Introduction
Ankylosing spondylitis (AS) is characterised by
inflammatory back pain, asymmetrical peripheral arthritis,
enthesitis and extra-articular features (1). AS is genetically associated with
human leucocyte antigen B27, and the average age at disease onset
is 27.7 years in B27− AS and 24.8 years in
B27+ AS (2). The
worldwide prevalence of AS varies from 0.1 to 1.1% in the adult
Caucasian population (3). Patients
with AS are likely to lose their physical function and ability to
work, which is likely to have a significant impact on the quality
of life unless an appropriate treatment is administered to the
patients.
Tumour necrosis factor-α (TNF-α) plays a crucial
role in the pathogenesis of AS, and TNF-α concentrations are
increased in the circulation (4)
and synovial tissue (5) in
patients with AS. Etanercept (ETN), which binds to TNF-α and blocks
its biological activity, is a recombinant, dimeric fusion protein.
It has been demonstrated that ETN is beneficial in the treatment of
rheumatic diseases, including rheumatoid arthritis (6), psoriatic arthritis (7) and polyarticular juvenile rheumatoid
arthritis (8). However, a number
of patients with AS are not suitable for ETN treatment due to
side-effects or a poor clinical response. Owing to the high
medication cost, ETN treatment is not generally favoured in the
developing world.
Non-steroidal anti-inflammatory drugs (NSAIDs) are
the first-line drugs for AS. Conventional disease-modifying
antirheumatic drugs (DMARDs) are occasionally recommended by
physicians; however, there is no evidence for the efficacy of these
drugs in the treatment of axial disease (9). At an early disease stage, patients
with AS with a higher erythrocyte sedimentation rate (ESR) and
peripheral arthritis may benefit from sulfasalazine (SSZ), as
concluded in a Cochrane review (10). Another meta-analysis (11) also indicated that SSZ was a safe
and effective drug for the short-term treatment of AS. SSZ may be
considered in patients with peripheral arthritis, based on the
Assessment in AS (ASAS)/European League Against Rheumatism
recommendations (9). In addition,
SSZ has the advantage over ETN in medical cost. Therefore, SSZ has
been extensively used in underdeveloped countries.
To the best of our knowledge, Li et al
(12) performed the first
systematic review concerning the effects of ETN in the treatment of
AS in 2009. Subsequent systematic reviews have been reported.
However, the previous reviews concentrated on comparing the total
effects of ETN. In the present study, two subgroup analyses were
performed to compare the effects of ETN with a placebo (PBO) or
SSZ. Continuous updates can collect data from new studies. Thus, a
novel comprehensive systematic review and overall meta-analysis are
requisite for drawing more reliable conclusions about the effects
of ETN in the treatment of AS.
Methods
To ensure the accuracy of the present systematic
review and meta-analysis, the results were designed and reported by
employing a checklist of items that was as consistent as possible
with the Preferred Reporting Items for Systematic Review and
Meta-Analyses statement (13).
Literature search strategy
The following digital databases were searched for
the identification of studies: PubMed, Embase, the Cochrane Library
and ClinicalTrials.gov. In addition, Chinese databases were
searched, including the China National Knowledge Infrastructure,
VIP, Chinese Biomedical Literature and WanFang Databases, and the
Chinese Clinical Trial Register. All the databases were searched
from the available date of inception to the latest issue
(2013).
Different search strategies were merged as follows.
For the English databases, free-text terms were used, including
‘Etanercept’, or ‘Enbrel’ and ‘Ankylosing Spondylitis’, ‘AS’ or
‘Bechterew Disease’. For the Chinese databases, free-text terms
were used, such as ‘Yi Na Xi Pu’, ‘Yi SaiPu’, ‘En Li’ or ‘QiangKe’
(which are the alternative names of ETN in Chinese) and ‘QiangZhi
Xing Ji Zhu Yan’ or ‘QiangZhi Xing JiZhui Yan’ (which means ‘AS’ in
Chinese). A filter for clinical trials was applied. To collect an
adequate number of trials, the reference lists of the relevant
publications were carefully read.
Inclusion and exclusion criteria
Regardless of blinding, publication status or
language, RCTs were included. Individual cases that were diagnosed
as exhibiting AS according to the modified New York criteria for AS
(14) were collected. For the
types of interventions, treatment with ETN alone in RCTs was
considered. The control groups consisted of treatments with a PBO
or SSZ. Studies were only included if the intervention was
administered for at least six weeks.
Case reports, reviews, retrospective studies,
open-label extension studies and studies without a control group
were excluded. Also excluded were RCTs without a clear description
of the required outcomes of interest, and particularly those
studies that did not describe the exact means and standard
deviations (SDs) of the outcomes. The studies comparing various TNF
inhibitors were also excluded since there is no evidence to support
a difference in their efficacies in treating the axial and
articular/entheseal disease manifestations (9).
Data extraction
The search strategy, application of inclusion
criteria, data extraction and statistical analyses were
independently executed by two of the present authors. Any
disagreements were resolved by consensus or mediation by a third
author. The methodological quality of each study was assessed
according to the following criteria: Baseline difference, method of
randomisation, degree of blinding, use of intention-to-treat (ITT)
analysis, and description of dropouts and withdrawals. The
validated Jadad scale was used to assess the quality of each study
(15).
The primary outcome was the proportion of patients
achieving the ASAS 20% response (ASAS 20) established by the ASAS
Working Group (16). The secondary
outcomes comprised the ASAS 50, ASAS 70, Bath AS Disease Activity
Index (BASDAI) (17), BASDAI 50,
Bath AS Functional Index (BASFI) (18), ASAS partial remission (ASAS PR)
(16), and levels of ESR and
C-reactive protein. Spinal mobility, assessed by the Schober’s test
(ST) and the occiput-to-wall (OW) distance, was also considered to
be a secondary outcome. The authors of the study were contacted if
any outcome was ambiguous or absent from the article. If the author
could not be reached, the data was extracted by consensus.
Statistical analysis
To summarise the effects of ETN, Review Manager
statistical software (version 5.2; Cochrane Collaboration,
Copenhagen, Denmark) was used to calculate weighted mean
differences (MDs), standard MDs (SMDs), and the 95% confidence
interval (CI) for the continuous data. MDs were used if outcomes
were measured in the same way between trials, while SMDs were used
if the same outcomes were measured by adopting different methods.
For dichotomous data, the data were pooled and expressed as odds
ratios (ORs) with a 95% CI. Heterogeneity was evaluated via
subgroup analysis using the χ2 and I2 tests.
Where the heterogeneity test was at P>0.10, the data were pooled
via a fixed effects model; otherwise, a random effects model was
used. Publication bias was evaluated by the Egger’s regression
asymmetry and Begg’s tests when the number of included trials
exceeded five (Stata 12.0 software; StataCorp LP, TX, USA). The
total effect was tested using a Z score, and P<0.05 was
considered to be statistically significant in all the analyses. To
minimise the clinical heterogeneity, two subgroup analyses were
performed: ETN compared with a PBO and ETN compared with SSZ.
Results
Study characteristics
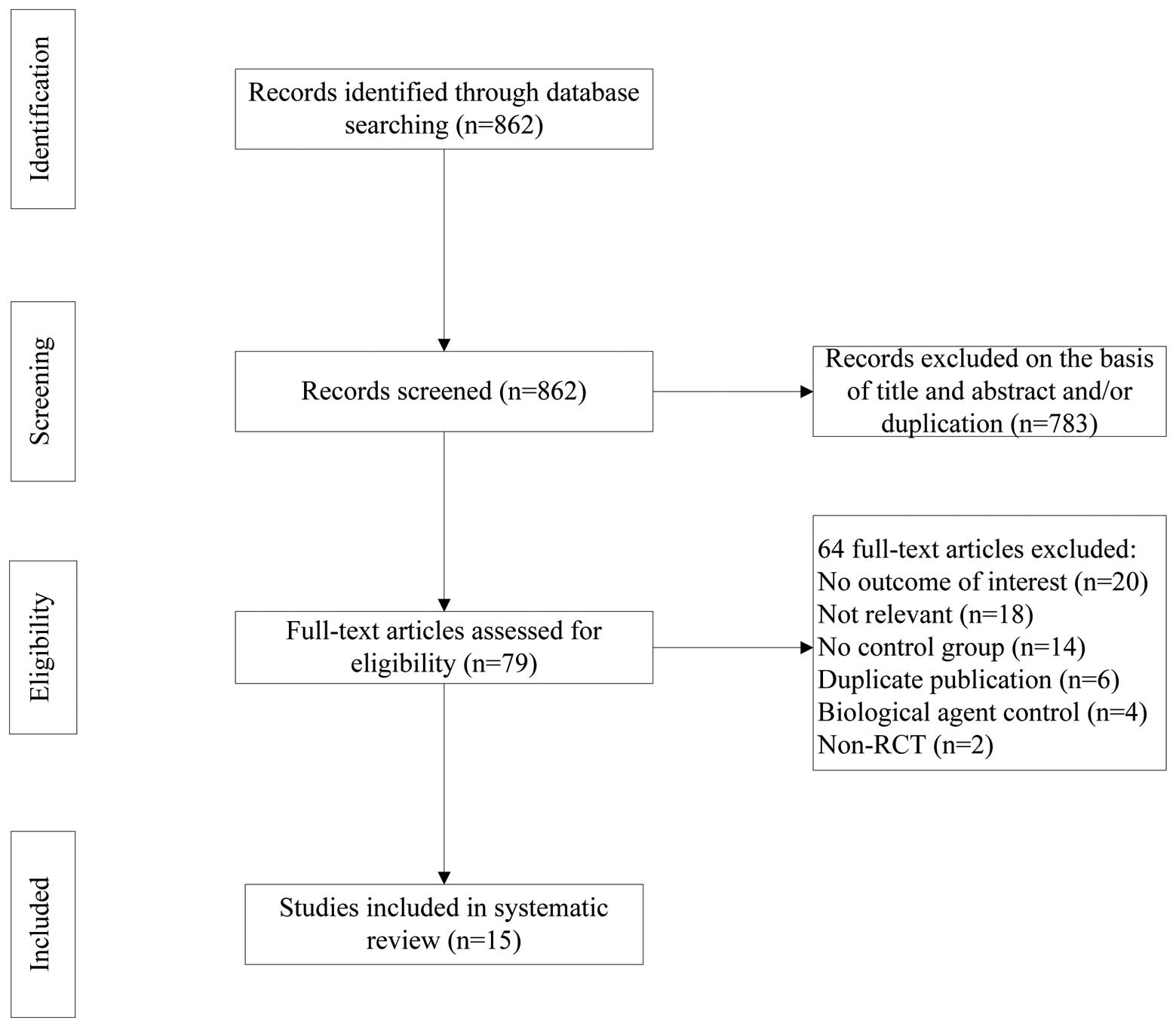
The process of study selection is shown in Fig. 1. As shown in Table I, the included studies were
published as full text between 2002 and 2011. Seven studies were
published in Chinese, and eight studies (19–26)
were published in English. Nine studies (19,20,22–28)
were multicentre trials, whereas the remaining six were performed
at a single centre. Together, those trials included a total of
2,194 participants.
 | Table ICharacteristics of the included
trials. |
Table I
Characteristics of the included
trials.
| Patients (n) | Intervention | |
|---|
|
|
| |
|---|
| Study | Experimental | Control | Experimental | Control | Duration (weeks) | Outcomes |
|---|
| Brandt et al
2003 (26) | 14 | 16 | ETN | PBO | 6 | ASAS 20, ASAS 50,
BASDAI, BASDAI 50, BASFI, AEs |
| Calin et al
2004 (24) | 45 | 39 | ETN | PBO | 8 | ASAS 20, ASAS 50,
ASAS 70, AEs |
| Davis Jr et
al 2003 (22) | 138 | 139 | ETN | PBO | 24 | ASAS PR, BASDAI,
BASFI, OW, CRP, AEs |
| Gorman et al
2002 (21) | 20 | 20 | ETN | PBO | 16 | OW, AEs |
| Deng et al
2009 (29) | 26 | 26 | ETN | PBO | 6 | ASAS 20, ASAS 50,
ASAS 70, BASDAI 50, AEs |
| Dougados et
al 2011 (19) | 39 | 43 | ETN | PBO | 12 | BASDAI, BASDAI 50,
BASFI, ASAS PR, CRP, AEs |
| van der Heijde
et al 2006 (25) | 150 | 51 | ETN | PBO | 12 | BASDAI 50, ASAS PR,
AEs |
| Huang et al
2010 (28) | 74 | 78 | ETN | PBO | 6 | ASAS 20, ASAS 50,
ASAS 70, BASDAI, BASFI, AEs |
| Huang et al
2011 (27) | 300 | 100 | ETN | PBO | 6 | ASAS 20, BASDAI 50,
ASAS PR, AEs |
| Lin et al
2010 (20) | 19 | 20 | ETN | PBO | 6 | ASAS 20, ASAS 50,
ASAS 70 |
| Zhang et al
2009 (30) | 43 | 43 | ETN | PBO | 6 | ASAS 20, ASAS 50,
ASAS 70, BASDAI 50, ASAS PR |
| Braun et al
2011 (23) | 378 | 187 | ETN | SSZ | 16 | ASAS 20, BASFI, ST,
CRP, AEs |
| Chen et al
2010 (35) | 20 | 20 | ETN | SSZ | 12 | ST |
| Zhao et al
2009 (31) | 30 | 30 | ETN | SSZ | 6 | ASAS 20, BASDAI,
ST, ESR, CRP, AEs |
| Zhao et al
2009 (32) | 43 | 43 | ETN | SSZ | 12 | BASDAI, BASFI, ESR,
CRP, AEs |
In addition to an initial double-blind trial, five
included trials (20,27–30)
conducted a subsequent open-label extension study in which the two
groups were treated with ETN (50 mg) per week. In the trial
reported by Zhao et al (31), the dose of ETN was reduced to 25 mg
per week after seven weeks. The trial conducted by Brandt et
al (26) had two phases: an
initial PBO-controlled period and an observational phase; only the
PBO-controlled period was selected for analysis. The majority of
the data in the study by Gorman et al (21) were expressed as the median ± SD and
were discarded.
The dose of ETN applied in the included trials was
25 mg twice weekly (BIW) or 50 mg once weekly (QW). In the study
reported by van der Heijde et al (25), three parallel groups were
established, which were ETN (50 mg QW), ETN (25 mg BIW) and PBO;
however, only ETN (25 mg BIW) was compared with the PBO. SSZ intake
ranged from 1.0 to 3.0 g per day. The duration of interventions in
the included trials was also different, ranging from 6 to 24 weeks.
To minimise heterogeneity, when compared with a PBO, the reported
outcomes of the ASAS 20, ASAS 50 and ASAS 70 were measured at six
weeks, with the exception of one trial (24) from which the data was extracted at
eight weeks. Twelve trials performed an ITT analysis, two (27,32)
performed a treated-per-protocol (TPP) analysis, and one (28) performed an ITT and TPP
analysis.
Quality of the included studies
Approximately three-quarters of included trials were
moderate quality (Jadad score ≥3), whereas four of the Chinese
trials were of low quality (Jadad score <3) due to unclear
randomisation, deficient allocation concealment, inadequate
blinding, and undisclosed withdrawal and dropouts. Two subgroup
analyses were performed to minimise the clinical heterogeneity.
Publication bias
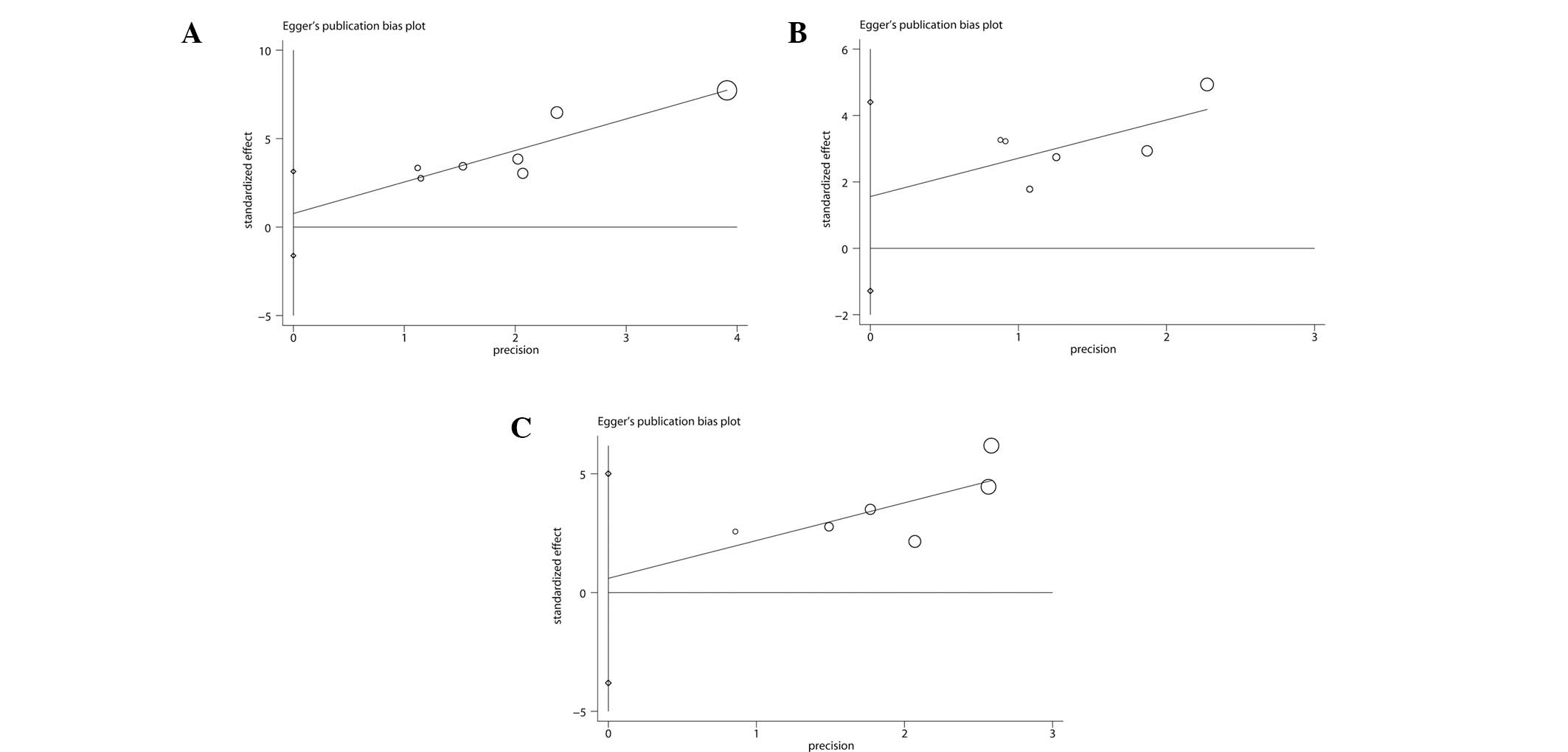
The Egger’s publication bias plots and Begg’s test
showed that there were no significant publication biases for three
outcomes with number of included trials ≥6 (Fig. 2). However, the results cannot be
regarded as convincing since three outcomes have <10 trials; the
power of a funnel plot is considered to be restricted unless
substantial bias is present and the number of trials is ≥10
(33).
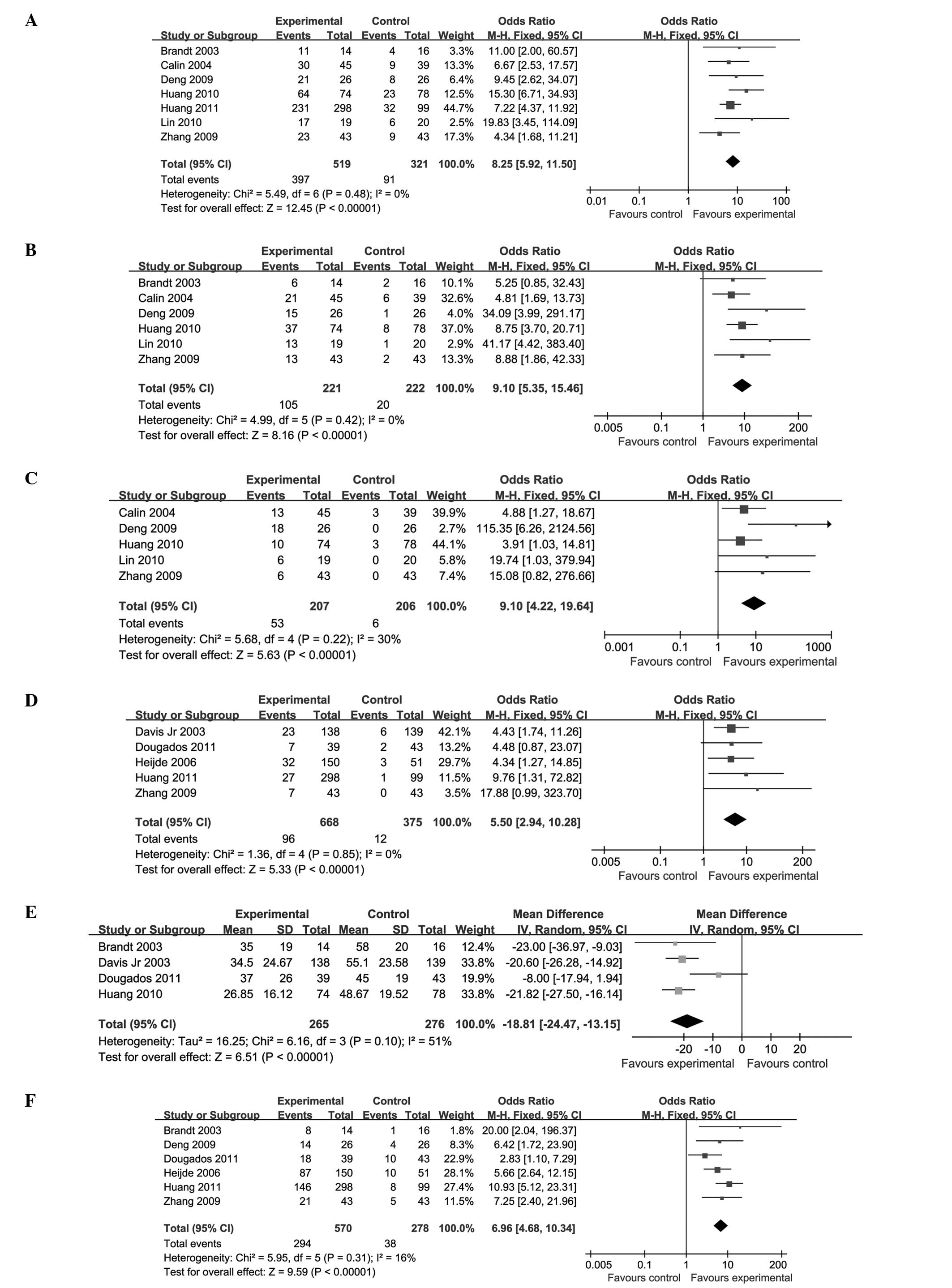
ETN compared with a PBO
Eleven trials (involving 1,443 patients) compared
the therapeutic effects of ETN and a PBO (19–22,24–30).
The number of trial participants ranged from 14 to 300, and the
trial duration varied from 6 to 24 weeks. There was no statistical
heterogeneity between the studies. The pooled results displayed a
significant difference between the ETN-treated and PBO groups, with
the exception of the OW distance (P=0.91; MD, −0.19; 95% CI,
−3.49–3.10). The ETN group was superior to the PBO group in terms
of improvements in the ASAS 20 (P<0.00001; OR, 8.25; 95% CI,
5.92–11.50), ASAS 50 (P<0.00001; OR, 9.10; 95% CI, 5.35–15.46),
ASAS 70 (P<0.00001; OR, 9.10; 95% CI, 4.22–19.64), BASDAI
(P<0.00001; MD, −18.81; 95% CI, −24.47 to −13.15) and ASAS PR
(P<0.00001; OR, 5.50; 95% CI, 2.94–10.28). In addition, the
BASDAI 50 (P<0.00001; OR, 6.96; 95% CI, 4.68–10.34) was
significantly enhanced and the BASFI (P<0.00001; SMD, −0.68; 95%
CI, −0.85 to −0.50) and CRP (P<0.00001; MD, −12.69; 95% CI,
−16.32 to −9.06) levels were decreased (Fig. 3; Table II).
 | Table IIResults of the meta-analysis for the
placebo control. |
Table II
Results of the meta-analysis for the
placebo control.
| Heterogeneity | Test for overall
effect |
|---|
|
|
|
|---|
| Outcomes | χ2 | P-value |
I2 (%) | Z | P-value | OR (95% CI) |
|---|
| ASAS 20 | 5.49 | 0.48 | 0 | 12.45 | <0.00001 | 8.25 (5.92,
11.50) |
| ASAS 50 | 4.99 | 0.42 | 0 | 8.16 | <0.00001 | 9.10 (5.35,
15.46) |
| ASAS 70 | 5.68 | 0.22 | 30 | 5.63 | <0.00001 | 9.10 (4.22,
19.64) |
| BASDAI | 6.16 | 0.10 | 51 | 6.51 | <0.00001 | −18.81 (−24.47,
−13.15) |
| BASDAI 50 | 5.95 | 0.31 | 16 | 9.59 | <0.00001 | 6.96 (4.68,
10.34) |
| BASFI | 5.96 | 0.11 | 50 | 7.61 | <0.00001 | −0.68 (−0.85,
−0.50) |
| ASAS PR | 1.36 | 0.85 | 0 | 5.33 | <0.00001 | 5.50 (2.94,
10.28) |
| CRP | 0.06 | 0.80 | 0 | 6.85 | <0.00001 | −12.69 (−16.32,
−9.06) |
| OW | 2.65 | 0.10 | 62 | 0.11 | 0.91 | −0.19 (−3.49,
3.10) |
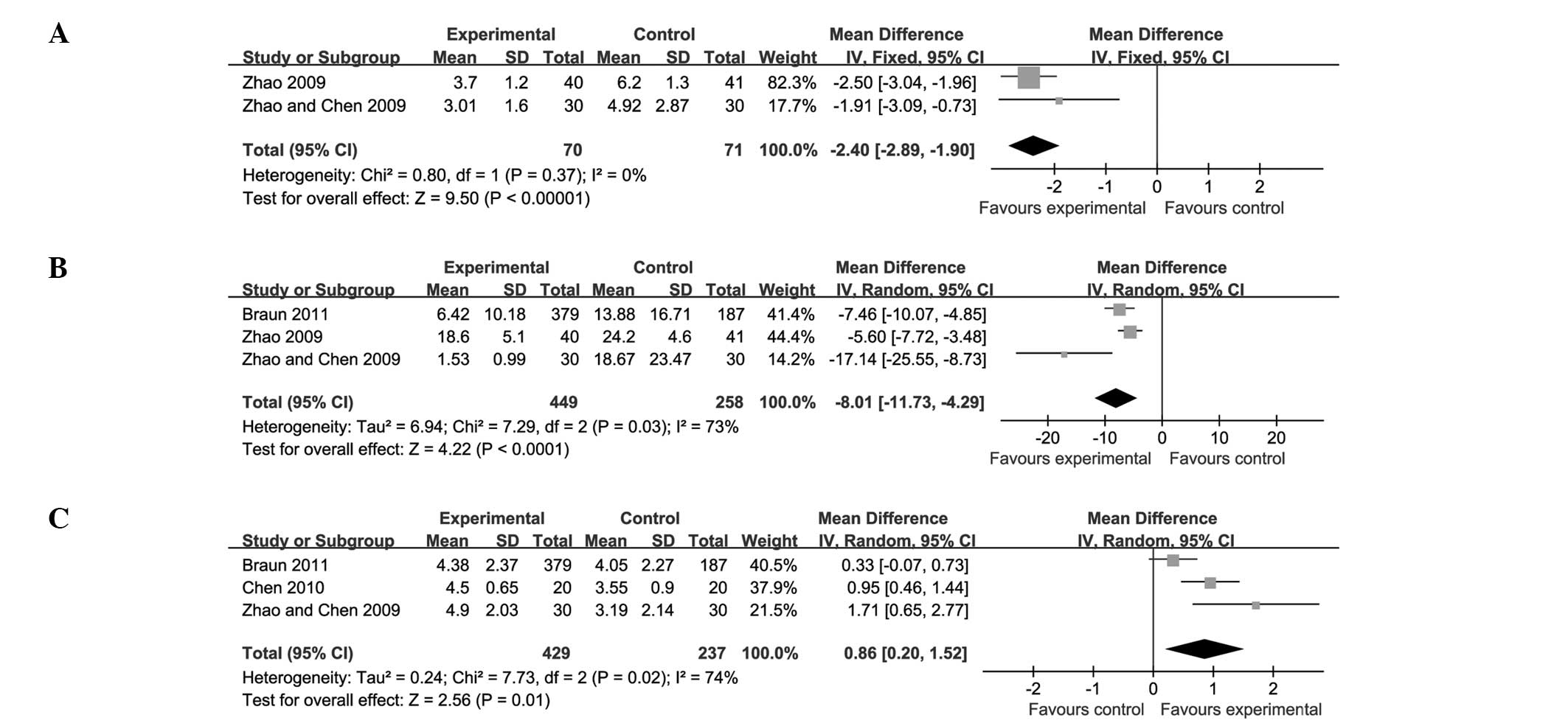
ETN compared with SSZ
Four trials (involving 751 patients) compared ETN
with SSZ (23,31,32,35).
The number of trial participants ranged from 20 to 378, and the
trial duration ranged from 6 to 16 weeks. In the combined results,
there was evident statistical heterogeneity between the comparisons
for the ASAS 20 (P<0.0001) and BASFI (P=0.0009), and
particularly for the ESR (P<0.00001). The above outcomes are
described separately in the present analysis, rather than being
subjected to combined analyses.
In two of these trials (32,35),
the difference in the ASAS 20 values between the ETN and SSZ groups
was not reported. The other two trials (23,31)
showed that ETN induced greater increases in the ASAS 20 levels
than SSZ did. In two trials (31,35),
the difference in the BASFI levels between the ETN and SSZ groups
was not reported. The other two trials (23,32)
indicated that ETN had a greater effect than SSZ in decreasing the
BASFI levels. Two of the trials (23,35)
did not compare the ESR levels in the ETN group with the levels in
the SSZ group. The other two trials (31,32)
revealed that ETN was more effective than SSZ in reducing the ESR
levels.
There was a significant difference between ETN and
SSZ in terms of the BASDAI (P<0.00001; MD, −2.40; 95% CI, −2.89
to −1.90) and CRP levels (P<0.0001; MD, −8.01; 95% CI, −11.73 to
−4.29). A small but significant increase in the ST score (P=0.01;
MD, 0.86; 95% CI, 0.20–1.52) was also found (Fig. 4; Table III).
 | Table IIIResults of meta-analysis for the
sulfasalazine control. |
Table III
Results of meta-analysis for the
sulfasalazine control.
| Heterogeneity | Test for overall
effect |
|---|
|
|
|
|---|
| Outcomes | χ2 | P-value |
I2 (%) | Z | P-value | OR (95% CI) |
|---|
| BASDAI | 0.80 | 0.37 | 0 | 9.50 | <0.00001 | −2.40 (−2.89,
−1.9) |
| ST | 7.73 | 0.02 | 74 | 2.56 | 0.01 | 0.86 (0.20
1.52) |
| CRP | 7.29 | 0.03 | 73 | 4.22 | <0.0001 | −8.01 (−11.73,
−4.29) |
Adverse effects
Twelve of the 15 trials reported outcomes for
adverse effects (AEs). The majority of the studies reported the
incidence of an injection site reaction (ISR); however, one trial
did not report the group in which the ISR occurred (29). Four trials reported abnormality of
liver function in the ETN group (24,27,28,31),
and in one trial, this was associated with concomitant indomethacin
treatment (24). In the trials of
Dougados et al (19) and
Deng et al (29),
neutropenia was detected during the ETN treatment. Non-neutralising
anti-ETN antibodies were found in the ETN group in the trials of
Davis Jr et al (22) and
van der Heijde et al (25).
In one study, one patient in the PBO group experienced aggravated
AS, which resulted in the withdrawal of the patient from the study,
and another patient in the ETN group withdrew due to a lung
neoplasm (19). Two neurologic
events were reported in a single patient treated with ETN: tinnitus
and benign fasciculations (34).
In the trial reported by Davis Jr et al (22), seven patients in the ETN group
discontinued the study: five due to serious AEs and two due to
gastrointestinal haemorrhage and ileitis. Additionally, one
life-threatening event occurred (a suicide attempt in the PBO
group) in the same trial. An ETN-treated patient with acute
myocardial infarction underwent angioplasty but continued to
participate in the study (24).
Discussion
Although several systematic reviews reporting the
efficacy and safety of ETN in the treatment of AS have been
conducted, they focused on evaluating the differences between ETN
and a PBO (12,36,37).
Unlike the previous reviews, the present review study included 15
trials and set two subgroups to minimise heterogeneity.
Furthermore, new studies were included that were published
subsequent to the previous reviews. Thus, the present systematic
review differs from the previous studies. For comparisons with a
PBO group, the present results are consistent with those of
previous reviews (12,36,37)
in terms of increasing ASAS 20. In addition, these results are
consistent with two previous reviews regarding the ASAS PR and
BASDAI (12,37) between ETN-treated and PBO groups.
Unlike one of the previous reviews (12), the present review shows that the
ETN group exhibits reduced BASFI and CRP levels compared with the
PBO group. Furthermore, the present study shows that compared with
SSZ, ETN is capable of decreasing the BASDAI and CRP level and
increasing the ST score. In addition, in the present review, it was
identified that the AEs of ETN generally appeared to be mild or
moderate. The most common side-effect was an ISR, and this reaction
was tolerable for the majority of the participants.
Several limitations exist in this systematic review.
Firstly, half of the included studies were conducted in Chinese
populations, which implies a high risk of selection bias. Secondly,
the majority of the studies published in Chinese were of poor
quality. Three studies (31,32,35)
did not use blinding and performed unclear allocation concealment.
Therefore, potential bias, such as in the selection of patients,
the administration of interventions and assessment of outcomes,
could have resulted in the overestimation of the therapeutic
efficacy of ETN. Finally, the limited number (two to seven) of
trials included in each subgroup dampened the positive evidence for
the efficacy of ETN in treating AS. Certain vital outcomes were not
reported for the SSZ control. Consequently, it was not possible to
draw a definitive conclusion concerning whether ETN monotherapy was
improved compared with SSZ. Therefore, it is necessary for all
outcomes to be carefully explained.
In conclusion, this meta-analysis suggests that ETN
exhibits beneficial effects in terms of improving the ASAS 20,
BASDAI and BASFI during the treatment of AS and exhibits
superiority in comparison with the effects of a PBO. Due to the
lack of high-quality clinical trials, whether ETN monotherapy is
improved compared with SSZ in disease activity control and symptom
relief remains to be validated. Therefore, it is necessary for
large and well-designed RCTs to be performed prior to recommending
the use of ETN to replace synthetic DMARD monotherapy or
combinations of synthetic DMARDs.
Acknowledgements
The present authors would like to acknowledge the
authors of the original studies included in the meta-analysis.
References
|
1
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007. View Article : Google Scholar
|
|
2
|
Feldtkeller E, Khan M, van der Heijde D,
et al: Age at disease onset and diagnosis delay in HLA-B27 negative
vs. positive patients with ankylosing spondylitis. Rheumatol Int.
23:61–66. 2003.PubMed/NCBI
|
|
3
|
Sieper J and Braun J: Anti-TNF agents for
the treatment of spondyloarthropathies. Expert Opin Emerg Drugs.
7:235–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gratacos J, Collado A, Filella X, et al:
Serum cytokines (IL-6, TNF-α, IL-1β and IFN-γ) in ankylosing
spondylitis: a close correlation between serum IL-6 and disease
activity and severity. Rheumatology. 33:927–931. 1994.
|
|
5
|
Braun J, Bollow M, Neure L, et al: Use of
immunohistologic and in situ hybridization techniques in the
examination of sacroiliac joint biopsy specimens from patients with
ankylosing spondylitis. Arthritis Rheum. 38:499–505. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Culy CR and Keating GM: Etanercept: an
updated review of its use in rheumatoid arthritis, psoriatic
arthritis and juvenile rheumatoid arthritis. Drugs. 62:2493–2537.
2002.PubMed/NCBI
|
|
7
|
Mease PJ, Goffe BS, Metz J, et al:
Etanercept in the treatment of psoriatic arthritis and psoriasis: a
randomised trial. Lancet. 356:385–390. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lovell DJ, Giannini EH, Reiff A, et al:
Etanercept in children with polyarticular juvenile rheumatoid
arthritis. N Engl J Med. 342:763–769. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Braun J, Van den Berg R, Baraliakos X, et
al: 2010 update of the ASAS/EULAR recommendations for the
management of ankylosing spondylitis. Ann Rheum Dis. 70:896–904.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J and Liu C: Sulfasalazine for
ankylosing spondylitis. Cochrane Database Syst Rev.
18:CD0048002005.
|
|
11
|
Ferraz MB, Tugwell P, Goldsmith CH, et al:
Meta-analysis of sulfasalazine in ankylosing spondylitis. J
Rheumatol. 17:1482–1486. 1990.PubMed/NCBI
|
|
12
|
Li SH, Ma B, Tan JY, et al: Efficacy and
safety of etanercept for patients with ankylosing spondylitis: a
systematic review. Chinese Journal of Evidence-Based Medicine.
9:423–429. 2009.(In Chinese).
|
|
13
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: the PRISMA statement. Ann Intern Med.
151:264–269. 2009. View Article : Google Scholar
|
|
14
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984.
|
|
15
|
Jadad AR, Moore RA, Carroll D, et al:
Assessing the quality of reports of randomized clinical trials: is
blinding necessary? Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
16
|
Anderson JJ, Baron G, van der Heijde D, et
al: Ankylosing spondylitis assessment group preliminary definition
of short-term improvement in ankylosing spondylitis. Arthritis
Rheum. 44:1876–1886. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garrett S, Jenkinson T, Kennedy LG, et al:
A new approach to defining disease status in ankylosing
spondylitis: the Bath Ankylosing Spondylitis Disease Activity
Index. J Rheumatol. 21:2286–2291. 1994.PubMed/NCBI
|
|
18
|
Calin A, Garrett S, Whitelock H, et al: A
new approach to defining functional ability in ankylosing
spondylitis: the development of the Bath Ankylosing Spondylitis
Functional Index. J Rheumatol. 21:2281–2285. 1994.PubMed/NCBI
|
|
19
|
Dougados M, Braun J, Szanto S, et al:
Efficacy of etanercept on rheumatic signs and pulmonary function
tests in advanced ankylosing spondylitis: results of a randomised
double-blind placebo-controlled study (SPINE). Ann Rheum Dis.
70:799–804. 2011. View Article : Google Scholar
|
|
20
|
Lin Q, Lin Z, Gu J, et al: Abnormal
high-expression of CD154 on T lymphocytes of ankylosing spondylitis
patients is down-regulated by etanercept treatment. Rheumatol Int.
30:317–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gorman JD, Sack KE and Davis JC Jr:
Treatment of ankylosing spondylitis by inhibition of tumor necrosis
factor α. N Engl J Med. 346:1349–1356. 2002.
|
|
22
|
Davis JC Jr, Van Der Heijde D, Braun J, et
al; Enbrel Ankylosing Spondylitis Study Group. Recombinant human
tumor necrosis factor receptor (etanercept) for treating ankylosing
spondylitis: a randomized, controlled trial. Arthritis Rheum.
48:3230–3236. 2003. View Article : Google Scholar
|
|
23
|
Braun J, van der Horst-Bruinsma IE, Huang
F, et al: Clinical efficacy and safety of etanercept versus
sulfasalazine in patients with ankylosing spondylitis: A
randomized, double-blind trial. Arthritis Rheum. 63:1543–1551.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calin A, Dijkmans BAC, Emery P, et al:
Outcomes of a multicentre randomised clinical trial of etanercept
to treat ankylosing spondylitis. Ann Rheum Dis. 63:1594–1600. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Heijde D, Da Silva JC, Dougados M,
et al; Etanercept Study 314 Investigators. Etanercept 50 mg once
weekly is as effective as 25 mg twice weekly in patients with
ankylosing spondylitis. Ann Rheum Dis. 65:1572–1577.
2006.PubMed/NCBI
|
|
26
|
Brandt J, Khariouzov A, Listing J, et al:
Six-month results of a double-blind, placebo-controlled trial of
etanercept treatment in patients with active ankylosing
spondylitis. Arthritis Rheum. 48:1667–1675. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang F, Zhang J, Zheng Y, et al: A
multicenter, double-blind, randomized, placebo-controlled clinical
trial of etanercept in the treatment of Chinese patients with
active ankylosing spondylitis. Zhonghua Nei Ke Za Zhi.
50:1043–1047. 2011.(In Chinese).
|
|
28
|
Huang F, Zhang J, Huang JL, et al: A
multicenter, double-blind, placebo-controlled, randomized clinical
study of etanercept in the treatment of ankylosing spondylitis.
Zhonghua Nei Ke Za Zhi. 49:741–745. 2010.(In Chinese).
|
|
29
|
Deng XH, Hang F, Zhang YM, et al:
Treatment of ankylosing spondylitis with recombinant human tumor
necrosis factor-Fc fusion protein (etanercept): a multicenter,
randomized, double-blind, placebo-controlled trial. Jun Yi Jin Xiu
Xue Yuan Xue Bao. 30:21–23. 2009.(In Chinese).
|
|
30
|
Zhang J, Zhang YM, Zhang JL, et al:
Efficacy of etanercept in patients with ankylosing spondylitis: A
double-blind, randomized, placebo controlled trial. Zhongguo Xin
Yao Za Zhi. 18:1846–1849. 2009.(In Chinese).
|
|
31
|
Zhao WM, Chen ZW and Wang MJ: Clinical
observation of etanercept in treatment of ankylosing spondylitis.
Suzhou Da Xue Xue Bao. 29:518–523. 2009.(In Chinese).
|
|
32
|
Zhao FT, Zhao H and Wang YL: Efficacy of
etanercept on ankylosing spondylitis. Shanghai Jiaotong Daxue
Xuebao Yixueban. 29:1506–1508. 2009.(In Chinese).
|
|
33
|
Sterne JA, Gavaghan D and Egger M:
Publication and related bias in meta-analysis: power of statistical
tests and prevalence in the literature. J Clin Epidemiol.
53:1119–1129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Davis JC Jr: The role of etanercept in
ankylosing spondylitis. Clin Exp Rheumatol. 20(Suppl 28):
S111–S115. 2002.PubMed/NCBI
|
|
35
|
Chen MZ, Zhao RGT, Wang HY, et al:
Clinical control study of recombinant human tumor necrosis
factor-Fc fusion protein and traditional immunity depressant in
treatment of ankylosing spondylitis (AS). Xinjiang Yi Ke Da Xue Xue
Bao. 33:913–915. 2010.(In Chinese).
|
|
36
|
Machado MA, Barbosa MM, Almeida AM, et al:
Treatment of ankylosing spondylitis with TNF blockers: a
meta-analysis. Rheumatol Int. 33:2199–2213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li ZH, Zhang Y, Wang J and Shi ZJ:
Etanercept in the treatment of ankylosing spondylitis: a
meta-analysis of randomized, double-blind, placebo-controlled
clinical trials, and the comparison of the Caucasian and Chinese
population. Eur J Orthop Surg Traumatol. 23:497–506. 2013.
View Article : Google Scholar : PubMed/NCBI
|