Introduction
Helicobacter pylori is a Gram-negative spiral
bacterium that colonizes the gastric mucosa. H. pylori
infection affects 70–90% the population in developing
countries, and 25–50% of the population in developed countries
(1). Standard triple-therapy
regimens with a proton-pump inhibitor (PPI) and two of amoxicillin,
clarithromycin and nitroimidazole have been used for the
eradication of H. pylori; however, their efficacy has been
declining with the increasing resistance of H. pylori to
antibiotics, and the H. pylori eradication failure rate
varies widely, from 10 to 45% (2).
At present, the administration of antibiotics for 10–14 days or
high-dose PPI (twice a day) has been recommended for H.
pylori eradication therapy by the Maastricht IV consensus
conference (3); this has resulted
in the increased incidence of undesirable side effects, such as
antibiotic-associated diarrhea, nausea or vomiting, during
anti-H. pylori therapy, which can lead to reduced compliance
(4). Among the alternative
anti-H. pylori options that have been considered, probiotics
have attracted substantial interest. Previous studies have shown
that probiotics, predominantly including
Lactobacillus, Saccharomyces boulardii and
Bifidobacterium, demonstrate anti-H. pylori activity
in vitro and in animal models of H. pylori infection
(5–8). Probiotics have also been used as an
adjuvant therapy to H. pylori infection in order to reduce
the side effects of antibiotics and improve the eradication rates
(9–11); however, the results have been
inconsistent, with certain studies showing that adjuvant probiotics
did not improve eradication rates or reduce the side effects
(12–14).
Previous meta-analyses have demonstrated that
probiotics, as adjuvant agents, have a positive effect on improving
eradication rates and reducing adverse events (15–20);
however, certain recent studies have produced results that are
inconsistent with those of the previous meta-analyses (21,22).
Furthermore, the appropriate timing and duration of probiotic
administration are indeterminate (23,24).
Miscellaneous probiotics may be used in an anti-H. pylori
treatment regimen, but it is unclear whether the efficacy of
different probiotics is similar. As such, an updated meta-analysis
of randomized controlled trials (RCTs) comparing the eradication
rates and adverse events of probiotics as an adjuvant treatment
with those of a placebo (or blank control) in participants with
H. pylori infection is required. The aim of the present
study was to evaluate, by meta-analysis, the efficacy and safety of
the administration of probiotics as adjuvant agents of standard
triple-therapy regimens for H. pylori infection, and to
investigate the appropriate timing and duration of the probiotic
administration in order to provide evidence to support this use of
probiotics in clinical practice.
Materials and methods
Study sources and search methods
The present meta-analysis was developed according to
the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses statement guidelines (25). Pubmed (1966 to November 2013),
Embase (1946 to November 2013), the Cochrane Central Register of
Controlled Trials (Issue 11, 2013) and the Science Citation Index
(SCI; 1945 to November 2013) were searched according to Medical
Subject Heading and text terms: (Helicobacter pylori OR
H. pylori) AND (probiotic OR probiotics OR yeast OR yeasts
OR yogurt OR Lactobacillus OR Bifidobacterium OR
Saccharomyces). Authors were also asked to provide
unpublished randomized trial results. In addition, the
ClinicalTrials.gov website (https://clinicaltrials.gov/) was searched for
registered RCTs whose results had not yet been published, and
relevant studies were identified from the references.
Inclusion and exclusion criteria
Articles that were eligible for inclusion in the
meta-analysis met the following inclusion criteria: i) RCTs; ii)
any age, endoscopic findings and symptoms at the time of
enrollment; iii) confirmation of eradication outcome by urea breath
test, histology or H. pylori stool antigen ≥4 weeks after
therapy; iv) trials comparing at least two branches of treatment
consisting of a control group (with placebo or no additional
intervention) and an experimental group (the standard
triple-therapy regimen plus probiotics); v) restriction of the
species of probiotics to Lactobacillus, Bifidobacterium,
Saccharomyces or a mixture of the three; vi) obtainable
eradication rates.
The exclusion criteria for the meta-analysis were as
follows: i) Undeterminable eradication rates; ii) use of agents
other than probiotics as the adjuvant therapy for H. pylori
infection in the experimental group; iii) articles without
full-text; iv) studies in languages other than English.
Validity assessment
Two reviewers independently, but not blinded to the
authors or journal, assessed the quality of the studies that met
the inclusion criteria. Any disagreements between the reviewers
were resolved by consulting a third reviewer. The quality of the
studies was assessed by the Jadad scale (26,27).
The scores, from 0 to 5, were evaluated according to three
criteria: Randomization, double blinding and description of
withdrawals and dropouts (26,27).
To avoid the duplication of data, if trials were published
repeatedly by the same authors or institutions, only the most
recently published article was included.
Data extraction
Standardized data abstraction sheets were prepared.
Data were extracted for study quality and type; the timing of
probiotic administration; duration of eradication treatment;
duration of probiotic treatment; species of probiotics; location of
trials; time of publication; anti-H. pylori regimens; number
and age of enrolled patients; diagnostic methods for detecting
H. pylori infection prior to enrollment and subsequent to
study completion; eradication rates by intention-to-treat (ITT)
analysis; rates of successful and failed eradication; and total
side effects (diarrhea, vomiting nausea, taste disturbance,
epigastric pain and total adverse effects) from all included
studies.
Statistical analysis
Statistical analysis was performed with the
Comprehensive Meta-Analysis Software (version 2; Biostat, Inc.,
Englewood, NJ, USA). The primary outcomes for the meta-analysis
were the H. pylori eradication rates and the side effects
among the trials comparing probiotic and control arms, based on ITT
and pro-protocol (PP) analysis. The efficacy of H. pylori
eradication was measured using relative risk (RR) to compare the
frequency of eradication in the probiotic arm with that in the
control arm.
The RRs for all studies were pooled into a summary
RR, using either a fixed- or random-effects model, based on inverse
variance methods. If the heterogeneity had a statistically
significant difference, the random-effects model was employed; if
not, the fixed-effects model was adopted. P-values and 95%
confidence intervals (CIs) were provided for the summary RRs. The
heterogeneity index (I2) was additionally calculated.
Other assessments of heterogeneity were accomplished using the
Q-test, and a Z-test was employed to assess the pooled effects.
Funnel plots, Egger’s test and Begg’s test were utilized to
estimate the publication bias. Meta-regression analyses were
performed to interpret the reasons for the heterogeneity.
Subgroup analysis
Subgroup analysis for the meta-analysis was
performed depending on the time that the probiotics were
administered [‘before’ (used prior to the eradication regimens),
‘same’ (simultaneously with the eradication regimens) and ‘after’
(beginning with the eradication regimens and continuing subsequent
to the eradication regimens)], the regimens utilized, the duration
of the probiotic treatment (≤2 weeks and >2 weeks), the species
of probiotics, the age of the subjects, the Jadad score (>2, and
≤2), the PPIs of the experimental group and the duration of the
eradication regimens.
Results
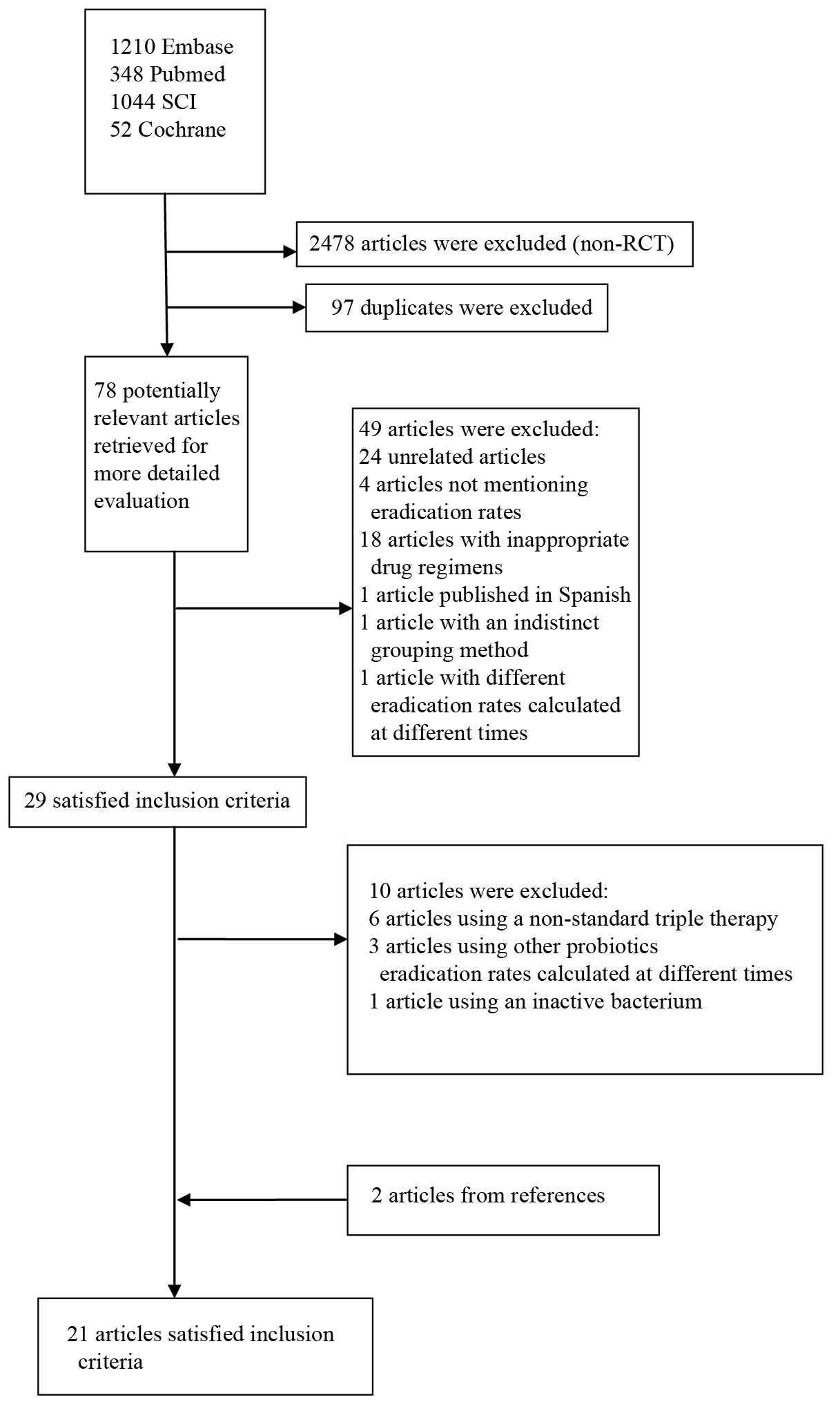
Description of the studies
The bibliographical search yielded a total of 2,653
studies. Among the studies from Pubmed, the Cochrane Central
Register of Controlled Trials, Embase and the SCI, another 2,478
articles were excluded subsequent to examining the article type.
Having excluded any duplicates, 78 potentially relevant articles
were retrieved for more detailed assessment. Following examinations
of the title and abstract, another 24 unrelated articles, four
articles that did not mention eradication rates, 18 articles with
inappropriate drug regimens and one study published in Spanish
(28) were excluded. The full-text
articles were then reviewed and another two articles were excluded,
one of which was excluded for indistinct grouping methods (29) and the other as a result of
eradication rates being calculated at different times. Twenty-nine
articles were further evaluated for details. Six articles were
excluded due to a non-standard triple therapy regimen (9,12,30–33),
three articles were excluded due to Lactobacillus,
Bifidobacterium or Saccharomyces not being used in the
eradication regimen (34–36) and one article due to an inactive
bacterium being used (37). Two
articles from the studied references were additionally included in
the meta-analysis (38,39). Twenty-one RCTs ultimately met the
inclusion criteria (11,38–57)
(Table I) (Fig. 1).
 | Table ICharacteristics of the included
studies. |
Table I
Characteristics of the included
studies.
| First author, year
(ref.) | Country,
language | Total cases
(treatment/control) | Patients | Eradication
regimen | Regimen duration
(days) | Species of
probiotics | Time of
probioticsa | Duration of
probiotics (days) | H. pylori
infection: Initial diagnosis re-checking | Jadad score |
|---|
| Armuzzia, 2001
(41) | Italy, English | 120 (60/60) | Adults | P: 40 mg
b.i.d.
C: 500 mg, b.i.d.
T: 500 mg b.i.d. | 7 |
Lactobacillus GG | 3 | 14 | 13C-UBT,
H. pylori IgG antibody measurements/13C-UBT | 3 |
| Armuzzia, 2001
(40) | Italy, English | 60 (30/30) | Adults | R: 20 mg
b.i.d.
C: 500 mg b.i.d.
T: 500 mg b.i.d. | 7 |
Lactobacillus GG | 3 | 14 | 13C-UBT,
H. pylori IgG antibody measurements/13C-UBT | 5 |
| Cremonini, 2002
(42) | Italy, English | 85 (64/21) | Adults | R: 20 mg
b.i.d.
C: 500 mg b.i.d.
T: 500 mg b.i.d. | 7 |
Lactobacillus GG; Saccharomyces
boulardii | 3 | 14 |
13C-UBT/13C-UBT | 5 |
| Sheu, 2002
(43) | Taiwan,
English | 160 (80/80) | Adults | L: 30 mg
b.i.d.
A: 1 g, b.i.d.
C: 500 mg, b.i.d. | 7 |
Lactobacillus;
Bifidobacterium | 3 | 35 | Histology and
RUT/13C-UBT | 2 |
| Myllyuioma, 2005
(44) | Finland,
English | 47 (23/24) | Adults | L: 30 mg
b.i.d.
C: 500 mg b.i.d.
A: 1 g b.i.d. | 7 |
Lactobacillus GG (ATCC 53103);
L. rhamnosus LC (DSM7061); P. freudenreichii ssp.
shermanii JS (DSM7076); B. breve Bb99 (DSM
13692) | 3 | 28 | 13C-UBT,
serology/13C-UBT, serology [IgG decrease by 40% (four
months)] | 5 |
| Sýkora, 2005
(45) | Czech Republic,
English | 86 (39/47) | Children | O: 10 mg (15–30
kg)
or 20 mg (30 kg) b.i.d.
A: 25 mg/kg b.i.d.
C: 7.5 mg/kg b.i.d. | 7 | Lactobacillus
casei (DN-114 001) | 3 | 14 | At least two of
three: RUT, histology and culture/HpSAT, 13C-UBT | 5 |
| Płewinska, 2006
(46) | Poland,
English | 60 (30/30) | Children | O: 0.5 mg/kg/24 h,
b.i.d.
A: 50 mg/kg/24 h b.i.d.
C: 15 mg/kg/24 h, b.i.d. | 30 | Lactobacillus
acidophilus; Lactobacillus rhamnosus | 3 | 28 | RUT, histology/RUT,
histology | 1 |
| de Bortoli, 2007
(47) | Italy, English | 206 (105/101) | Adults | E: 20 mg
b.i.d.
C: 500 mg b.i.d.
A: 1 g b.i.d. | 7 | Lactobacillus
reuteri | 1 | 7 | HpSAT (99),
13C-UBT (107)/13C-UBT | 2 |
| Cindoruk, 2007
(48) | Turkey,
English | 124 (62/62) | Adults | L: 30 mg
b.i.d.
C: 500 mg b.i.d.
A: 1000 mg b.i.d. | 14 | Lactobacillus
plantarum; L. reuterii; L. casei subsp.
rhamnosus; Bifidobacterium infantis; B.
longum; L. salivarius; L. acidophilus;
Streptococcus termophilus; L. sporogenes
(Lactobacillaceae) | 1 | 14 | HE or Giemsa
stain/13C-UBT | 5 |
| Kim, 2008 (49) | Korea, English | 347 (168/179) | Adults | PPI
b.i.d.
C: 500 mg b.i.d.
A: 1 g b.i.d. | 7 | L.
acidophilus HY 2177; L. casei HY 2743; B. longum
HY 8001; S. thermophilus B-1 | 2 | 28 | RUT,
13C-UBT, histology/13C-UBT | 3 |
| Hurduc, 2009
(50) | Romania,
English | 90 (48/42) | Children | O/E: 1 mg/kg/day,
b.i.d.
A: 50 mg/kg/day, b.i.d.
C: 15 mg/kg/day, b.i.d. | 7 or 10 | Saccharomyces
boulardii | 3 | 28 | Histology,
RUT/histology, RUT | 3 |
| Szajewska, 2009
(51) | Poland,
English | 83 (44/39) | Children | O: 0.5 mg/kg
b.i.d.
A: 25 mg/kg b.i.d.
C: 10 mg/kg b.i.d. | 7 |
Lactobacillus GG | 1 | 7 | Two of three tests
(13C-UBT, histopathology, RUT)/13C-UBT | 5 |
| Song, 2010
(52) | Korea, English | 991 (660/331) | Adults | O: 20 mg,
b.i.d.
A: 1 g, b.i.d.
C: 500 mg, b.i.d. | 7 | S.
boulardii | 3 | 28 | Histology/UBT | 3 |
| Yaşar, 2010
(53) | Turkey,
English | 76 (38/38) | Adults | P: 40 mg,
b.i.d.
A: 1 g b.i.d.,
C: 500 mg b.i.d. | 14 |
Bifidobacterium | 1 | 14 | HE and modified
Giemsa staining/13C-UBT | 1 |
| Medeiros, 2011
(54) | Portugal,
English | 62 (31/31) | Adults | E: 20 mg
b.i.d.
A: 1 g, b.i.d.
C: 500 mg, b.i.d. | 8 | L.
acidophilus | 1 | 8 |
Culture/13C-UBT | 2 |
| Ozdil, 2011
(11) | Turkey,
English | 285 (98/187) | Adults | Group 1: L: 30 mg
b.i.d.
C: 500 mg b.i.d.
A: 1 g b.i.d.
Group 2: E: 40 mg b.i.d.
Lev: 500 mg q.d.
A: 1000mg b.i.d.
Group3b: E: 40 mg
b.i.d.
A: 1000 mg b.i.d. for 5 days
E: 40 mg b.i.d.
L: 500 mg q.d.
T: 500 mg t.i.d. for 5 days | 14 | Saccharomyces
boulardii | 1 | 14 |
Giemsa-staining/monoclonal HpSAT | 1 |
| Deguchi, 2012
(55) | Japan, English | 229 (115/114) | Adults | R: 10 mg
b.i.d.
A: 750 mg b.i.d.
C: 200 mg b.i.d. | 7 | Lactobacillus
gasseri OLL2716 | 2 | 28 | Culture, RUT,
histology/13C-UBT, HpSAT or culture | 2 |
| Du, 2012 (56) | China, English | 234 (155/79) | Adults | O: 20 mg
b.i.d.
C: 500 mg b.i.d.
A: 1g b.i.d. | 7 | Lactobacillus
acidophilus; S. faecalis; B. subtilis | 2 or 3 | 21 | RUT, 13C
or 14C-UBT, pathology/13C or
14C-UBT | 2 |
| Tolone, 2012
(57) | Italy, English | 68 (34/34) | Children | O: 1 mg/kg (before
breakfast) b.i.d.
A: 50 mg/kg (after meals), b.i.d.
C: 15 mg/kg (after meals) b.i.d. | 7 | Lactobacillus
plantarum; L. reuterii; L. casei. subsp.
rhamnosus; Bifidobacterium infantis ; B.
longum; L. salivarius; L. acidophilus;
Streptococcus termophilus; L. sporogenes | 1 | 7 |
13C-UBT/13C-UBT | 2 |
| Wang, 2014
(38) | China, English | 100 (49/51) | Children | PPI: 0.6–0.8 mg/kg
b.i.d.
C: 10–15 mg/kg b.i.d.
A: 30–50 mg/kg b.i.d. | 14 | L.
acidophilus; Bifidobacterium bifidum | 3 | 42 |
13C-UBT/13C-UBT | 2 |
| Dajani, 2013
(39) | Italy, English | 301 (195/106) | Children | PPI: NR
C: 500 mg b.i.d.
A: 1000 mg b.i.d. | 7 | Bifidobacterium
infantis | 1 or 2 | 7 or 21 |
14C-UBT/14C-UBT | 2 |
Two institutions published two similar articles
(40,41), in Italy. This triggered a concern
about duplication of data; however, following a careful review of
the articles in question, it was decided that the two articles were
separate trials.
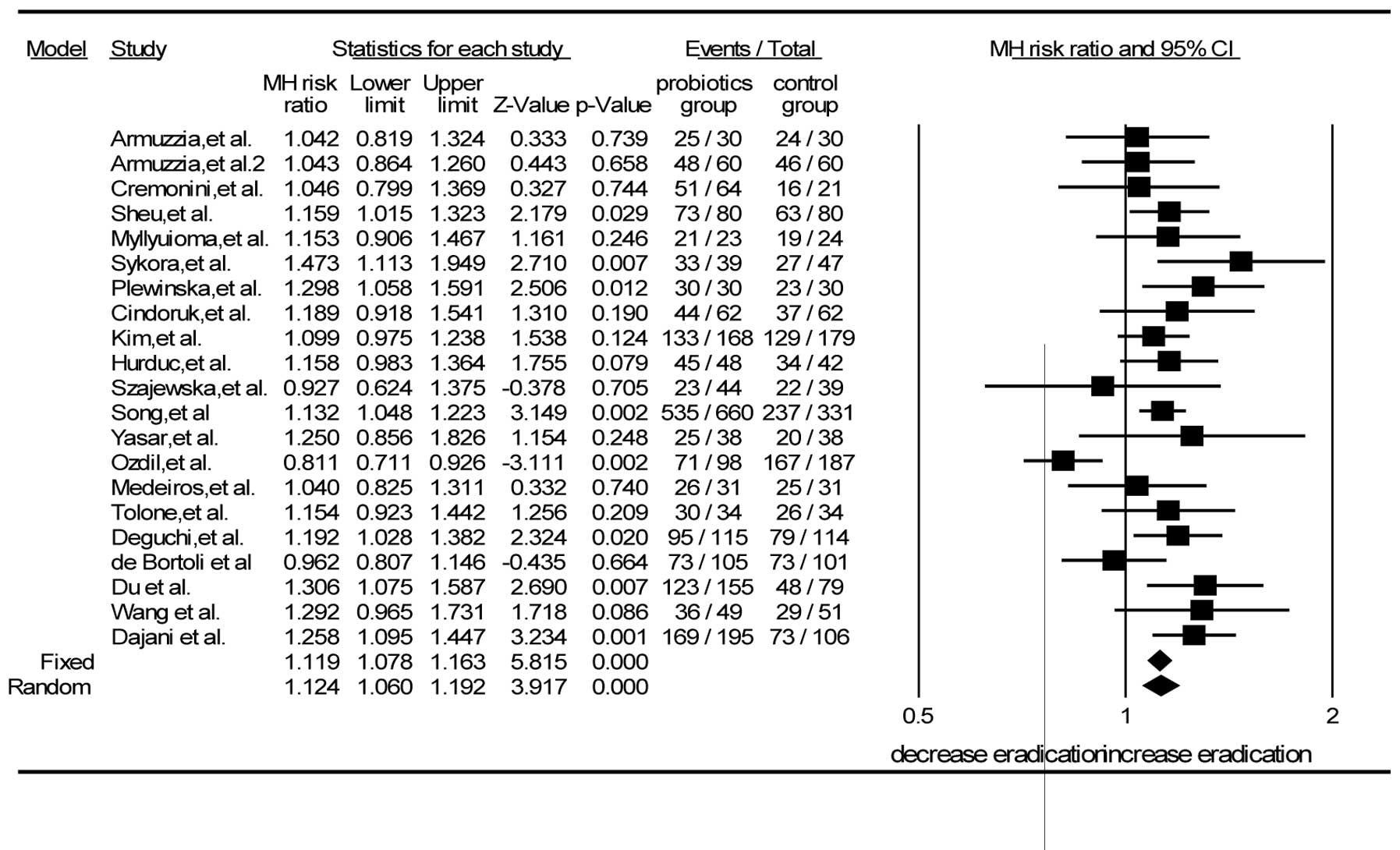
Efficacy of H. pylori eradication
The 21 RCTs included 3,814 patients in total, of
whom 21 patients were in the probiotic group and 1,529 in the
control group. The pooled eradication rate of the probiotic group
was 80.3% (1,709/2,128) by intention-to-treat (ITT) and 83.8%
(1,709/2,039) by PP analysis; the eradication rate in the probiotic
group was higher than that in the control group (80.3 vs. 72.2%)
with a statistically significant difference (Z=3.917, P<0.001).
Using the random-effects model, the values of I2=52.3%
and P=0.003 were obtained. The RR from a pooled analysis of the
selected studies was 1.12 (95% CI, 1.06–1.19) by ITT analysis
(Fig. 2).
Subgroup analyses
Multiple subgroup analyses were carried out to
explain the heterogeneity by stratifying the studies based on the
timing of probiotic supplementation (‘before’, ‘same’ and ‘after’),
eradication regimens, duration of probiotic supplementation,
species of probiotics, age of patients (adults and children) and
Jadad scores.
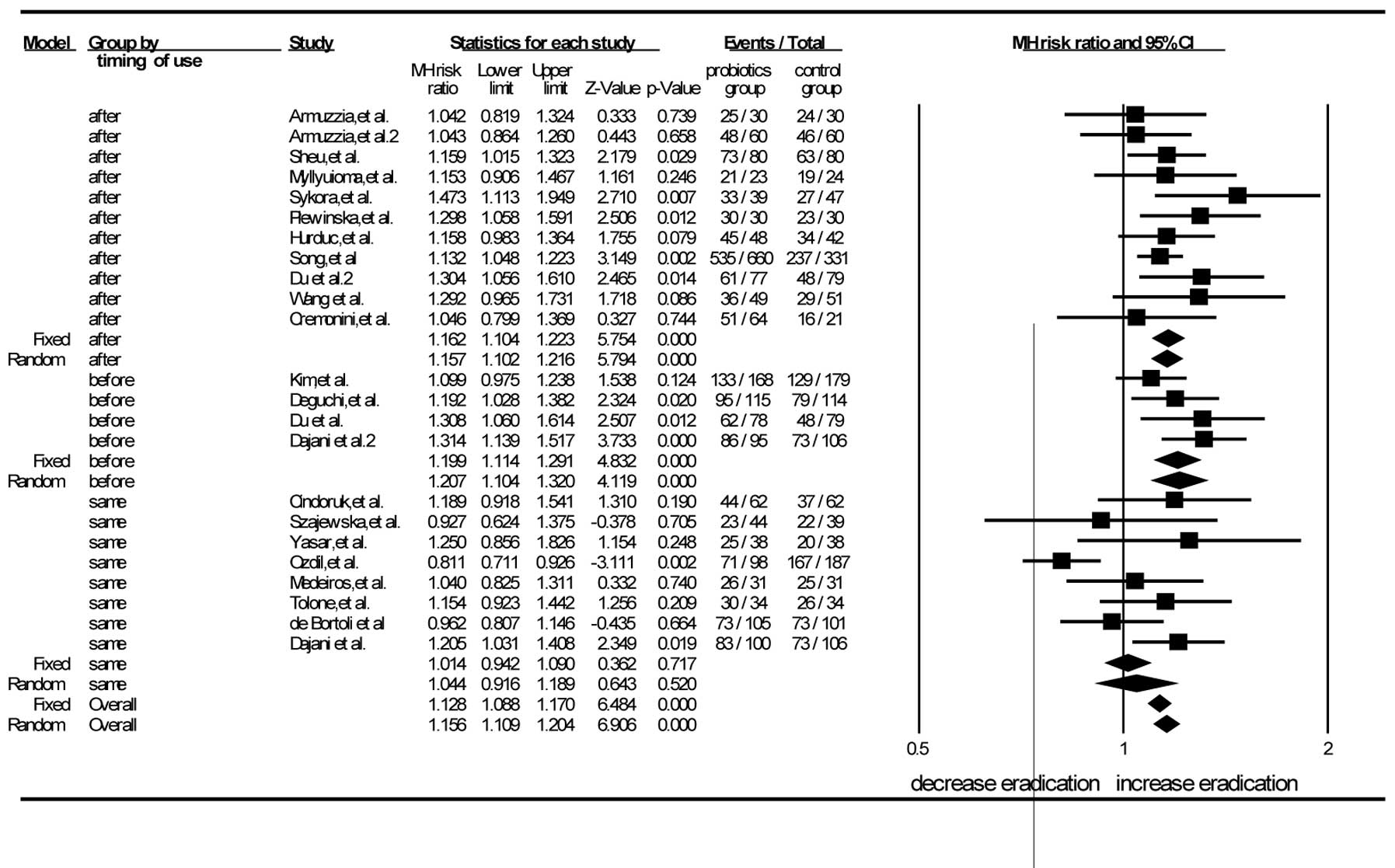
There were 11 trials in which the probiotics were
administered subsequent to the eradication regimens (38,40–46,50,52,56).
The pooled analysis showed that the RR was 1.15 (95% CI, 1.10–1.21)
according to the random-effects model. The RR of the eight studies
in which probiotics were used simultaneously with the eradication
regimens was 1.04 (95% CI, 0.92–1.19) by the random-effects mode
(11,51,39,47,48,53,54,57).
The RR for the probiotics used prior to the eradication regimens in
the four RCTs was 1.21 (95% CI, 1.10–1.32) by the random-effects
model (39,49,55,56).
When the probiotics were administered prior or subsequent to the
standard triple therapy, the differences between the experimental
and control groups were statistically significant. When probiotics
were used concurrently with the eradication regimens, no
significant difference was found between the experimental group and
the control group. This demonstrated that the timing of probiotic
supplementation, i.e. prior or subsequent to the standard
triple-therapy regimen, could improve the eradication rate
(Fig. 3).
Subgroup analyses were additionally performed
according to the eradication regimens (PPI plus amoxicillin and
clarithromycin, PPI plus clarithromycin and tinidazole). The RRs
analyzed in the random-effects model were 1.14 (95% CI, 1.07–1.21)
and 1.04 (95% CI, 0.92–1.19) for the two regimens, respectively.
The combination of the standard triple-therapy regimen (PPI,
amoxicillin and clarithromycin) with probiotics achieved a
significantly higher eradication rate than that obtained without
probiotics; however, the combination of the PPI plus clarithromycin
and tinidazole triple therapy with probiotics did not significantly
improve the eradication rate for H. pylori.
According to the subgroup analyses of the duration
of probiotic supplementation, the RR for durations of ≤2 weeks was
1.04 (95% CI, 0.98–1.11) by random-effects model, and the RR for
durations of ≤2 weeks was 1.17 (95% CI, 1.12–1.23) by
random-effects model. The results showed that probiotic
supplementation for >2 weeks could improve the eradication of
H. pylori.
According to subgroup analyses of species, the RR
for Lactobacillus was 1.14 (95% CI, 1.08–1.25), the RR of
Saccharomyces boulardii was 1.06 (95% CI, 0.89–1.23) and
that of Bifidobacterium was 1.25 (95% CI, 0.86–1.82) (all by
random-effects model). The RR in the multiple strains subgroup was
1.15 (95% CI, 1.08–1.22). The findings demonstrated that the use of
Lactobacillus and multiple probiotic strains as adjuvant
agents could improve the effectiveness of the H. pylori
eradication to a greater extent than the control treatment;
however, the administration of Bifidobacterium or
Saccharomyces boulardii did not appear to improve
eradication during anti-H. pylori treatment.
In the adult and children subgroup analyses, the RRs
were 1.08 (95% CI, 1.01–1.16) and 1.22 (95% CI, 1.11–1.34),
respectively, by the random-effects model. The results showed the
enhanced efficacy of probiotic supplementation relative to that of
the control treatment (P<0.001) in both adults and children.
There were 11 trials in which the Jadad scores were
≥3, indicating that their quality was high (40–42,44,45,48–52,56).
The Jadad scores were <3 in 10 studies, which indicated that
their quality was low (11,30,38,39,42,44–53,
56,57). According to the pooled analysis of
the high-quality trials, the summary RR was 1.13 (95% CI,
1.08–1.19) by random-effects model. The RR of the studies of low
quality was 1.12 (95% CI, 1.00–1.25) by random-effects model. The
high-quality studies all showed the benefit of probiotic
supplementation compared with the control treatment (P<0.001),
but no significant difference was found between the treatments in
the low-quality studies (P=0.053).
Subgroup analyses were also performed based on the
different PPIs of the trial groups and the duration of the
eradication regimens. In the omeprazole and rabeprazole subgroups,
the differences between the probiotic and control groups were
statistically significant (P<0.001 and P=0.034, respectively).
Significant differences were also found for eradication durations
of seven and 10 days (P<0.001 and P=0.012, respectively).
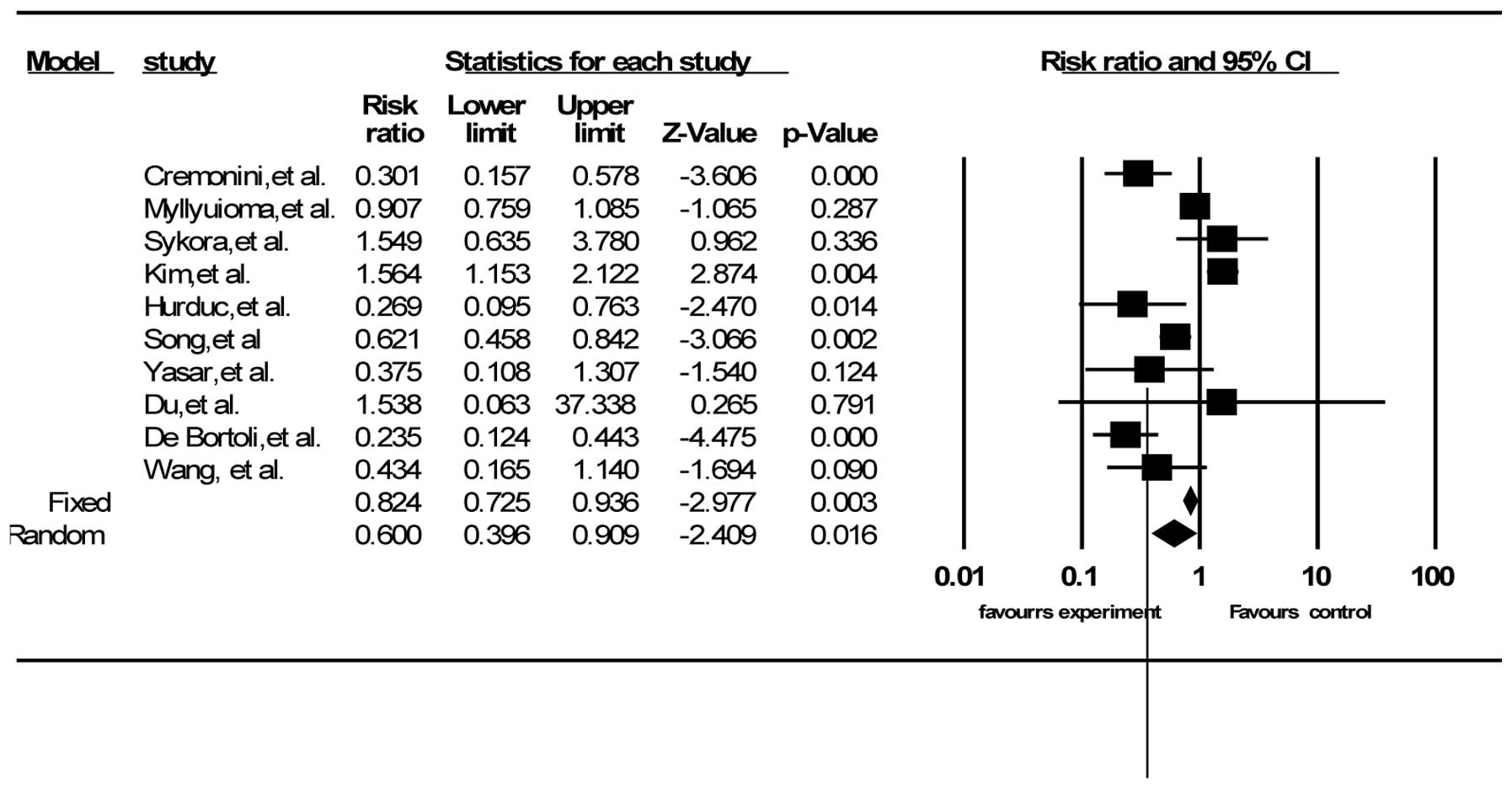
Adverse events
A total of 16 out of the 21 trials described side
effects, including diarrhea, vomiting and nausea, and epigastric
pain (30,38,39,42,44–53,56,57).
Ten RCTs had data on total side effects (38,42,44,45,47,49,50,52,53,56).
The summary RR was 0.60 (95% CI, 0.40–0.91) according to
random-effects model analysis (I2=83.72%, P<0.001)
(Fig. 4). Twelve RCTs reported the
data for diarrhea (30,38,39,42,44,46–49,51,52,57),
10 for vomiting and nausea (30,38,42,44,46–49,52,57)
and eight for epigastric pain (42,44,46–49,52,57).
The pooled RRs were 0.42 for diarrhea (95% CI, 0.24–0.73) and 0.56
for vomiting and nausea (95% CI, 0.27–1.16) by random-effects model
(I2=61.75%, P=0.002 and I2=60.05%, P=0.007,
respectively), and 0.58 for epigastric pain (95% CI, 0.34–0.97) by
the fixed-effects model (I2=34.00%, P=0.157). The
majority of the studies did not provide details on how they
estimated the severity of adverse events.
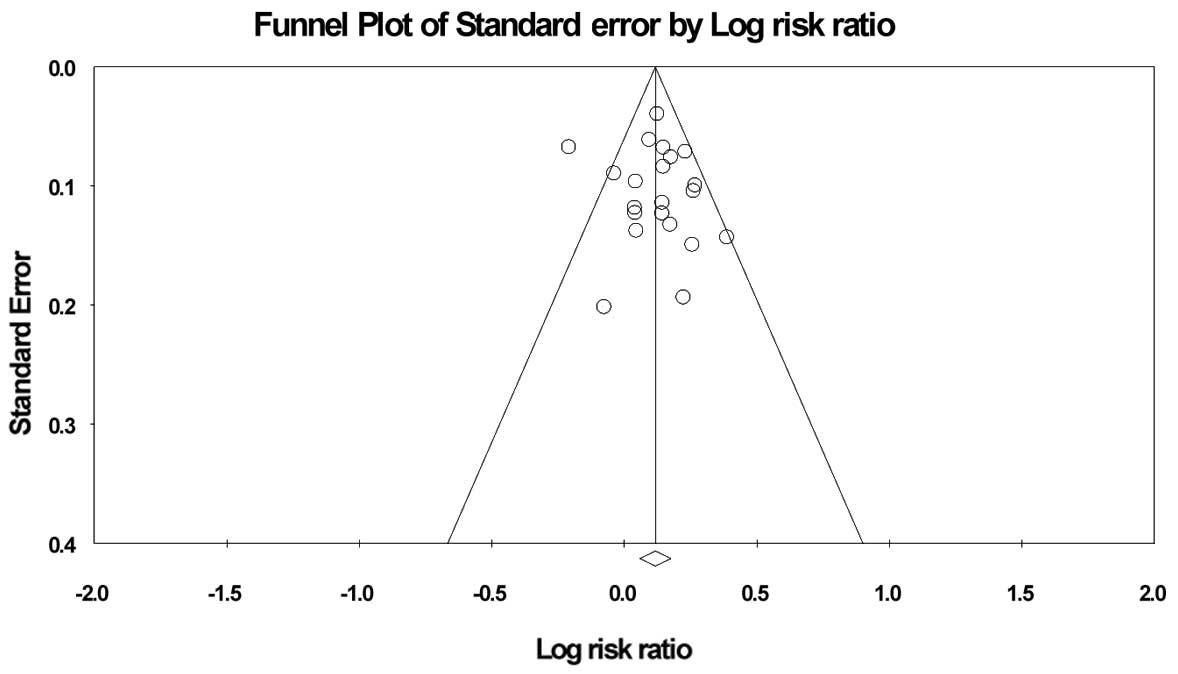
Risk of bias in publication
Funnel plot analyses by ITT analysis revealed slight
asymmetry, but Egger’s test and Begg’s test showed no significant
asymmetry of the funnel plot (Fig.
5).
Sensitivity analysis
A sensitivity analysis was performed to interpret
the reliability of the outcomes of the meta-analysis. Based on ITT
analysis, the pooled RR values were established though the fixed-
and random-effects models. The RRs were 1.12 (95% CI, 1.08–1.16)
and 1.12 (95% CI, 1.06–1.19), respectively. No significant
difference was found (overlapping CIs). When the largest study
(52) was excluded from the
sensitivity analysis, the RR did not change significantly
(RR=1.12). The RRs were therefore steady.
Heterogeneity
To interpret heterogeneity in the meta-analysis, a
meta-regression analysis was performed. The results showed that the
timing and duration of probiotic supplementation, the duration of
the eradication regimen and the quality of study were the main
causes of heterogeneity.
Discussion
Probiotics, according to the World Health
Organization, are defined as ‘live microorganisms, which, when
administered in adequate amounts, confer a health benefit on the
host’. They consist of bacteria and yeasts. It has been recognized
that probiotics can exhibit an inhibitory ability against H.
pylori (5). The effects of
probiotics on H. pylori may be due to immunologic as well as
non-immunologic mechanisms: i) Competition at the site of the
stomach mucosal epithelium (6);
ii) production of substances against H. pylori, such as
acetic, propionic or butyric acid (58); iii) regulation of immune function
and secretion of immunoglobulin A to improve mucosal defensive
ability (59–61); and iv) strengthening tight
junctions between epithelial cells (61,62).
The current results showed that probiotics could
improve the eradication rate and decrease adverse events during
anti-H. pylori treatment. The RRs were 1.12 (95% CI,
1.06–1.19) and 0.62 (95% CI, 0.40–0.91), respectively. The outcomes
of the present meta-analysis were similar with several previous
meta-analyses (15,17,18).
The optimal timing of probiotic administration is
still unknown (23,24). It is generally believed that better
efficacy occurs when probiotic supplementation occurs concurrently
with or subsequent to antibiotic regimens. When probiotics are
administered prior to antibiotic regimens, H. pylori is
converted from a spiral to a coccoid form, which can lead to
eradication failure. The timing of the addition of probiotics has
been different in clinical trials (38,56,57).
Whether the variability affected the eradication rate of H.
pylori is not clear. The results of the current meta-analysis
suggested that probiotic supplementation could improve eradication
rates when provided prior or subsequent to the standard treatment
regimens, but that supplementation supplied concurrently with the
regimen did not significantly improve the eradication rate. The
reason behind this may be that, when probiotics and antibiotics are
administered simultaneously, it is inevitable that the antibiotics
restrain the growth of the probiotics, resulting in a decrease in
the anti-H. pylori substances produced by the probiotics
(23,24).
An additional undetermined factor in studies to date
is the appropriate duration of probiotic administration (23,24).
In the present meta-analysis, the duration of probiotic
administration varied from 7 days to months (38,45,47,51).
The results suggested that a duration of >2 weeks could
significantly improve the eradication rate for H. pylori
infection, while a duration of ≤2 weeks could not. This indicated
that the long-term administration of probiotics could be beneficial
during anti-H. pylori treatment; however, further
investigation is required to confirm this.
Based on the subgroup analyses of probiotic species
employed, it was shown that the regimens with Lactobacillus
were superior to the control group regimens (RR, 1.15; 95% CI,
1.05–1.25); however, only two RCTs used Bifidobacterium
alone for adjuvant therapy during anti-H. pylori treatment.
The effectiveness was slightly better than that of the control
group regimens, but the difference was not statistically
significant. Further investigation is therefore required to draw a
definite conclusion. The use of Saccharomyces boulardii as a
single supplement did not improve the eradication rate during
anti-H. pylori treatment (RR, 1.05; 95% CI, 0.89–1.23). This
suggests that the administration of Saccharomyces boulardii
alone may not be suitable for adjuvant treatment during anti-H.
pylori therapy (11,42,48,50,52).
Subgroup analyses were also performed according to
different PPIs and durations of eradication regimens. It was of
note that the omeprazole and rabeprazole subgroups achieved
significant eradication success, while the esomeprazole,
lansoprazole and pantoprazole subgroups did not, as compared with
the control group. The current meta-analysis also demonstrated that
the triple-therapy regimens with PPIs, amoxicillin and
clarithromycin could achieve significantly higher eradication rates
than the control group regimens, but the triple-therapy regimens
consisting of PPIs, clarithromycin and nitroimidazole could
not.
A number of studies have indicated that the
administration of probiotics can ameliorate the symptoms and reduce
the adverse effects associated with eradication therapy for H.
pylori, such as diarrhea, vomiting, nausea and epigastric pain
(14,35,47);
however, certain investigations have suggested that probiotic
supplementation does not reduce the incidence of side effects
(21,49). The side effects experienced during
anti-H. pylori regimens were therefore examined in the
current meta-analysis, which showed that the supplementation of
probiotics had a substantial effect on reducing H. pylori
therapy-related adverse reactions, particularly diarrhea and
epigastric pain. The results were consistent with those of previous
meta-analyses (15,17,18).
We believe that the application of probiotics has a beneficial
effect and diminishes the discomfort during anti-H. pylori
therapy.
To decrease bias in the present meta-analysis, the
study selection, data extraction and assessment of study quality
were performed by two reviewers. Another strength of the current
meta-analysis was that it identified the majority of the RCTs
published in English that used different species of probiotics as
adjuvant agents for H. pylori treatment. The efficacy and
safety of probiotics in anti-H. pylori treatment were
comprehensively analyzed. The meta-regression analysis made the
outcomes of the present meta-analysis reliable.
There were several limitations to the
meta-analysis. Firstly, some evident heterogeneity was noted in the
meta-analysis, although sub-analysis and meta-regression analysis
were conducted to decrease the effects. Secondly, the language
restriction could have influenced the results. There could have
been a bias in the published languages, so it is likely that the
present meta-analysis does not reflect all the outcomes of
probiotics used for anti-H. pylori treatment. Finally,
certain authors were asked for unpublished data, so the
introduction of bias on that basis is possible. The Egger’s and
Begg’s tests suggested that there could have been publication
biases, and these could have affected the results of the
meta-analysis.
In conclusion, the present meta-analysis showed
that probiotic supplementation can improve eradication rates and
reduce the adverse effects experienced during eradication therapy.
In addition, probiotics appear to have enhanced effects on
eradication rates when administered prior or subsequent to the
standard regimens. Long-term probiotic treatment may have a
superior effect to short-term probiotic administration.
Lactobacillus and probiotic supplementation with multiple
species appear to improve the eradication rate for H. pylori
infection.
Acknowledgements
This study was supported by a grant from the
National Science and Technology Major Projects for ‘Major New Drug
Innovation and Development’ of China (no. 2011ZX09302-007-03). The
original manuscript was edited and proofread by Medjaden Bioscience
Limited. The abstract of the present study has previously been
published in the proceedings for the XXVIIth International Workshop
on Helicobacter and Microbiota in Chronic Digestive Inflammation
and Gastric Cancer (11–13 September 2014), and in the Journal of
Gastroenterology and Hepatology (vol 29, supplement S3, 2014).
References
|
1
|
Go MF: Review article: natural history and
epidemiology of Helicobacter pylori infection. Aliment Pharmacol
Ther. 16(Suppl 1): 3–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chey WD and Wong BC; Practice Parameters
Committee of the American College of Gastroenterology. American
College of Gastroenterology guideline on the management of
Helicobacter pylori infection. Am J Gastroenterol. 102:1808–1825.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malfertheiner P, Megraud F, O’Morain CA,
et al: Management of Helicobacter pylori infection - the Maastricht
IV/Florence consensus report. Gut. 61:646–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bell GD, Powell K, Burridge SM, et al:
Experience with ‘triple’ anti-Helicobacter pylori eradication
therapy: side effects and the importance of testing the
pre-treatment bacterial isolate for metronidazole resistance.
Aliment Pharmacol Ther. 6:427–435. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhatia SJ, Kochar N, Abraham P, Nair NG
and Mehta AP: Lactobacillus acidophilus inhibits growth of
Campylobacter pylori in vitro. J Clin Microbiol. 27:2328–2330.
1989.PubMed/NCBI
|
|
6
|
Mukai T, Asasaka T, Sato E, et al:
Inhibition of binding of Helicobacter pylori to the glycolipid
receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med
Microbiol. 32:105–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson-Henry KC, Mitchell DJ, Avitzur Y,
et al: Probiotics reduce bacterial colonization and gastric
inflammation in H. pylori-infected mice. Dig Dis Sci. 49:1095–1102.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsieh PS, Tsai YC, Chen YC, et al:
Eradication of Helicobacter pylori infection by the probiotic
strains Lactobacillus johnsonii MH-68 and L. salivarius ssp
salicinius AP-32. Helicobacter. 17:466–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tursi A, Brandimarte G, Giorgetti GM and
Modeo ME: Effect of Lactobacillus casei supplementation on the
effectiveness and tolerability of a new second-line 10-day
quadruple therapy after failure of a first attempt to cure
Helicobacter pylori infection. Med Sci Monit. 10:CR662–CR666.
2004.PubMed/NCBI
|
|
10
|
Lionetti E, Indrio F, Pavone L, et al:
Role of probiotics in pediatric patients with Helicobacter pylori
infection: a comprehensive review of the literature. Helicobacter.
15:79–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ozdil K, Calhan T, Sahin A, et al:
Levofloxacin based sequential and triple therapy compared with
standard plus probiotic combination for Helicobacter pylori
eradication. Hepatogastroenterology. 58:1148–1152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoon H, Kim N, Kim JY, et al: Effects of
multistrain probiotic-containing yogurt on second-line triple
therapy for Helicobacter pylori infection. J Gastroenterol Hepatol.
26:44–48. 2011. View Article : Google Scholar
|
|
13
|
Scaccianoce G, Zullo A, Hassan C, et al:
Triple therapies plus different probiotics for Helicobacter pylori
eradication. Eur Rev Med Pharmacol Sci. 12:251–256. 2008.PubMed/NCBI
|
|
14
|
Ahmad K, Fatemeh F, Mehri N and Maryam S:
Probiotics for the treatment of pediatric Helicobacter pylori
infection: a randomized double blind clinical trial. Iran J
Pediatr. 23:79–84. 2013.PubMed/NCBI
|
|
15
|
Tong JL, Ran ZH, Shen J, Zhang CX and Xiao
SD: Meta-analysis: the effect of supplementation with probiotics on
eradication rates and adverse events during Helicobacter pylori
eradication therapy. Aliment Pharmacol Ther. 25:155–168. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szajewska H, Horvath A and Piwowarczyk A:
Meta-analysis: the effects of Saccharomyces boulardii
supplementation on Helicobacter pylori eradication rates and side
effects during treatment. Aliment Pharmacol Ther. 32:1069–1079.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou J, Dong J and Yu X: Meta-analysis:
Lactobacillus containing quadruple therapy versus standard triple
first-line therapy for Helicobacter pylori eradication.
Helicobacter. 14:97–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang ZH, Gao QY and Fang JY: Meta-analysis
of the efficacy and safety of Lactobacillus-containing and
Bifidobacterium-containing probiotic compound preparation in
Helicobacter pylori eradication therapy. J Clin Gastroenterol.
47:25–32. 2013. View Article : Google Scholar
|
|
19
|
Sachdeva A and Nagpal J: Effect of
fermented milk-based probiotic preparations on Helicobacter pylori
eradication: a systematic review and meta-analysis of
randomized-controlled trials. Eur J Gastroenterol Hepatol.
21:45–53. 2009. View Article : Google Scholar
|
|
20
|
Zheng X, Lyu L and Mei Z:
Lactobacillus-containing probiotic supplementation increases
Helicobacter pylori eradication rate: evidence from a
meta-analysis. Rev Esp Enferm Dig. 105:445–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shavakhi A, Tabesh E, Yaghoutkar A, et al:
The effects of multistrain probiotic compound on bismuth-containing
quadruple therapy for Helicobacter pylori infection: a randomized
placebo-controlled triple-blind study. Helicobacter. 18:280–284.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Navarro-Rodriguez T, Silva FM, Barbuti RC,
et al: Association of a probiotic to a Helicobacter pylori
eradication regimen does not increase efficacy or decreases the
adverse effects of the treatment: a prospective, randomized,
double-blind, placebo-controlled study. BMC Gastroenterol.
13:562013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsushima M and Takagi A: ‘Is it
effective?’ to ‘How to use it?’: the era has changed in probiotics
and functional food products against Helicobacter pylori infection.
J Gastroenterol Hepatol. 27:851–853. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boyanova L and Mitov I: Coadministration
of probiotics with antibiotics: why, when and for how long? Expert
Rev Anti Infect Ther. 10:407–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liberati A, Altman DG, Tetzlaff J, et al:
The PRISMA statement for reporting systematic reviews and
meta-analyses of studies that evaluate healthcare interventions:
explanation and elaboration. BMJ. 339:b27002009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jadad AR, Moore RA, Carroll D, et al:
Assessing the quality of reports of randomized clinical trials: is
blinding necessary? Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Downs SH and Black N: The feasibility of
creating a checklist for the assessment of the methodological
quality both of randomised and non-randomised studies of health
care interventions. J Epidemiol Community Health. 52:377–384. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sahagún-Flores JE, López-Peña LS, de la
Cruz-Ramírez Jaimes J, et al: Eradication of Helicobacter pylori:
triple treatment scheme plus Lactobacillus vs. triple treatment
alone. Cir Cir. 75:333–336. 2007.(In Spanish).
|
|
29
|
Ziemniak W: Efficacy of Helicobacter
pylori eradication taking into account its resistance to
antibiotics. J Physiol Pharmacol. 57(Suppl 3): 123–141.
2006.PubMed/NCBI
|
|
30
|
Sheu BS, Cheng HC, Kao AW, et al:
Pretreatment with Lactobacillus- and Bifidobacterium-containing
yogurt can improve the efficacy of quadruple therapy in eradicating
residual Helicobacter pylori infection after failed triple therapy.
Am J Clin Nutr. 83:864–869. 2006.PubMed/NCBI
|
|
31
|
Lionetti E, Miniello VL, Castellaneta SP,
et al: Lactobacillus reuteri therapy to reduce side-effects during
anti-Helicobacter pylori treatment in children: a randomized
placebo controlled trial. Aliment Pharmacol Ther. 24:1461–1468.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Manfredi M, Bizzarri B, Sacchero RI, et
al: Helicobacter pylori infection in clinical practice: probiotics
and a combination of probiotics + lactoferrin improve compliance,
but not eradication, in sequential therapy. Helicobacter.
17:254–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu C, Xiao L and Zou H: Effect of birid
triple viable on peptic ulcer patients with Helicobacter pylori
infection. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 35:1000–1004.
2010.(In Chinese). PubMed/NCBI
|
|
34
|
Park SK, Park DI, Choi JS, et al: The
effect of probiotics on Helicobacter pylori eradication.
Hepatogastroenterology. 54:2032–2036. 2007.
|
|
35
|
Bekar O, Yilmaz Y and Gulten M: Kefir
improves the efficacy and tolerability of triple therapy in
eradicating Helicobacter pylori. J Med Food. 14:344–347. 2011.
View Article : Google Scholar
|
|
36
|
Nista EC, Candelli M, Cremonini F, et al:
Bacillus clausii therapy to reduce side-effects of
anti-Helicobacter pylori treatment: randomized, double-blind,
placebo controlled trial. Aliment Pharmacol Ther. 20:1181–1188.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Canducci F, Armuzzi A, Cremonini F, et al:
A lyophilized and inactivated culture of Lactobacillus acidophilus
increases Helicobacter pylori eradication rates. Aliment Pharmacol
Ther. 14:1625–1629. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang YH and Huang Y: Effect of
Lactobacillus acidophilus and Bifidobacterium bifidum
supplementation to standard triple therapy on Helicobacter pylori
eradication and dynamic changes in intestinal flora. World J
Microbiol Biotechnol. 30:847–853. 2014. View Article : Google Scholar
|
|
39
|
Dajani AI, Abu Hammour AM, Yang DH, et al:
Do probiotics improve eradication response to Helicobacter pylori
on standard triple or sequential therapy? Saudi J Gastroenterol.
19:113–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Armuzzi A, Cremonini F, Bartolozzi F, et
al: The effect of oral administration of Lactobacillus GG on
antibiotic-associated gastrointestinal side-effects during
Helicobacter pylori eradication therapy. Aliment Pharmacol Ther.
15:163–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Armuzzi A, Cremonini F, Ojetti V, et al:
Effect of Lactobacillus GG supplementation on antibiotic-associated
gastrointestinal side effects during Helicobacter pylori
eradication therapy: a pilot study. Digestion. 63:1–7. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cremonini F, Di Caro S, Covino M, et al:
Effect of different probiotic preparations on anti-Helicobacter
pylori therapy-related side effects: a parallel group, triple
blind, placebo-controlled study. Am J Gastroenterol. 97:2744–2749.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sheu BS, Wu JJ, Lo CY, et al: Impact of
supplement with Lactobacillus- and Bifidobacterium-containing
yogurt on triple therapy for Helicobacter pylori eradication.
Aliment Pharmacol Ther. 16:1669–1675. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Myllyluoma E, Veijola L, Ahlroos T, et al:
Probiotic supplementation improves tolerance to Helicobacter pylori
eradication therapy - a placebo-controlled, double-blind randomized
pilot study. Aliment Pharmacol Ther. 21:1263–1272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sýkora J, Valecková K, Amlerová J, et al:
Effects of a specially designed fermented milk product containing
probiotic Lactobacillus casei DN-114 001 and the eradication of H.
pylori in children: a prospective randomized double-blind study. J
Clin Gastroenterol. 39:692–698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Plewińska EM, Płaneta-Małecka I,
Bąk-Romaniszyn L, Czkwianianc E and Małecka-Panas E: Probiotics in
the treatment of Helicobacter pylori infection in children.
Gastroenterologia Polska. 13:315–319. 2006.
|
|
47
|
de Bortoli N, Leonardi G, Ciancia E, et
al: Helicobacter pylori eradication: a randomized prospective study
of triple therapy versus triple therapy plus lactoferrin and
probiotics. Am J Gastroenterol. 102:951–956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cindoruk M, Erkan G, Karakan T, Dursun A
and Unal S: Efficacy and safety of Saccharomyces boulardii in the
14-day triple anti-Helicobacter pylori therapy: a prospective
randomized placebo-controlled double-blind study. Helicobacter.
12:309–316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim MN, Kim N, Lee SH, et al: The effects
of probiotics on PPI-triple therapy for Helicobacter pylori
eradication. Helicobacter. 13:261–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hurduc V, Plesca D, Dragomir D, Sajin M
and Vandenplas Y: A randomized, open trial evaluating the effect of
Saccharomyces boulardii on the eradication rate of Helicobacter
pylori infection in children. Acta Paediatr. 98:127–131. 2009.
View Article : Google Scholar
|
|
51
|
Szajewska H, Albrecht P and
Topczewska-Cabanek A: Randomized, double-blind, placebo-controlled
trial: effect of Lactobacillus GG supplementation on Helicobacter
pylori eradication rates and side effects during treatment in
children. J Pediatr Gastroenterol Nutr. 48:431–436. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Song MJ, Park DI, Park JH, et al: The
effect of probiotics and mucoprotective agents on PPI-based triple
therapy for eradication of Helicobacter pylori. Helicobacter.
15:206–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yaşar B, Abut E, Kayadıbı H, et al:
Efficacy of probiotics in Helicobacter pylori eradication therapy.
Turk J Gastroenterol. 21:212–217. 2010.
|
|
54
|
Medeiros JA, Gonçalves TM, Boyanova L, et
al: Evaluation of Helicobacter pylori eradication by triple therapy
plus Lactobacillus acidophilus compared to triple therapy alone.
Eur J Clin Microbiol Infect Dis. 30:555–559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Deguchi R, Nakaminami H, Rimbara E, et al:
Effect of pretreatment with Lactobacillus gasseri OLL2716 on
first-line Helicobacter pylori eradication therapy. J Gastroenterol
Hepatol. 27:888–892. 2012. View Article : Google Scholar :
|
|
56
|
Du YQ, Su T, Fan JG, et al: Adjuvant
probiotics improve the eradication effect of triple therapy for
Helicobacter pylori infection. World J Gastroenterol. 18:6302–6307.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tolone S, Pellino V, Vitaliti G, Lanzafame
A and Tolone C: Evaluation of Helicobacter pylori eradication in
pediatric patients by triple therapy plus lactoferrin and
probiotics compared to triple therapy alone. Ital J Pediatr.
38:632012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gotteland M, Brunser O and Cruchet S:
Systematic review: are probiotics useful in controlling gastric
colonization by Helicobacter pylori? Aliment Pharmacol Ther.
23:1077–1086. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang YJ and Sheu BS: Probiotics-containing
yogurts suppress Helicobacter pylori load and modify immune
response and intestinal microbiota in the Helicobacter
pylori-infected children. Helicobacter. 17:297–304. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kwon HK, Kim GC, Kim Y, et al:
Amelioration of experimental autoimmune encephalomyelitis by
probiotic mixture is mediated by a shift in T helper cell immune
response. Clin Immunol. 146:217–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sultana R, McBain AJ and O’Neill CA:
Strain-dependent augmentation of tight-junction barrier function in
human primary epidermal keratinocytes by Lactobacillus and
Bifidobacterium lysates. Appl Environ Microbiol. 79:4887–4894.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yeung CY, Chiang Chiau JS, Chan WT, et al:
In vitro prevention of Salmonella lipopolysaccharide-induced
damages in epithelial barrier function by various Lactobacillus
strains. Gastroenterol Res Pract. 2013:9732092013. View Article : Google Scholar : PubMed/NCBI
|