
concentration and renal potassium excretion velocity in the
tailored rapid treatment group were higher than those in the
conventional treatment group (P<0.05), the total renal potassium
excretion was lower than that in the conventional treatment group
(P<0.05; Table II).
Comparison of transcellular potassium
shift during the potassium supplement treatment
Prior to supplementation, no significant difference
in the ECF potassium concentration was present between the two
groups (P>0.05). After potassium supplementation, the ECF
potassium concentration in the tailored rapid treatment group was
significantly higher than that in the conventional group
(P<0.05). Although the rate of transcellular potassium shift in
the tailored rapid treatment group was higher than that in the
conventional treatment group, the total amount of the transcellular
potassium shift in the tailored rapid treatment group was lower
than that in the conventional treatment group (P<0.05; Table II).
Comparison of the occurrence of
arrhythmia after potassium supplementation between the two
groups
When cardiac arrhythmia occurred, the serum
potassium concentration was not significantly different between the
two groups (P>0.05). However, the duration of cardiac arrhythmia
in the conventional treatment group was longer than that in the
tailored rapid treatment group (P<0.05; Table II).
Comparison of the changes in
circulatory and respiratory parameters pre- and post-potassium
supplementation
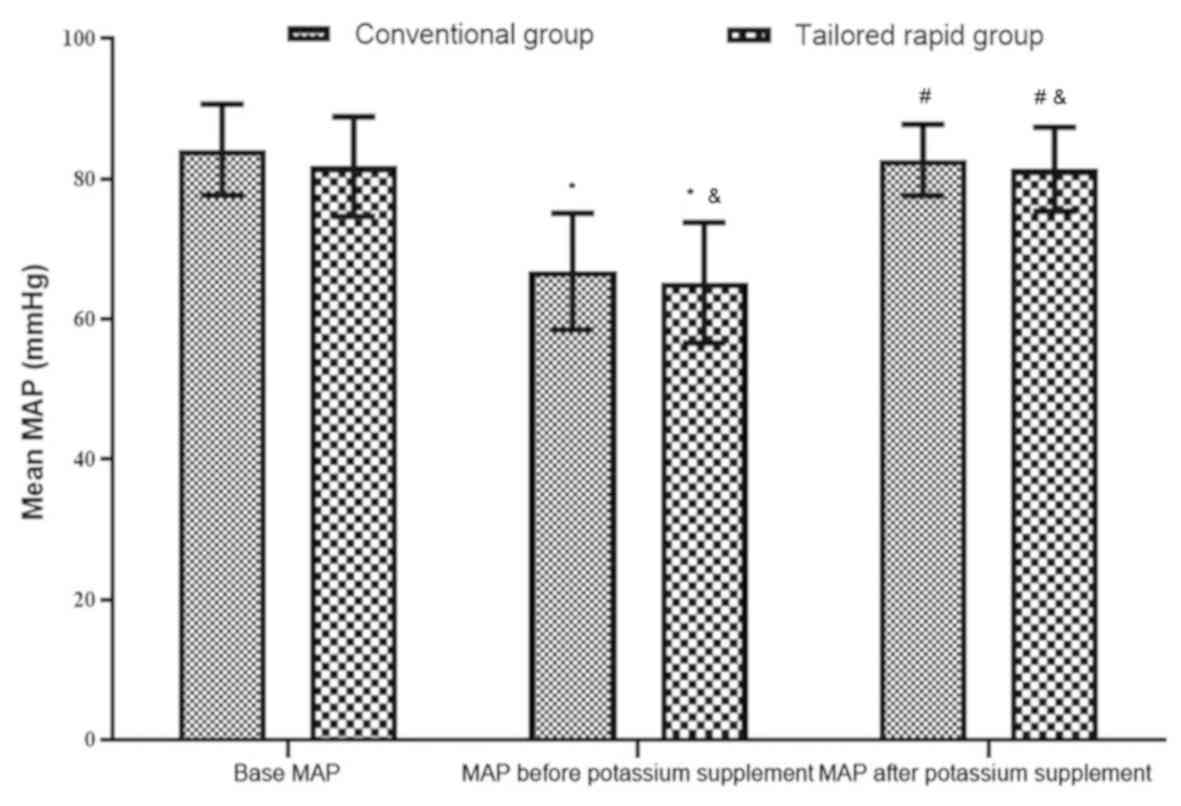
At the three time-points, namely at baseline, and
pre- and post-potassium supplementation, the MAP and RR exhibited
no significant difference between the two groups (P>0.05).
Furthermore, the MAP prior to the potassium supplementation for
each of the two groups was lower than the basal MAP (P>0.05),
and the MAP was elevated after the potassium supplementation
(P<0.05) and became almost equal to the basal MAP (P>0.05;
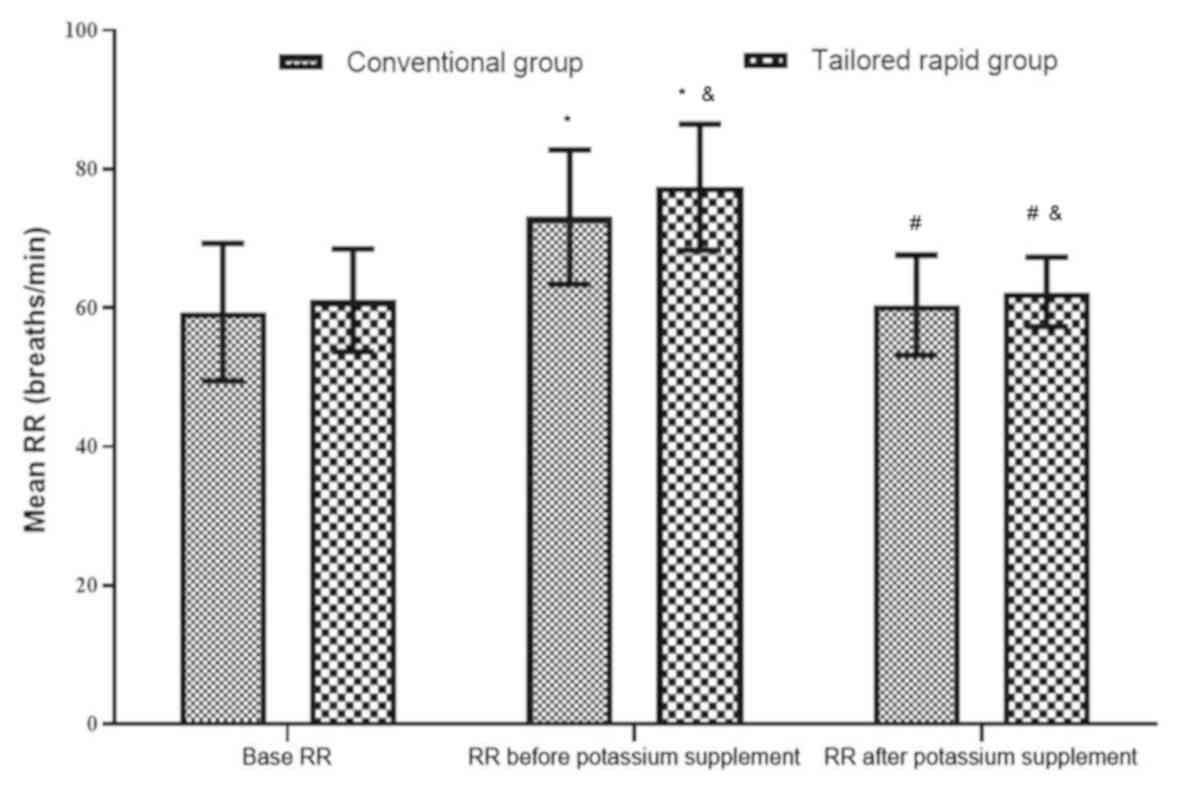
Fig. 3). The RR prior to the
potassium supplementation was higher than the basal RR (P<0.05),
while the RR decreased after potassium supplementation (P<0.05)
and was almost equal to the basal RR (P>0.05; Fig. 4).
Comparison of adverse events and death
during the experiment
In each of the two groups, no hyperkalemia occurred
during the potassium supplementation process. At the end of the
experiment, all of the rabbits (100%) in the tailored rapid
treatment group were alive, while only six rabbits (60%) were alive
in the conventional treatment group; the cause of death was
associated with hypokalemia. Two rabbits died during the course of
potassium supplementation, as the increase in the serum potassium
concentration was too slow, leading to the occurrence of
arrhythmia, respiratory failure and death. Another two rabbits died
at 1 day after the end of the potassium supplementation, as the
serum potassium concentration decreased, leading to death. In
addition, in the 14-day observation period, the total number of
bleeds for the rabbits in the conventional treatment group was
18.4±7.2 times and the total blood volume withdrawn during multiple
blood draws was 0.004±0.001 l/kg, but those in the tailored rapid
treatment group were 25.7±3.7 times and 0.005±0.001 l/kg,
respectively (Tables II and
III).
 | Table III.Mortality of rabbits in the
conventional treatment group. |
Table III.
Mortality of rabbits in the
conventional treatment group.
| Subject no. | Duration of
potassium infusion (h) | Time of death
(h) | Serum potassium
concentration at death (mmol/l) | Cause of death |
|---|
| 1 | 3.6 | 3.6 | 2.5 | VT, VF, RF |
| 4 | 4 | 24.3 | 2.4 | VF, RF |
| 8 | 3.6 | 3.6 | 2.9 | VT, VF |
| 10 | 4.3 | 28.5 | 2.2 | VT, VF, RF |
Discussion
In clinical practice, it is difficult to correct
fatal severe hypokalemia via conventional potassium
supplementation. In the potassium supplement experiments of the
current study, the dose/speed of potassium administered in the
conventional treatment group may have been too low, leading to the
mortality of 4/10 animals. This serves as a limitation of
conventional therapy and should be considered when designing a
novel strategy in future experimentation. The feasibility to pursue
the strategy of ‘tailored rapid potassium supplementation’ to
achieve rapid reversal of the fatal condition of severe hypokalemia
was explored in the present study. For this, a novel rabbit model
of BaCl2-induced severe hypokalemia was established,
which was subjected to conventional treatment or a novel and more
rapid treatment strategy comprised of initial bolus injections,
followed by maintenance infusion.
First, the required dose of potassium supplement
that rapidly increases the serum potassium concentration to the
required level through the circulation was calculated. The
occurrence of cardiac arrest is determined by the extracellular
potassium concentration of cardiomyocytes, which is more likely to
be influenced by the speed and location of potassium infusion
(2,8,10,11), and
it also determines the tolerance of the heart to rapid potassium
supplementation (11). The body's
circulation volume is ~7% of the body weight (20). For an adult with a body weight of
60–100 kg, the circulation volume would be 4–7 l. The stroke volume
of a normal adult in the resting state is 65–70 ml; thus, for a
heart rate of 60–100 beats/min, the cardiac minute volume is 4–7 l.
However, the circulatory blood volume of rabbits is ~7-10% of body
weight (21) and the cardiac output
of rabbits weighing 2.6 kg is ~0.28 l/min (22), which is close to the proportions of
humans. In general, the cardiac output is approximately equal to
the circulation volume; thus, theoretically, injection of potassium
supplement at a dose of [(target serum potassium concentration -
actual serum potassium concentration) × circulation volume] within
1 min does not cause hyperkalemia. Therefore, to ensure uniform
distribution of potassium in the blood, the experimental design of
the present study comprised an intravenous bolus of potassium
supplement over 5 min to ensure safety to the heart.
According to the principles of hemodynamics,
circulating blood has an obvious dilution effect on a high
potassium concentration. Before the potassium chloride solution
infused from the peripheral vein or the central vein reaches the
left heart, it follows the blood circulation path involving the
vena cava, right atrium and right ventricle, pulmonary artery,
pulmonary vein and left heart, as well as the aorta and its
branches. Due to the presence of a capillary network in the entire
body, together with the elasticity of blood vessels, muscle
compression, pump suction and pressure changes due to the
respiratory movement of the thorax, the potassium chloride solution
in the blood flows via blood vessels, and it is repeatedly diluted
and mixed with the blood. Therefore, even if the potassium chloride
solution is directly injected, following which it flows from small
blood vessels, a time lag occurs before it reaches the heart; by
the time the solution reaches the heart, the potassium chloride
concentration of the initial injection is reduced. In addition, in
the circulating blood, a certain amount of the potassium ions is
taken up by red blood cells and alveolar cells to compensate for
potassium deficits; furthermore, certain potassium ions are taken
up by other tissue cells for energy metabolism, and a part of the
potassium ions is discharged in situ. This continuous
process and the process of dynamic reduction further gradually
reduces the concentration of potassium from the initial injection.
Thus, while the concentration of potassium at the time of injection
may be relatively high, it becomes diluted by the time it reaches
the heart and does not cause any hyperkalemia. For instance, in the
present study, the lowest cardiac output per minute of a normal
adult in the resting state (4 l) was assumed to make a calculation
for continuous intravenous infusion of potassium solution at the
infusion rate of 20 mmol/h, in theory, the amount of potassium that
reaches the heart is increased by only 0.083 mmol/l. The same
cardiac output, which was injected with 20 mmol potassium for 5
min, reached the heart at only 0.42 mmol/l. The dilution effect of
circulating blood to reduce the high potassium concentration is
thereby sufficiently explained. With this regard, for calculating
the appropriate load to treat severe hypokalemia, the following
formula was used: (Target potassium concentration - measured
potassium concentration) ×0.07. An intravenous injection of this
dose was given over 5 min, which is a feasible approach. The lowest
limit of the normal serum potassium concentration is 3.5 mmol/l,
which is a relatively safe concentration (1,23).
Therefore, it may be reasonable to increase the serum potassium
concentration to 3.5 mmol/l as soon as possible, in order to
abrogate all types of fatal complications, including arrhythmia,
and reduce the risk of mortality for the patient in a timely
manner. Although the tailored rapid potassium supplementation
strategy of the current study violates the traditional speed and
dosage of potassium administration, it is feasible for use in fatal
severe hypokalemia. Furthermore, as the circulatory blood volume
and cardiac output of rabbits are similar to that of humans the
current study may be converted for human use.
After the serum potassium concentration of 3.5
mmol/l was reached, a maintenance dose of potassium supplement was
given for continued correction of the extracellular potassium
deficiency and to achieve a balance of potassium in the body
fluids. The maintenance dose was calculated using the following
formula: (Target serum potassium concentration - actual serum
potassium concentration) × weight (kg) ×0.2. The normal amount of
fluid in the human body accounts for ~60% of the body weight, and
the body fluid is distributed as intracellular and extracellular
fluids, which account for 40 and 20%, respectively. Most of the
intracellular fluids are present in the skeletal muscle, while the
extracellular fluids comprise plasma and interstitial fluid (5 and
15% of the body weight, respectively). Potassium ions are mainly
distributed in three relatively independent regions: Intracellular
fluid pool, blood circulation pool and tissue space pool. As the
osmotic equilibrium of potassium between the blood circulation pool
and the tissue space is adjusted rapidly, occurring within a few
minutes, these areas are considered as one. In clinical practice,
the ratio of the potassium concentration pre-potassium injection
vs. baseline was calculated from the serum potassium concentration
and the body weight, assuming that the ECF accounts for 20% of the
body weight. The amount of potassium deficiency in the ECF was
thereby determined and subsequently corrected.
Real-time monitoring and real-time decision making
were performed during the present study. With the micro-injection
pump and ECG monitor, and the extensive use of point-of-case
testing, the infusion rate and concentration of the infused
potassium solution was adjusted according to real-time serum
potassium concentration to pursue the ‘tailored rapid potassium
supplementation’ strategy. In clinical practice, the amount of
potassium supplement is calculated by the potassium deficiency in
the ECF, and due to the urinary excretion of a proportion of the
potassium dose, it is actually still difficult to reach the set
target (1,24,25). In
the present study, it was not possible to measure or estimate the
amount of intracellular and transcellular potassium shift directly,
and in previous clinical studies, the effect on potassium excretion
from the kidneys and the clinical manifestations and severity of
hypokalemia were different, and the excretion and distribution of
potassium may have been influenced by drugs. Therefore, after the
calculated supplementary dose is given, the serum potassium
concentration should be detected in the peripheral blood vessels in
a different location from that of the potassium injection, and the
dose should be accordingly adjusted until it reaches the
target.
The three major factors that affect potassium
supplementation are potassium intake, potassium excretion and
transcellular potassium shift. Intravenous potassium
supplementation is still the first choice to rescue patients with
fatal hypokalemia. Usually, critical patients who cannot tolerate
oral potassium and have poor cardiopulmonary function are
encountered in the clinic, which restricts the quantity and speed
of infusion. Therefore, these conventional methods are not able to
elevate the serum potassium concentration to a safe level in a
short time, but rather increase the heart capacity load. More
importantly, due to the slow infusion according to current
restrictions, it takes a long time to treat patients with fatal
hypokalemia, and the potassium concentration may not be
sufficiently increased within the time-window during which rescue
is possible. In the present experimental animal study, the duration
of potassium supplementation in the tailored rapid treatment group
(2.1±0.7 h) was significantly shorter than that in the conventional
treatment group (4.7±1.4 h). The actual infusion rate (1.9±0.5
mmol/h) and the net infusion rate (1.4±0.4 mmol/h) of the potassium
supplementation in the tailored rapid group were significantly
higher than those in the conventional group (0.9±0.1 and 0.6±0.1
mmol/h, respectively). This implies that the tailored rapid
potassium supplementation is superior to the conventional method in
restoring the serum potassium concentration.
The kidney is the major organ for potassium
excretion and balance. The body's potassium intake and excretion
are always balanced under normal conditions. The amount of
potassium excreted by the kidneys fluctuates daily, with variations
of >10-fold (23); this is mainly
determined by the amount of intake. When the intake is reduced, the
urinary excretion of potassium decreases, and vice versa; however,
even under the condition of severe potassium deficiency, the kidney
still pursues the activity of potassium excretion (26–28). In
the present study, the renal function in the two groups of rabbits
was normal. The urine potassium concentration (164.9±18.1 mmol/l)
and the potassium excretion rate (0.5±0.1 mmol/h) in the tailored
rapid group were higher than those in the conventional group
(108.4±19.7 mmol/l, 0.3±0.1 mmol/h; P<0.05), which was
associated with the higher dose of potassium supplement in the
former group. This result is similar to those of Bundgaard and
Kjeldsen (26) and Lin et al
(27). However, in the present
study, the total urine volume (6.4±1.8 ml) and the total renal
potassium excretion (1.0±0.3 mmol) in the tailored rapid treatment
group were significantly lower than those in the conventional
treatment group (13.6±4.7 ml, 1.5±0.5 mmol; P<0.05). Due to the
shortened time of potassium supplementation in the tailored rapid
group, the urine volume and the total amount of potassium excreted
from the kidney were reduced; thus, the net amount of potassium in
5 min after rapid potassium supplementation was greatly improved,
and therefore, this novel strategy improves the serum potassium
concentration more effectively.
The transcellular potassium shift is another
important factor that affects potassium supplementation. As
numerous factors are involved in the shift, the potassium
concentration in the ECF and the amount of potassium excretion from
the kidney cannot completely reflect the total potassium loss;
therefore, it is scientifically inappropriate to calculate the
required total amount of potassium only on the basis of the lack of
potassium in the ECF. The present study also indicated that even
after potassium supplementation according to the above, the serum
potassium concentration did not reach the pre-set target level,
which is not only associated with the renal excretion of potassium,
but also with the transcellular potassium shift. When the body
lacks potassium, the transcellular potassium shift rate increases,
and the speed and amplitude of serum potassium concentration
increase are reduced; consequently, the potassium injection volume
increases significantly, and the rate of potassium infusion is
probably underestimated (26). In
the present study, the total amount of transcellular potassium
shift (1.4±0.2 mmol) in the tailored rapid treatment group was
significantly lower than that in the conventional treatment group
(1.8±0.6 mmol), which was due to the shortened time of potassium
supplementation. The extracellular potassium concentration and the
efficiency of potassium supplementation at the end of the
experiment were also increased in the tailored rapid treatment
group.
In the present study, complications and the risk of
mortality were compared between rabbits with severe hypokalemia
subjected to the two treatment strategies. It was observed that the
tailored rapid treatment group (19.6±8.9 min) had a significantly
shorter duration of arrhythmia induced by hypokalemia than the
conventional group (71.8±9.8 min) and an improved prognosis. At the
end of the experiment, the survival rate in the tailored rapid
treatment group was 100%, while that in the conventional treatment
group was only 60% (P<0.05). Certain retrospective studies
suggested that intravenous infusion of potassium chloride at high
concentrations (1.5-9%) with high speed (20 mmol/h) to correct mild
to moderate hypokalemia is relatively safe and efficient, and no
cases died of low potassium or hyperkalemia in the process of
potassium supplementation (10,17,28,29).
Hamill et al (10) reported
48 cases of critical adult patients with potassium levels of
<3.5 mmol/l who received potassium chloride infusions (20, 30 or
40 mmol in 100 ml normal saline over 1 h) for patients with serum
potassium levels of <3.5 but >3.2 mmol/l, 3.0–3.2 mmol/l, and
<3.0 mmol/l, respectively. All hypokalemia patients with normal
renal function were effectively treated to increase the serum
potassium levels in a safe and dose-dependent manner. In a study by
He et al (18), 128 critical
patients with hypokalemia (serum potassium <3.5 mmol/l) were
randomly divided into the therapy group and the control group,
which were given 9 and 1.5% of potassium chloride solution,
respectively, intravenously with the aid of a micropump, with an
hourly equal quantity of KCl in the two groups. All patients
tolerated the infusion without evidence of hemodynamic changes,
hyperkalemia or acute heart dysfunction, and hypokalemia was
efficiently treated. Kruse et al (29) observed the effects of potassium
chloride infusion in 495 subjects at a medical intensive care unit
with a mean pre-infusion potassium level of 3.2 mmol/l. The
infusion sets consisted of 1–8 consecutive individual infusions,
each containing 1.5% potassium chloride solution at the infusion
rate of 20 mmol/h. The mean post-infusion potassium level in all
patients was 3.9 mmol/l. No temporally associated life-threatening
arrhythmias were noted; however, there were 10 instances of mild
hyperkalemia. Kruse et al (30) examined the difference between the
infusions through the central and the peripheral vein at the same
speed with an average concentration of potassium chloride of 2.9
mmol/l in 40 patients with hypokalemia. In all subjects, the
potassium concentration significantly increased in comparison with
the baseline. The mean peak potassium concentration was 3.5 mmol/l.
No complications, including arrhythmias or changes in cardiac
conduction intervals, occurred (30). In order to observe whether this
conventional treatment method is equally effective in the treatment
of fatal severe hypokalemia, the present study was performed, and
the dose and speed of injection used in the previous patient study
were converted according to the animal weight. The present study
used the same concentration range (3% KCl) and speed of intravenous
infusion of potassium chloride (0.4 mmol/kg/h) in the conventional
group as that recommended by previous studies (1.5-9% at 20
mmol/h); however, the results are different from those of the
previous clinical study. In the conventional group, the death of
two rabbits occurred in the process of potassium supplementation,
as the increase of serum potassium was too slow. In the present
study, a lower level of serum potassium (<2.5 mmol/l) was used
to define severe hypokalemia; however, the previous conventional
method dealing with fatal severe hypokalemia remains insufficient.
Similar results have been reported in certain case reports. In a
study on 13 children with severe hypokalemia (serum potassium,
0.7–1.5 mmol/l), Welfare et al (2) indicated that hypokalemia was not
adjusted and 7 cases died of low serum potassium levels even though
the dose and speed of potassium supplement were enhanced by 30% of
the conventional recommendation. Furthermore, the previous studies
reported no deaths during potassium supplementation, and did not
observe whether hypokalemia or hyperkalemia occurred after
potassium supplementation (10,18,29,30).
However, the present study observed not only the death of animals
during potassium supplementation in the conventional treatment
group, but also at 1 day after the end of treatment, which was due
to a low serum concentration of potassium. Despite the experimental
design of the present study, animal death in the conventional
treatment group occurred in the 14-day observation period,
indicating that there was still a rebound of hypokalemia after
conventional potassium supplementation. As the potassium balance in
the intracellular and extracellular fluid after intravenous
potassium supplementation requires to be maintained for 15 h or
even longer (1), the kidney
continues to excrete potassium. If potassium intake is
insufficient, hypokalemia symptoms reoccur. The 2000 Guidelines for
Cardiopulmonary Resuscitation and Emergency Cardiovascular Care
recommended that if cardiac arrest from hypokalemia is imminent
(i.e., malignant ventricular arrhythmias), rapid empirical
injection of potassium is required (9), which is supported by several successful
clinical case reports (2,31). Welfare et al (2) reported that one case of an 8 month-old
patient with the severe hypokalemia (0.7 mmol/l) survived after he
was given a 5 times faster than conventional (2 mmol/kg/h)
intravenous potassium supplement and an extremely high dosage of
potassium (14.5 mmol/kg/day), without any sign of hyperkalemia
during potassium supplementation (2). In cases of hypokalemia caused by
aldosterone-producing adenoma, which were treated with potassium
infusion at a speed of 36 mmol/h for 2 h and for 5 consecutive
days, hyperkalemia did not occur (31). The present experimental design is
inspired by the inadequacy of the conventional treatment method and
guidelines to improve the efficiency of rescuing fatal severe
hypokalemia. The potassium supplementation strategy was designed
such that it would increase the concentration of serum potassium
rapidly to a safe level (3.5 mmol/l) by intravenous bolus load of
potassium solution, and reverse the fatal condition rapidly, and
after that, to decrease the speed of continued supplementation to
make up for the loss of extracellular potassium, so that the
balance of potassium in the intracellular fluids and ECF is
restored. The experimental results of the tailored rapid treatment
group of the present study are consistent with these reports
(2,31) and the previous hypothesis. In
addition, in the present study, blood was repeatedly collected from
the central ear artery of rabbits, and the volume of blood taken
each time, the total number of bleeds and the total blood volume
withdrawn during several blood draws over a 14-day period in the
two groups were within the safety margins for rabbits, no shock
occurred and no fluid replacement was required, and the design of
the present study was suitable.
The safety of intravenous rapid infusion of
potassium must be ensured, as it may be linked with a risk of
hyperkalemia and associated fatal arrhythmia. In the present
experimental study, no hyperkalemia occurred in any of the two
groups subjected to different potassium supplementation strategies.
However, the conventional potassium supplementation strategy was
not safe for fatal hypokalemia. The animal experiments of the
current study revealed that the mortality rate of the conventional
group was 40%, the cause of which is associated with low serum
potassium levels and insufficient potassium infusion. Certain
studies suggest that aberrations in the serum potassium
concentration cause fatal ventricular arrhythmia, which is mainly
determined by the potassium levels at the heart, and also depends
on the heart's tolerance to potassium, which is closely associated
with the speed of potassium supplementation and the location of the
infusion, but there is no significant association with the
concentration of the potassium infusion (2,10,11). In
the present study, potassium solution was injected into rabbits
through the ear vein, which is located far from the heart; the
step-wise dilution by the circulating blood reduced the potassium
concentration that reached the heart. When potassium is rapidly
infused, the kidney also rapidly drains it simultaneously; this
phenomenon is termed ‘more intake, more discharge’. The potassium
trans-cellular shift, the non-renal mechanism of the potassium
balance, was also another important factor. To maintain the balance
of potassium in intracellular fluids and ECF, the speed of
transcellular potassium shift may increase rapidly after rapid
potassium supplementation. During the process of the dynamic
reduction of potassium in the circulation, the concentration of the
potassium solution was gradually reduced; thus, no hyperkalemia
occurred. The present results indicated that the rapid injection of
a high concentration of potassium solution may prevent
hypokalemia-associated mortality over a short time, while the
low-dose, slow infusion strategy is currently implemented to avoid
hyperkalemia, as 40% of the animals in the conventional group died
due to the slow speed of potassium supplementation. It may be
indicated that the conventional potassium supplementation strategy
overestimates the risk of injecting potassium rapidly, particularly
for the treatment of fatal severe hypokalemia.
The treatment mode of potassium supplementation
cannot be generalized; specific patients require treatment
decision-making based on specific circumstances. It is required to
determine an appropriate balance between a low and a high potassium
concentration, closely monitor ECG and serum potassium
concentration, calculate the dose of potassium supplement and
reasonably adjust the treatment; this may save affected patients
from life-threatening conditions in a timely manner and improve the
success rate of the treatment. The tailored rapid potassium
supplementation strategy was also confirmed to be effective and
safe in the aforementioned experiment.
In conclusion, the present study demonstrated that
the tailored rapid potassium supplementation strategy shortens the
time to treat hypokalemia and is a safe and better treatment option
to reverse life-threatening arrhythmia caused by severe
hypokalemia, with a high rescue success rate. Thus, the present
study provided a novel concept on intravenous potassium
supplementation, termed ‘the tailored rapid potassium
supplementation strategy’, which provides a more efficient, safer
and more reasonable method for clinicians to treat patients with
fatal severe hypokalemia.
Acknowledgements
Not applicable.
Funding
This study was supported by the fund for applied and
popular scientific research projects of the Health and Family
Planning Commission of Sichuan, China (grant no. 16PJ255).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
YD and JL conceived the current study. YD and YM
performed the experiments, collected the data, performed
statistical analysis and wrote the manuscript. The final version of
the manuscript was read and approved by all authors, and each
author believes that the manuscript represents their honest
work.
Ethical approval and consent to
participate
The Animal Ethics Committee of Sichuan University
(Chengdu, China) approved all of the procedures performed in this
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gennari FJ: Hypokalemia. N Engl J Med.
339:451–458. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Welfare W, Sasi P and English M:
Challenges in managing profound hypokalaemia. BMJ. 324:269–270.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bradberry SM and Vale JA: Disturbances of
potassium homeostasis in poisoning. J Toxicol Clin Toxicol.
33:295–310. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu D, Yi M and Jin L: Incorrigible
hypokalemia caused by barium chloride ingestion. Am J Med Sci.
349:279–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schaber DE, Uden DL, Stone FM, Singh A,
Katkov H and Bessinger FB: Intravenous KCl supplementation in
pediatric cardiac surgical patients. Pediatr Cardiol. 6:25–28.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prasad A, Ghodsizad A, Pae W, Singbartl K,
Boone J, Zeriouh M, Ruhparwar A, Loebe M, Khorrami GS, Koerner MM
and Brehm C: Non-cardiac symptoms of moderate to severe hypokalemia
in a patient with a syncardia™ total artificial heart. Heart Surg
Forum. 19:E12–E13. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stedwell RE, Allen KM and Binder LS:
Hypokalemic paralyses: A review of the etiologies, pathophysiology,
presentation, and therapy. Am J Emerg Med. 10:143–148. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mandal AK: Hypokalemia and hyperkalemia.
Med Clin North Am. 81:611–639. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
No authors listed, . Guidelines 2000 for
cardiopulmonary resuscitation and emergency cardiovascular care.
Part 8: advanced challenges in resuscitation: Section 1:
Life-threatening electrolyte abnormalities. The American heart
association in collaboration with the international liaison
committee on resuscitation. Circulation. 102 (Suppl 8):I217–I222.
2000.PubMed/NCBI
|
|
10
|
Hamill RJ, Robinson LM, Wexler HR and
Moote C: Efficacy and safety of potassium infusion therapy in
hypokalemic critically ill patients. Crit Care Med. 19:694–699.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choy AM, Lang CC, Chomsky DM, Rayos GH,
Wilson JR and Roden DM: Normalization of acquired QT prolongation
in humans by intravenous potassium. Circulation. 96:2149–2154.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choudhury H and Cary R: Barium and barium
compounds. World Health Organisation; Geneva: 2001
|
|
13
|
Flomenbaum NE: The clinical basis of
medical toxicology, section 1, case studies in toxicologic
emergencies, pesticides, rodenticides. In: Goldfrank's Toxicologic
Emergencies. Goldfrank LR, Flomenbaum NE, Lewin NA, et al: McGraw
Hill Medical Publishing Division; pp. 1379–1392. New York, NY:
2002
|
|
14
|
Tsai CY, Tseng CC, Liu SF, Lin MC and Fang
WF: Acute barium intoxication following accidental inhalationof
barium chloride. Intern Med J. 41:293–295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jourdan S, Bertoni M, Sergio P, Michele P
and Rossi M: Suicidal poisoning with barium chloride. Forensic Sci
Int. 119:263–265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bowen LN, Subramony SH, Cheng J, Wu SS and
Okun MS: Elementary, my dear Dr. Allen: The case of barium toxicity
and Pa Ping. Neurology. 74:1546–1549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rispin A, Farrar D, Margosches E, Gupta K,
Stitzel K, Carr G, Greene M, Meyer W and McCall D: Alternative
methods for the median lethal dose (LD(50)) test: The up-and-down
procedure for acute oral toxicity. ILAR J. 43:233–243. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He Q, Wang JH, Liu YL, Tang PX, Chang ZG,
Du LQ and Huang XF: Study on safety and efficacy of concentrated
potassium chloride infusions in critically ill patients with
hypokalemia. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 20:416–418.
2008.PubMed/NCBI
|
|
19
|
Rastergar A and Soleimani M: Hypokalaemia
and hyperkalaemia. Postgrad Med J. 77:759–764. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuming W: Blood circulation. Physiology.
Tinghuai W: 4. 9th. People's Health Press; Beijing, BJ: pp. 85–146.
2018
|
|
21
|
Experimental Animal Anatomy Writing Group
of Nankai University, . Anatomy of Experimental Animals. China
People's Education Press; pp. 120–121. 1979
|
|
22
|
Guyton AC, Jones CE and Coleman TG: Normal
cardiac output and its variations. Chap 1. Circulatory Physiology:
Cardiac Output and its Regulation. WB Sauders; London: pp. 3–20.
1973
|
|
23
|
Halperin ML and Kamel KS: Potassium.
Lancet. 352:135–140. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thompson CB, Choi C, Youn JH and McDonough
AA: Temporal responses of oxidative vs. glycolytic skeletal muscles
to K+ deprivation: Na+ pumps and cell
cations. Am J Physiol. 276:C1411–C1419. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muto S: Potassium transport in the
mammalian collecting duct. Physiol Rev. 81:85–116. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bundgaard H and Kjeldsen K: Potassium
depletion increases potassium clearance capacity in skeletal
muscles in vivo during acute repletion. Am J Physiol Cell Physiol.
283:C1163–C1170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin SH, Cheema-Dhadli S, Gowrishankar M,
Marliss EB, Kamel KS and Halperin ML: Control of excretion of
potassium: Lessons from studies during prolonged total fasting in
human subjects. Am J Physiol. 273:F796–F800. 1997.PubMed/NCBI
|
|
28
|
Hoskote SS, Joshi SR and Ghosh AK:
Disorders of potassium homeostasis: Pathophysiology and management.
J Assoc Physicians India. 56:685–693. 2008.PubMed/NCBI
|
|
29
|
Kruse JA and Carlson RW: Rapid correction
of hypokalemia using concentrated intravenous potassium chloride
infusions. Arch Intern Med. 150:6131990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kruse JA, Clark VL, Carlson RW and Geheb
MA: Concentrated potassium chloride infusion in critically ill
patients with hypokalemia. J ClinPharmacol. 34:1077–1082. 1994.
|
|
31
|
Coruzzi P, Gualerzi M, Parati G, Brambilla
L, Brambilla V, Di Rienzo M and Novarini A: Potassium
supplementation improves the natriuretic response to central volume
expansion in primary aldosteronism. Metabolism. 52:1597–1600. 2003.
View Article : Google Scholar : PubMed/NCBI
|