Introduction
Ischemic heart disease is a major cause of mortality
worldwide, and the World Health Organization predicts that it will
be the leading cause of mortality by 2030 (1). For patients that present with acute
myocardial infarction, the most effective therapeutic strategy to
preserve their myocardial tissue is timely reperfusion; however,
the process of reperfusion may prompt further injury, which is a
major feature of morbidity and mortality following infarction and
has a direct correlation with the occurrence of coronary heart
disease (CHD) (2). At present, there
is no effective treatment that protects the heart against
reperfusion injury. Cyclosporine-A acts by inhibiting the opening
of the mitochondrial permeability transition pore (mPTP) and phase
II trials are exploring the use of cyclosporine-A immediately prior
to percutaneous transluminal coronary intervention (PCI) (3); however, the severe adverse reactions
associated with cyclosporine-A limit its clinical use. β-blocker
agents have been in use for numerous years. Metoprolol was reported
to reduce the infarct area in patients with anterior ST-elevation
myocardial infarction undergoing PCI (4); however, it was also previously reported
that metoprolol increases mortality in patients with a systolic
blood pressure of <120 mmHg (5).
Therefore, novel therapies to reduce myocardial
ischemia/reperfusion (I/R) injury are required to improve clinical
outcomes for patients with CHD.
Although the molecular mechanisms mediating
reperfusion injury remain to be fully elucidated, the underlying
pathophysiology of myocardial I/R injury may involve reactive
oxygen species (ROS) generation, cytosolic and mitochondrial
Ca2+ overload, cell apoptosis and inflammatory responses
(6). Numerous studies have
demonstrated the importance of mitochondrial dysfunction due to
opening of the mPTP in I/R injury. Opening of the mPTP results in
the non-selective permeability of the inner mitochondrial membrane
to small molecules, resulting in the collapse of the mitochondrial
membrane potential (ΔΨm) and uncoupling of oxidative
phosphorylation. Finally, cell death occurs due to ATP depletion
(7). Therefore, inhibition of mPTP
opening may be an important cardioprotective strategy. Increasing
evidence has indicated that Ca2+ overload in the cytosol
and mitochondria, and mitochondrial oxidative stress are the key
inducers of mPTP opening, with ROS generation from the electron
transport chain appearing within the first few minutes after
myocardial reperfusion (8). Akt and
extracellular signal-regulated kinase 1/2 (Erk1/2) are components
of the reperfusion injury salvage kinase (RISK) pathway, which is
thought to be the major signaling pathway involved in
cardioprotection following myocardial reperfusion. The pathway is
activated by ischemic pre-conditioning (IPC) and post-conditioning,
and may be targeted by various pharmacological agents. An
association between the activation of the phosphoinositide 3-kinase
(PI3K)/Akt and Erk1/2 pathways, and the inhibition of mPTP opening
has been previously suggested (9).
Activation of the RISK pathway stimulates mPTP, enhances
cardiomyocyte survival and reduces I/R injury (10).
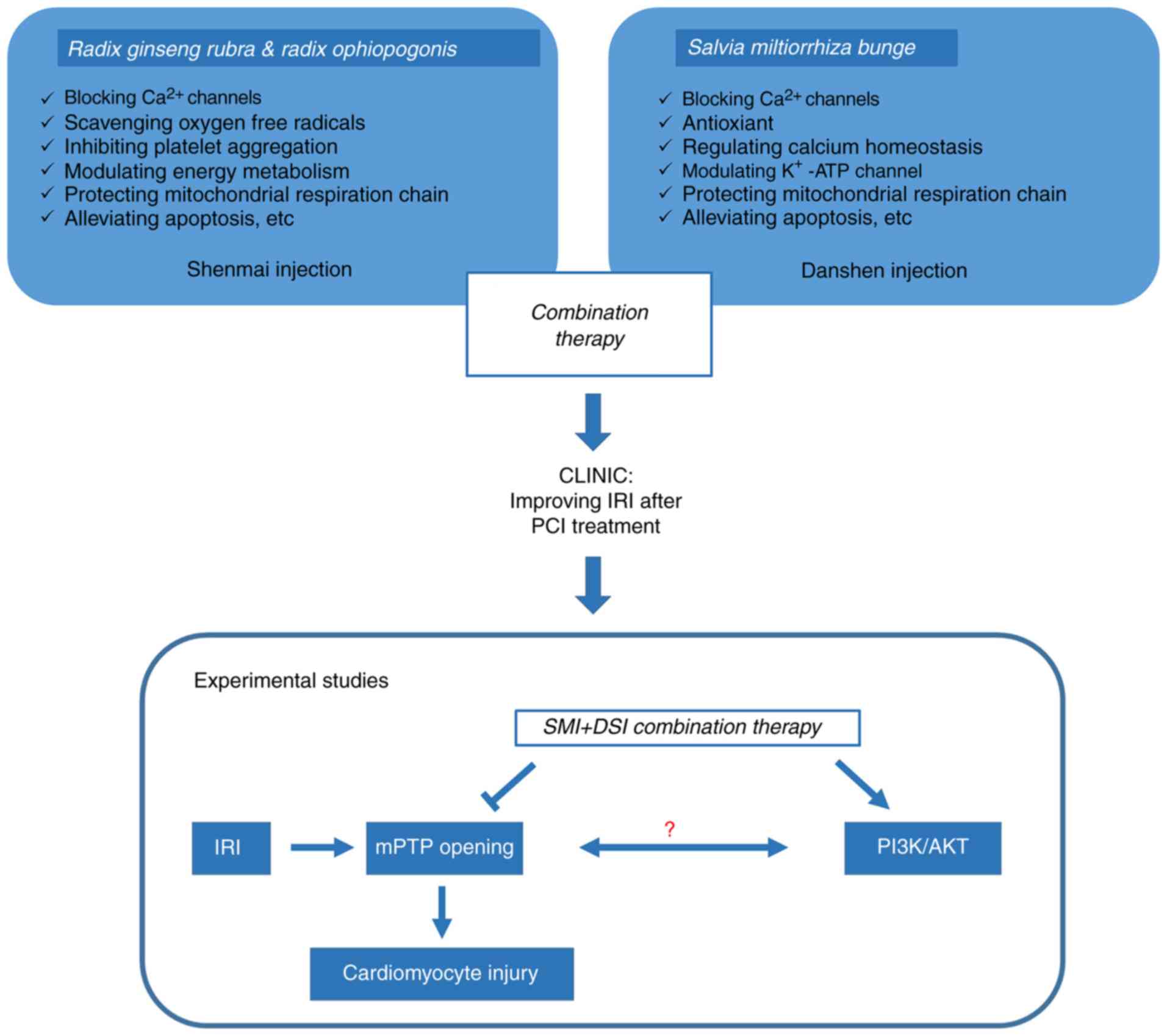
Traditional Chinese Medicine (TCM) has received
increasing attention regarding applications of multi-target
therapies. Radix Ginseng Rubra, Radix Ophiopogonis and Salvia
miltiorrhiza Bunge are well-known Chinese herbal medicines
frequently used together to enhance their therapeutic efficacy.
Shenmai injection (SMI) is composed of water-soluble extracts from
Radix Ginseng Rubra and Radix Ophiopogonis. Danshen injection (DSI)
is composed of aqueous extracts of S. miltiorrhiza Bunge.
Ginsenosides, including protopanaxatriol-type ginsenosides (Re, Rf,
Rg1), protopanaxadiol-type ginsenosides (Rb2, Rb1, Rd, Rc) and
oleanolic acid-type ginsenosides (Ro), are the major active
components of SMI (11). Previous
studies by our and other groups have isolated and identified >15
phenolic acids in the water-soluble constituents of S.
miltiorrhiza, including salvianic acid, protocatechuic acid,
protocatechuic aldehydrate and caffeic acid, as well as salvianolic
acid A and B (12,13).
SMI, DSI and their combination, termed Yiqi Yangyin
Huoxue (YYH), are clinically used to treat cardiovascular diseases,
including CHD, myocardial infarction, congestive heart failure and
myocardial I/R injury. Ginsenosides are the primary bioactive
components of SMI, and have been confirmed to have various effects,
including blocking Ca2+ channels, scavenging oxygen free
radicals (14,15), and attenuating I/R injury in
cardiovascular and cerebrovascular diseases. Furthermore, SMI has
also been demonstrated to inhibit apoptosis and Ca2+
influx in neurocytes subjected to hypoxia-reoxygenation (H/R)
(15,16). In vitro and in vivo
studies suggest that DSI may be vasoactive, able to scavenge ROS,
promote circulation and inhibit platelet aggregation (17). Clinical studies have reported that
the combination of SMI and DSI therapy improved myocardial
reperfusion injury following IPC in patients with acute myocardial
infarction by reducing oxidative stress (18). A previous study by our group has
demonstrated the protective effect of pretreatment with YYH,
against myocardial I/R injury via the PI3K/Akt and Erk1/2 signaling
pathways in isolated rat hearts (Fig.
1) (19). However, the
mechanisms underlying the cardioprotective effect of YYH have
remained elusive. The present study aimed to investigate the
underlying mechanisms by which YYH attenuates H/R- and hydrogen
peroxide (H2O2)-induced cardiomyocyte injury,
focusing on the inhibition of mPTP opening via the PI3K/Akt and
Erk1/2 signaling pathways.
Materials and methods
Reagents
SMI and DSI were donated by Chiatai-Qing-Chun-Bao
Pharmaceutical Co., Ltd. (Hangzhou, China).
Chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
(CM-H2DCFDA), fluo-4 acetoxymethyl (Fluo-4/AM) and
calcein acetoxymethyl (Calcein/AM) were purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Collagenase II,
bromodeoxyuridine (BrdU), rhodamine123 (Rh123), MTT,
H2O2 and PD98059 were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Rhodamine-2
acetoxymethyl (Rhod-2/AM) was purchased from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). LY294002 was purchased from
Apollo Scientific Ltd. (Stockport, UK). Fetal bovine serum (FBS)
and Dulbecco's modified Eagle's medium were obtained from Hyclone
(GE Healthcare Life Sciences, Logan, UT, USA). Creatine kinase
[(CK); cat. no. A0032] and lactate dehydrogenase [(LDH); cat. no.
C0016] assay kits were purchased from Beyotime Institute of
Biotechnology (Shanghai, China). Trypsin (1:25) was purchased from
Beijing Solarbio Science & Technology Co., Ltd. (Beijing,
China).
Isolation and culture of neonatal rat
cardiomyocytes
A total of 30 neonatal Sprague Dawley rats (age, 1–3
days; body weight, 10–15 g) were obtained from Beijing HFK
Bioscience Co. Ltd. (Beijing, China). Without further housing,
these neonatal rats were anaesthetized in a container with
metofane-saturated gauze and ventricular cardiomyocytes were
isolated (20,21). In brief, after administration of
metofane, the newborn rats exhibited no sign of consciousness,
indicated by absence of reaction to soft poking and disappearance
of skin pinch reaction, which indicated full anesthesia.
Furthermore, vital signs and absence of any indications of toxicity
were confirmed. The neonatal rats were disinfected with 75% ethanol
and the hearts were rapidly harvested. The ventricular myocardium
was minced into 1 mm3 pieces using scissors in PBS.
Samples were digested in PBS containing 0.0625% (w/v) trypsin and
0.1% (w/v) collagenase II with gentle agitation at 37°C for 5 min.
Digestion was repeated for 5–8 cycles in total, and the digestion
was stopped by addition of medium containing 10% (v/v) FBS.
Subsequently, the cell suspension was filtered through a 200-mesh
sieve and centrifuged at 560 × g at 4°C for 10 min. The harvested
cell pellet was re-suspended in basic medium containing 10% (v/v)
FBS and incubated at 37°C for 90 min to allow for fibroblast
adhesion. Non-adherent cells were collected and seeded in a culture
flask at 3×105 cells/mm2. Cells were
incubated with 95% air and 5% CO2 for 1 h. To enhance
the purity of the cardiomyocytes, BrdU (0.1 mM) was added to the
culture medium for the first 3 days according to previously
described methods (22).
Cardiomyocytes were then used for subsequent experiments.
H/R injury in cardiac myocytes
H/R was simulated as previously described (23). In brief, the cardiomyocyte medium for
hypoxia was deprived of glucose and serum. Then the cells were
deposited into a hypoxia chamber (Stemcell Technologies, Inc.,
Vancouver, BC, Canada) containing with 95% (v/v) N2 and
5% (v/v) CO2 at 37°C for 20 h of hypoxia. The medium was
replaced with high-glucose medium and the cells were transferred to
the regular incubator and maintained for 4 h for reoxygenation.
H2O2-induced
oxidative stress injury
In the clinic, adult patients receive an i.v.
infusion of 30 ml Shenmai injection and 30 ml Danshen injection
(18,24), which are diluted with 250 ml saline
solution containing 5% glucose. As the maximum concentration, 10
µl/ml [SMI/DSI/culture medium, 5:5:990 (v/v/v)] is equivalently
used in the clinic. In the present study, cells were pre-treated
with a combination of SMI and DSI (2.5, 5 and 10 µl/ml) for 10 h.
Oxidative stress was induced in cultured cardiac myocytes by adding
100 µM H2O2 for 120 min, or 90 min for the
mitochondrial Ca2+ experiment. Cells were pre-treated
with specific probes and with Hank's solution in the absence or
presence of 100 µM H2O2, and fluorescence was
monitored.
Cell viability, CK and LDH activity
assays
Following reoxygenation, the medium was removed and
cells were incubated with a solution of 1.2 mM MTT for 4 h at 37°C.
Subsequently, 150 µl dimethylsulfoxide was added to each well
following removal of the medium. Mitochondrial dehydrogenase
activity, which reflects cell viability, was measured at 490 nm.
Cell viability was expressed as a percentage of the control. CK and
LDH activity in the medium was measured using the CK or LDH assay
kits according to the manufacturer's protocol, respectively.
Assessment of ΔΨm
Fluorescence quenching of Rh123 was used to assess
ΔΨm as previously described (25).
Cardiomyocytes were cultured with 5 µM Rh123 at 37°C for 30 min and
then washed three times with PBS. Fluorescence was measured using a
Multimode plate reader (PerkinElmer, Inc., Waltham, MA, USA) at
excitation/emission (ex/em) wavelengths of 488/535 nm. Values are
expressed as a percentage of the control. Images of the cells were
captured under a fluorescence microscope (Olympus IX73; Olympus
Corp., Tokyo, Japan).
Measurement of intracellular ROS
Intracellular ROS were detected using the
fluorescent dye CM-H2DCFDA. In brief, following the
specific treatments, samples were washed with PBS and incubated
with 5 µM CM-H2DCFDA for 20 min at 37°C, and then washed
again twice with PBS. Fluorescence intensity was measured at ex/em
wavelengths of 488/525 nm using a Multimode plate reader
(PerkinElmer, Inc.).
Determination of cytosolic and
mitochondrial Ca2+
To monitor cytosolic Ca2+, cells were
loaded with 4 µM Fluo-4/AM at 37°C for 30 min and then washed three
times with dye-free buffer. Following further incubation with 90 µl
Hank's solution at 37°C for 20 min, cells were exposed to
H2O2 (10 µl H2O2 added
to 90 µl Hank's solution) at 37°C for 2 h, and the fluorescence was
measured at ex/em wavelengths of 494/516 nm using a Multimode plate
reader (PerkinElmer, Inc.).
The Ca2+-sensitive dye Rhod-2AM was used
to monitor mitochondrial Ca2+ (26,27).
Cells were washed with Hank's solution, followed by incubation with
4 µM dihydro Rhod-2/AM containing 0.05% (v/v) Pluronic F-127 at
37°C for 45 min. Rhod-2 fluorescence was measured at ex/em
wavelengths of 552/581 nm as a baseline value. Subsequently, cells
were treated with 100 µM H2O2 (diluted with
Hank's solution) at 37°C for 90 min and the fluorescence was
measured at a series of time-points. Results are expressed as a
percentage of the baseline fluorescence intensity.
Monitoring of mPTP opening
mPTP opening was monitored by co-loading cells with
Calcein/AM and CoCl2 as previously described (28,29). In
brief, cardiomyocytes were incubated with 2 µM Calcein/AM and 1 mM
CoCl2 at room temperature for 35 min, and then washed
with 1 mM CoCl2 for 25 min. Calcein fluorescence was
measured at ex/em wavelengths of 488/515 nm as the baseline value.
Subsequently, cells were treated with 100 µM
H2O2 (diluted with Hank's solution) at 37°C
for 120 min and the fluorescence was measured at a series of
time-points. The abrupt loss of fluorescence was regarded as an
indicator of mPTP opening. Results are expressed as a percentage of
the baseline fluorescence intensity.
Statistical analysis
Statistical analyses were performed using the SPSS
15.0 software (SPSS, Inc., Chicago, IL, USA). All values are
expressed as the mean ± standard deviation (number of replicate
wells, n=6). Differences between two groups were assessed using
Student's t-test and one-way analysis of variance followed by the
least-significant differences method was employed for comparison
between multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
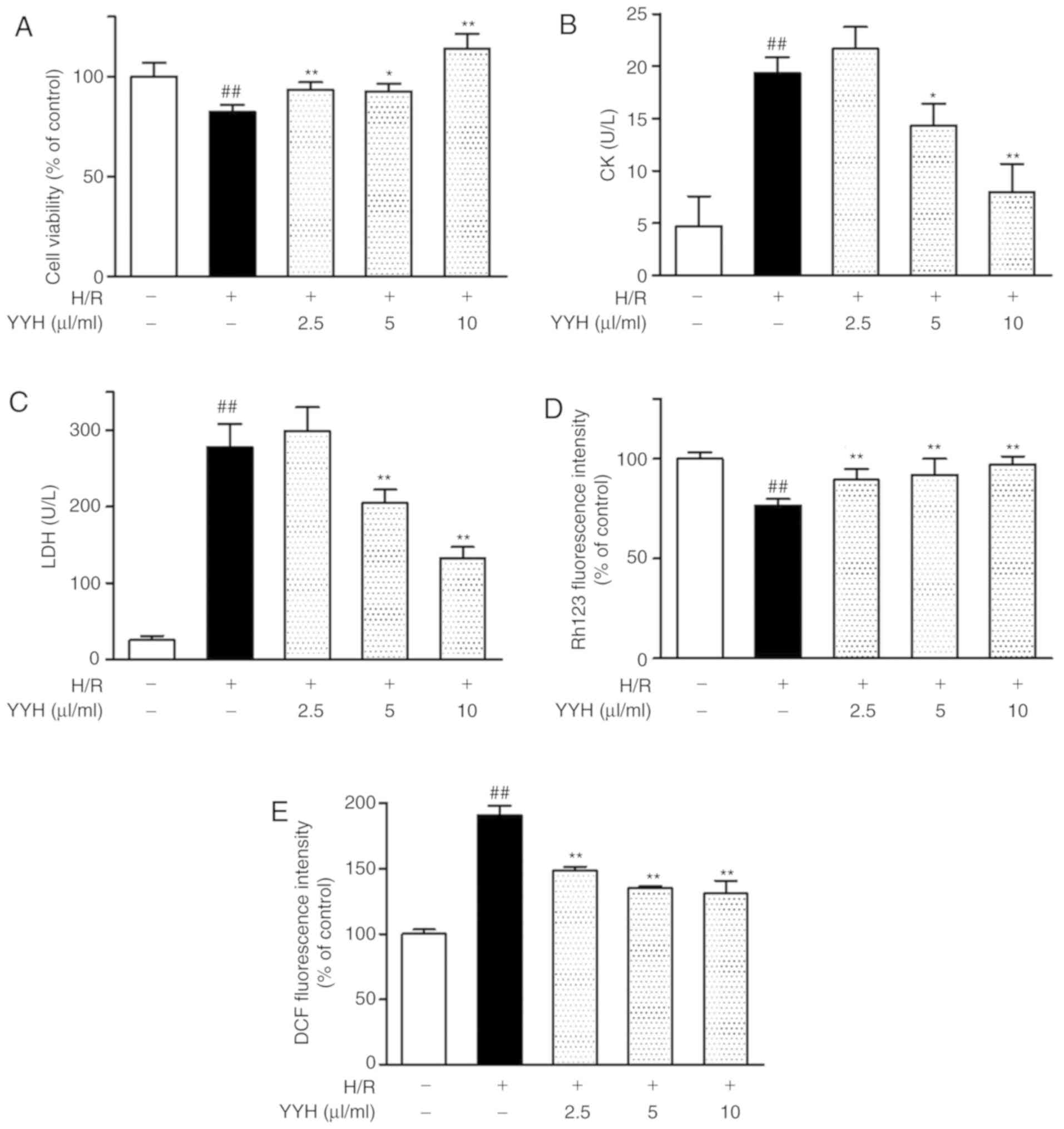
Cardioprotective effects of YYH in
cardiomyocytes subjected to H/R. YYH improves the viability of
cardiomyocytes subjected to H/R injury
H/R resulted in an 82.46±3.52% reduction in
cardiomyocyte viability compared with that in the control group.
YYH pretreatment at increasing concentrations (2.5, 5 and 10 µl/ml)
enhanced the cell viability following H/R injury (93.40±3.81,
92.66±3.66 and 113.83±7.57% of the control, respectively; Fig. 2A).
YYH prevents H/R-induced CK and LDH
release in cardiomyocytes
The CK and LDH levels were higher in the H/R group
(19.3±1.5 and 278.3±30.0 U/l, respectively) than those in the
control group (4.6±2.8 and 25.3±5.5 U/l, respectively; P<0.01).
YYH pretreatment (5 and 10 µl/ml) significantly reduced the levels
of CK and LDH in a concentration-dependent manner (Fig. 2B and C).
YYH preserves ΔΨm in cardiomyocytes
subjected to H/R
H/R increased ΔΨm depolarization to 76.61±3.33% of
that in the control group (P<0.01). YYH pretreatment (2.5, 5 and
10 µl/ml) increased mitochondrial depolarization to 89.4±5.4,
91.9±8.1 and 97.2±4.0% of the control, respectively (P<0.01;
Fig. 2D).
YYH reduces H/R-induced ROS
generation
H/R resulted in increased generation of
intracellular ROS compared with the control group (190.82±7.24%;
P<0.01). Pretreatment with YYH (2.5, 5 and 10 µl/ml)
significantly reduced intracellular ROS generation compared with
the H/R treatment group (148.35±2.82, 134.88±1.58 and 130.74±10.01%
of the control, respectively) after 4 h of reperfusion following
hypoxia (P<0.01; Fig. 2E).
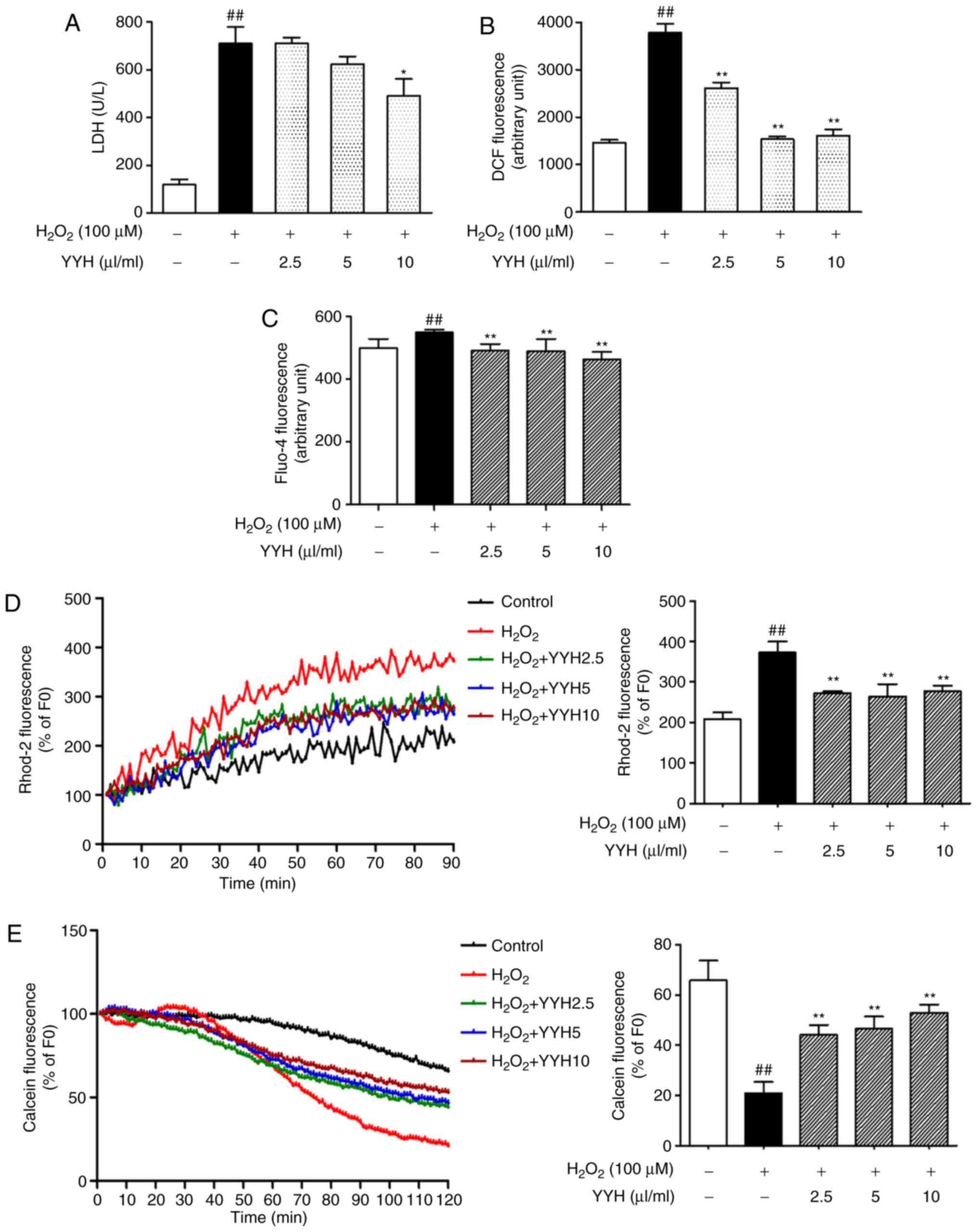
Cardioprotective effect of YYH determined
in cardiomyocytes challenged with H2O2
YYH reduces
H2O2-induced LDH release in
cardiomyocytes
LDH activity was detected at 2 h after treatment
with H2O2. Exposure to
H2O2 induced an increase in LDH release from
117.5±21.9 to 709.0±37.0 U/l, which was significantly suppressed by
YYH preincubation (10 µl/ml) for 10 h (490.0±31.4 U/l; P<0.05;
Fig. 3A).
YYH attenuates
H2O2-induced ROS generation
Aberration in DCF fluorescence reflects the change
in ROS levels. H2O2 induced a marked increase
in ROS levels compared with those in the control group (P<0.01).
Treatment with YYH (2.5, 5 and 10 µl/ml) resulted in a significant
decrease in ROS levels compared with those in the
H2O2 group (P<0.01; Fig. 3B). ROS levels in middle and high dose
of YYH (5 and 10 µl/ml) were closed to those in the control group,
indicating a better inhibitory effect on ROS overproduction.
YYH reduces cytosolic and
mitochondrial Ca2+ overload in
H2O2-challenged cardiomyocytes
The cytosolic Ca2+ level was monitored
using Fluo-4AM. The Ca2+ level in the
H2O2 group was significantly increased
compared with that in the control group (P<0.01), which was
reversed by the pretreatment with YYH (P<0.01; Fig. 3C). Among these, the high dose of YYH
significantly decreased cytosolic Ca2+ levels, beyond
that of the control group (P<0.01).
Mitochondrial Ca2+ overload induced by
H2O2 was evaluated using time-lapse
fluorescence microscopy, monitoring changes in Rhod-2 fluorescence.
There was an obvious increase of mitochondrial fluorescence
appeared at 30 min after exposure to H2O2.
The high fluorescence was maintained for the remaining time period
after 60 min. Pretreatment with YYH did not alter the baseline
level of mitochondrial Ca2+. Of note, after 30 min of
exposure to H2O2, the associated increases in
the mitochondrial Ca2+ levels were reduced in the groups
pretreated with YYH (2.5, 5 and 10 µl/ml; Fig. 3D). Rhod-2 fluorescence at 90 min
after exposure to H2O2 is presented in the
bar graph. Compared with the control group,
H2O2 exposure significantly increased
fluorescence intensity (P<0.01), indicating increased
mitochondrial Ca2+ levels. Pretreatment with YYH (2.5, 5
and 10 µl/ml) significantly inhibited
H2O2-induced mitochondrial Ca2+
overload (P<0.01). However, levels of mitochondrial
Ca2+ in the pretreatment groups remain higher than those
in the control group. With the increasing concentration of YYH, the
fluctuating fluorescence intensity indicated that the inhibitory
effect of YYH (10 µl/ml) on mitochondrial Ca2+
overloading may be reach the level of saturation.
YYH inhibits mPTP opening induced by
H2O2
mPTP opening in intact cells was analyzed by
monitoring the fluorescence of mitochondrial-entrapped calcein. A
rapid decrease in calcein fluorescence was detected at ~35 min
after exposure to H2O2, indicating mPTP
opening. YYH pretreatment (2.5, 5 and 10 µl/ml) suppressed the
sudden drop of the fluorescence value and the 10 µl/ml dose exerted
the greatest effect (Fig. 3E).
Differences in calcein fluorescence at 120 min after exposure to
H2O2 are presented in the bar graph. Compared
with the control group, H2O2 exposure
decreased the fluorescence intensity from 65.8±7.8 to 21.0±4.3% of
the baseline value (P<0.01), indicating increased mPTP opening.
By contrast, YYH pretreatment (2.5, 5 and 10 µl/ml) inhibited mPTP
opening, as indicated by the increase in fluorescence from 21.0±4.3
to 44.1±3.9, 46.6±4.7 and 52.9±3.2% of the baseline value,
respectively (P<0.01). Therefore, YYH inhibited
H2O2-induced mPTP opening.
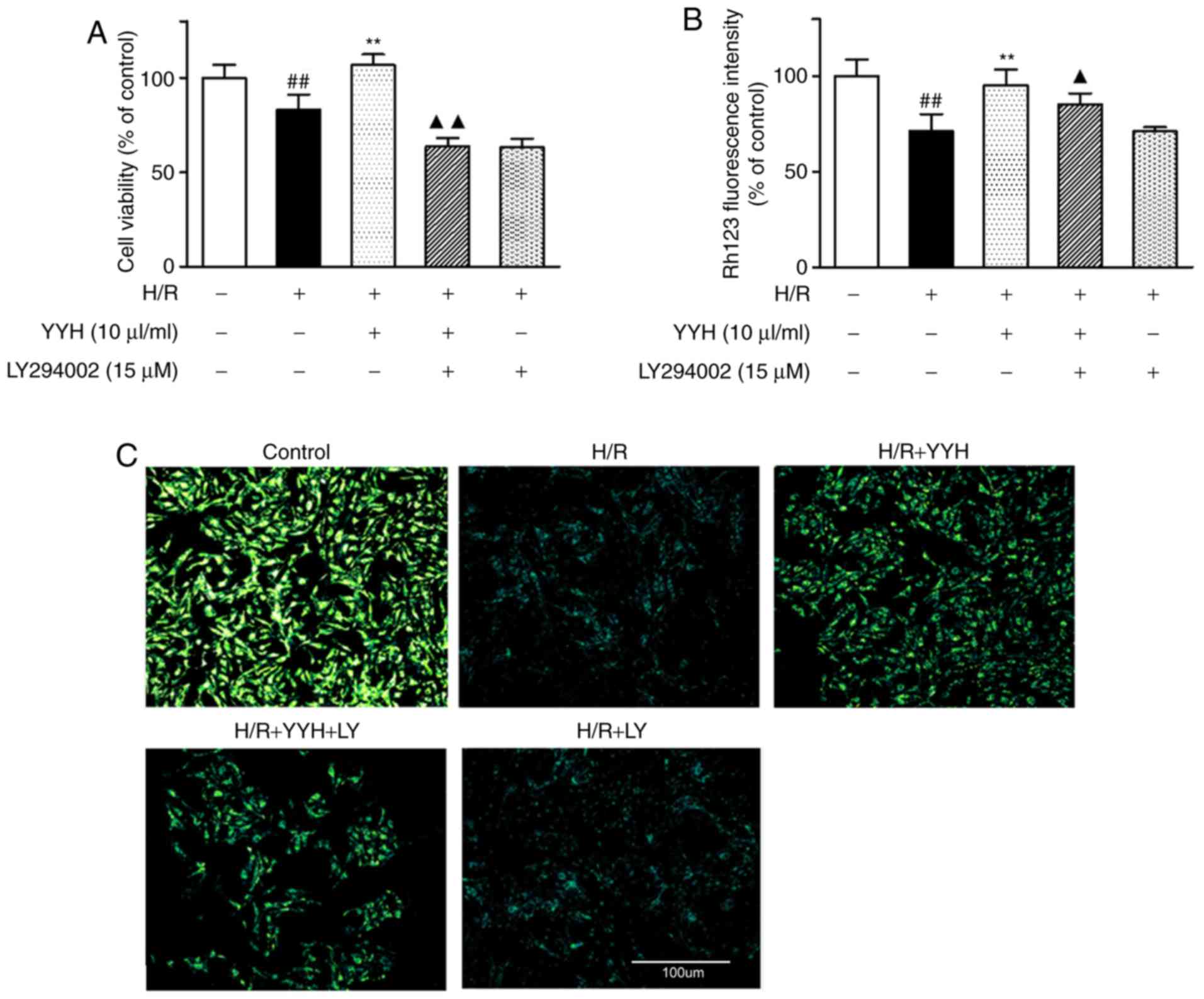
Inhibition of PI3K/Akt and ERK1/2
pathways attenuates YYH cardioprotection from H/R injury
To further explore whether the cardioprotective
effect of YYH is associated with the activation of PI3K/Akt and
Erk1/2 signaling, a PI3K-specific inhibitor, LY294002, and an
ERK1/2-specific inhibitor, PD98059, were used to investigate cell
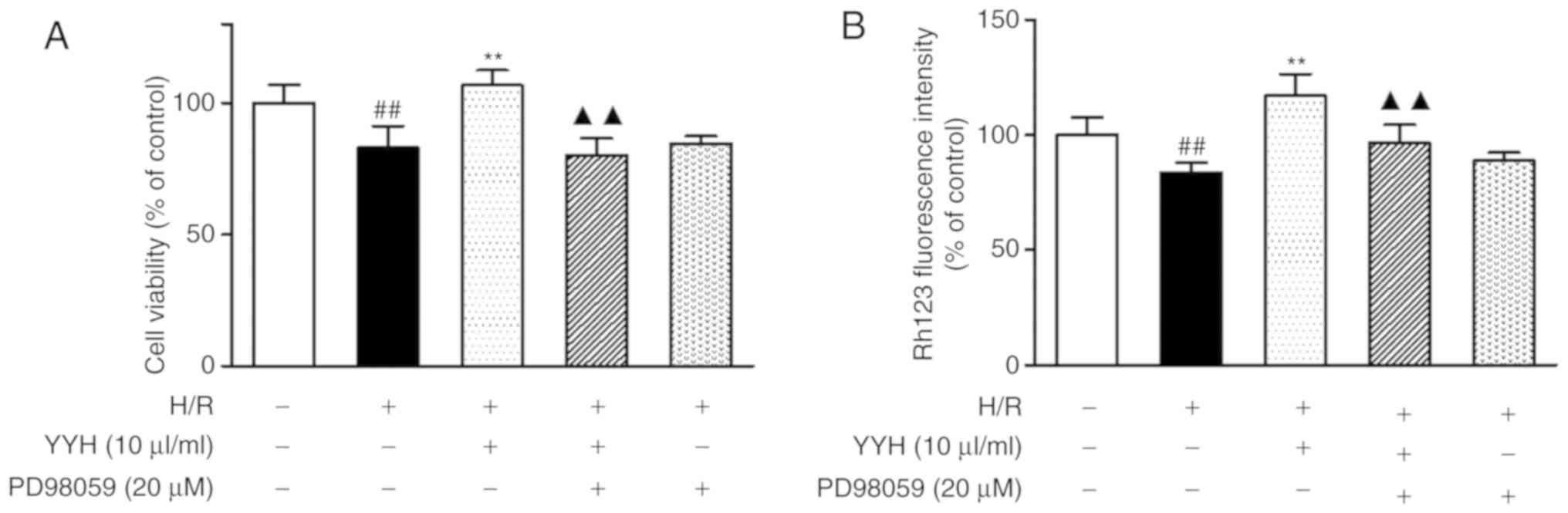
viability and ΔΨm. Compared with that in the H/R group,
pre-treatment with YYH (10 µl/ml) resulted in a marked increase in
cell viability (P<0.01; Figs. 4A
and 5A). However, these effects were
partially attenuated by LY294002 (P<0.01) or PD98059 (P<0.01)
compared with combination group LY294002 reduced cell viability
compared with that in the H/R group, indicating that the protective
effect of the PI3K signaling pathway was inhibited by LY294002.
Compared with that in the H/R group, pretreatment
with YYH (10 µl/ml) resulted in a marked increase in the ΔΨm
(P<0.01; Fig. 4B and C, Fig. 5B). However, these effects were
partially abolished by LY294002 (P<0.01) or PD98059 (P<0.01).
LY294002 or PD98059 alone exerted no effect on cell viability and
ΔΨm. The results indicate that the PI3K/Akt pathway and the ERK1/2
pathway may be involved in the protective effect of YYH.
Discussion
Blockage of cardiac blood flow deprives the heart of
its oxygen supply, resulting in myocardial injury. Timely
restoration of the blood flow effectively attenuates ischemic
injury; however, subsequent reperfusion induces secondary damage to
the ischemic myocardium, known as reperfusion injury (30). The mechanisms underlying reperfusion
injury are complex and multifactorial, with ROS generation,
Ca2+ overload, opening of the mPTP, endothelial
dysfunction and pronounced inflammatory responses all implicated in
causing the damage (31). Radix
Ginseng Rubra, Radix Ophiopogonis and S. miltiorrhiza Bunge,
which are included in YYH, have been investigated to determine
their potential combined pharmaceutical properties, including
anti-inflammatory, anti-oxidant, microcirculation promotion and
cardioprotective abilities (32,33). In
the clinic, it has been reported that YYH improved myocardial
reperfusion injury following PCI in patients with acute myocardial
infarction (18).
In the present study, cardiomyocytes were subjected
to H/R- and H2O2-induced injury. YYH was
administered at three concentrations (2.5, 5 and 10 µl/ml) to
investigate its cardioprotective actions. The results of the
present study indicated that H/R reduced cell viability and ΔΨm,
suggesting disruption of mitochondrial integrity and function. Of
note, pre-treatment with YYH inhibited these decreases. The
protective effects of YYH were abolished by LY294002, a specific
inhibitor of PI3K, and PD98059, a specific inhibitor of the Erk1/2
pathway, suggesting that the PI3K/Akt and Erk1/2 signaling pathways
are involved in the cardioprotective effects of YYH. A previous
study by our group demonstrated that ginsenoside Rb1, a principal
active component of SMI, directly inhibited mPTP opening on
mitochondria isolated from rat hearts in vitro (34). Its effect on mPTP opening is mediated
via phosphorylation of Akt and glycogen synthase kinase-3β, as
demonstrated in a Langendorff-perfused rat heart model and in
experiments using a H/R induced cardiomyocyte injury model that was
subjected to H/R (34). Increasing
evidence suggests that the PI3K/Akt and Erk1/2 pathways are
involved in signaling cascades in myocardial IPC and have important
roles in cardioprotection following myocardial I/R injury (35,36).
Activation of the PI3K/Akt and Erk1/2 signaling stimulates mPTPs
downstream, enhances cardiomyocyte survival and reduces morbidity
and mortality following I/R injury (37).
mPTP is a non-selective conductance pore located in
the inner mitochondrial membrane. mPTP opening contributes to the
transition from reversible to irreversible myocardial I/R injury
(38) by inducing mitochondrial
swelling and outer membrane rupture, subsequently facilitating
activation of caspases and release of pro-apoptotic proteins, and
ultimately contributing to the induction of apoptosis. This process
is initiated shortly after ischemia and amplified by reperfusion.
ROS and Ca2+ are also elevated during myocardial I/R,
following activation of mPTP opening, particularly during
reperfusion. I/R injury induces mitochondria to produce high level
of ROS, including superoxide anion (O2−·),
hydroxyl radical (OH−·) and hydrogen peroxide
H2O2 (39).
Excessive amounts of ROS cause damage to mitochondria, inducing
mPTP opening and mitochondrial depolarization (40). Furthermore, an increased
concentration of cytosolic Ca2+ activates a variety of
cell death-associated processes following I/R. Ca2+ is
then transported into the mitochondria of cardiomyocytes via
mitochondrial Ca2+ uniporters. Once mitochondrial
Ca2+ overloading occurs, the mPTP response is triggered.
Therefore, numerous cardioprotective processes primarily function
via direct inhibition of mPTP opening or upstream factors (23,41).
The results of the present study revealed that YYH
protects cardiomyocytes by blocking H/R- and
H2O2-induced CK and LDH release, inhibiting
ROS production, reducing oxidative stress-induced cytosolic and
mitochondrial Ca2+ overload, and subsequently
suppressing mPTP opening. Inhibition of mPTP opening may be a key
event in mediating myocardial protection against I/R injury. This
beneficial effect is associated with activation of the PI3K/Akt and
Erk1/2 signaling pathways (19).
However, it has not been established whether the mechanisms that
mediate the effects of YYH in vitro are also involved in the
therapeutic effects in vivo, and further studies are
required to support this. The mitochondrial protection mechanism of
YYH therapy also requires validation using animal models. Further
research is also required to explore mechanisms of potential
mPTP-targeting strategies and other mechanisms that may be involved
in the effects of SMI and DSI combination using in vitro and
in vivo analyses.
In summary, the present study demonstrated that YYH
protects cardiomyocytes against H/R- and
H2O2-induced injury through activation of the
PI3K/Akt and Erk1/2 signaling pathway and inhibition of mPTP
opening resulting from ROS generation and calcium overload
(Fig. 6). The in vitro
molecular mechanisms of action of YYH therapy and their protective
effects against myocardial I/R in vivo require further
exploration.
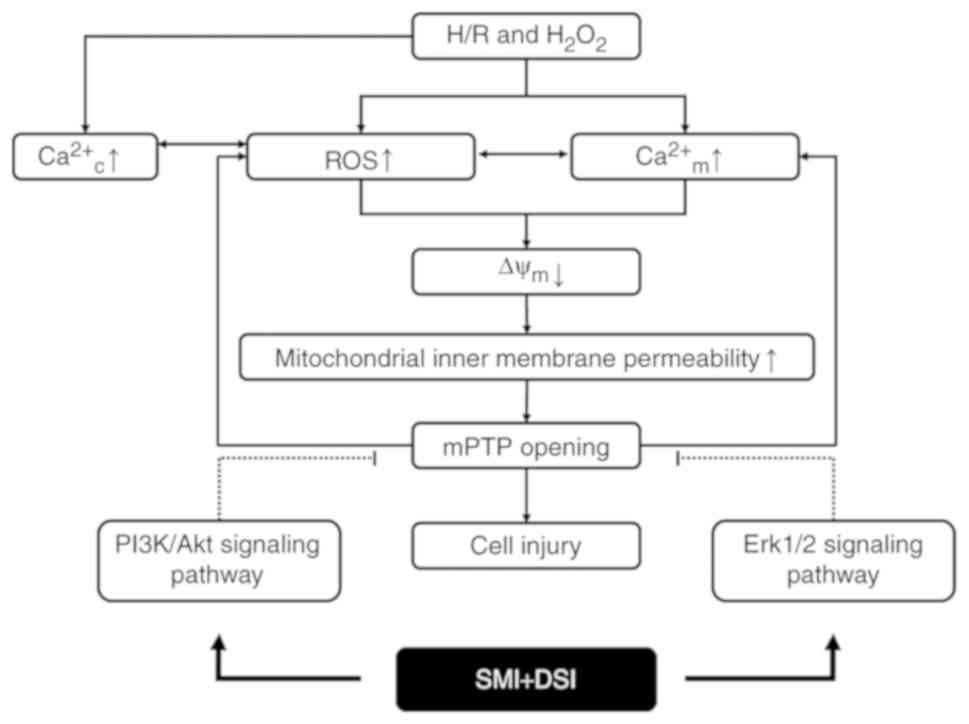 | Figure 6.Summary scheme of the mechanism
underlying inhibition of SMI plus DSI combination on H/R and
H2O2-induced mPTP opening. H/R and
H2O2 treatment induces accumulation of ROS,
Ca2+m overload and an increase of mitochondrial inner membrane
permeability. SMI plus DSI combination therapy activates the
PI3K/Akt and Erk1/2 signaling pathways, therefore inhibiting mPTP
opening and cardiomyocyte injury. H/R, hypoxia/reoxygenation;
Ca2+c, cytosolic calcium; Ca2+m, mitochondrial calcium; ROS,
reactive oxygen species; ΔΨm, mitochondrial membrane potential;
mPTP, mitochondrial permeability transition pore; PI3K,
phosphoinositide 3-kinase; Erk1/2, extracellular signal-regulated
kinase; SMI, Shenmai injection; DSI, Danshen injection. |
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from the
National Natural Science Foundation of China (grant nos. 81774017
and 81202779) and the Scientific Research Project of Tianjin
Education Commission (grant no. ٢٠١٧KJ١٤٠).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are included in this published paper.
Authors' contributions
LL, ZS and YW performed the experiments and wrote
the paper. ZD revised the paper. DY, JL and HW performed the
experiments. YL designed the experiments. All of the authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was performed in strict accordance with
the recommendations in the Guidance Suggestions for the Care and
Use of Laboratory Animals issued by the Ministry of Science and
Technology of China. The protocols were approved by the Laboratory
Animal Ethics Committee of Tianjin University of Traditional
Chinese Medicine (Tianjin, China; permit no. TCM-LAEC20160035).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BrdU
|
bromodeoxyuridine
|
|
CHD
|
coronary heart disease
|
|
CK
|
creatine kinase
|
|
DSI
|
Danshen injection
|
|
H/R
|
hypoxia/reoxygenation
|
|
IPC
|
ischemic pre-conditioning
|
|
I/R
|
ischemia/reperfusion
|
|
LDH
|
lactate dehydrogenase
|
|
mPTP
|
mitochondrial permeability transition
pore
|
|
PCI
|
percutaneous transluminal coronary
intervention
|
|
Rh123
|
Rhodamine123
|
|
ROS
|
reactive oxygen species
|
|
SMI
|
Shenmai injection
|
|
TCM
|
Traditional Chinese Medicine
|
|
YYH
|
YiqiYangyinHuoxue
|
|
ΔΨm
|
mitochondrial membrane potential
|
References
|
1
|
Mathers CD and Loncar D: Projections of
gobal mortality and burden of disease from 2002 to 2030. PLoS Med.
3:e4422006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Investig. 123:92–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piot C, Croisille P, Staat P, Thibault H,
Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant
D, et al: Effect of cyclosporine on reperfusion injury in acute
myocardial infarction. N Engl J Med. 359:473–481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ibanez B, Macaya C, Sánchez-Brunete V,
Pizarro G, Fernández-Friera L, Mateos A, Fernández-Ortiz A,
García-Ruiz JM, García-Álvarez A, Iñiguez A, et al: Effect of early
metoprolol on infarct size in ST-segment-elevation myocardial
infarction patients undergoing primary percutaneous coronary
intervention: The effect of metoprolol in cardioprotection during
an acute myocardial infarction (METOCARD-CNIC) trial. Circulation.
128:1495–1503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roolvink V, Rasoul S, Ottervanger JP,
Dambrink JH, Lipsic E, van der Horst IC, de Smet B, Kedhi E, Marcel
Gosselink AT, Piek JJ, et al: Rationale and design of a
double-blind, multicenter, randomized, placebo-controlled clinical
trial of early administration of intravenous β-blocker in patients
with ST-elevation myocardial infarction before primary percutaneous
coronary intervention: EARLY β-blocker administration before
primary PCI in patients with ST-elevation myocardial infarction
trial. Am Heart J. 168:661–666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murphy E and Steenbergen C: Mechanisms
underlying acute protection from cardiac ischemia-reperfusion
injury. Physiol Rev. 88:581–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Halestrap AP and Richardson AP: The
mitochondrial permeability transition: A current perspective on its
identity and role in ischaemia/reperfusion injury. J Mol Cell
Cardiol. 78:129–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ong SB, Samangouei P, Kalkhoran SB and
Hausenloy DJ: The mitochondrial permeability transition pore and
its role in myocardial ischemia reperfusion injury. J Mol Cell
Cardiol. 78:23–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hausenloy DJ, Ong SB and Yellon DM: The
mitochondrial permeability transition pore as a target for
preconditioning and postconditioning. Basic Res Cardiol.
104:189–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Zhou YF, Li YL, Wang LL, Aria H, Qi
Y and Xu Y: Aqueous extract of Cortex Dictamni protects H9c2
cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress
and apoptosis by PI3K/Akt signaling pathway. Biomed Pharmacother.
89:233–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Gu LQ, Xin YF, Gao HY, Xu XZ, Zhuang
S, Zhou GL, You ZQ, Huo LR and Xuan YX: Simultaneous determination
and pharmacokinetics of eight ginsenosides by LC-MS/MS after
intravenously infusion of ‘SHENMAI’ injection in dogs. Pak J Pharm
Sci. 30:421–427. 2017.PubMed/NCBI
|
|
12
|
Chang YX, Ding XP, Qi J, Cao J, Kang LY,
Zhu DN, Zhang BL and Yu BY: The antioxidant-activity-integrated
fingerprint: An advantageous tool for the evaluation of quality of
herbal medicines. J Chromatogr A. 1208:76–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang JL, Cui M, He Y, Yu HL and Guo DA:
Chemical fingerprint and metabolic fingerprint analysis of Danshen
injection by HPLC-UV and HPLC-MS methods. J Pharm Biomed Anal.
36:1029–1035. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Y, Chu S, Shao Q, Zhang M, Xia C, Wang
Y, Li Y, Luo Y, Huang H and Chen N: Antioxidant activities of
ginsenoside Rg1 against cisplatin-induced hepatic injury through
Nrf2 signaling pathway in mice. Free Radic Res. 51:1–13. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan SM: Potential cardioprotective
effects of Ginseng preparations. Pak J Pharm Sci. 28:963–968.
2015.PubMed/NCBI
|
|
16
|
Ye LF, Zheng YR and Wang LH: Effects of
Shenmai injection and its bioactive components following
ischemia/reperfusion in cardiomyocytes. Exp Ther Med. 10:1348–1354.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han JY, Horie Y, Miura S, Akiba Y, Guo J,
Li D, Fan JY, Liu YY, Hu BH, An LH, et al: Compound Danshen
injection improves endotoxin-inducedmicrocirculatory disturbance in
rat mesentery. World J Gastroenterol. 13:3581–3591. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geng QX, Zhu XL and Zhang XH: Effect of
combined therapy of Shenmai and compound Danshen injection on
myocardial reperfusion injury after percutaneous coronary
intervention in patients with acute myocardial infarction. Chin J
Integr Med. 24:496–499. 2004.
|
|
19
|
Li YH, Li YY, Fan GW, Zhou K, Duan ZZ, Yu
JH and Gao XM: Cardioprotective effects of YiqiYangyinHuoxue
injection against ischemia-reperfusion injury in isolated hearts of
rats. Chinese Traditional and Herbal Drugs. 47:281–289. 2016.
|
|
20
|
Ren J, Poon BY, Tang Y, Funk GD and Greer
JJ: Ampakines alleviate respiratory depression in rats. Am J Respir
Crit Care Med. 174:1384–1391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pagliardini S, Ren J and Greer JJ:
Ontogeny of the pre-botzinger complex in perinatal rats. J
Neurosci. 23:9575–9584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang KK, Ding RR, Ha YP, Jia YN, Liao XM,
Wang SS, Li RJ, Shen ZH, Xiong H, Guo JL and Jie W:
Hypoxia-stressed cardiomyocytes promote early cardiac
differentiation of cardiac stem cells through HIF-1α/Jagged1/Notch1
signaling. Acta Pharm Sin B. 8:795–804. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan ZZ, Li YH, Li YY, Fan GW, Chang YX,
Yu B and Gao XM: Danhong injection protects cardiomyocytes against
hypoxia/reoxygenation- and H2O2-induced
injury by inhibiting mitochondrial permeability transition pore
opening. J Ethnopharmacol. 175:617–625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao SX: Study on the therapeutic effect of
Shenmai injection combined with compound Danshen injection on acute
myocardial infarction. Chin J Med Guide. 19:728–730. 2017.
|
|
25
|
Baracca A, Sgarbi G, Solaini G and Lenaz
G: Rhodamine 123 as a probe of mitochondrial membrane potential:
Evaluation of proton flux through F(0) during ATP synthesis.
Biochim Biophys Acta. 1606:137–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukumori R, Takarada T, Kambe Y, Nakazato
R, Fujikawa K and Yoneda Y: Possible involvement of mitochondrial
uncoupling protein-2 in cytotoxicity mediated by acquired
N-methyl-D-aspartate receptor channels. Neurochem Int. 61:498–505.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carrasco-Pozo C, Pastene E, Vergara C,
Zapata M, Sandoval C and Gotteland M: Stimulation of cytosolic and
mitochondrial calcium mobilization by indomethacin in Caco-2 cells:
Modulation by the polyphenols quercetin, resveratrol and rutin.
Biochim Biophys Acta. 1820:2052–2061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Petronilli V, Miotto G, Canton M, Brini M,
Colonna R, Bernardi P and Di Lisa F: Transient and long-lasting
openings of the mitochondrial permeability transition pore can be
monitored directly in intact cells by changes in mitochondrial
calcein fluorescence. Biophys J. 76:725–734. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharov VG, Todor A, Khanal S, Imai M and
Sabbah HN: Cyclosporine A attenuates mitochondrial permeability
transitionand improves mitochondrial respiratory function in
cardiomyocytes isolated from dogs with heart failure. J Mol Cell
Cardiol. 42:150–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu SD, Xia F, Lin XM, Duan KL, Wang F, Lu
QL, Cao H, Qian YH and Shi M: Ginsenoside-Rd promotes neurite
outgrowth of PC12 cells through MAPK/ERK- and PI3K/AKT-dependent
pathways. Int J Mol Sci. 17:E1772016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou Q, Sun Y, Tan W, Liu X, Qian Y, Ma X,
Wang T, Wang X and Gao X: Effect of Shenmai injection on preventing
the development of nitroglycerin-induced tolerance in rats. PLoS
One. 12:e01767772017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YH, Li YY, Fan GW, Yu JH, Duan ZZ, Wang
LY and Yu B: Cardioprotection of Ginsenoside Rb1 against
ischemia/reperfusion injury is associated with mitochondrial
permeability transition pore opening inhibition. Chin J Integr Med.
2016.(Epub ahead of print). View Article : Google Scholar
|
|
35
|
Yang B, Yan P, Gong H, Zuo L, Shi Y, Guo
J, Guo R, Xie J and Li B: TWEAK protects cardiomyocyte against
apoptosis in a PI3K/AKT pathway dependent manner. Am J Transl Res.
8:3848–3860. 2016.PubMed/NCBI
|
|
36
|
Cheng XY, Gu XY, Gao Q, Zong QF, Li XH and
Zhang Y: Effects of dexmedetomidine postconditioning on myocardial
ischemia and the role of the PI3K/Akt-dependent signaling pathway
in reperfusion injury. Mol Med Rep. 14:797–803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Crompton M: The mitochondrial permeability
transition pore and its role in cell death. Biochem J. 341:233–249.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li JZ, Yu SY, Wu JH, Shao QR and Dong XM:
Paeoniflorin protects myocardial cell from doxorubicin-induced
apoptosis through inhibition of NADPH oxidase. Can J Physiol
Pharmacol. 90:1569–1575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li L, Zhou YF, Li YL, Wang LL, Arai H and
Xu Y: In vitro and in vivo antioxidative and hepatoprotective
activity of aqueous extract of Cortex Dictamni. World J
Gastroenterol. 23:2819–3010. 2017.PubMed/NCBI
|
|
41
|
Feng Y, Madungwe NB, da Cruz Junho CV and
Bopassa JC: Activation of G protein-coupled estrogen receptor 1 at
the onset of reperfusion protects the myocardium against
ischemia/reperfusion injury by reducing mitochondrial dysfunction
and mitophagy. Br J Pharmacol. 174:4329–4344. 2017. View Article : Google Scholar : PubMed/NCBI
|