Introduction
Urothelial bladder cancer is the most common type of
malignancy of the urinary tract, and its incidence and the number
of associated mortalities are increasing. According to the
estimates of the American Cancer Society for 2018, bladder cancer
is the fourth most common cancer type in men, but is less common in
women in the USA (1). Based on the
Annual Report of the Taiwan Cancer Registry for 2015, the incidence
of bladder cancer cases account for 2.07% of all cancer cases and
~50% of all urinary tract cancer cases, with 1.93% bladder
cancer-related mortalities (2).
Stratified by gender, the incidence of bladder cancer amongst all
cancer types ranked 10th and 16th and the mortality rates ranked
12th and 15th in males and females, respectively. It has been
reported that drinking water contamination and agricultural
exposure to arsenic may be associated with the occurrence of
bladder cancer (3–5). The southwest coast of Taiwan is an
endemic area for bladder cancer with an increased incidence rate
due to the contamination of drinking water with arsenic, also
causing the symptoms of black foot disease (6). The pathological type of urothelial
carcinoma (transitional cell carcinoma) accounts for ~94% of
bladder cancers, and 60–80% of these tumors are superficial disease
and confined to the urothelium or the lamina propria at diagnosis.
According to the 2018 National Comprehensive Cancer Network
guidelines, total endoscopic resection of visible superficial tumor
[transurethral resection (TUR)] may be the only treatment required
for low-grade pTaG1 low-grade papillary tumors (7). However, tumor recurrence is a major
obstacle in patients with higher-grade Ta and T1 lesions. At 1 year
post-TUR, ~20% of patients with low-risk and 40% of those with
medium-risk disease have developed tumor recurrence. Patients with
high-risk disease have an even greater recurrence rate of >90%
at 1–2 years following TUR. In order to prevent local recurrence,
the most commonly used therapeutic approach is intravesical
instillation, whereby the agents are instilled into the bladder to
delay or prevent tumor recurrence (8). Chemoradiation-based bladder
preservation therapy is currently an alternative option for
non-metastatic muscle-invasive bladder cancer patients (9). However, the current treatment
strategies have limited efficacy with regards to inhibiting bladder
cancer progression.
Chinese herbal medicines have been widely used as
traditional and functional remedies in oriental medicine for
thousands of years. Magnolia officinalis, also named Houpo
in Chinese or Saiboku-tu in Japanese, is an ancient genus mainly
distributed in Southeast Asia and Eastern North America and is
known to improve allergic and asthmatic reactions (10,11).
Magnolia has been reported to contain several biologically
active components, including magnolol, honokiol (International
Union of Pure and Applied Chemistry name,
5,3′-diallyl-2,4′-dihydroxybiphenyl), 4-O-methylhonokiol,
obovatol and certain other neolignan compounds, which have numerous
and diverse actions (12). Honokiol
is a biphenolic bioactive component of the bark of Magnolia,
and it has been indicated to have multifunctional activities,
including anti-oxidant, anti-inflammatory and chemopreventive
effects (13,14).
It has been reported that honokiol has anti-cancer
effects against glioblastoma multiforme, as well as head and neck,
lung, breast, stomach and colorectal cancers, and exerts
synergistic effects in combination with chemotherapy for cancer
treatment (15–19). However, only a small amount of
evidence is available for bladder cancer. Previous studies
suggested that honokiol is a potential agent against bladder cancer
cell invasion and epithelial-to-mesenchymal transition (20); however, the capacity of honokiol to
induce cell death and the underlying mechanisms remain unknown. In
the present study, it was indicated that honokiol induced obvious
reactive oxygen species (ROS) accumulation and transient
hyperpolarization of the mitochondrial membrane, which resulted in
cell cycle arrest and further led to apoptotic cell death. These
pre-clinical data indicate that honokiol-based novel intravesical
remedies have promising potential to be applied for bladder cancer
treatment in the future.
Materials and methods
Cells and materials
Honokiol, sulforhodamine B (SRB), dimethyl sulfoxide
(DMSO), trichloroacetic acid (TCA) and 3,3′-dihexyloxacarbocyanine
iodide (DiOC6) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). A fluorescein isothiocyanate (FITC) Annexin V
Apoptosis Detection kit (cat. no. 556547) was obtained from BD
Biosciences (Franklin Lakes, NJ, USA).
2′,7′-dichlorodihydrofluorescein diacetate (DCFH2-DA)
was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). The Bioresource Collection and Research Center (BCRC; Food
Industry Research and Development Institute, Hsin Chu, Taiwan)
provided the human bladder cancer cell lines, including T24 (BCRC
no. 60062), BFTC-905 (BCRC no. 60068) and TSGH 8301 (BCRC no.
60145). The other reagents were of high grade and purchased from
commercial companies.
Cell culture
T24 bladder cancer cells were cultured in McCoy's 5a
basal medium supplemented with 1.5 g/l sodium bicarbonate, 100 U/ml
penicillin/streptomycin and 10% fetal bovine serum (FBS; all Gibco;
Thermo Fisher Scientific, Inc.). BFTC-905 and TSGH 8301 bladder
cancer cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 1.5 g/l sodium
bicarbonate, 100 U/ml penicillin/streptomycin and 15% or 10% FBS,
respectively. All cells were maintained at 37°C in a 5%
CO2-humidified incubator (Steri-Cycle CO2
incubator; Thermo Fisher Scientific, Inc.).
SRB cell viability assay
The cytotoxic effects of honokiol were determined
using a modified SRB colorimetric drug-screening assay (21). In brief, cells were seeded into
96-well flat-bottomed plates (1,500 cells/well) in 200 µl medium.
After 24-h incubation, the cells were treated with different
concentrations of honokiol (0, 25, 50 and 100 µM; stock solution in
DMSO, final DMSO concentration was ≤0.05%), followed by further
incubation for 3 days. Subsequently, the medium was aspirated and
the cells were fixed by addition of 100 µl 10% TCA for 2 h at 4°C.
The plates were washed five times with water to remove TCA and
air-dried for at least 1 h. This was followed by staining with 200
µl of 0.065% SRB for 30 min at room temperature, washing for five
times with 1% acetic acid to remove any unbound dye and subsequent
drying in a chemical hood. The bound dye was dissolved in 200 µl 10
mM Tris base (pH 10.5). The absorbance was read using a
spectrophotometer (SpectraMax M2e; Molecular Devices,
Sunnyvale, CA, USA) at 570 nm. Cell morphology was examined under a
light microscope (Olympus CKX41; Olympus, Tokyo, Japan).
Cell cycle analysis
Cells were treated with various concentrations (0,
12.5, 25, 50 and 75 µM) of honokiol for 72 h. The floating and
adherent cells were then harvested prior to centrifugation at 600 ×
g for 10 min at 4°C. Subsequently, cells were washed twice with
cold PBS and fixed with 70% ethanol and stored immediately at 4°C
overnight. Following incubation, ethanol was removed and cells were
incubated with a solution containing 25 µg/ml PI, 0.5% Triton X-100
and 0.2 mg/ml RNase A (Thermo Fisher Scientific, Inc.) for 30 min
after washing with cold PBS. A positive control group was
established by exposing the cells to ultraviolet radiation for 1 h.
Finally, the cells were re-suspended in 500 µl PBS and then
analyzed by flow cytometry (BD Accuri™ C6 Plus; BD Biosciences).
Data were collected from 10,000 cells and evaluated by using BD
Accuri™ C6 software (version 1.0; BD Biosciences).
Measurement of intracellular ROS
The cells were treated with various concentrations
of honokiol for 24 h. At the end of the incubation, the floating
and the adherent cells were harvested and then incubated with
DCFH2-DA (final concentration, 20 µM) for 30 min after
washing with cold PBS. Finally, the cells were re-suspended in 500
µl PBS and analyzed by flow cytometry (BD Biosciences). The data
for 10,000 cells were collected and evaluated with BD Accuri™ C6
software (BD Biosciences).
Annexin V/PI double staining
assay
The cells were treated with various concentrations
of honokiol for 48 h. At the end of the incubation, the floating
and the adherent cells were harvested and then incubated with
Annexin V-FITC and PI for 15 min at 37°C in the dark after washing
with cold PBS. Finally, the cells were re-suspended in 500 µl PBS
and then analyzed by flow cytometry (BD Biosciences). A positive
control group was established by exposing the cells to ultraviolet
radiation for 30 min for adjusting for fluorescence spectral
overlap compensation. The data for 10,000 cells were collected and
evaluated with BD Accuri™ C6 software (BD Biosciences).
Mitochondrial membrane potential (ΔΨm)
evaluation
Cells were treated with various concentrations of
honokiol for 48 h. At the end of the incubation, the floating and
the adherent cells were harvested and then incubated with DiOC6
(final concentration, 40 µM) for 15 min at 37°C in the dark after
washing with cold PBS. Finally, the cells were resuspended in 500
µl PBS and then analyzed in the FL-1 channel by flow cytometry (BD
Biosciences). Data were collected from 10,000 cells and evaluated
with BD Accuri™ C6 software (BD Biosciences).
Caspase 3/7 activity analysis
The activity of caspase-3/7 was assessed using a
fluorometric Caspase 3/7 assay kit (cat. no. 556547; Abcam,
Cambridge, UK), according to the manufacturer's protocol. The
caspase-3/7 assay is based on the detection of cleaved fluorescent
DEVD-7-amino-4-trifluoromethyl coumarin (AFC). In brief, the
treated cells were re-suspended in 50 µl chilled cell lysis buffer
and incubated on ice for 10 min. Following incubation, 1 ml of 2X
reaction buffer (containing 10 µM DTT) was added to each sample and
incubated in the dark for 1–2 h at 37°C in a 96-well plate. The
fluorescence was measured with a fluorescence reader (SpectraMax
M2e; Molecular Devices) using an excitation wavelength
of 400 nm and an emission wavelength of 505 nm. Fold-increases in
caspase-3/7 activity were determined by comparing the results for
the treated groups with those of the untreated control and
normalized to the respective protein concentration. Total protein
was quantified using the Bradford dye-binding method (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All assays were performed as three independent
experiments with n≥3 in each test. Values are expressed as the mean
± standard error of the mean. Statistically significant differences
between treatment group and control group were assessed by one-way
analysis of variance followed by Tukey's multiple-comparisons test
using Prism 6.1d (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Honokiol significantly inhibits
bladder cancer cell growth
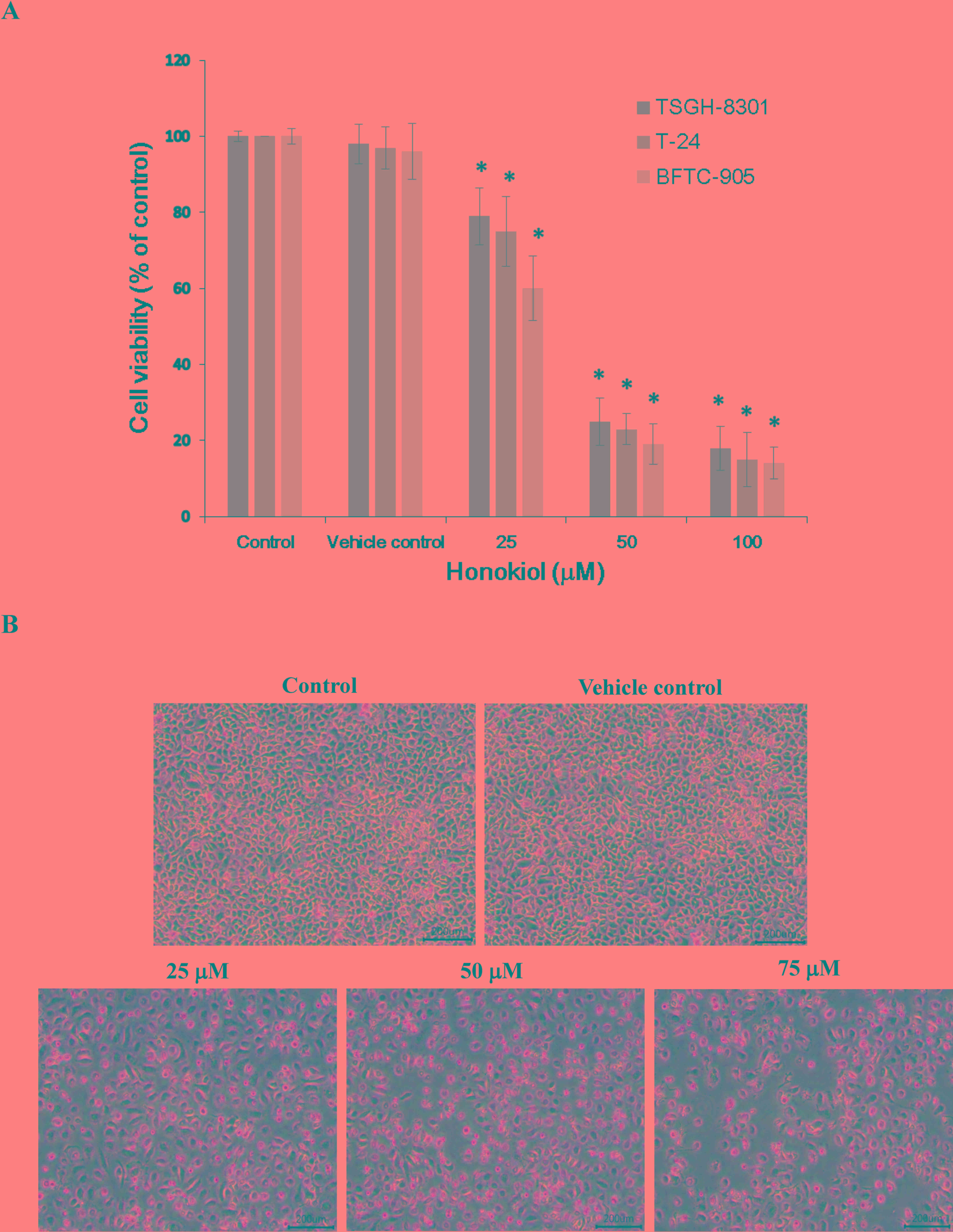
First, the inhibitory effect of honokiol on bladder
cancer cells was investigated. The human bladder cancer cell lines
T-24 (human transitional cell carcinoma), TSGH-8301 (human urinary
bladder carcinoma) and BFTC-905 (human bladder papillary
transitional cell carcinoma grade III) were examined. It was
revealed that honokiol exhibited a marked cytotoxic effect against
all three bladder cancer cell lines, and BFTC-905 cells were more
sensitive to honokiol at the lower concentration (25 µM;
IC50=30±2.8 µM at 72 h of incubation; Fig. 1A), while no obvious difference in
cytotoxicity was observed between the cell lines at high
concentrations of honokiol. Of note, DMSO at 0.05% as the vehicle
control did not exert any inhibitory effect on any of the three
cell lines. Furthermore, cell morphological observation revealed
that honokiol obviously reduced the amount of cells and induced
cell detachment after 48 h of incubation (Fig. 1B). Due to its high sensitivity to
honokiol, the subsequent experiments were performed using BFTC-905
as the model cells.
Honokiol induces cell cycle arrest and
apoptotic cell death of bladder cancer cells
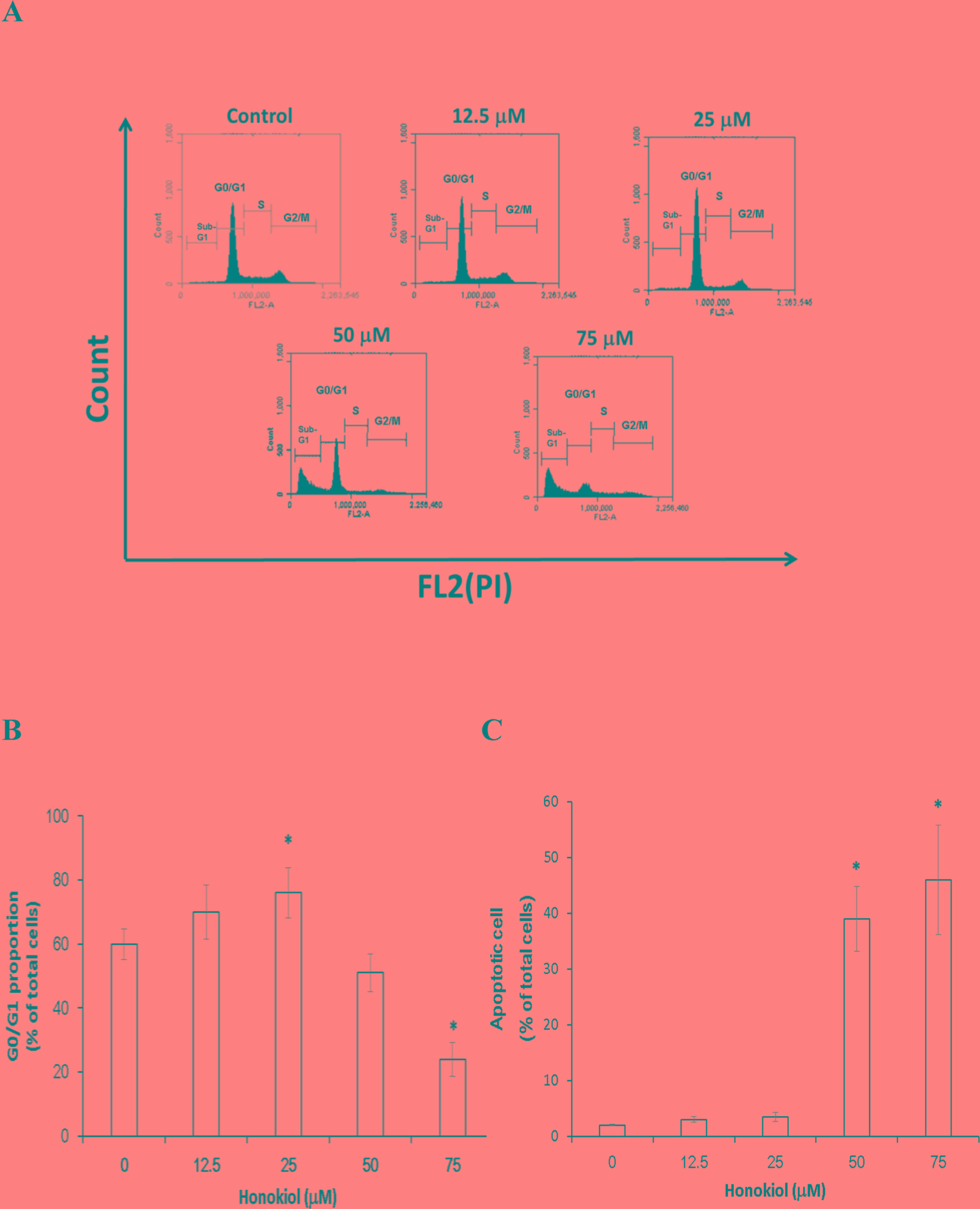
The effect of honokiol on cell cycle progression was
then investigated. It was revealed that low doses of honokiol
induced significant cell cycle arrest in
G0/G1 phase (P<0.05), with the population
in that phase demonstrating an increase from 60±4.8% in the control
group to 70±8.4 and 76±7.9% in the groups treated with 12.5 and 25
mM honokiol, respectively, for 72 h incubation (Fig. 2A and B). Furthermore, the high dose
of honokiol increased the population in sub-G1 phase that resembles
apoptotic cells (P<0.05); the sub-G1 phase population increased
from 3±1.8% in the control group to 38±3.7 and 46±2.8% following
treatment with 50 and 75 µM honokiol, respectively, for 72 h
(Fig. 2C).
Apoptotic effect of honokiol on
bladder cancer cells
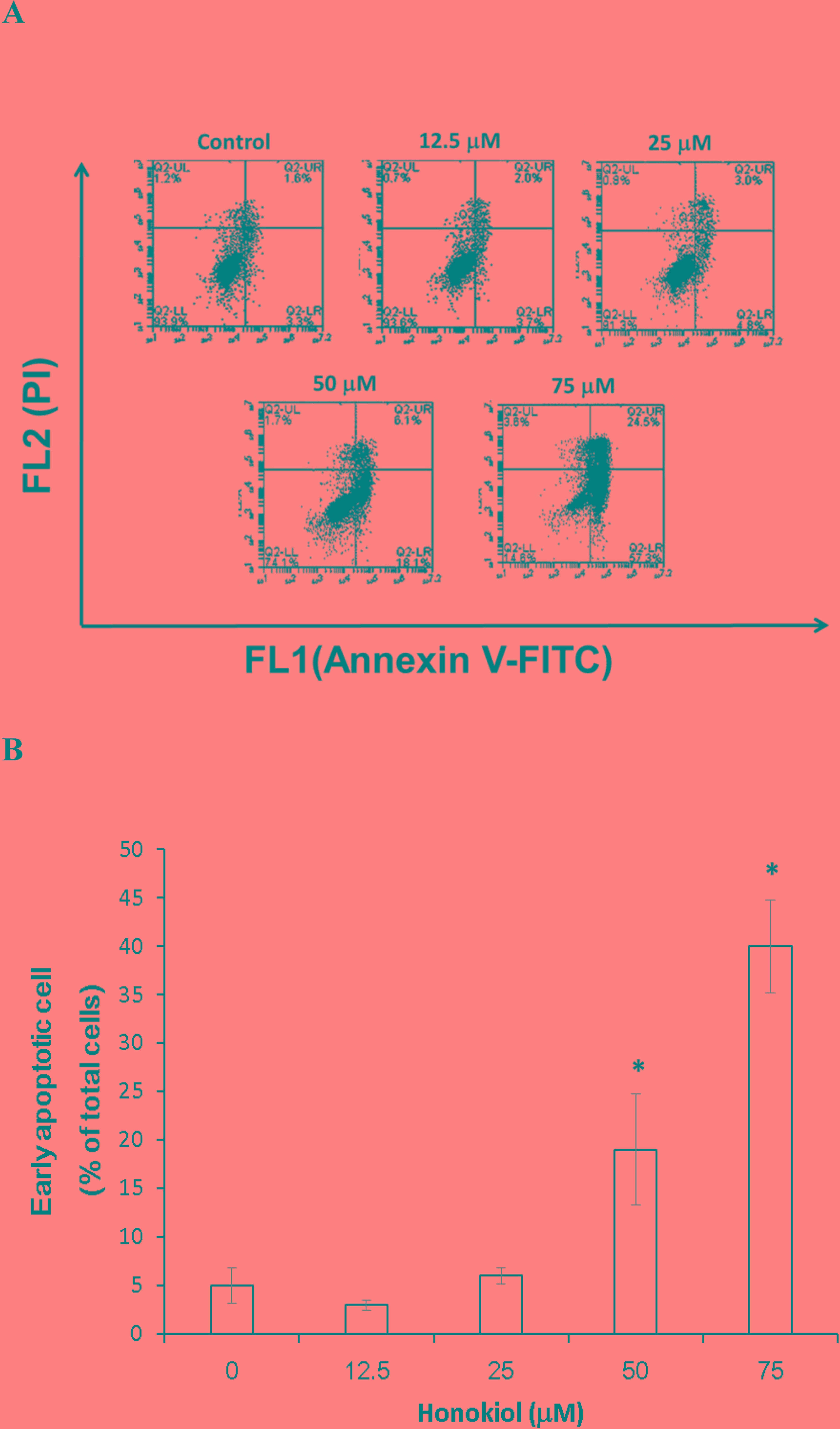
In the present study, the detailed effect of
honokiol on apoptotic cell death was assessed. It was revealed that
50 and 75 µM honokiol significantly increased the proportion of
cells in early apoptosis (Annexin V+/PI−
cells; 19±5.7 and 40±4.8%) compared with the control group
(5±1.8%), following 48 h of incubation (P<0.05; Fig. 3). In addition, high-dose honokiol (75
µM) significantly induced late apoptotic cell death (21±6.7%)
compared with the control group (3±2.8%; P<0.05).
Oxidative burst in honokiol-treated
cells
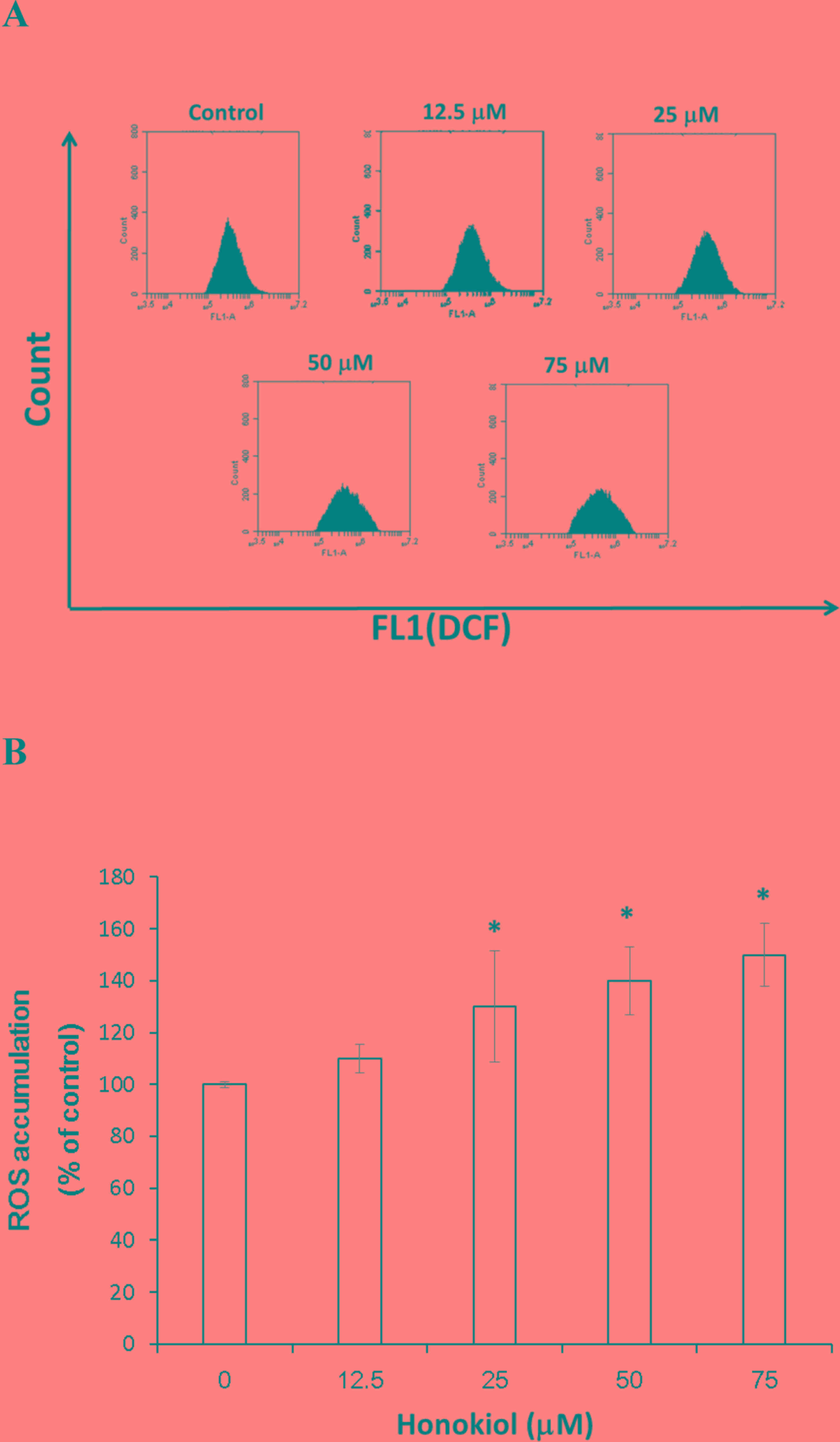
The effect of honokiol on the redox status of
bladder cancer cells was investigated. It was identified that
high-dose honokiol (50 and 75 µM) induced a significant increase in
ROS in bladder cancer cells (140±13.2 and 150±12.3%, respectively)
compared with the control group, following 24 h incubation
(P<0.05; Fig. 4).
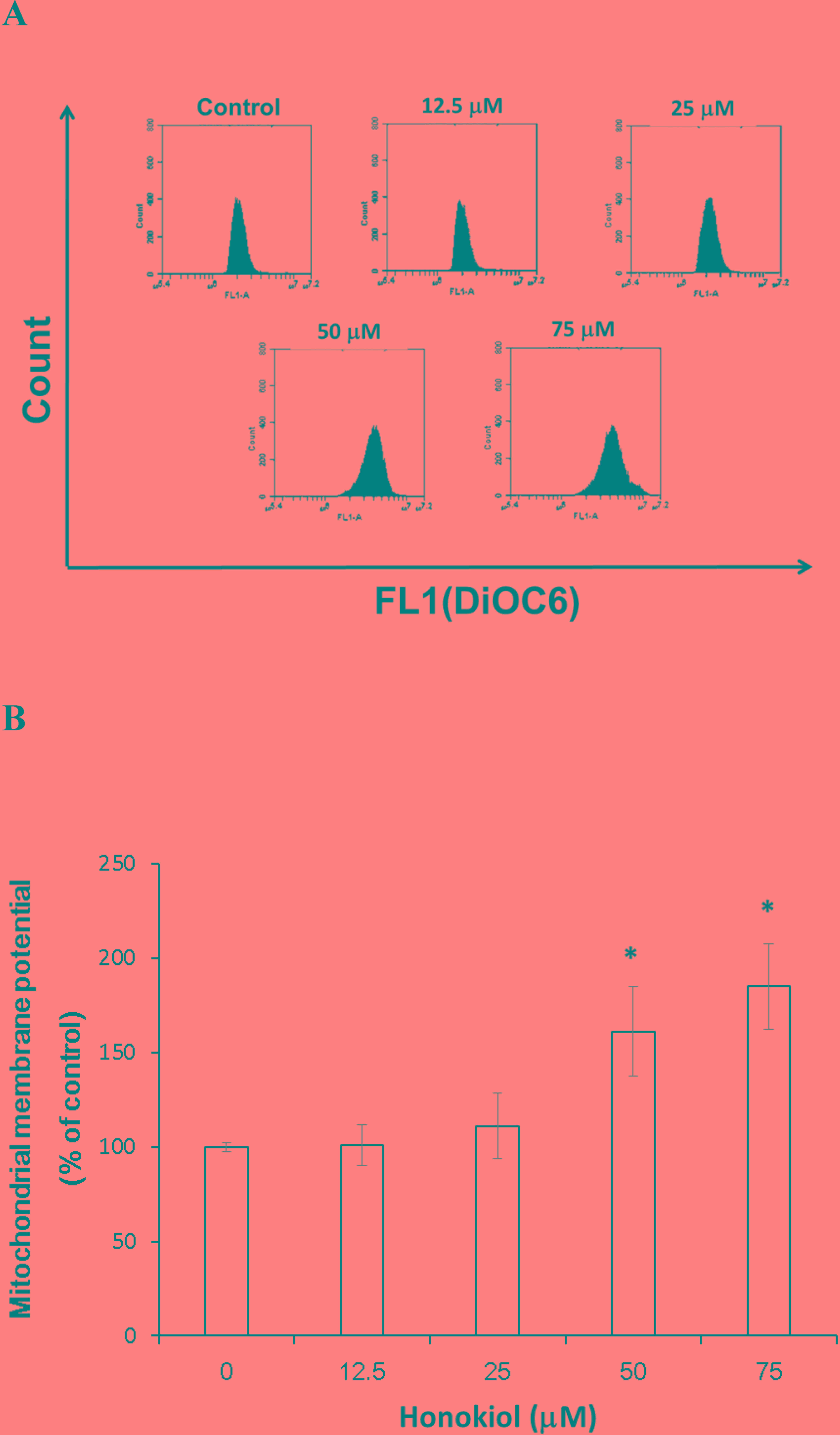
Reduction of ΔΨm in honokiol-induced
cell death
The present study further investigated the ΔΨm in
honokiol-treated bladder cancer cells. By using the DiOC6 staining
assay, it was identified that honokiol induced transient
hyperpolarization of the mitochondrial membrane in bladder cancer
cells (Fig. 5). Higher doses of
honokiol (50 and 75 µM) induced a significant increase in ΔΨm
(161±23.7 and 185±22.8%, respectively) compared with the control
group after 24 h of incubation (P<0.05; Fig. 5).
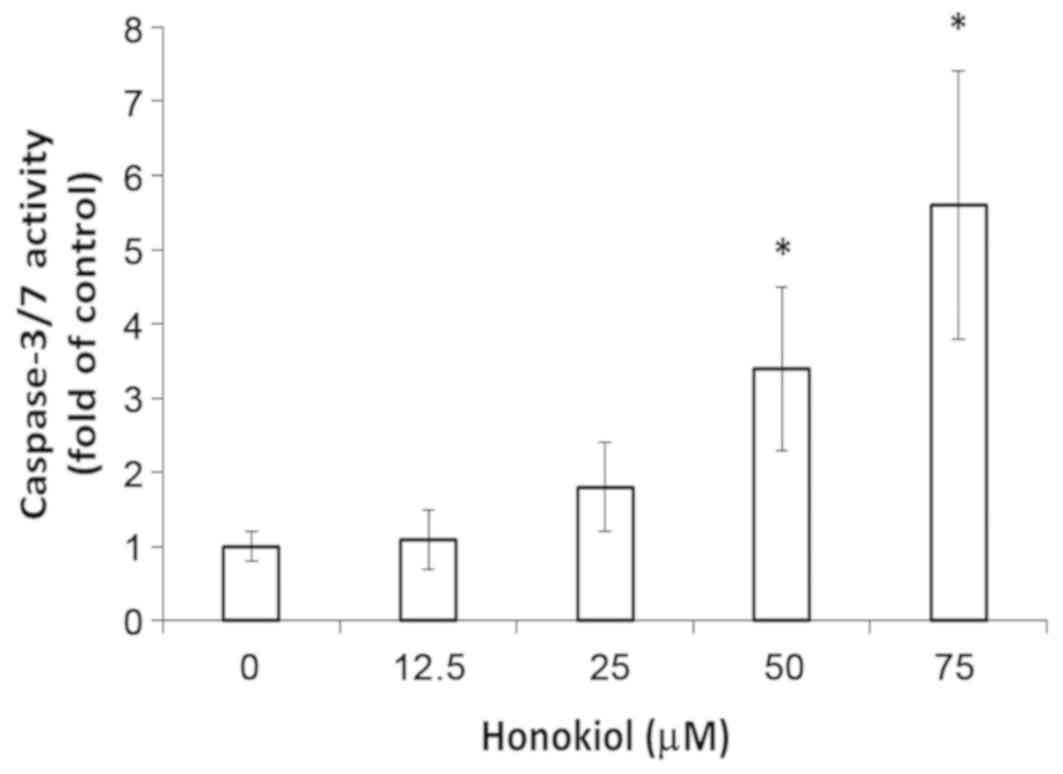
Caspase-3/7 activation in
honokiol-induced apoptotic cell death
Finally, the caspase activities in honokiol-treated
bladder cancer cells were investigated. As presented in Fig. 6, caspase-3/7 was significantly
activated in high-dose honokiol-treated bladder cancer cells in
comparison with untreated control cells after 36 h of incubation
(P<0.05). These results indicated that the intrinsic apoptotic
pathway may have a critical role in honokiol-triggered apoptosis in
bladder cancer cells.
Discussion
Flow cytometry is a well-known laser-based
technology for cell characterization, functional assays and disease
diagnosis utilized in basic research and for clinical examinations.
Thus, it is a convenient and effective method for clinicians to
investigate the molecular mechanisms of specific targets. Flow
cytometric immunophenotyping is another sensitive, reproducible and
low-cost quantitative auxiliary diagnostic technique with a fast
turnaround time, particularly for the detection and classification
of hematolymphoid neoplasms (22).
DNA indexing, also named the Vindelov method, is recommended for
single-parameter DNA studies by flow cytometry on fresh as well as
frozen clinical samples, and various novel multicolour staining
techniques for cell characterization and identification by flow
cytometry have been developed for clinical application (23). Magnolia officinalis is a plant
used in traditional Chinese and Japanese medicine that provides
multiple benefits. In the present study, the anti-cancer properties
of honokiol, a bioactive component derived from Magnolia
officinalis, on bladder cancer cells were investigated by flow
cytometric technology. It has been previously reported that
honokiol reduced bladder tumor growth by blocking the enhancer of
zeste 2 polycomb repressive complex 2 subunit/microRNA-143 axis and
inhibited bladder cancer cell invasion through repressing SRC-3
expression and epithelial-to-mesenchymal transition (19,20). In
the present study, the cytotoxicity of honokiol against various
human bladder cancer cell lines was first assessed. An SRB cell
viability assay was performed to evaluate the cytotoxic effect of
honokiol on three human transitional cell carcinoma cell lines. It
was revealed that the human BFTC-905 cell line with grade-III
malignancy exhibited the highest sensitivity to honokiol.
Therefore, the subsequent experiments were performed with this cell
line to investigate the mechanisms of honokiol-induced
cytotoxicity. It was revealed that honokiol exerted its anti-cancer
activity through induction of growth arrest, apoptosis and
necrosis. Low-dose honokiol (<25 µM) induced cell cycle arrest
in G0/G1 phase, while high-dose honokiol (50
and 75 µM) induced apoptotic cell death, and further induced
necrotic cell death at concentrations of >100 µM (data not
shown). It has been previously revealed that honokiol treatment had
no obvious cytotoxicity on kidney epithelial cells at <15 µM and
honokiol (50 µM) also protected the pancreatic β cells from
intermittent hypoxia and high glucose-induced cytotoxicity
(24). Honokiol also acts as a
potent neuroprotective agent against the cognitive defects after
traumatic brain injury in rats (25). It has also been reported that
honokiol had an obvious cytotoxic effect on murine hepatic AML12
cells at concentrations of up to 40 µM, while at lower
concentrations (<20 µM), honokiol exerted a cytoprotective
effect against tert-butyl hydroperoxide-induced oxidative stress
(26). Recently, it has been
reported that honokiol acts as a potential antagonist against
forkhead box M1, which may partially explain for its anti-cancer
activity in several solid cancer cell lines (27). Based on the above, honokiol may have
cell- and dosage-dependent anti-cancer and cytoprotective effects
by targeting diverse pathways. The dosage-dependent effects of
honokiol revealed in these in vitro studies may serve as a
reference for animal in vivo studies in the future. To the
best of our knowledge, the present study was the first to provide
evidence of honokiol-induced apoptotic death of bladder cancer
cells. Based on the present results, it is likely that honokiol
induces cell cycle arrest and apoptotic cell death by causing
oxidative burst and hyperpolarization of the mitochondrial
membrane. It has been previously revealed that honokiol induced
apoptosis/autophagy of human glioma cells by ROS-mediated signaling
transduction pathways and enhanced caspase activation (15,16). In
line with this, the present study also indicated that honokiol
induced significant ROS accumulation and stimulated caspase-3/7
activation. Honokiol may thus have an impact on several molecular
pathways and have various biological functions. The ΔΨm is a
decisive factor that determines the cell fate between survival and
death (28). Of note, to the best of
our knowledge, the present study was the first to indicate that
honokiol induced hyperpolarization of the mitochondrial membrane in
bladder cancer cells. It has been proposed that under pro-apoptotic
conditions, the closed state of the voltage-dependent anion channel
may bring about a transient mitochondrial membrane
hyperpolarization, followed by osmotic imbalance, outer membrane
rupture, release of the intermembrane space proteins and ultimately
cell death (29). The effects of
honokiol-induced dysfunction of mitochondria may be exerted in a
time-, dosage- and cell type-dependent manner. Although the present
study provided critical insight into the apoptosis-inducing effect
of honokiol on bladder cancer cells via hyperpolarization of
mitochondria and the induction of ROS burst, the synergistic
efficacy of honokiol in combination with chemoradiation-based
therapies used in bladder cancer treatment requires assessment by
further studies. Of note, a limitation of the present study was the
lack of cell viability assessment of honokiol-treated normal
bladder cells as a safety evaluation. However, a study by Hua et
al (30) revealed that only a
high concentration (100 µM) of honokiol suppressed the
proliferation of normal colon epithelial cells.
In conclusion, the present study suggested that
honokiol has potential use as an adjuvant for urothelial bladder
cancer treatment. In the future, the detailed underlying mechanisms
require to be elucidated and the efficacy requires evaluation in
pre-clinical in vivo studies.
Acknowledgements
The authors would like to thank Miss Tsu-Yi Yi
(Cancer Center, Wan Fang Hospital, Taipei, Taiwan) for her
technical support.
Funding
The present study was supported by grants from the
Research Fund of the Department of Medical Research, China Medical
University Hospital (grant nos. DMR-107-153 and DMR-CELL-1809) and
the National Science Council of Taiwan (grant no. NSC
105-2320-B-039-068).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CHH, CJY, GML and PHS made contributions to the
conception and design of the study and prepared the manuscript.
CHH, CJY and PHS performed the experiments and data analysis. GML,
LML and JWP reviewed the literature and interpreted the results.
CHH and PHS revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SRB
|
sulforhodamine B
|
|
PI
|
propidium iodide
|
|
DCFH2-DA
|
2′,7′-dichlorodihydrofluorescein
diacetate
|
|
DiOC6
|
3,3′-dihexyloxacarbocyanine iodide
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
American Cancer Society. Cancer Facts
& Figures. 2018.
|
|
2
|
Cancer Prevention Group: Annual Report of
Taiwan Cancer Registry for 2016. December 27–2018, https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=10227
|
|
3
|
Huang CY, Lin YC, Shiue HS, Chen WJ, Su
CT, Pu YS, Ao PL and Hsueh YM: Comparison of arsenic methylation
capacity and polymorphisms of arsenic methylation genes between
bladder cancer and upper tract urothelial carcinoma. Toxicol Lett.
295:64–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boulanger M, Tual S, Lemarchand C, Guizard
AV, Velten M, Marcotullio E, Baldi I, Clin B and Lebailly PP:
Agricultural exposure and risk of bladder cancer in the AGRIculture
and CANcer cohort. Int Arch Occup Environ Health. 90:169–178. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mendez WM Jr, Eftim S, Cohen J, Warren I,
Cowden J, Lee JS and Sams R: Relationships between arsenic
concentrations in drinking water and lung and bladder cancer
incidence in U.S. counties. J Expo Sci Environ Epidemiol.
27:235–243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shibata A, Ohneseit PF, Tsai YC, Spruck CH
III, Nichols PW, Chiang HS, Lai MK and Jones PA: Mutational
spectrum in the p53 gene in bladder tumors from the endemic area of
black foot disease in Taiwan. Carcinogenesis. 15:1085–1087. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
NNational Comprehensive Cancer Network
(NCCN) Clinical Practice Guidelines in Oncology (NCCN GuidelinesÒ).
Bladder cancer Version 2.2018. February 7–2018.https://oncolife.com.ua/doc/nccn/Bladder_Cancer.pdf
|
|
8
|
Shelley MD, Mason MD and Kynaston H:
Intravesical therapy for superficial bladder cancer: A systematic
review of randomised trials and meta-analyses. Cancer Treat Rev.
36:195–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koga F, Takemura K and Fukushima H:
Biomarkers for predicting clinical outcomes of chemoradiation-based
bladder preservation therapy for muscle-invasive bladder cancer.
Int J Mol Sci. 19:E27772018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rauf A, Patel S, Imran M, Maalik A, Arshad
MU, Saeed F, Mabkhot YN, Al-Showiman SS, Ahmad N and Elsharkawy E:
Honokiol: An anticancer lignan. Biomed Pharmacother. 107:555–562.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zubair H, Azim S, Ahmad A, Khan MA, Patel
GK, Singh S and Singh AP: Cancer chemoprevention by phytochemicals:
Nature's healing touch. Molecules. 22:E3952017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee YJ, Lee YM, Lee CK, Jung JK, Han SB
and Hong JT: Therapeutic applications of compounds in the magnolia
family. Pharmacol Ther. 130:157–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar A, Kumar Singh U and Chaudhary A:
Honokiol analogs: A novel class of anticancer agents targeting cell
signaling pathways and other bioactivities. Future Med Chem.
5:809–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasad R and Katiyar SK: Honokiol, an
active compound of Magnolia plant, inhibits growth, and progression
of cancers of different organs. Adv Exp Med Biol. 928:245–265.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chio CC, Chen KY, Chang CK, Chuang JY, Liu
CC, Liu SH and Chen RM: Improved effects of honokiol on
temozolomide-induced autophagy and apoptosis of drug-sensitive and
-tolerant glioma cells. BMC Cancer. 18:3792018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chio CC, Tai YT, Mohanraj M, Liu SH, Yang
ST and Chen RM: Honokiol enhances temozolomide-induced apoptotic
insults to malignant glioma cells via an intrinsic
mitochondrion-dependent pathway. Phytomedicine. 49:41–51. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai IC, Shih PH, Yao CJ, Yeh CT,
Whang-Peng J, Lui TN, Chuang SE, Hu TS, Lai TY and Lai GM:
Elimination of cancer stem-like cells and potentiation of
temozolomide sensitivity by Honokiol in glioblastoma multiforme
cells. PLoS One. 10:e01148302015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang MT, Lee SP, Fang CY, Hsieh PL, Liao
YW, Lu MY, Tsai LL, Yu CC and Liu CM: Chemosensitizing effect of
honokiol in oral carcinoma stem cells via regulation of IL-6/Stat3
signaling. Environ Toxicol. 33:1105–1112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, Zhao W, Ye C, Zhuang J, Chang C,
Li Y, Huang X, Shen L, Li Y, Cui Y, et al: Honokiol inhibits
bladder tumor growth by suppressing EZH2/miR-143 axis. Oncotarget.
6:37335–37348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen L, Zhang F, Huang R, Yan J and Shen
B: Honokiol inhibits bladder cancer cell invasion through
repressing SRC-3 expression and epithelial-mesenchymal transition.
Oncol Lett. 14:4294–4300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Monappa V, Reddy SM and Kudva R:
Hematolymphoid neoplasms in effusion cytology. Cytojournal.
15:152018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danova M, Torchio M, Comolli G, Sbrana A,
Antonuzzo A and Mazzini G: The role of automated cytometry in the
new era of cancer immunotherapy. Mol Clin Oncol. 9:355–361.
2018.PubMed/NCBI
|
|
24
|
Li CG, Ni CL, Yang M, Tang YZ, Li Z, Zhu
YJ, Jiang ZH, Sun B and Li CJ: Honokiol protects pancreatic beta
cell against high glucose and intermittent hypoxia-induced injury
by activating Nrf2/ARE pathway in vitro and in vivo. Biomed
Pharmacother. 97:1229–1237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Liao Z, Sun X, Shi Q, Huo G, Xie
Y, Tang X, Zhi X and Tang Z: Intravenous administration of Honokiol
provides neuroprotection and improves functional recovery after
traumatic brain injury through cell cycle inhibition.
Neuropharmacology. 86:9–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu JX, Shen SN, Tong Q, Wang YT and Lin
LG: Honokiol protects hepatocytes from oxidative injury through
mitochondrial deacetylase SIRT3. Eur J Pharmacol. 834:176–187.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Halasi M, Hitchinson B, Shah BN, Váraljai
R, Khan I, Benevolenskaya EV, Gaponenko V, Arbiser JL and Gartel
AL: Honokiol is a FOXM1 antagonist. Cell Death Dis. 9:842018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Green DR and Llambi F: Cell death
signaling. Cold Spring Harb Perspect Biol. 7:a0060802015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hua H, Chen W, Shen L, Sheng Q and Teng L:
Honokiol augments the anti-cancer effects of oxaliplatin in colon
cancer cells. Acta Biochim Biophys Sin (Shanghai). 45:773–779.
2013. View Article : Google Scholar : PubMed/NCBI
|