Introduction
Colorectal cancer (CRC) is one of the most lethal
cancers in the world. In Japan, cancer-related death from CRC was
estimated to be the most frequent cause of female cancer-related
death, and the third most frequent cause of male cancer-related
death in 2008 (1). Recent advances
in chemotherapy have been improving the overall survival of
patients with metastatic CRC, however, the prognosis of patients
with metastatic CRC remains relatively low. Therefore,
identification of prognostic factors that could select CRC patients
at a high risk of recurrence would be helpful for planning better
treatment strategies.
Cancer cells generally are hyper-proliferative, have
a high rate of glycolysis and exert anti-apoptotic effects compared
to surrounding normal cells. Glycolysis is a key step for the
acquisition of ATP in all mammalian cells, including cancer
tissues. Metabolism of glucose via glycolysis results in the
production of high concentrations of lactate, which must be
transported out of cells (2). This
transport of lactate across the plasma membrane is mediated by a
family of protone-coupled monocarboxylate transporters (MCTs)
(3).
The MCT family has 14 members (3). Of these members, only MCT1-MCT4
catalyze the proton-coupled transport of lactate (4–8). The
distribution of MCTs is different by cell type. While MCT1 is
expressed in most cells (3,4), the
highest expression of MCT2 is observed in the testis (9), and MCT3 expression is largely
restricted to the retinal pigment epithelium (3,4). On
the other hand, MCT4 is strongly expressed in highly glycolytic
cells, such as white muscle (10),
white blood cells (4,7) and tumors (11–13).
Recently, we reported that MCT4 expression of lung cancer cell
lines are strongly correlated with invasion activity in Matrigel
(14).
Cancer cells produce a large amount of lactic acid
(2), which is generated through
glucose metabolism and inefficient vascular clearing, resulting in
an acidic microenvironment within many solid tumors (15). The partial pressure of oxygen
within human cancer is frequently much lower than that of the
surrounding normal tissue, and intratumoral hypoxia is associated
with an increased risk of local spread, metastasis and patient
mortality (16).
In order to prevent apoptosis due to cellular
acidosis, tumor cells increase their proton efflux through pH
regulators, such as proton pumps, sodium-proton exchangers,
bicarbonate transporters and MCTs, which have been described as
being up-regulated in tumor cells (17). However, only few studies have
evaluated the immunohistochemical expression of MCT4 in cancer
specimens (11–13). The significance of MCT4 expression
in CRC has not been fully evaluated, especially with regard to its
relationship to prognosis. Therefore, the present study
investigated the relationship between clinicopathological factors
and the immunohistochemical MCT4 expression on the plasma membrane
of the primary CRC cells, and prognostic factors of primary CRC
patients.
Patients and methods
Patients
A total of 105 patients with primary CRC who
underwent surgery at the Department of Surgery 1 of the University
Hospital of Occupational and Environmental Health, Japan, from 1997
to 2000, were recruited for this study. The clinical data of these
patients are summarized in Table
I. Informed consent was obtained from all patients prior to the
study. No patients had received chemotherapy or radiotherapy before
surgery. The clinicopathological findings were determined according
to UICC tumor-node-metastasis (TNM) classifications (18).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| No. of
patients | 105 |
| Gender (M/F) | 59/46 |
| Age (years; mean ±
SD) | 65.3±12.1 |
| Tumor size (cm;
mean ± SD) | 5.3±2.1 |
| Histological
type | |
|
Differentiated | 99 |
|
Undifferentiated | 6 |
| Depth of
invasion | |
|
T1/T2/T3/T4a/T4b | 1/19/40/17/28 |
| Lymph node
metastasis (−/+) | 50/55 |
| Distant metastasis
(−/+) | 82/23 |
| TNM stage | |
| I/II/III/IV | 17/31/34/23 |
| Lymphatic invasion
(weak/strong) | 64/41 |
| Venous invasion
(−/+) | 62/43 |
Antibody
For the immunohistochemical staining of
monocarboxylate transporter 4 (MCT4), an anti-MCT4 rabbit
polyclonal antibody (H-90) (sc-50329) was purchased from Santa Cruz
Biotechnology, Inc.
Immunohistochemical staining of MCT4
The immunohistochemical staining (IHC) of MCT4 was
performed on formalin-fixed 2-μm sections of tissues
embedded in paraffin. The 2-μm sections were deparaffinized
in xylene and then rehydrated. Endogenous peroxidase was blocked
with 3% hydrogen peroxidase in methanol for 10 min. After washing
with phosphate-buffered saline (PBS), the sections were
pre-incubated with 10% rabbit serum albumin in PBS for 10 min at
room temperature. The slides were then incubated with the MCT4
antibody for 1 h at room temperature (dilution 1:100). Antibody
binding was visualized using the EnVision+ Dual link system and
diaminobenzidine as chromogen (Dako Cytomation, Kyoto, Japan). The
slides were counterstained with methyl green and mounted.
Staining evaluation
Immunostained slides were analyzed independently by
two authors. Slight differences were resolved by simultaneous
viewing. The expression of MCT4 in the CRC specimens was evaluated
according to the methods described by Pinheiro et al
(13). Sections were scored
semi-quantitatively for the extent of immunoreaction as follows: 0,
0% immunoreactive cells; 1, <5% immunoreactive cells; 2, 5–50%
immunoreactive cells; and 3, >50% immunoreactive cells. In
addition, the intensity of staining was scored semi-quantitatively
as 0, negative; 1, weak; 2, intermediate; and 3, strong. The final
immunoreactions score was defined as the sum of both parameters
(extension and intensity), and samples were grouped as negative
(0), weak staining (1–2), moderate staining (3) and strong staining (4–6). For
statistical purposes, only moderate and strong final immunoreaction
scores were considered to be positive. The other final scores were
considered to be negative.
Clinicopathological assessment
The tumors were staged by two pathologists, who had
no prior knowledge of the results of the assays, according to UICC
TNM classifications 7th edition (18). Clinicopathological factors, such as
age, gender, tumor size, histological type, depth of invasion,
lymph node metastasis, distant metastasis and TNM staging, were
analyzed for an association with MCT4 expression.
Statistical analysis
The relationships between the parameters were also
statistically assessed using the Chi-square test with the Stat
View-J statistical package (version 5.0; SAS Institute, Inc., Cary,
NC, USA). The Kaplan-Meier method was applied to determine
survival, and statistical significance was calculated using the
log-rank test. Both univariate and multivariate analyses of
survival were conducted using the Cox proportional hazards model.
Since the number of patients with TNM stage IV was the same as the
number of patients with distant metastasis, distant metastasis was
excluded as a variable from the multivariate analysis. Statistical
significance was established at p≤0.05.
Results
Table I shows the
profiles of the 105 patients diagnosed with primary CRC recruited
for the present study.
IHC staining of endogenous MCT4 was performed on 105
CRC specimens. Expression of MCT4 was mainly observed on the plasma
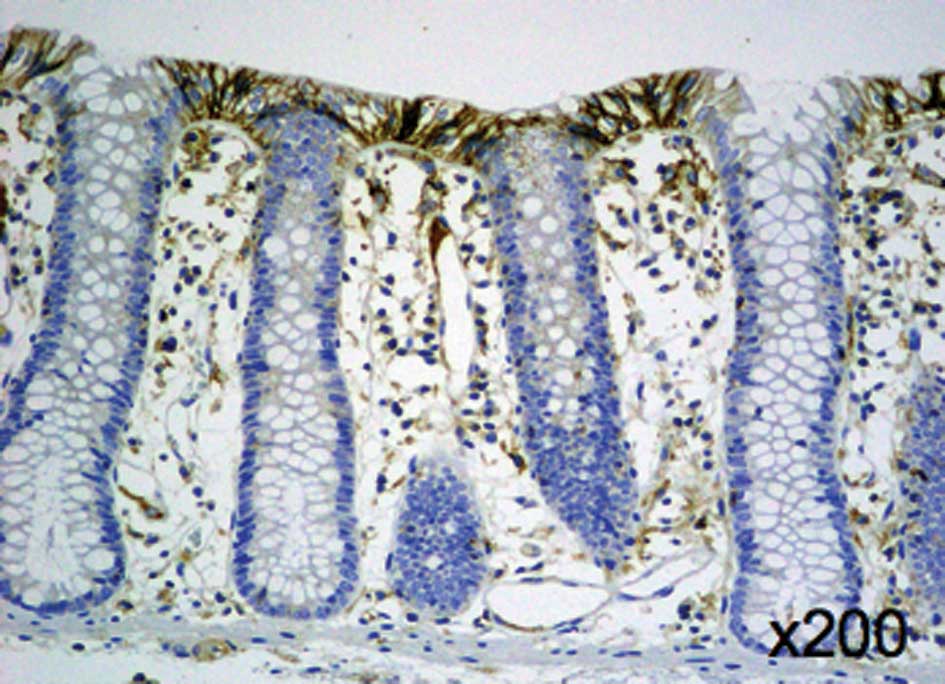
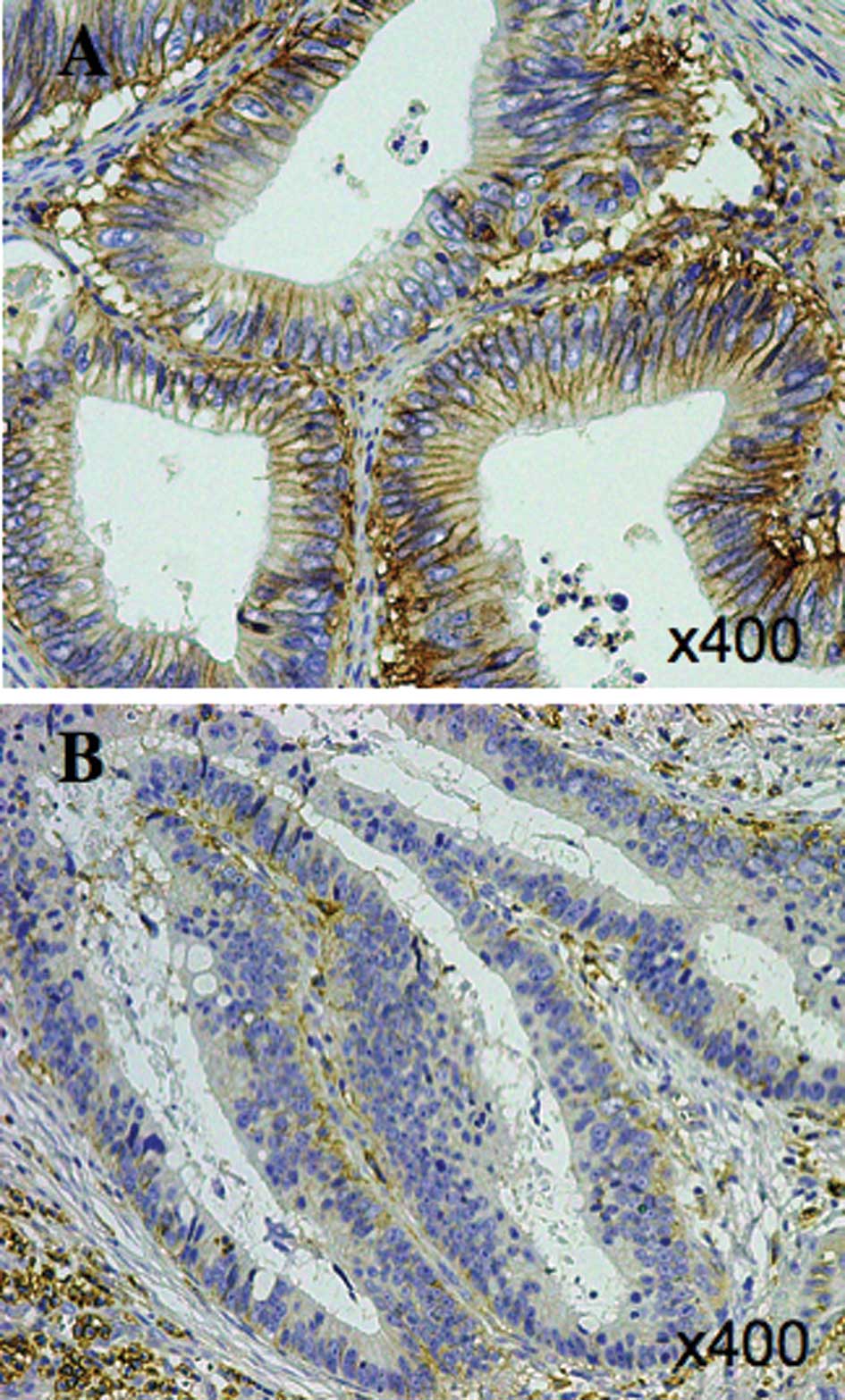
membrane of the normal colonic mucosa (Fig. 1). Positive signals for MCT4 were
mainly observed on the plasma membrane and slightly observed in the
cytoplasm in the cancer cells (Fig.
2A). The MCT4 expression on the plasma membrane was graded as
weak in 49.5% of all cases, moderate in 8.6% and strong in 41.9% of
all cases, and none of the patients had negative staining. A total
of 53 (50.5%) of the 105 patients with CRC were determined to have
positive MCT4 expression, and 52 cases (49.5%) had negative MCT4
expression.
According to the evaluation of the MCT4
immunostaining, the expression of MCT4 was found to significantly
correlate with the tumor size, depth of invasion, lymph node
metastasis, distant metastasis and TNM staging. However, the
expression of MCT4 did not correlate with age, gender or
histopathological type (Table
II).
 | Table II.Association between the expression of
MCT4 and clinicopathological factors of all patients with
colorectal cancer. |
Table II.
Association between the expression of
MCT4 and clinicopathological factors of all patients with
colorectal cancer.
| MCT4 expression
| p-value |
|---|
| Negative | Positive |
|---|
| Age (years) | | | 0.7763 |
| Young | 24 | 23 | |
| Old | 28 | 30 | |
| Gender | | | 0.7587 |
| Male | 30 | 29 | |
| Female | 22 | 24 | |
| Tumor size
(cm) | | | 0.0220 |
| Small | 36 | 25 | |
| Large | 16 | 28 | |
| Histological
type | | | 0.9808 |
|
Differentiated | 49 | 50 | |
|
Undifferentiated | 3 | 3 | |
| Depth of invasion
(T) | | | 0.0096 |
| ≤T4a | 44 | 33 | |
| T4b | 8 | 20 | |
| Lymph node
metastasis (N) | | | 0.0148 |
| (−) | 31 | 19 | |
| (+) | 21 | 34 | |
| Distant metastasis
(M) | | | 0.0110 |
| (−) | 46 | 36 | |
| (+) | 6 | 17 | |
| TNM stage | | | 0.0110 |
| I, II, III | 46 | 36 | |
| IV | 6 | 17 | |
| Lymphatic
invasion | | | 0.0850 |
| Weak | 36 | 28 | |
| Strong | 16 | 25 | |
| Venous
invasion | | | 0.3623 |
| (−) | 33 | 29 | | |
| (+) | 19 | 24 | | |
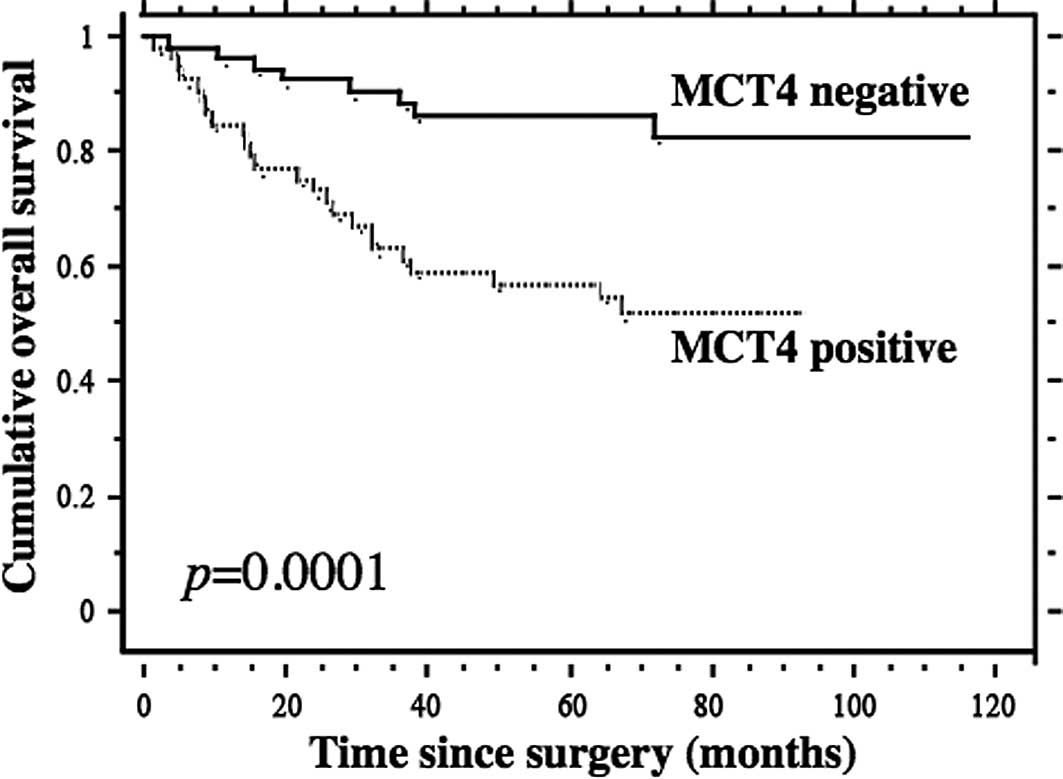
The results of the Kaplan-Meier analyses for overall
survival based on MCT4 expression are shown in Fig. 3. The median follow-up time was 71
months (range 1.4–115.9). The survival rate of the patients who had
negative MCT4 expression was significantly higher than that of
patients with positive MCT4 expression (5-year survival rate, 88.2
vs. 55%, p=0.0001; Fig. 3).
Univariate analysis indicated that the TNM stage,
depth of invasion, presence of lymph node metastasis, presence of
distant metastasis, MCT4 expression and histological type were
significant prognostic factors for CRC (Table III). As the data for TNM staging
were related to the presence of distant metastasis, the data for
the distant metastasis were omitted from the multivariate
analysis.
 | Table III.Univariate analysis of the
clinicopathological factors and the expression of MCT4 in patients
with colorectal cancer. |
Table III.
Univariate analysis of the
clinicopathological factors and the expression of MCT4 in patients
with colorectal cancer.
| Factor | p-value of
univariate analysis |
|---|
| TNM stage (I, II,
III vs. IV) | <0.0001 |
| T (T1, T2, T3,
T4a vs. T4b) | <0.0001 |
| N (− vs. +) | <0.0001 |
| M (− vs. +) | <0.0001 |
| Lymphatic invasion
(weak vs. strong) | <0.0001 |
| Venous invasion (−
vs. +) | <0.0001 |
| MCT4 expression
(negative vs. positive) | 0.0001 |
| Histological type
(diff. vs. undiff.) | 0.0003 |
| Gender (M vs.
F) | 0.0936 |
| Tumor size (small
vs. large) | 0.3086 |
| Age (young vs.
old) | 0.5735 |
Therefore, the multivariate analysis indicated that
the TNM stage, lymph node metastasis and MCT4 expression were
significant prognostic factors for patients with CRC (Table IV).
 | Table IV.Multivariate analysis of the
clinicopathological factors and the expression of MCT4 in patients
with colorectal cancer. |
Table IV.
Multivariate analysis of the
clinicopathological factors and the expression of MCT4 in patients
with colorectal cancer.
| Factor | p-value | Hazard ratio | 95% CI |
|---|
| TNM stage (I, II,
III vs. IV) | <0.0001 | 0.108 | 0.040–0.290 |
| N (− vs. +) | 0.0120 | 0.124 | 0.024–0.632 |
| MCT4 expression
(negative vs. positive) | 0.0201 | 3.163 | 1.198–8.356 |
| Histological type
(diff. vs. undiff.) | 0.1302 | 0.438 | 0.150–1.276 |
| Venous invasion (−
vs. +) | 0.1820 | 0.544 | 0.223–1.330 |
| T (T1, T2, T3, T4a
vs. T4b) | 0.7348 | 1.160 | 0.491–2.744 |
| Lymphatic invasion
(weak vs. strong) | 0.8877 | 0.934 | 0.361–2.417 |
Discussion
Only a few studies that have evaluated the IHC
expression of MCT4 in cancer specimens have been reported (11–13).
In the present study, we demonstrated that 53 (50.5%) of 105
patients with CRC were clearly determined to have positive MCT4
expression, and 52 cases (49.5%) had negative MCT4 expression (no
or weak IHC staining) on the plasma membrane. The other reports
indicated controversial results concerning the IHC expression of
MCT4 in CRC (11–13). One report indicated an absence of
MCT4 expression in CRC (11),
while another report indicated the presence of weak MCT4 expression
in the tumor environment (12).
The third report indicated that 96% of 126 patients with CRC were
determined to have positive MCT4 expression in the cytoplasm or on
the plasma membrane, and 38.1% of 126 patients were determined to
have positive expression on the plasma membrane (13). These discrepancies may be due to
the different antibodies which were used in these studies. Cellular
protein recognized by our antibody against MCT4 was completely
abolished by transfection of two different types of specific MCT4
siRNA (14).
Our study indicated that positive MCT4 expression on
the plasma membrane significantly correlated with tumor size, depth
of invasion, lymph node metastasis, distant metastasis and TNM
staging. However, positive MCT4 expression did not correlate with
age, gender, lymphatic invasion, venous invasion or the
histopathological type (Table II).
These data indicate that positive MCT4 expression on the plasma
membrane may reflect the tumor progression in CRC. Several studies
have indicated that the behavior of cancer cells which express MCT4
is different from cells not expressing MCT4. According to an
experimental study using lung cancer cell lines, the invasion of
the lung cancer cells was reduced by knockdown of MCT4 using MCT4
siRNA (14). Gallagher et
al indicated that the silencing of MCT4 in MDA-MB-231 cells
impaired their migration (19).
Since lactate is co-transported out of the cells with a proton,
silencing MCT would be expected to decrease the intracellular pH
and increase the extracellular pH. Integrin-mediated cell migration
is pH-sensitive, and is inhibited by alkalization of the
extracellular environment (20,21).
Therefore, these studies indicated that a high expression of MCT4
may induce tumor progression.
Our study indicated that the TNM stage, depth of
invasion, lymph node metastasis, distant metastasis, MCT4
expression, lymphatic invasion, venous invasion and histological
type were significant prognostic factors by univariate analysis
(Table III). Moreover,
multivariate analysis indicated that the TNM stage, lymph node
metastasis and MCT4 expression were significant prognostic factors
for patients with CRC (Table IV).
These data indicate that positive MCT4 expression on the plasma
membrane correlates with the tumor progression of CRC and the poor
survival of patients with CRC.
The accumulation of lactate within tumors has been
reported to be associated with a poor clinical outcome (22,23).
It is well established that increased rates of glycolysis in
hypoxia are associated with greater expression of glycolytic
enzymes, including the glucose transporter, Glut-1, due to a
transcriptional mechanism involving a hypoxia inducible factor
(HIF-1α) (24). Ullah et al
indicated that MCT4, like other glycolytic enzymes, is up-regulated
by hypoxia through a HIF-1α-mediated mechanism (25). Chronic hypobaric hypoxia has been
reported to up-regulate MCT4, but not MCT1, in rat skeletal muscle
(26), zebra fish gills (27) and certain tumor cells (28), at least in part, through a
transcriptional mechanism. These studies suggest that the
accumulation of lactate within tumors up-regulates MCT4 through a
HIF-1α-mediated mechanism and that this correlates with a poor
clinical outcome.
Certain studies have indicated the relationship
between MCT4 and drug resistance. Overexpression of CD44v3-10, MDR1
and MCT4 was found in most primary prostate cancer tissues, and was
significantly associated with prostate cancer progression (29). Another study indicated that the
overexpression of CD147, MCT1 and MCT4 is correlated with the
progression of epithelial ovarian cancer (EOC) (30). This suggests that the
overexpression of MCT1/MCT4 is related to drug resistance during
EOC metastasis, and that these proteins could be useful therapeutic
targets to prevent the development of incurable, recurrent and
drug-resistant EOC (30). These
studies indicate that the overexpression of MCT4 may be related to
drug-resistance through induction of the expression of MDR.
Many inhibitors of the MCT family have been reported
(31). DDS is an anion transporter
inhibitor and suppresses the function of all proteins in the MCT
family. Quercetin and simvastatin inhibit human MCT1 and human
MCT4, respectively. Simvastatin has been studied for the treatment
of several malignancies, such as colon cancer, prostate cancer and
chronic lymphocytic leukemia (32–35).
In fact, a phase II study of simvastatin plus irinotecan,
5-fluoruracil and leucovorin as first-line chemotherapy has been
carried out (33).
The mechanisms of MCT4 regulation are not fully
understood. However, the regulation of MCT4 expression may be
important, since CRC patients with negative MCT4 immuno-staining
had a more favorable prognosis. More extensive studies concerning
the regulation of MCT4 are therefore required.
Acknowledgements
The authors thank Ms. Yuko Ueda,
Hitomi Arimasu and Miyuki Warashina for the technical assistance.
This study was supported in part by a Grant-in-Aid for Scientific
Research from the Ministry of Education, Science and Culture of
Japan.
References
|
1.
|
Center for Cancer Control and Information
Services, National Cancer Center, Japan: Vital Statistics Japan
(Ministry of Health, Labour and Welfare).
|
|
2.
|
PM GullinoSH ClarkFH GranthamThe
interstitial fluid of solid tumorsCancer
Res24780796196414190544
|
|
3.
|
AP HalestrapAP MeredithThe SLC16 gene
family – from monocarboxylate transporters (MCTs) to aromatic amino
acid transporters and beyondPflugers Arch Eur J
Physiol4476196282004
|
|
4.
|
AP HalestrapNT PriceThe proton-linked
monocarboxylate transporter (MCT) family: structure, function and
regulationBiochem
J343281299199910.1042/0264-6021:343028110510291
|
|
5.
|
S BröerA BröerHP SchneiderC StegenAP
HalestrapJW DeitmerCharacterization of the high-affinity
monocarboxylate transporter MCT2 in Xenopus laevis
oocyteBiochem J3415295351999
|
|
6.
|
EF GrollmanNJ PhilpP McPhieRD WardB
SauerDetermination of tranport kinetics of chick MCT3
monocarboxylate transporter from retinal pigment epithelium by
expression in genetically modified
yeastBiochemistry3993519357200010.1021/bi000464+10924129
|
|
7.
|
KS DimmerB FriedrichF LangJW DeitmerS
BröerThe low-affinity monocarboxylate transporter MCT4 is adapted
to the export of lactate in highly glycolytic cellsBiochem
J350219227200010.1042/0264-6021:350021910926847
|
|
8.
|
JE Manning FoxD MeredithAP
HalestrapCharacterisation of human monocarboxylate transporter 4
substantiates its role in lactic acid efflux from skeletal muscleJ
Physiol529285293200011101640
|
|
9.
|
RY LinJC VeraRS ChagantiDW GoldeHuman
monocarboxylate transporter 2 (MCT2) is a high affinity pyruvate
transporterJ Biol
Chem2732895928965199810.1074/jbc.273.44.289599786900
|
|
10.
|
C JuelAP HalestrapLactate transporter in
skeletal muscle-role of regulation of the monocarboxylate
transporterJ
Physiol517633642199910.1111/j.1469-7793.1999.0633s.x
|
|
11.
|
DW LambertIS WoodA EllisSP
Shirazi-BeecheyMolecular changes in the expression of human colonic
nutrient transporters during the transition from normality to
malignancyBr J
Cancer8612621269200210.1038/sj.bjc.660026411953883
|
|
12.
|
MI KoukourakisA GiatromanolakiAI HarrisE
SivridisComparison of metabolic pathways between cancer cells and
stromal cells in colorectal carcinomas: a metabolic survival role
for tumor-associated stromaCancer
Res66632637200610.1158/0008-5472.CAN-05-3260
|
|
13.
|
C PinheiroA Longatto-FilhoC ScapulatempoL
FerreiraS MartinsL PellerinM RodriguesVAF AlvesF SchmittF
BaltazarIncreased expression of monocarboxylate transpoerters 1, 2
and 4 in colorectal carcinomasVirchows
Arch452139146200810.1007/s00428-007-0558-518188595
|
|
14.
|
H IzumiM TakahashiH UramotoY NakayamaT
OyamaK-Y WangY SasaguriS NishizawaK KohnoMonocarboxylate
transporter 1 and 4 involved in the invasion activity of human lung
cancer cellsCancer
Sci10210071013201110.1111/j.1349-7006.2011.01908.x21306479
|
|
15.
|
RA GatenbyRJ GilliesWhy do cancers have
high aerobic glycolysis?Nat Rev
Cancer4891899200410.1038/nrc147815516961
|
|
16.
|
P VaupelA MayerHypoxia and cancer:
significance and impact on clinical outcomeCancer Metastasis
Rev26225239200710.1007/s10555-007-9055-117440684
|
|
17.
|
H IzumiT TorigoeH IshiguchiH UramotoY
YoshidaM TanabeT IseT MurakamiT YoshidaM NomotoK KohnoCellular pH
regulators: potentially promising molecular targets for cancer
chemotherapyCancer Treat
Rev29541549200310.1016/S0305-7372(03)00106-314585264
|
|
18.
|
LH SobinMK GospodarowiczC
WittekindInternational Union Against Cancer (UICC) TNM
Classification of Malignant Tumors7th editionWiley-LissNew
York2010
|
|
19.
|
SM GallagherJJ CastorinoD WangNJ
PhilpMonocarboxylate transporter 4 regulates maturation and
trafficking of CD147 to the plasma membrane in the metastatic
breast cancer cell line MDA-MB-231Cancer
Res6741824189200710.1158/0008-5472.CAN-06-318417483329
|
|
20.
|
V BetapudiLS LicataTT EgelhoffDistinct
roles of nonmuscle myosin II isoforms in the regulation of
MDA-MB-231 breast cancer cell spreading and migrationCancer
Res6647254733200610.1158/0008-5472.CAN-05-423616651425
|
|
21.
|
C StockB GassnerCR HauckH ArnoldS MallyJA
EbleP DieterichA SchwabMigration of human melanoma cells depends on
extracellular pH and Na−/H+ exchangeJ
Physiol567225238200510.1113/jphysiol.2005.08834415946960
|
|
22.
|
KM KennedyMW DewhirstTumor metabolism of
lactate: the influence and therapeutic potential for MCT and CD147
regulationFuture Oncol6127148201010.2217/fon.09.14520021214
|
|
23.
|
S WalentaM WetterlingM LehrkeG SchwickertK
SundforEK RofstadW Mueller-KlieserHigh lactate levels predict
likelihood of metastasis, tumor recurrence, and restricted patient
survival in human cervical cancersCancer
Res60916921200010706105
|
|
24.
|
GL SemenzaHypoxia-inducible factor 1:
oxygen homeostasis and disease pathophysiologyTrend Mol
Med7345350200110.1016/S1471-4914(01)02090-111516994
|
|
25.
|
MS UllahAJ DeviesAP HalestrapThe plasma
membrane lactate transporter MCT4, but not MCT1, is upregulated by
hypoxia through a HIF-1α-dependent mechanismJ Biol
Chem281903090372006
|
|
26.
|
G PyN EydouxK LambertR ChapotN KoulmannH
SanchesL BahiA PeinnequinJ MercierAX BigardRole of hypoxia-induced
anorexia and right ventricular hypertrophy on lactate transporter
and MCT expression in rat
muscleMetabolism54634644200510.1016/j.metabol.2004.12.00715877294
|
|
27.
|
DL Van der MeerGE van den ThillartF
WitterMA de BakkerJ BesserMK RichardsonHP SpainkJT LeitoCP
BagowskiGene expression profiling of the long-term adaptive
response to hypoxia in the gills of adult zebrafishAm J Physiol
Regul Integr Comp Physiol289R1512R1519200515994372
|
|
28.
|
JJ OrdEH StreeterISD RobertsD CranstonAL
HarrisComparison of hypoxia transcriptome in vitro with in vivo
gene expression in human bladder cancerBr J
Cancer93346354200510.1038/sj.bjc.660266616052224
|
|
29.
|
J HaoH ChenMC MadiganPJ CozziJ BeretovWJ
DelpradoPJ RussellCo-expression of CD147 (EMMPRIN), CD44v3-10, MDR1
and monocarboxylate transporters is associated with prostate cancer
drug resistance and progressionBr J
Cancer10310081018201010.1038/sj.bjc.660583920736947
|
|
30.
|
H ChenL WangJ BeretovJ HaoW XiaoY
LiCo-expression of CD147/EMMPRIN with monocarboxylate transporters
and multiple drug resistance proteins is associated with epithelial
ovarian cancer progressionClin Exp
Metastasis27557569201010.1007/s10585-010-9345-920658178
|
|
31.
|
ME MorrisMA FelmleeOverview of the
proton-coupled MCT (SLC16A) family of transporters:
characterization, function and role in the transport of the drug of
abuse γ-hydroxybutyric acidAAPS J10311321200818523892
|
|
32.
|
SJ ChoJS KimJM KimJY LeeHC JungIS
SongSimvastatin induces apoptosis in human colon cancer cells and
in tumor xenografts, and attenuates colitis-associated colon cancer
in miceInt J Cancer123951957200810.1002/ijc.2359318521906
|
|
33.
|
J LeeKH JungYS ParkJB AhnSJ ShinSA OmY
OhdoDB ShinTW KimN LeeJH ByunYS HongJO ParkSH ParkHY LimWK
KangSimvastatin plus irinotecan, 5-fluorouracil, and leucovorin
(FOLFIEI) as first-line chemotherapy in metastatic colorectal
patients: a multicenter phase II studyCancer Chemother
Pharmacol64657663200910.1007/s00280-008-0913-519169686
|
|
34.
|
ST KochuparambilB Al-HuseinA GocS
SolimanPR SomanathAnticancer efficacy of simvastatin on prostate
cancer cells and tumor xenografts is associated with inhibition of
Akt and reduced prostate-specific antigen expressionJ Pharmacol Exp
Ther336496505201010.1124/jpet.110.174870
|
|
35.
|
M PodhoreckaD HalickaP KlimekM KowalS
ChocholskaA DmoszynskaSimvastatin and purine analogs have a
synergic effect on apoptosis of chronic lymphocytic leukemia
cellsAnn Hematol8911151124201010.1007/s00277-010-0988-z20499237
|