Introduction
Osteonecrosis of the femoral head (ONFH) is an
ischemic disease that may result in femoral head collapse. It is
caused by multiple factors, such as trauma, alcoholism, long-term
use of hormones and Legg-Calve-Perthes disease. The incidence of
ONFH in adults is increasing, resulting in significant problems
worldwide (1). Although surgical
intervention is often used for the treatment of ONFH (2–4), the
efficacy is not sufficient for patients. Furthermore, the surgical
methods and curative effects are difficult to define as the
pathogenesis of ONFH remains unclear (5,6).
Although numerous surgical and non-surgical animal
models of ONFH have been established (5–7),
there is not a reliable animal model of the early stages of ONFH
that may be used for the evaluation of novel therapeutic
approaches. Therefore, the aim of the present study was to
establish a defined rabbit ONFH model with partial necrosis of the
femoral heads using an argon-helium freeze-thaw method under the
guidance of magnetic resonance imaging (MRI).
Materials and methods
Animal experiments
A total of 48 New Zealand rabbits (weight, 3.50±0.30
kg) were purchased from Xilingjiao Aquaculture Breeding Center
(Jinan, China) and used to generate the ONFH models. The rabbits
were maintained in standard conditions with free access to food and
water. In group I, the left femoral head of every rabbit received
two cycles of argon-helium freezing-thawing, while in group II, the
right femoral head of each rabbit received only one cycle of
argon-helium freezing-thawing. The study was performed in
accordance with the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health and the protocol was
approved by the Animal Care and Use Committee of Shandong
University (Jinan, China).
Using a CRYO-HIT system (Galil Medical Ltd.,
Yokneam, Israel), the model was established in an MRI
interventional unit (Shandong Medical Imaging Research Institute,
Jinan, China), which used a 0.23-T open-configuration MRI system
mounted with an iPath 200 optical tracking system (Panorama;
Philips Medical Systems, Vantaa, Finland). A hole was drilled from
the lateral side of the proximal femur into the center of the two
femoral heads under MRI guidance. The diameter of the drill track
was 2.0 mm and the depth was 5.0 mm under the cartilage (Fig. 1A).
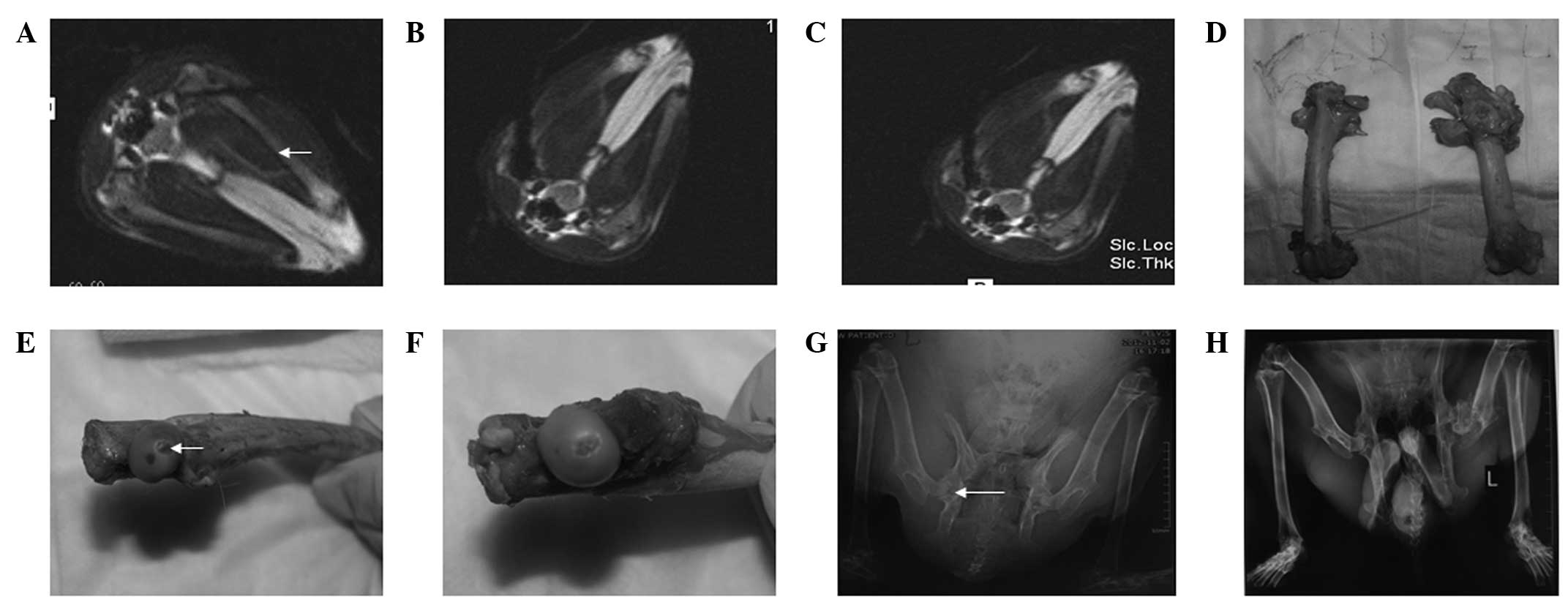 | Figure 1Surgery of the rabbits (groups I and
II) was performed in an open 0.23-T MRI system. (A) As guided by
MRI, the guide pin was inserted into the bones and located at a
position 5 mm below the articular cartilage. (B) In group I
rabbits, an ice ball appeared following two freeze-thaw cycles. (C)
In group II, one freeze-thaw cycle was performed. (D) At week 4
following the establishment of the model, the femoral head contour
was smooth and the cartilage surface was intact, without any
defects detected (left, group I left femoral head; right, group II
right femoral head). At week 12 following surgery the (E) left
femoral head cartilage was defected, as indicated with the arrow,
and (F) the right femoral head cartilage was almost integral. (G)
At week 8 following surgery, X-ray images showed that the left
femoral head had cystic lesions, as indicated with the arrow, while
the right femoral head had no abnormal changes. (H) At week 12
following surgery, X-ray images showed that the left femoral head
had collapsed, but the right femoral head remained intact. MRI,
magnetic resonance imaging. |
All the femoral heads were evaluated with X-ray
scans (X-ray units and DR radiographic systems; General Medical
Merate S.p.A, Seriate, Italy) at weeks 4, 8 and 12 following
surgery. In total, 16 animals were sacrificed by air embolism at
weeks 4, 8 and 12 after surgery and the surface of the cartilage
and bone tunnel of the femoral heads were observed.
Hematoxylin and eosin (HE) histological
analyses
A total of 16 rabbits from each group were
sacrificed at weeks 4, 8 and 12 following surgery. The two femoral
heads of each rabbit were fixed, decalcified, embedded and cut into
5-μm sections. Staining of the samples with HE was then performed.
A total of 50 fields were randomly selected and at least 200
lacunae were counted. The percentage of empty lacunae was defined
as the ratio of empty lacuna number to the total lacuna count. The
mean values of three independent experiments were calculated. The
histological images were converted to grayscale images using a
computer to calculate the percentage of empty lacunae.
Statistical analysis
All numerical data are presented as the mean ± SD.
The differences between the two groups were calculated using the
Student’s t-test. Differences between multiple groups were
calculated with one-way analysis of variance (SAS 8.1; SPSS version
17.0, SPSS, Inc., Chicago, IL, USA). Values were considered to
indicate a statistically significant difference when P<0.05.
Statistical analyses were performed using the SPSS
statistical package, version 17.0 (SPSS, Inc., Chicago, IL, USA).
The incidence of femoral collapse in groups I and II were compared
using the χ2-test. Comparisons of the percentage of
empty lacunae between weeks 4, 8 and 12 were performed using an
unpaired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
General data of the animals
To establish a novel ONFH animal model using an
MRI-guided argon-helium cryotherapy system, 48 rabbits were used.
In group I, the left femoral head of every rabbit received two
cycles of argon-helium freezing-thawing (Fig. 1B), while in group II, the right
femoral head of each rabbit received only one cycle of argon-helium
freezing-thawing (Fig. 1C).
In the experiments, none of the rabbits exhibited
skin necrosis or infection and there were no mortalities. The
femoral head contours of the rabbits in the two groups were smooth
and the cartilage surfaces were integral, without any defects
detected at week 4 following surgery (Fig. 1D). The femoral head contours of the
rabbits in group I became pale, flat and mushroom-shaped, with some
bones collapsing at week 12 following surgery (Fig. 1E). Three months after surgery, the
right femoral head cartilage was almost intact (Fig. 1F). These results indicated that a
novel ONFH animal model using an MRI-guided argon-helium
cryotherapy system was successfully established.
Radiological analysis
To further determine the differences between groups
I and II, radiological analyses were performed. In group I, the
bone densities of the femoral heads in 10 rabbits were decreased at
week 4 following surgery. Cystic changes appeared at week 8
(Fig. 1G) and seven femoral heads
were collapsed at week 12 following surgery (Fig. 1H; Table I). In group II, the bone densities
of the femoral heads in nine rabbits were reduced at week 4
following surgery. Cystic changes and narrowed hip joint space were
observed at week 8 and two femoral heads were collapsed at week 12
(Table I).
 | Table ICollapse rates of the femoral heads in
groups I and II at week 12 following surgery (n=16). |
Table I
Collapse rates of the femoral heads in
groups I and II at week 12 following surgery (n=16).
| Group | Collapsed, n | Non-collapsed, n | Collapse rate
(%) |
|---|
| I | 7 | 9 | 43.7a |
| II | 2 | 14 | 12.5 |
Histological analysis
HE analyses were performed. A normal femoral head is
shown in Fig. 2A and the cases in
group I are shown in Fig. 2B–E. At
week 4 following the induction of necrosis, a number of osteocytes
were necrotic (Fig. 2B),
hematopoietic cells were absent and a large number of erythrocytes
had died in the marrow cavities. At week 8, the majority of the
marrow cavities were filled with fibrous tissue, characterized by a
high cellularity with numerous macrophages (Fig. 2C). The chondrocytes were
disorganized and the articular surface was rough (Fig. 2D). As observed in an additional
group I case, chondrocytes were dispersed (Fig. 2E). Group II cases are shown in
Fig. 2F–H. In group II at week 4
following surgery, the percentage of cell lacunae was significantly
less when compared with group I (Fig.
2F). In addition, at week 8 following surgery, lacunae cells
were easily identifiable and fibrous tissues had formed (Fig. 2G). At week 12 following surgery,
the cartilage cells of group II were well arranged and no evident
collapses were identified in the cartilage surface (Fig. 2H). As presented in Table II, the percentages of lacunae in
the femoral heads of group I at weeks 4, 8 and 12 following surgery
(49.75±3.17, 62.06±4.12 and 48.25±2.76%, respectively) were higher
than those in group II (39.13±4.48, 50.69±3.84 and 37.50±3.86%,
respectively). The percentage of empty lacunae in group I was
62.06% at week 8 following surgery, indicating that bone resorption
plays a predominant role. Therefore, the results indicate that the
percentage of empty lacunae in group I was higher than that in
group II at weeks 4, 8 and 12 following surgery.
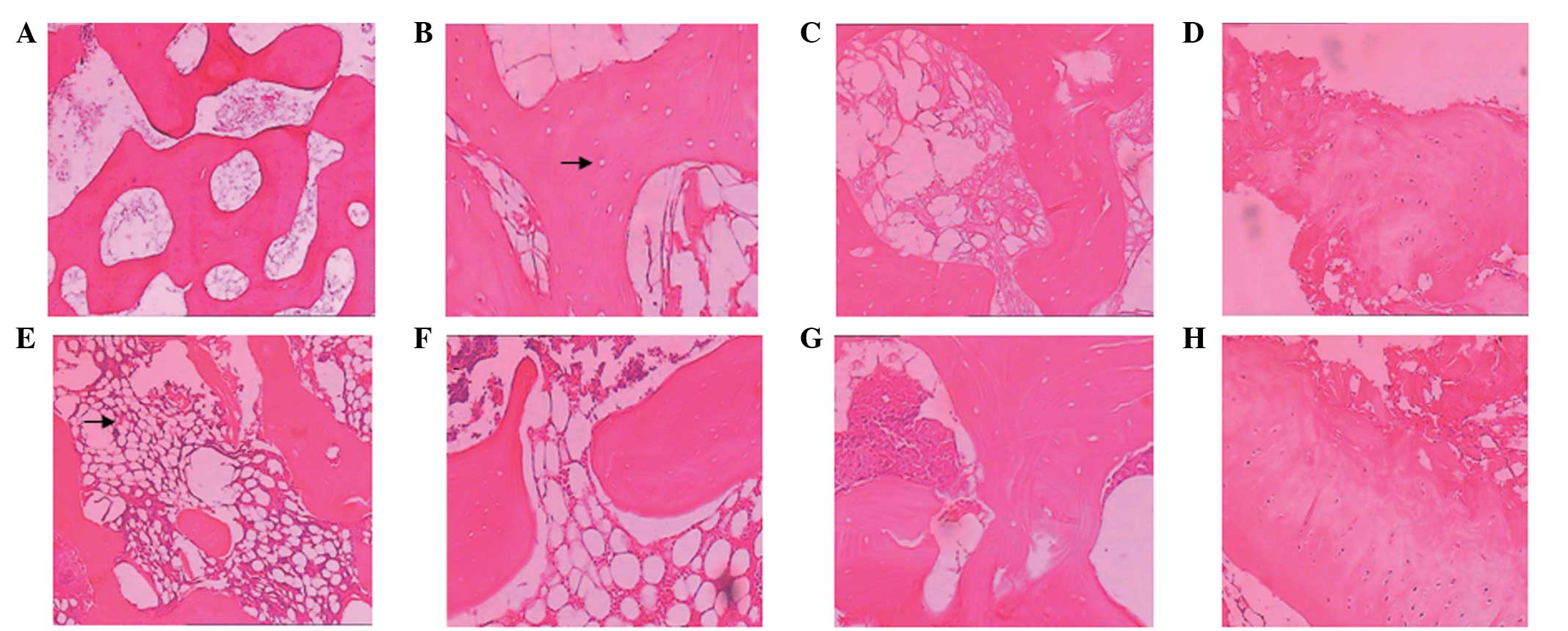 | Figure 2HE staining. (A) A normal femoral head
(magnification, ×40). In group I at (B) week 4 following surgery,
lacunae (as indicated by the arrow) were observed (magnification,
×100); (C) week 8, the lacunae size increased significantly
(magnification, ×100); and (D) week 12, the chondrocytes were
disorganized, the articular surface was rough and (E) new bones
were formed in the bone necrosis areas, as indicated by the arrow.
In group II at (F) week 4 following surgery, the number of cell
lacunae was significantly lower than that in group I; (G) week 8,
the lacunae cells were identifiable and fibrous tissues had formed;
and (H) week 12, cartilage cells were well arranged and no evident
collapses were identified in the cartilage surface. HE, hematoxylin
and eosin. |
 | Table IIPercentages of lacunae in the femoral
heads of groups I and II at weeks 4, 8 and 12 following surgery
(n=16). |
Table II
Percentages of lacunae in the femoral
heads of groups I and II at weeks 4, 8 and 12 following surgery
(n=16).
| Weeks after
surgery |
|---|
|
|
|---|
| Group | 4 | 8 | 12 |
|---|
| I | 49.75±3.17 | 62.06±4.12a | 48.25±2.76 |
| II | 39.13±4.48b | 50.69±3.84b | 37.50±3.86b |
Discussion
Osteonecrosis can be induced by liquid nitrogen and
heat (7–11). To the best our knowledge, there are
yet to be any studies on the use of cryoablation in establishing an
ONFH animal model. The present study used an argon-based system, as
discussed in a number of previous studies (12–15),
to establish an ONFH animal model. Under the guidance of MRI, the
probes can be maintained in the correct position in every femoral
head. All the femoral heads of the rabbits used in the present
study received precise and well-controlled treatment and two or
three freezing cycles led to complete interface sterilization. The
differences between a single freezing cycle and two freezing cycles
have been demonstrated to be significant (16). The present study divided the
rabbits into two groups; group I received two freeze-thaw cycles
and group II received one freeze-thaw cycle. The percentage of
empty lacunae in group I was higher than that in group II at weeks
4, 8 and 12 following surgery. In addition, a statistically
significant difference was observed in the femoral head collapse
rates between the two groups. Therefore, the results of the present
study indicate that an animal model of ONFH was successfully
established using an argon-helium cryotherapy system. Furthermore,
MRI-guided argon-helium cryotherapy system may provide animal
models of ONFH with high reliability, good repeatability and a
precisely controlled necrotic region, which may be of great
importance for the study of ONFH
Acknowledgements
The study was supported by a grant from the National
Natural Science Foundation of China (no. 81271966).
References
|
1
|
Etienne G, Mont MA and Ragland PS: The
diagnosis and treatment of nontraumatic osteonecrosis of the
femoral head. Instr Course Lect. 53:67–85. 2004.PubMed/NCBI
|
|
2
|
Mont MA, Carbone JJ and Fairbank AC: Core
decompression versus nonoperative management for osteonecrosis of
the hip. Clin Orthop Relat Res. 324:169–178. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choy WS, Kim KJ, Lee SK, et al:
Ceramic-on-ceramic total hip arthroplasty: minimum of six-year
follow-up study. Clin Orthop Surg. 5:174–179. 2013.PubMed/NCBI
|
|
4
|
Gao YS, Chen SB, Jin DX, et al: Modified
surgical techniques of free vascularized fibular grafting for
treatment of the osteonecrosis of femoral head: Results from a
series of 407 cases. Microsurgery. Aug 1–2013.(Epub ahead of
print). View Article : Google Scholar
|
|
5
|
Lieberman JR, Berry DJ, Mont MA, et al:
Osteonecrosis of the hip: management in the 21st century. Instr
Course Lect. 52:337–355. 2003.PubMed/NCBI
|
|
6
|
Mont MA and Hungerford DS: Non-traumatic
avascular necrosis of the femoral head. J Bone Joint Surg Am.
77:459–474. 1995.PubMed/NCBI
|
|
7
|
Takaoka K, Yoshioka T, Hosoya T, et al:
The repair process in experimentally induced avascular necrosis of
the femoral head in dogs. Arch Orthop Trauma Surg. 99:109–115.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cetik O, Cift H, Comert B and Cirpar M:
Risk of osteonecrosis of the femoral condyle after arthroscopic
chondroplasty using radiofrequency: a prospective clinical series.
Knee Surg Sports Traumatol Arthrosc. 17:24–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonutti PM, Seyler TM, Delanois RE, et al:
Osteonecrosis of the knee after laser or radiofrequency-assisted
arthroscopy: treatment with minimally invasive knee arthroplasty. J
Bone Joint Surg Am. 88(Suppl 3): 69–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Han R, Geng C, et al: A new
osteonecrosis animal model of the femoral head induced by microwave
heating and repaired with tissue engineered bone. Int Orthop.
33:573–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan M, Peng J, Wang A, et al: Emu model of
full-range femoral head osteonecrosis induced focally by an
alternating freezing and heating insult. J Int Med Res. 39:187–198.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yanagawa B, Holmes SD, Henry L, Hunt S and
Ad N: Outcome of concomitant Cox-maze III procedure using an
argon-based cryosurgical system: a single-center experience with
250 patients. Ann Thorac Surg. 95:1633–1639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albåge A, Péterffy M and Källner G:
Learning what works in surgical cryoablation of atrial
fibrillation: results of different application techniques and
benefits of prospective follow-up. Interact Cardiovasc Thorac Surg.
13:480–484. 2011.PubMed/NCBI
|
|
14
|
Ad N, Henry L and Hunt S: The concomitant
cryosurgical Cox-maze procedure using argon based cryoprobes: 12
month results. J Cardiovasc Surg (Torino). 52:593–599.
2011.PubMed/NCBI
|
|
15
|
Wu B, Xiao YY, Zhang X, Zhao L and Carrino
JA: CT-guided percutaneous cryoablation of osteoid osteoma in
children: an initial study. Skeletal Radiol. 40:1303–1310. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robinson D, Halperin N and Nevo Z: Two
freezing cycles ensure interface sterilization by cryosurgery
during bone tumor resection. Cryobiology. 43:4–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
















