Introduction
Apoptosis, a genetically programmed form of cell
death, is a tightly regulated process to maintain tissue
homeostasis and eliminate unwanted or dysfunctional cells in
multicellular organisms. The dysregulation of apoptosis can lead to
a variety of heart diseases including heart failure and myocardial
infarction with or without reperfusion injury (1,2).
The role of apoptosis in heart diseases is becoming increasingly
clear. Therefore, it is vital to develop a potential therapeutic
strategy to block or prevent inappropriate apoptosis in heart
diseases.
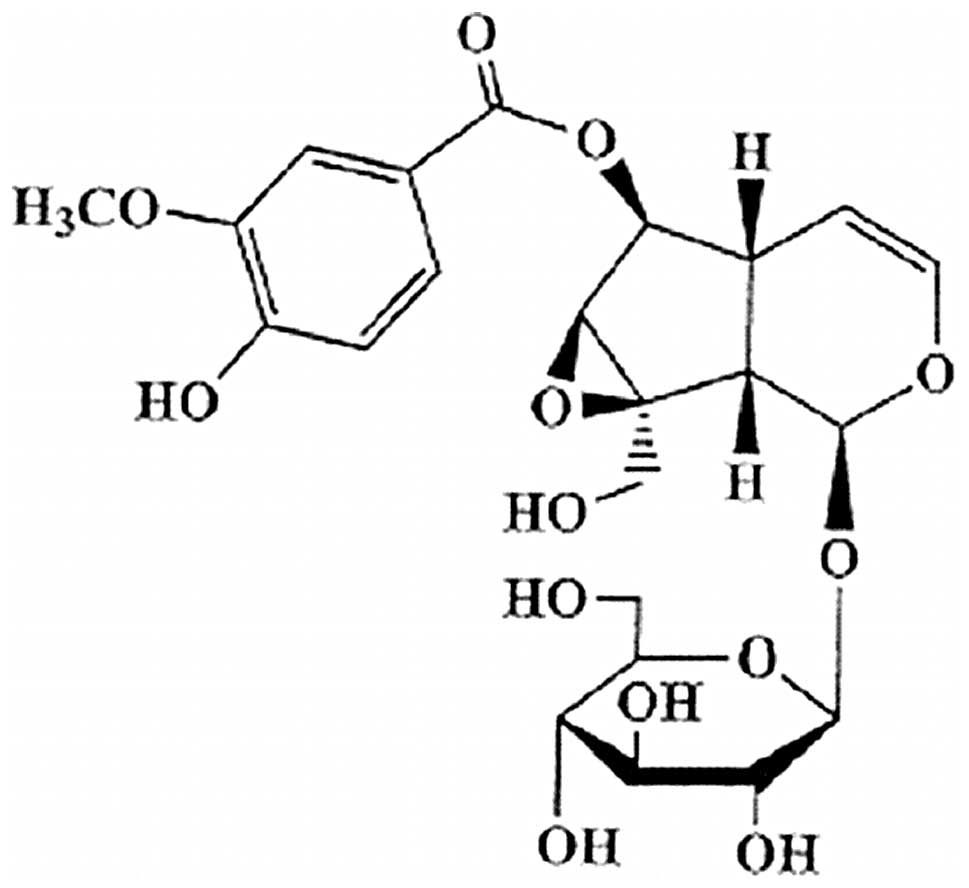
Picroside II
(h-D-glucopyranoside,1a,1b,2,5a,6,6a-hexa-hydro-6-[(4-hydroxy-3-methoxybenzoyl)oxy]-1a(hydroxymethyl)
oxireno[4,5]cyclopenta[1,2-c]pyran-2-yl) is a major iridoid
glucoside isolated from Picrorhiza scrophulariiflora Pennell
(Scrophulariaceae) (Fig. 1)
(3). Previous studies have shown
that picroside II has a wide range of pharmacological effects,
including neuroprotective (4,5),
hepatoprotective (6),
anti-oxidation (7),
anticholestatic, anti-inflammatory and immunemodulating activities
(8,9). In addition, picroside II is also
reported to possess potential anti-apoptotic activities in
hepatocytes (6), and rat model of
focal cerebral ischemia (5).
Therefore, it seems reasonable to investigate whether picroside II
is able to protect cardiomyocytes against apotosis.
Akt, a serine/threonine kinase, is the primary
mediator of Phosphoinositide 3-kinase (PI3K)-initiated signaling.
The PI3K/Akt pathway regulates the process of cell survival through
phosphorylation of a variety of downstream targets such as the
Bcl-2 family member Bad, caspase-9 and CREB (10). The pro- and anti-apoptotic members
of the Bcl-2 family are intrinsic to the apoptotic pathway; Bcl-2
and Bcl-xL induce cell survival, whereas Bax and Bad promote cell
death (11,12). It is widely accepted that Bcl-2
family, located in the outer membranes of the mitochondria, can
regulate mitochondrial outer membrane permeability and trigger
apoptosis (13). The Bcl-2
protein inhibits apoptosis by preventing the release of cytochrome
c and the subsequent activation of caspases (14).
In the present study, we investigated the effect of
picroside II on hypoxia/reoxygenation-induced apoptosis in
cardiomyocytes and, most importantly, explored the possible
underlying mechanisms.
Materials and methods
Animals
The animal use in this study was performed according
to the Guide for the Care and Use of Laboratory Animals published
by the National Institutes of Health (NIH publication no. 85-23,
revised 1996). The use of animals was also reviewed and approved by
the China Medical University Animal Care Review Committee. Neonatal
Sprague-Dawley 1-to 3-day-old rats [Grade II, Certificate Number
SCXK-(Liao) 2009-0001] were purchased from China Medical University
(Shenyang, China).
Chemicals and reagents
Picroside II (purity >99%), was purchased from
the Chinese National Institute for the Control of Pharmaceutical
and Biological products (Beijing, China). Dulbecco’s modified
Eagle’s medium (DMEM), DMEM glucose-free, new-born calf serum and
trypsin were purchased from Gibco-BRL (Carlsbad, CA, USA).
Collagenase II was purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). ECL reagent kit was purchased from Pierce
Biotechnology, Inc. (Rockford, IL, USA). Antibodies against Akt,
phospho-Akt (Ser-473), CREB and phospho-CREB, were obtained from
Bioworld Technology, Inc. (Minneapolis, MN, USA). anti-Bcl-2
antibody, anti-Bax antibody and anti-β-actin antibody was purchased
from Boster Bilogical Technology, Ltd. (Wuhan, China). The
detection kits of lactate dehydrogenase (LDH), were purchased from
the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
bicinchoninic acid (BCA) protein assay kit,
2-(4-Morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride
(LY294002) and wortmannin were supplied by Byeotime (Jianshu,
China). Annexin V-FITC apoptosis detection kits were obtained from
Nanjing KeyGen Biotech, Co, Ltd. (Nanjing, China). All other
chemicals were purchased from Sigma-Aldrich. The purity of all
reagents was at least analytical grade.
Primary culture of neonatal rat
cardiomyocytes
The primary culture of neonatal rat cardiac
ventricular myocytes was prepared from Sprague-Dawley rats (1- to
3-day-old) based on a previously published protocol with some
modifications (15). Briefly,
hearts were harvested and placed in ice-cold D-Hank’s buffer.
Ventricles were separated and cut into 1-mm3 pieces. The
tissue fragments were digested by treatment with 0.06% trypsin once
(37°C) and subsequently with 0.08% collagenase II 4–5 times (37°C).
The cell suspension was then filtered and centrifuged for 8 min
(120 × g, 4°C), and finally resuspended in Dulbecco’s modifed
Eagle’s media supplemented with 10% fetal calf serum, penicillin
(100 U/ml) and streptomycin (100 U/ml). Resuspended cells were
plated onto 6-well plates in a humidified incubator (5%
CO2, 95% air, 37°C) for 2 h to exclude fibroblasts cells
based on the fact that the fibroblasts cells attach to the surface
more rapidly. To prevent proliferation of fibroblasts, 0.1 mmol/l
5-bromo-2′-deoxyuridine (BrdU) was added to the culture medium.
Non-adherent cells were then replated onto fresh 96- and 6-well
plates (1.2×105 and 2×106 cells/well,
respectively) and incubated for 3–4 days before the experiment.
Hypoxia-reoxygenation model
In the present study, we used the model of
hypoxia-reoxygenation in vitro which was similar to that
described by Zhu et al (16). Briefly, before starting the
experiments, the cultured cardiomyocytes were carefully washed 3
times with Hank’s solution (5 mM HEPES, 137 mM NaCl, 4 mM KCl, 1 mM
MgCl2, 1.5 mM CaCl2; pH 7.2). The cells were incubated
in a glucose-free DMEM base medium and then subjected to hypoxia to
mimic the in vivo condition of myocardial ischemia. The
cells were placed in an incubator at 37°C. N2 (95%) and
CO2 (5%) were flushed into the incubator to bring the
oxygen content down to 1% monitored by an oxygen probe. After 3 h
of hypoxia, the cells were reoxygenated by immediately replacing a
glucose-free DMEM base medium with a DMEM base medium with 5.5 mM
glucose (pH 7.4) followed by incubation under normoxia for 2 h.
Experimental groups and protocols
After cardiomyocytes had been cultured for ∼72 h,
they were in the state of confluence and beat synchronously. The
cultured cardiomyocytes were randomly divided into 7 groups: i)
control group: cardiomyocytes were incubated under normoxic
conditions at 37°C during the entire experimental period; ii)
hypoxia/reoxygenation group: cardiomyocytes were incubated with a
glucose-free DMEM base medium for 3 h of hypoxia followed by
reoxygenation for 2 h as described above; iii) picroside II-50
group: picroside II (50 μg/ml) was applied 48 h prior to
hypoxia/reoxygenation; iv) picroside II-100 group: picroside II
(100 μg/ml) was applied 48 h prior to hypoxia/reoxygenation;
v) picroside II (200 μg/ml) group: picroside II (200
μg/ml) was applied 48 h prior to hypoxia/reoxygenation;
vi–vii) cells co-treated with hypoxia/reoxygenation and picroside
II (200 μg/ml) were challenged with the alternative PI3K
inhibitor wortmannin (wortmannin, 50 nM: picroside II + wortmannin
group) or 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
(LY294002, 15 μM: picroside II + LY294002 group).
Cell viability assays
Cell viability was assessed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay. The cardiomyocytes were seeded on 96-well plates at
1.2×105 cells/well. Following exposure to
hypoxia-reoxygenation, cardiomyocytes were treated by the addition
of 20 μl MTT solution (5 mg/ml phosphate buffer) for 4 h and
the media were removed. The formazan blue crystals, formed by
oxidation of the MTT dye, were dissolved in 150 μl DMSO for
10 min at the condition of vibration. The absorbance at 490 nm was
measured using a microplate reader (Sunrise RC; Tecan Group, Ltd.,
Mannedorf, Switzerland) and cell viability was expressed as a
percentage of the control culture value.
LDH activity assay
LDH activity was measured as the LDH content was
released in the culture medium. After hypoxia/reoxygenation, 0.2 ml
of culture medium was taken and assayed for LDH activity using
commercial kits (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) with a microplate reader (Sunrise RC; Tecan Group,
Ltd.), following the manufacturer’s instructions.
Caspase-3 activity assay
Caspase-3 activity was measured using the caspase-3
assay kit/colorimetric according to the manufacturer’s
instructions. Briefly, at the end of hypoxia/reoxygenation,
2×106 cells were collected, centrifuged 1,000 × g for 10
min at 4°C. The cells were resuspended in the lysis buffer,
incubated for 5 min on ice bath and centrifuged at 10,000 × g for
10 min at 4°C. The supernatant was then reacted with Ac-DEVD-pNA (2
mM), which were the substrates of caspase-3, at 37°C for 2 h. The
protein concentration in the supernatant was determined by the BCA
method. The absorbance at 405 nm was measured using a microplate
reader (Sunrise RC; Tecan Group, Ltd.).
Nuclear staining for assessment of
apoptosis
The nuclei of cardiomyocytes were stained with the
chromatin dye Hoechst 33258. Cells were fixed with 4%
paraformaldehyde for 10 min at room temperature. After three washes
with PBS, the cells were incubated with Hoechst 33258 (5
μg/ml in PBS) at room temperature for 15 min. After rinsing
with PBS again, the cells were examined under a fluorescence
microscope.
Analysis of flow cytometry
The apoptotic cells were measured by Annexin
V-FITC/PI staining. At the end of hypoxia/reoxygenation, cells were
harvested, washed with PBS, resuspended in binding buffer (10 mM
Hepes/NaOH pH 7.4, 140 mM NaCl and 2.5 mM CaCl2) and
incubated with Annexin-V at room temperature in dark for 15 min.
Then the cells were centrifuged, resuspended in the binding buffer
and incubated with Propidium iodide (PI). After incubation, 400
μl of binding buffer was added and the cells were analyzed
by flow cytometry (FACScalibur; BD Biosciences, USA).
Western blot analysis
Cardiomyocytes with various treatments were lysed in
an ice-cold RIPA buffer [1% Triton, 0.1% SDS, 0.5% deoxycholate, 1
mmol/l EDTA, 20 mmol/l Tris (pH 7.4), 150 mmol/l NaCl, 10 mmol/NaF
and 0.1 mmol/l phenylmethylsulfonyl fluoride (PMSF)]. The lysates
were centrifuged at 12,000 rpm for 15 min at 4°C to remove debris.
The protein concentration was determined with BCA protein assay.
Equal amounts of denatured protein samples (50–100 μg
protein/lane) were separated by 10–12% sodium dodecyl
sulfate-polyacrylamide gelelectrophoresis (SDS-PAGE) and
transferred to polyvinylidene difluoride (PVDF) membranes.
Membranes were then washed with TTBS 3 times and incubated further
with horseradish peroxidase-conjugated secondary antibody (1:2,000)
for 2 h at room temperature. The blots were processed using an
electrochemiluminescence (ECL) kit and and light emission was
captured on X-ray film. The blots were visualized with ECL-plus
reagent and then subjected to densitometric analysis. β-actin was
used as the internal loading control.
Statistical analysis
The data are expressed as the mean ± SD. One-way
analysis of variance (ANOVA) followed by the Student-Newman-Keuls
test. Differences were considered to be statistically significant
at P<0.05.
Results
Effect of picroside II on the viability
of cardiomyocytes subjected to hypoxia/reoxygenation
The results show that pre-incubation of
cardiomyocytes with different concentrations of picroside II (0,
6.25, 12.5, 25, 50, 100, 200, 400 and 800 μg/ml) prevented
the loss of viability that resulted from hypoxia/reoxygenation in a
dose-dependent manner (data not shown). Maximum viability was
apparent at a concentration of 200 μg/ml. However, higher
concentrations (400 and 800 μg/ml) of picroside II did not
cause any enhancement of this preventive effect. We analyzed the
effective half-maximal concentration for protection (EC50) was 50
μg/ml. Therefore, we used concentrations of picroside II
(50, 100 or 200 μg/ml) for our subsequent experiments. As
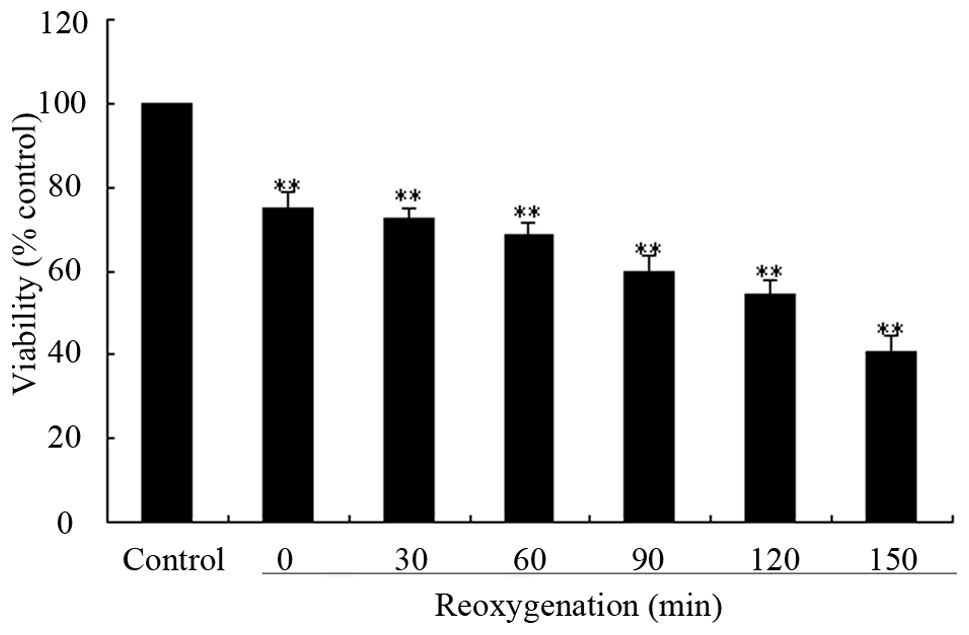
shown in Fig. 2, hypoxia 3
h/reoxygenation for 30-150 min significantly decreased the
percentage of survival cells in a time-dependent manner. Subjected
to hypoxia 3 h/reoxygenation for 120 min, there were only
54.29±3.4% viable cells as compared to the control cells. Thus,
hypoxia 3 h/reoxygenation 120 min was used as a standard apoptosis
induction in the subsequent experiments.
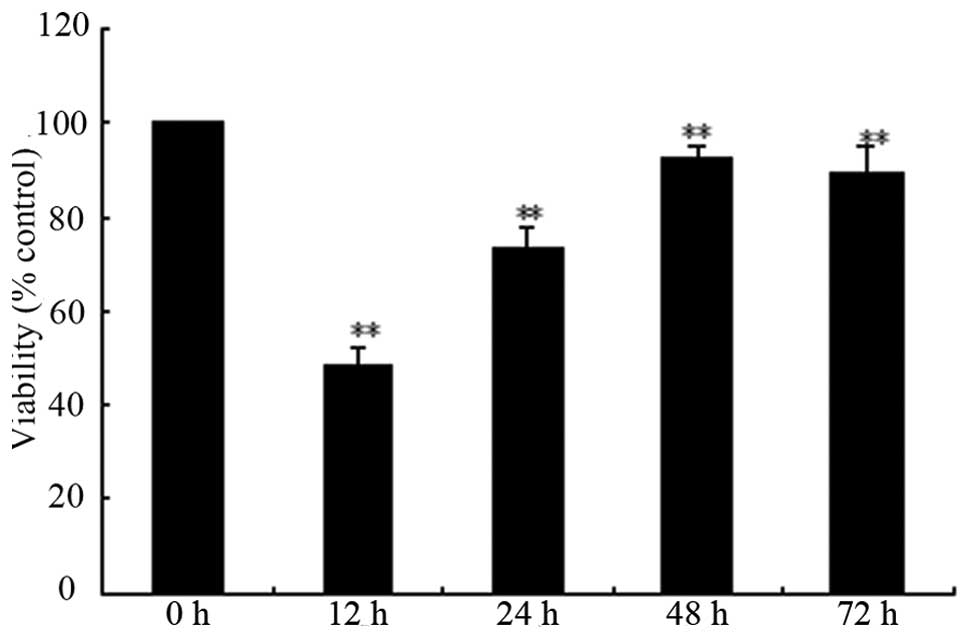
Fig. 3 shows the
results of the time-response investigation during which cells were
exposed to 200 μg/ml picroside II for ≤72 h. The magnitude
of cell survival peaked at 48 h, when cell viability was
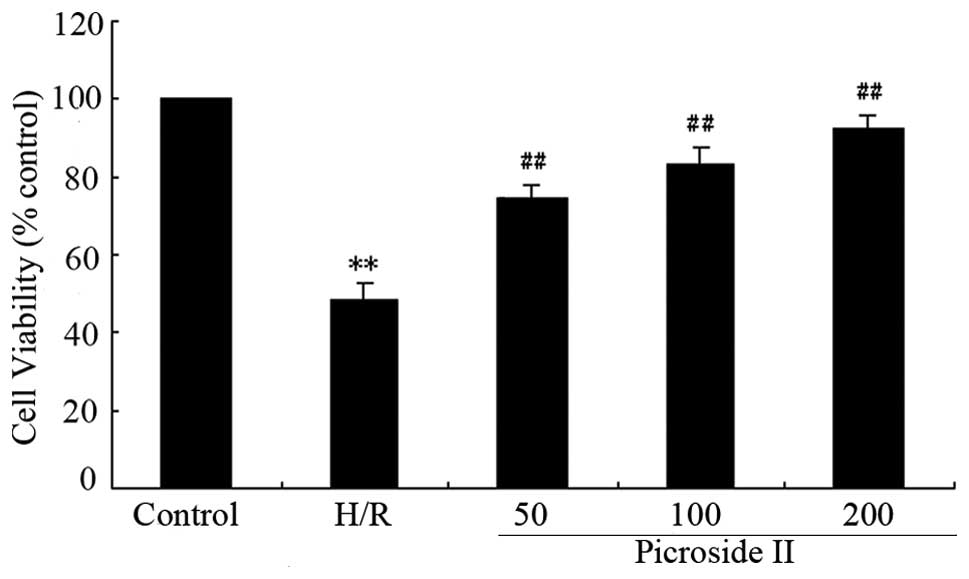
92.3±3.02%. Fig. 4 shows that
after exposure to hypoxia 3 h/reoxygenation for 2 h the survival
rate of cardiomyocytes was 48.25±4.31%. Pre-incubation with
picroside II (50, 100 and 200 μg/ml) prevented
cardiomyocytes from hypoxia/reoxygenation-induced damage, and
caused a dose-dependent attenuation in cell survival to 74.17±
3.51, 83 ±4.5 and 92.02±3.6%, respectively (all P<0.01 vs.
hypoxia/reoxygenation).
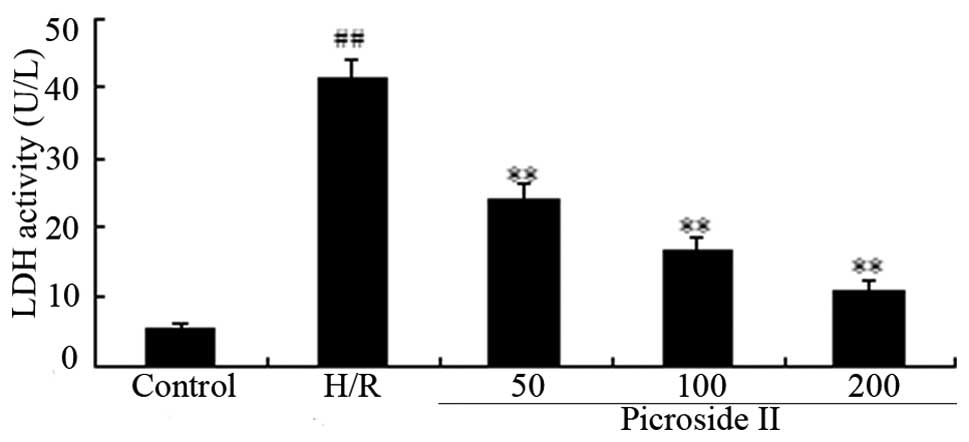
Effect of picroside II on the levels of
LDH in cardiomyocytes subjected to hypoxia/reoxygenation
The release of LDH in the medium is widely known as
an indicator of cardiomyocyte injury. As shown in Fig. 5, LDH concentrations in medium from
control group cells were minimal (5.63±0.39 U/l), while LDH
activity induced by hypoxia/reoxygenation increased up to
(41.5±2.71 U/l). Treatment with picroside II (50, 100 and 200
μg/ml) significantly attenuated LDH activity in a
dose-dependent manner (concentrations were 24.02±2.0 U/l,
16.69±1.92 U/l and 10.09±1.38 U/l, respectively).
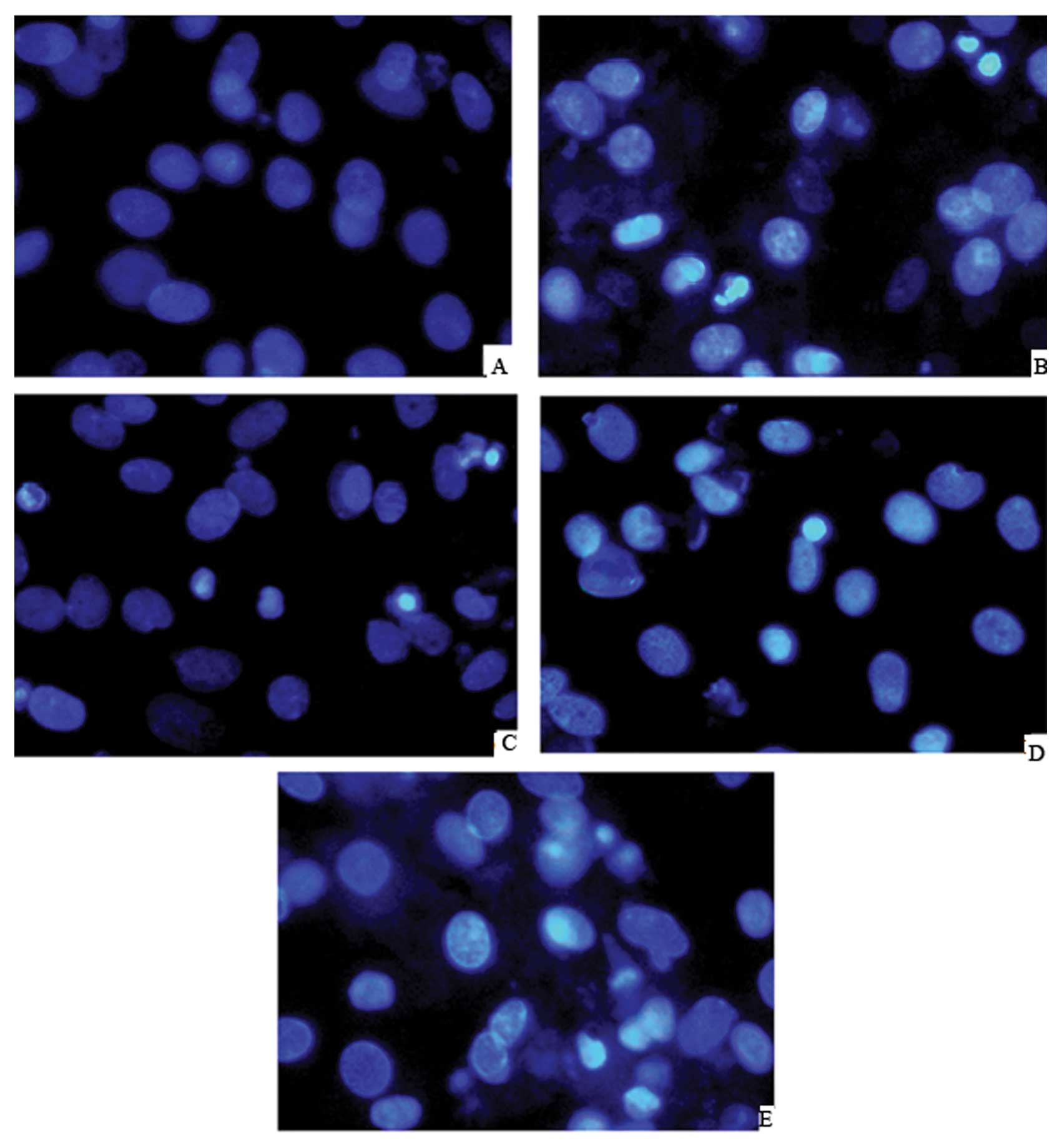
Effect of picroside II on apoptosis in
cardiomyocytes subjected to hypoxia/reoxygenation
Hoechest 33258 staining assay showed that
cardiomyocytes exposed to hypoxia/reoxygenation featured typical
characteristics of apoptosis, including the condensed chromatin and
the fragmented apoptotic nuclei. However, the development of these
apoptotic features were significantly suppressed when cells were
treated with picroside II (200 μg/ml) (Fig. 6).
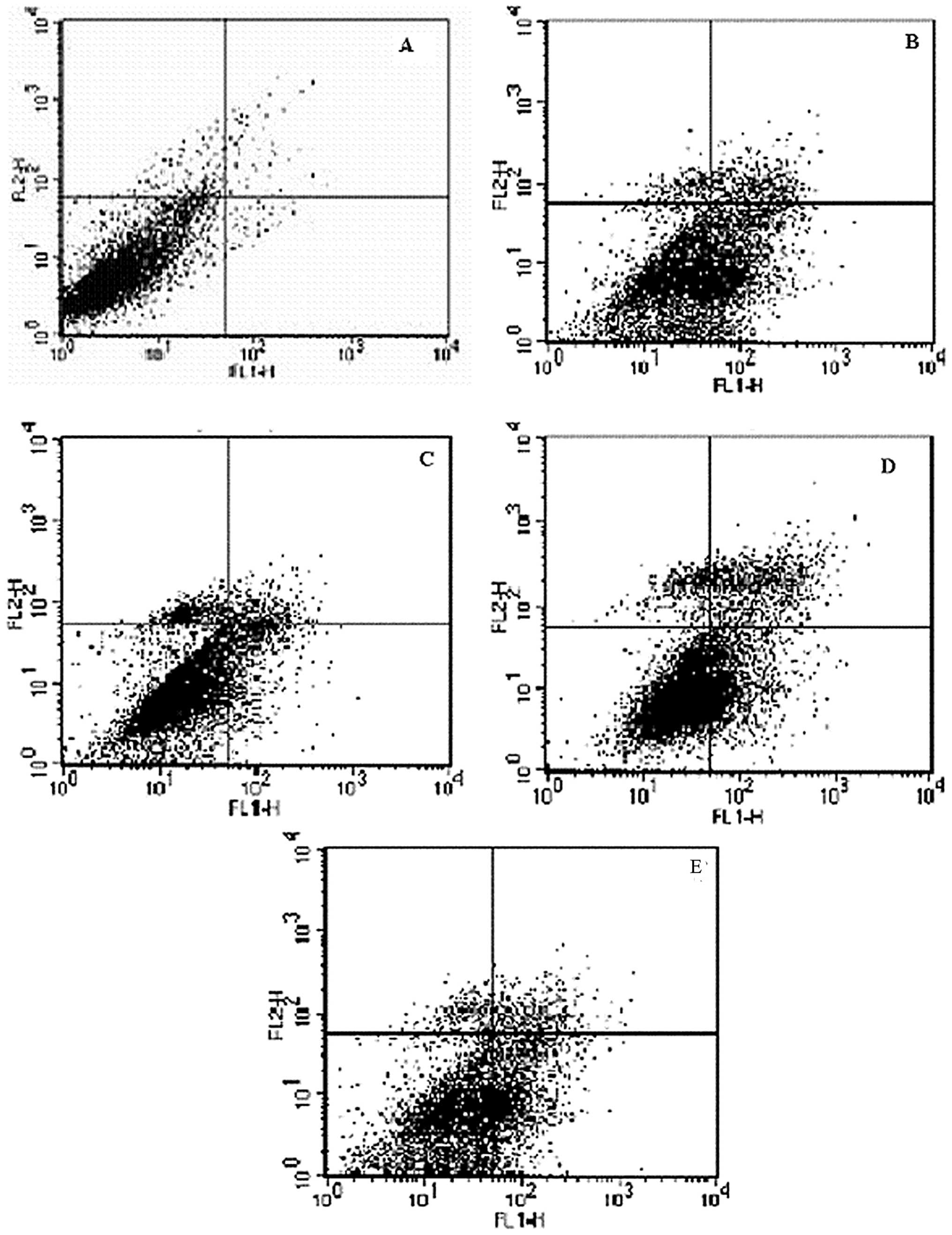
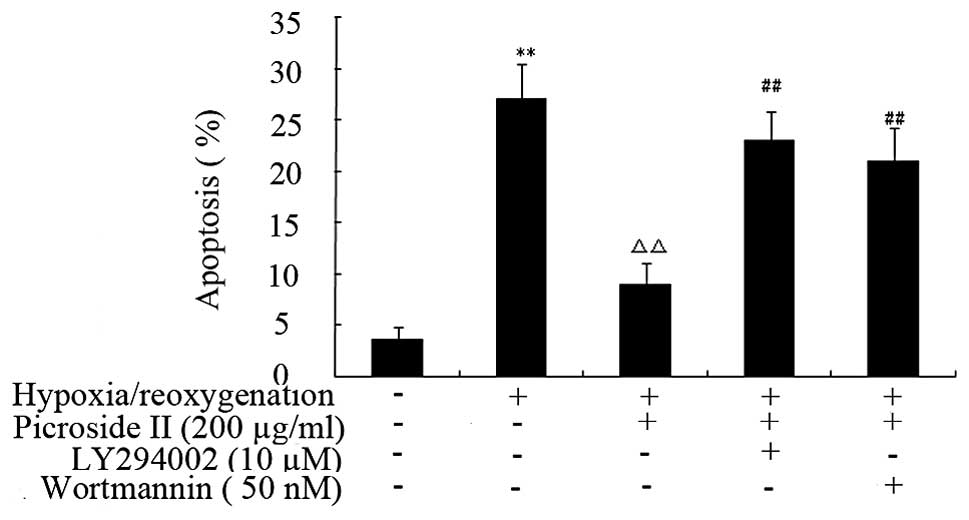
Flow cytometry revealed that hypoxia-reoxygenation
caused a significant increase in the percentage of apoptotic cells
from 3.3±0.95% in control cells to 27.09±3.42% (P<0.01)
(Fig. 7). However, when picroside
II (200 μg/ml) was applied 48 h prior to
hypoxia-reoxygenation, it caused a reduction in the percentage of
apoptotic cells to 9.05±2.0% (P<0.01) (Fig. 8).
Cardiomyocytes exposed to hypoxia/reoygenation
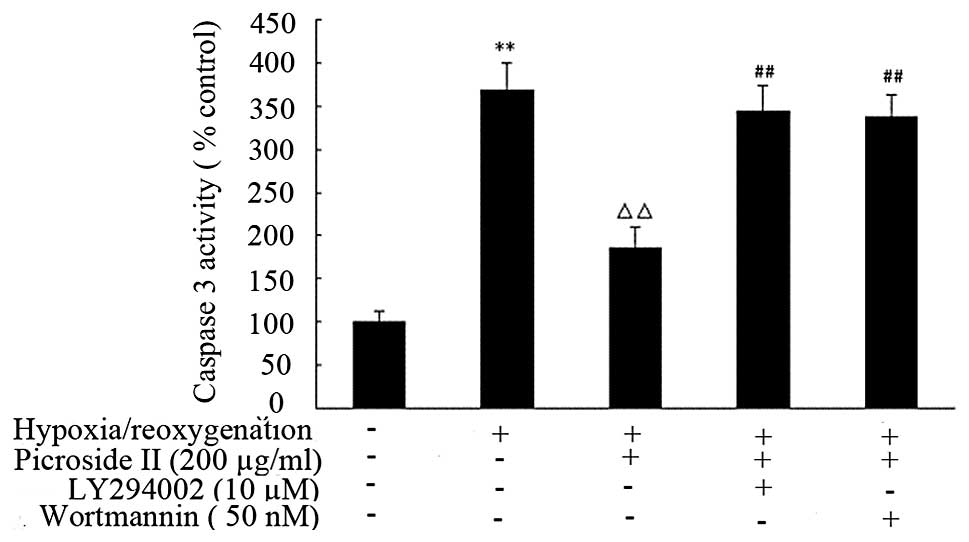
insults had an increase in caspase-3 activation (368.3±32.12)
comparing to the control group (100±12.01). Treatment with
picroside II (200 μg/ml) for 48 h significantly decreased
this hypoxia/reoygenation-induced caspase-3 activation (Fig. 9).
However, the protection of picroside II was
partially blocked by pretreating cells with wortmannin or LY294002.
These results provide direct evidence that picroside II can
significantly inhibit apoptosis in cardiomyocytes induced by
hypoxia/reoygenation, and that this effect is partially inhibited
by concurrent wortmannin or LY294002 treatment.
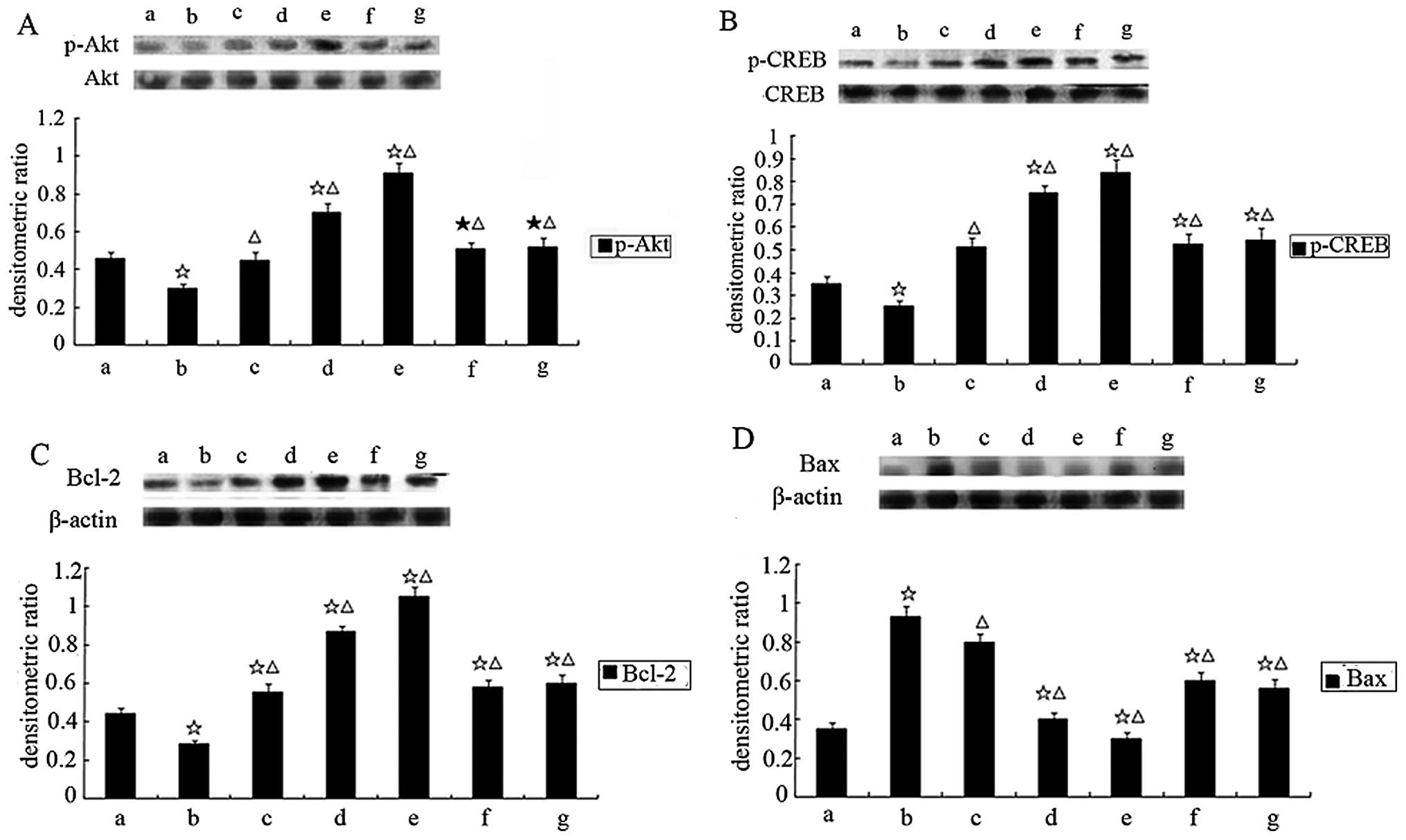
Effect of picroside II on the
phosphorylation of Akt and CREB in cardiomyocytes exposed to
hypoxia/reoxygenation
The kinetics of Akt activation were examined, to
evaluate a possible mechanism for the anti-apoptotic effect of
picroside II. As shown in Fig.
10A, hypoxia/reoxygenation significantly reduced the
phosphorylation of Akt without alteration of the total Akt
expression. Pretreatment of cardiomyocytes with picroside II, prior
to hypoxia/reoxygenation exposure, resulted in a significant
increase in the phosphorylation of Akt. In addition, CREB, a
downstream target of Akt, was also markedly phosphorylated
(Fig. 10B). Both 50 nM
wortmannin and 15 μM LY294002 markedly inhibited picroside
II-induced Akt and CREB phosphorylation.
Effect of picroside II on the
phosphorylation of Bcl-2 and Bax in cardiomyocytes exposed to
hypoxia/reoxygenation
Akt phosphorylates the transcription factor CREB,
implicated in the transcription of the anti-apoptotic Bcl-2 gene
(17). We investigated the
protein expression of the anti-apoptotic Bcl-2 and the
pro-apoptotic Bax. As shown in Fig.
10C, the expression of Bcl-2 protein decreased in the
hypoxia/reoxygenation group compared to the control group. However,
cells treated with picroside II showed an increase in Bcl-2 protein
expression as detected by western blot analysis. Picroside II
pretreatment downregulated Bax levels in cells exposed to
hypoxia/reoxygenation comparing to untreated cells (Fig. 10D). This effect was partially
inhibited by concurrent wortmannin or LY294002 treatment. Picroside
II treatment mediated phosphorylation of Akt and CREB, increased
Bcl-2 protein expression, and decreased Bax protein expression and
caspase-3 activity in cardiomyocytes hypoxia/reoxygenation, which
was abolished by LY294002 or wortmannin treatment.
Discussion
Through examination, we firstly proved the
cardioprotective effects of picroside II on apoptosis induced by
hypoxia/reoxygenation in a neonatal rat cardiomyocyte. In the
present study, we demonstrated that pretreatment of cardiomyocytes
with picroside II (50–200 μg/ml) for 48 h significantly
reduced the decrease in viability and LDH activities associated
with exposure to hypoxia/reoxygenation as determined by MTT and LDH
assays. Picroside II decreased hypoxia/reoxygenation-induced
apoptosis in cardiomyocytes as indicated by nuclear morphological
Hoechst staining, flow cytometry and caspase-3 activity. These
results indicate that picroside II protects against
hypoxia/reoxygenation-induced apoptosis in that it not only
involves enhanced phosphorylation of Akt and CREB, but also
upregulation of Bcl-2 and downregulation of Bax via the
PI3-kinase/Akt signaling pathway.
Picroside II is a major iridoid glucoside isolated
from Picrorhiza scrophulariiflora Pennell (Scrophulariaceae)
(3). Previous studies have shown
that picroside II has a number of pharmacological effects,
including neuroprotective (4,5),
hepatoprotective (6),
antioxidant, anticholestatic, anti-inflammatory and
immunemodulating activities (7,8).
In addition, picroside II is also reported to possess potent
anti-apoptotic activities (5,6).
It has been reported to stimulate anti-apoptotic proteins such as
Bcl-2 (6) and inhibit proteins
with pro-apoptotic properties such as caspase-3 and poly ADP-ribose
polymerase (PARP) (5). The most
demonstrated findings are from studies in neuronal cells (4,5).
In the present study, we examined the effect of picroside II on the
viability of cardiomyocytes subjected to hypoxia/reoxygenation by
MTT and LDH assays. Apoptosis was also assessed by Hoechst 33258
staining, Annexin V-FITC staining and caspase-3 activity. We found
that picroside II protected cardiomyocytes from
hypoxia/reoxygenation-induced decrease in cell viability and
apoptosis.
The mechanism by which picroside II protects
cardiomyocytes from hypoxia/reoxygenation-induced apoptosis is an
important issue raised by the results presented in this study.
Multiple signaling pathways participate in regulation of cell
survival. Our study emphasized on examining potential changes in
the Akt-CREB pathway. Akt is a critical regulator of
PI3-kinase-mediated cell proliferation and survival (18). Akt is involved in many cell
anabolic processes, including glucose transport, glycogen
synthesis, and protein synthesis (19). Akt can also regulate the process
of cell survival through phosphorylation of diverse target
molecules including the Bcl-2-family member Bad, Forkhead,
caspase-9, NF-κB and CREB (20,21).
Akt also activates cAMP response element binding
(CREB) protein, stimulates recruitment of CBP to the promoter and
promotes cellular survival through a CRE-dependent mechanism
(22,23). The transcription factor CREB
mediates survival in many cells, including cardiomyocytes. It has
been shown that Bcl-2 is one of the most important CREB-regulated
genes, playing a critical role in the cell survival (24). The promoter region of Bcl-2
contains a cAMP-response element site and CREB is known as a
positive regulator of Bcl-2 expression (25–27). Furthermore, activated CREB can
also inhibit caspase-3 activation and finally attenuate myocyte
apoptosis (28). In line with
this notion, our results showed that picroside II upregulated Bcl-2
protein expression and inhibited caspase-3 activation in the
hypoxia/reoxygenation myocytes, which was accompanied by a
significant increase of CREB phosphorylation. In this study, we
found that Akt predominantly existed in an inactivated form in
cardiomyocytes that were not undergoing hypoxia/reoxygenation, but
was activated and phosphorylated when cells were co-treated with
hypoxia/reoxygenation and picroside II. Picroside II markedly
increased the phosphorylation of Akt and CREB. This activation was
significantly blocked by the PI3K inhibitor wortmannin and
LY294002. These results suggest that picroside II may protect
cardiomyocytes from hypoxia/reoxygenation-induced apoptosis at
least in part by activating the PI3K/Akt-CREB signaling
pathway.
Many genes have been reported to be involved in the
regulation of apoptosis, in which Bcl-2 and Bax genes are suggested
to play a major role in maintaining the balance of cell death and
survival (29,30). The main site of action of Bcl-2
family proteins appears to be the mitochondrion. Several studies
have shown that the anti-apoptotic protein Bcl-2 and the
proapoptotic protein Bax of the Bcl-2 family are associated with
the mitochondrial membrane and affect membrane permeability
(31). Bcl-2 prevent the release
of cytochrome c from the mitochondria by binding to the
pro-apoptotic proteins Bad, Bax and Bak (32,33). Thus, it can inhibit activation of
caspases, such as caspase-9 and caspase-3, and prevent apoptosis
(34,35). Moreover, it has been shown that
upregulation of Bcl-2 can antagonize the pro-apoptotic activities
of Bax and Bak (36). To further
support our findings, the effects of picroside II on expression of
Bcl-2 and Bax proteins were detected by western blot analysis. In
the present study, we found that hypoxia/reoxygenation
downregulated the expression of Bcl-2, whereas upregulated Bax
expression. Pretreatment with picroside II ameliorated these
changes in Bax and Bcl-2 protein expression. Furthermore, these
effects were partially abolished by the PI3K inhibitor wortmannin
and LY294002. Our findings suggest that Bcl-2 and Bax are involved
in mediating the anti-apoptotic effects associated with picroside
II treatment in cardiomyocytes exposed to
hypoxia/reoxygenation.
In conclusion, our study for the first time
demonstrates, that picroside II protects cardiomyocytes from
hypoxia/reoxygenation-induced apoptosis. Our results also suggest
that the anti-apoptotic effects associated with picroside II
pretreatment are at least in part due to inhibited caspase-3
activation, activation of the PI3K/Akt/CREB signaling pathway and
modulated Bcl-2 and Bax expression. Picroside II may hold promise
as a therapeutic intervention for the treatment of myocardial
ischemia/reperfusion. Further detailed investigation is needed in
these fields.
References
|
1.
|
MR BennettApoptosis in the cardiovascular
systemHeart87480487200210.1136/heart.87.5.480
|
|
2.
|
PM KangS IzumoApoptosis in heart: basic
mechanisms and implications in cardiovascular diseasesTrends Mol
Med9177182200310.1016/S1471-4914(03)00025-X12727144
|
|
3.
|
P LiK MatsunagaT YamakuniY
OhizumiPotentiation of nerve growth factor- action by picrosides I
and II, natural iridoids, in PC12D cellsEur J
Pharmacol406203208200010.1016/S0014-2999(00)00662-211020482
|
|
4.
|
T LiJW LiuXD ZhangMC GuoG JiThe
neuroprotective effect of picroside II from hu-huang-lian against
oxidative stressAm J Chin
Med35681691200710.1142/S0192415X0700517X17708634
|
|
5.
|
Q LiZ LiXY XuYL GuoF DuNeuroprotective
properties of picroside II in a rat model of focal cerebral
ischemiaInt J Mol Sci1145804590201010.3390/ijms1111458021151457
|
|
6.
|
H GaoYW ZhouInhibitory effect of picroside
II on hepatocyte apoptosisActa Pharmacol
Sin26729736200510.1111/j.1745-7254.2005.00729.x15916740
|
|
7.
|
Y CaoJW LiuYJ YuPY ZhengXD ZhangT LiMC
GuoSynergistic protective effect of picroside II and NGF on PC12
cells against oxidative stress induced by
H2O2Pharmacol Rep59573579200718048958
|
|
8.
|
HF SmitBH KroesAJ van den BergD van der
WalE van den WormCJ BeukelmanH van DijkRP LabadieImmunomodulatory
and anti-inflammatory activity of Picrorhiza
scrophulariifloraJ
Ethnopharmacol73101109200010.1016/S0378-8741(00)00268-3
|
|
9.
|
LJ HeM LiangFF HouZJ GuoD XieX
ZhangEthanol extraction of Picrorhiza scrophulariiflora
prevents renal injury in experimental diabetes via
anti-inflammation actionJ Endocrinol2003473552009
|
|
10.
|
I HersEE VincentJM TavaréAkt signalling in
health and diseaseCellular
Signal2315151527201110.1016/j.cellsig.2011.05.00421620960
|
|
11.
|
M KvansakulH YangWD FairliePE CzabotarSF
FischerMA PeruginiDC HuangPM ColmanVaccinia virus anti-apoptotic
F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly
selective subset of BH3-containing death ligandsCell Death
Differ1515641571200810.1038/cdd.2008.8318551131
|
|
12.
|
RJ YouleA StrasserThe Bcl-2 protein
family: opposing activities that mediate cell deathNat Rev Mol Cell
Biol94759200810.1038/nrm230818097445
|
|
13.
|
J EstaquierD ArnoultInhibiting
Drp1-mediated mitochondrial fission selectively prevents the
release of cytochrome c during apoptosisCell Death
Differ1410861094200710.1038/sj.cdd.440210717332775
|
|
14.
|
S CoryDC HuangJM AdamsThe Bcl-2 family:
roles in cell survival and
oncogenesisOncogene2285908607200310.1038/sj.onc.120710214634621
|
|
15.
|
H ReineckeM ZhangT BartosekCE
MurrySurvival, integration, and differentiation of cardiomyocyte
grafts: A study in normal and injured rat
heartsCirculation100193202199910.1161/01.CIR.100.2.19310402450
|
|
16.
|
D ZhuL WuCR LiXW WangYJ MaZY ZhongHB ZhaoJ
CuiSF XunXL HuangGinsenoside Rg1 protects rat cardiomyocyte from
hypoxia/reoxygenation oxidative injury via antioxidant and
intracellular calcium homeostasisJ Cell
Biochem108117124200910.1002/jcb.2223319530220
|
|
17.
|
S Willaime-MorawekN ArbezJ MarianiB
BruggIGF-I protects cortical neurons against ceramide-induced
apoptosis via activation of the PI-3K/Akt and ERK pathways; is this
protection independent of CREB and Bcl-2?Mol Brain
Res14297106200510.1016/j.molbrainres.2005.09.02016290312
|
|
18.
|
B WangJ ShravahH LuoK RaedscheldersDD
ChenDM AnsleyPropofol protects against hydrogen peroxide-induced
injury in cardiac H9c2 cells via Akt activation and Bcl-2
up-regulationBiochem Biophys Res
Commun389105111200910.1016/j.bbrc.2009.08.09719703415
|
|
19.
|
KL NathanW KennethA ZoltanMetabolic
benefits of resistance training and fast glycolytic skeletal
muscleAm J Physiol Endocrinol
Metab300E3E10201110.1152/ajpendo.00512.201021045171
|
|
20.
|
T MatsuiA RosenzweigConvergent signal
transduction pathways controlling cardiomyocyte survival and
function: the role of PI3-kinase and AktJ Mol Cell
Cardiol386371200510.1016/j.yjmcc.2004.11.00515623422
|
|
21.
|
F GaoE GaoTL YueEH OhlsteinBL LopezTA
ChristopherXL MaNitric oxide mediates the antiapoptotic effect of
insulin in myocardial ischemia reperfusion: the roles of
PI3-kinase, Akt, and endothelial nitric oxide synthase
phosphorylationCirculation10514971502200210.1161/01.CIR.0000012529.00367.0F11914261
|
|
22.
|
K DuM MontminyCREB is a regulatory target
for the protein kinase Akt/PKBJ Biol
Chem2733237732379199810.1074/jbc.273.49.323779829964
|
|
23.
|
H DudekSR DattaTF FrankeMJ BirnbaumR YaoGM
CooperRA SegalDR KaplanME GreenbergRegulation of neuronal survival
by the serine-threonine protein kinase
AktScience275661665199710.1126/science.275.5300.6619005851
|
|
24.
|
MR WaltonI DragunowIs CREB a key to
neuronal survival?Trends
Neurosci234853200010.1016/S0166-2236(99)01500-310652539
|
|
25.
|
S PugazhenthiA NesterovaC SableKA
HeidenreichLM BoxerLE HeasleyJE ReuschAkt/protein kinase B
up-regulates Bcl-2 expression through cAMP-response element-binding
proteinJ Biol
Chem2751076110766200010.1074/jbc.275.15.1076110753867
|
|
26.
|
K FreelandLM BoxerDS LatchmanThe cyclic
AMP response element in the Bcl-2 promoter confers inducibility by
hypoxia in neuronal cellsBrain Res Mol Brain
Res9298106200110.1016/S0169-328X(01)00158-911483246
|
|
27.
|
R MellerM MinamiJA CameronS ImpeyD ChenJQ
LanDC HenshallRP SimonCREB-mediated Bcl-2 protein expression after
ischemic preconditioningJ Cereb Blood Flow
Metab2234246200510.1038/sj.jcbfm.960002415647742
|
|
28.
|
T TokudomeT HorioM FukunagaH OkumuraJ
HinoK MoriF YoshiharaS SugaY KawanoM KohnoK KangawaVentricular
nonmyocytes inhibit doxorubicin-induced myocyte apoptosis:
involvement of endogenous endothelin-1 as a paracrine
factorEndocrinology14524582466200410.1210/en.2003-1322
|
|
29.
|
JC ReedDouble identity for proteins of the
Bcl-2 familyNature387773776199710.1038/428679194558
|
|
30.
|
S LiuNA PereiraJJ TeoP MillerP ShahZ
SongMitochondrially targeted Bcl-2 and Bcl-X(L) chimeras elicit
different apoptotic responsesMol Cells24378387200718182854
|
|
31.
|
EA TannerTA BluteCB BrachmannK McCallBcl-2
proteins and autophagy regulate mitochondrial dynamics during
programmed cell death in the Drosophila
ovaryDevelopment138327338201110.1242/dev.05794321177345
|
|
32.
|
PA ParoneDI JamesSD CruzY MattenbergerO
DonzéF BarjaJC MartinouInhibiting the mitochondrial fission
machinery does not prevent Bax/Bak-dependent apoptosisMol Cell
Biol2673977408200610.1128/MCB.02282-0517015472
|
|
33.
|
ZZ ChongJQ KangK MaieseApaf-1, Bcl-xL,
Cytochrome c, and caspase-9 form the critical elements for cerebral
vascular protection by erythropoietinJ Cereb Blood Flow
Metab23320330200310.1097/00004647-200303000-0000712621307
|
|
34.
|
D SolangeJC MartinouMitochondria as the
central control point of apoptosisTrends Cell
Biol10369377200010.1016/S0962-8924(00)01803-1
|
|
35.
|
B LamotheBB AggarwalEctopic expression of
Bcl-2 and Bcl-xL inhibits apoptosis induced by TNF-related
apoptosisinducing ligand (TRAIL) through suppression of caspases-8,
7, and 3 and BID cleavage in human acute myelogenous leukemia cell
line HL-60J Interferon Cytokine
Res22269279200210.1089/107999002753536248
|
|
36.
|
M GiamDC HuangP BouilletBH3-only proteins
and their roles in programmed cell
deathOncogene27S128S136200810.1038/onc.2009.5019641498
|