Introduction
Breast cancer is a common malignant disease in women
and is the second leading cause of cancer-related deaths in the
United States (1,2). Although the mortality rate has
declined in developed countries, a significant number of patients
(3–8), whose features include the negative
expression of estrogen receptor-α (ER-α), progesterone receptor
(PR), and human epidermal growth factor receptor 2 (Her2)
demonstrate aggressive clinical behavior and poor outcome. This
subgroup of breast cancer patients are defined as triple-negative
breast cancer patients (TNBC).
Notably, TNBC represents approximately 15% of all
breast cancers (3), and is
diagnosed more frequently in younger and premenopausal women
(4–8). Patients with TNBC have the
disadvantage of not benefiting from currently available
receptor-targeted systemic therapy, such as hormonal and
trastuzumab-based therapies. Thus, chemotherapeutic regimens have
been the mainstay available treatments which always bring variable
efficacy. Therefore, the development of novel molecular targeted
therapeutic strategies is required.
Genistein (Gen), one of the major soy isoflavones in
soy products, was reported as a protective factor against breast
cancer (9). Epidemiological
evidence strongly supports that Asian women, who consume a diet
high in soy products, have a relatively low incidence of breast
cancer and risk of breast cancer recurrence (10–13). Previous studies have suggested
that Gen can inhibit the cell cycle, induce cell apoptosis, and
promote cell differentiation for TNBC breast cancer cells (14,15). Gen, a relative nontoxic natural
agent, may become another therapeutic option for the treatment of
breast cancer, especially for TNBC. However, the mechanisms of its
action remain unclear.
Notch-1 signaling pathway plays a critical role in
breast cancer (16). Importantly,
the study indicated that targeting Notch signaling pathway with γ
secretase inhibitors (GSI) should be explored to further improve
the survival rate of TNBC patients. Nonetheless, GSI may have
widespread adverse effects in vivo because proteases
participate in a wide array of cellular functions (17). It is essential to investigate
whether Gen is an efficient agent substituting for GSI to overcome
these limitations. Additionally, nuclear factor-κB (NF-κB) is a
putative target gene of activated Notch-1 (18), which is another major cell growth
and apoptosis regulatory pathway. In the present study, we
investigated whether Gen inhibits triple-negative breast cancer
MDA-MB-231 cells growth via the Nocth-1 signaling pathway.
The MDA-MB-231 cell line is a typical TNBC line. Our
data demonstrate for the first time, to our knowledge, that Gen
inhibits triple-negative breast cancer MDA-MB-231 cell growth by
inhibiting NF-κB activity via the Nocth-1 signaling pathway. Our
study suggests that Gen may be a potential therapeutic agent for
TNBC.
Materials and methods
Cell culture
The human breast cancer cell line MDA-MB-231 was
obtained from the American Tissue Culture Collection (ATCC). The
cells were routinely cultured in complete medium (DMEM supplemented
with 10% fetal bovine serum, 100 U/ml penicillin, and 100
μg/ml streptomycin) in a 5% CO2 37°C incubator.
Gen (Sigma, USA) was dissolved in dimethyl sulfoxide (DMSO), and
was added directly to the culture media at different
concentrations. The concentration of DMSO (0.05%) in the final
working solution did not interfere with cell growth. The same
concentration of DMSO was present in all control cultures.
siRNA plasmid and transfection
MDA-MB-231 cells were transfected with Notch-1
siRNA, NF-κB siRNA and siRNA controls (GenePharma, China), using
Lipofectamine 2000. One day before transfection, cells were plated
in 2 ml DMEM without antibiotics. siRNA of 5 µl (final
concentration 100 pM) and 5 µl Lipofectamine 2000 were
diluted in 250 µl opti-MEM medium without serum separately,
mixed gently and incubated for 5 min at room temperature.
Subsequently, the diluted oligomer was combined with the diluted
Lipofectamine 2000. The combination was incubated for 20 min at
room temperature, and added to each well containing cells and
medium. The medium were changed after 6 h.
Cell growth inhibition studies by the MTT
assay
MDA-MB-231 cells were seeded at a density of
5×103 cells/well in 200 μl of medium in a 96-well
culture plates, and cultured for 24 h. The cells were treated with
5, 10, 20 μM Gen for 24, 48 and 72 h, while control cells
were cultured in medium with 0.05% DMSO. After treatment, the cells
were incubated with MTT (0.5 mg/ml) at 37°C for 4 h and with DMSO
at room temperature for 15 min. The absorbance of the samples was
measured at 490 nm on a scanning multi-well spectrophotometer. The
experiment was repeated 3 times.
Quantitative apoptotic cell death
assay
MDA-MB-231 cells were seeded in 6-well culture
plates, and subsequently treated with 5, 10 and 20 μM Gen
for 72 h, while control cells received 0.05% DMSO in culture
medium. After treatment, the floating and adherent cells were
collected. Pooled cells were washed with phosphate-buffered saline
(PBS). The cells were suspended in 100 μl PBS and mixed with
5 μl of Annexin V-FITC and 10 μl of propidium iodide.
The cells were analyzed with a flow cytometer after 15 min of
incubation in the dark.
Cell cycle analyses
MDA-MB-231 cells were synchronized in G0 by serum
starvation for 24 h in serum-free medium. Subsequently, cells were
incubated in complete phenol red-free DMEM containing 10% fetal
bovine serum for 72 h with or without various concentrations of
Gen. After treatment, cells were trypsinized and washed in cold PBS
twice, fixed in 70% ethanol over 6 h at 4°C. The distribution of
cells at different stages of the cell cycle was estimated by flow
cytometric DNA analysis. The percentage of cells in each cell cycle
phase (G0/G1, S or G2/M) was calculated by using Lysis II
software.
Determination of NF-κB activity
MDA-MB-231 cells were seeded in a 6-well culture
plate, and treated with 0, 5, 10 and 20 μM Gen for 72 h. The
cells were immunofluorescence-labeled according to the
manufacturer’s instructions using the NF-κB activation-nuclear
translocation assay kit (Beyotime Biotech, China). After washing
and fixing, cells were incubated with a blocking buffer for 1 h to
suppress non-specific binding. Next, cells were incubated with the
primary NF-κB p65 antibody at 4°C overnight, followed by incubation
with a Cy3-conjugated secondary antibody for 1 h, then with DAPI
for 5 min before observation. NF-κB p65 protein and nuclei
fluoresce red and blue respectively, and can be simultaneously
viewed by laser confocal microscope at an excitation wavelength of
350 nm for DAPI and 540 nm for Cy3. To create a two-color image,
the red and blue images were overlaid, producing pink fluorescence
in areas of co-localization.
For the siRNA transfected effect, the assay was
performed as described above 72 h after the cells were transfected
with Notch-1 siRNA, NF-κB siRNA or siRNA control.
Western blotting
After treatment, cells were washed twice with cold
PBS and then scraped off in 500 μl of buffer RIPA (with
PMSF) and incubated on ice for 30 min. Then, cell lysates were
centrifuged at 14,000 x g for 15 min, and the supernatants were
stored at −80°C. Protein concentrations were measured by use of the
BCA protein assay. The prepared proteins were boiled for 5 min, and
separated by 12% SDS-PAGE. Proteins were transferred to PVDF
membranes, which were activated in methanol. The membranes were
immunoblotted with anti-rabbit Notch-1 (Abcam), cyclin B1, Bcl-2,
Bcl-xL (Cell Signaling Technology) and GAPDH (Santa Cruz
Biotechnology, Inc.) antibody, and anti-mouse NF-κB antibody,
followed by secondary anti-mouse antibody and anti-rabbit antibody.
GAPDH was used to normalize for protein loading. The membranes were
probed using ECL and autoradiographed. All experiments were
performed three times. The intensity of the bands was determined
using densitometric analysis.
Statistical analyses
Data are expressed as means ± standard deviation
(SD). Comparisons were made between control and treatment groups.
Statistical differences were analyzed using one-way ANOVA. P-value
<0.05 was considered to indicate statistical significance.
Results
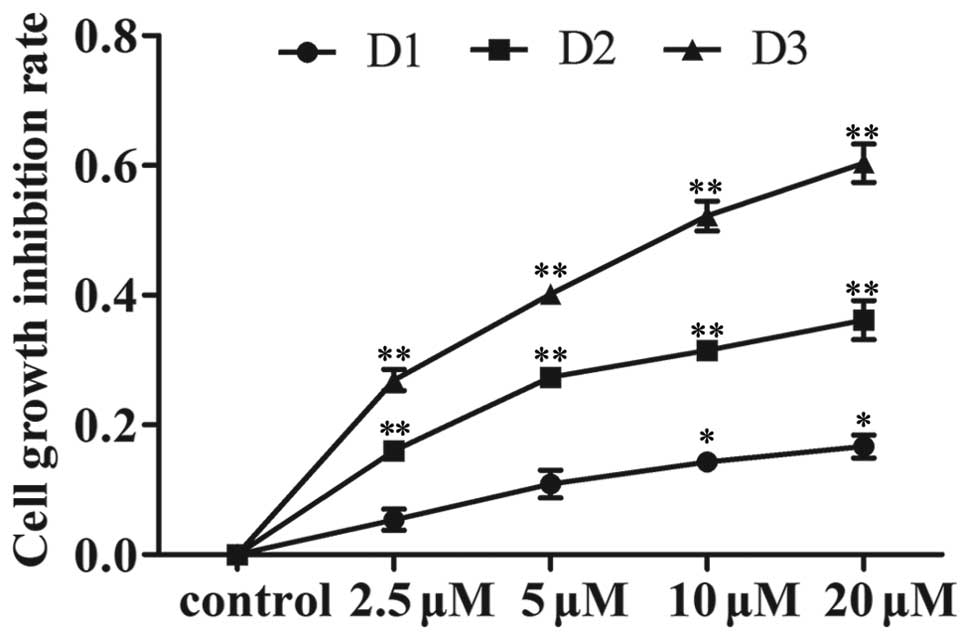
Gen inhibits proliferation in MDA-MB-231
cells
MDA-MB-231 cells were exposed to Gen at
concentrations of 5, 10 and 20 μM, while the control cells
received 0.05% DMSO in the medium. Subsequently, cell proliferation
was determined by the MTT assay at 24, 48 and 72 h. A
time-dependent and dose-dependent inhibition of cell growth was
observed (Fig. 1).
Gen induces apoptosis in MDA-MB-231
cells
MDA-MB-231 cells (0, 5, 10 and 20 μM Gen for
72 h) were collected, and stained with Annexin V and propidium
iodide. The Gen-induced apoptosis was investigated by flow
cytometry. The result showed that Gen induced apoptosis in a
dose-dependent manner (Fig. 2A).
The apoptosis percentages of all MDA-MB-231 cells treated with 0,
5, 10 or 20 μM Gen were 6.78, 18.98, 30.45 and 60.64%,
respectively.
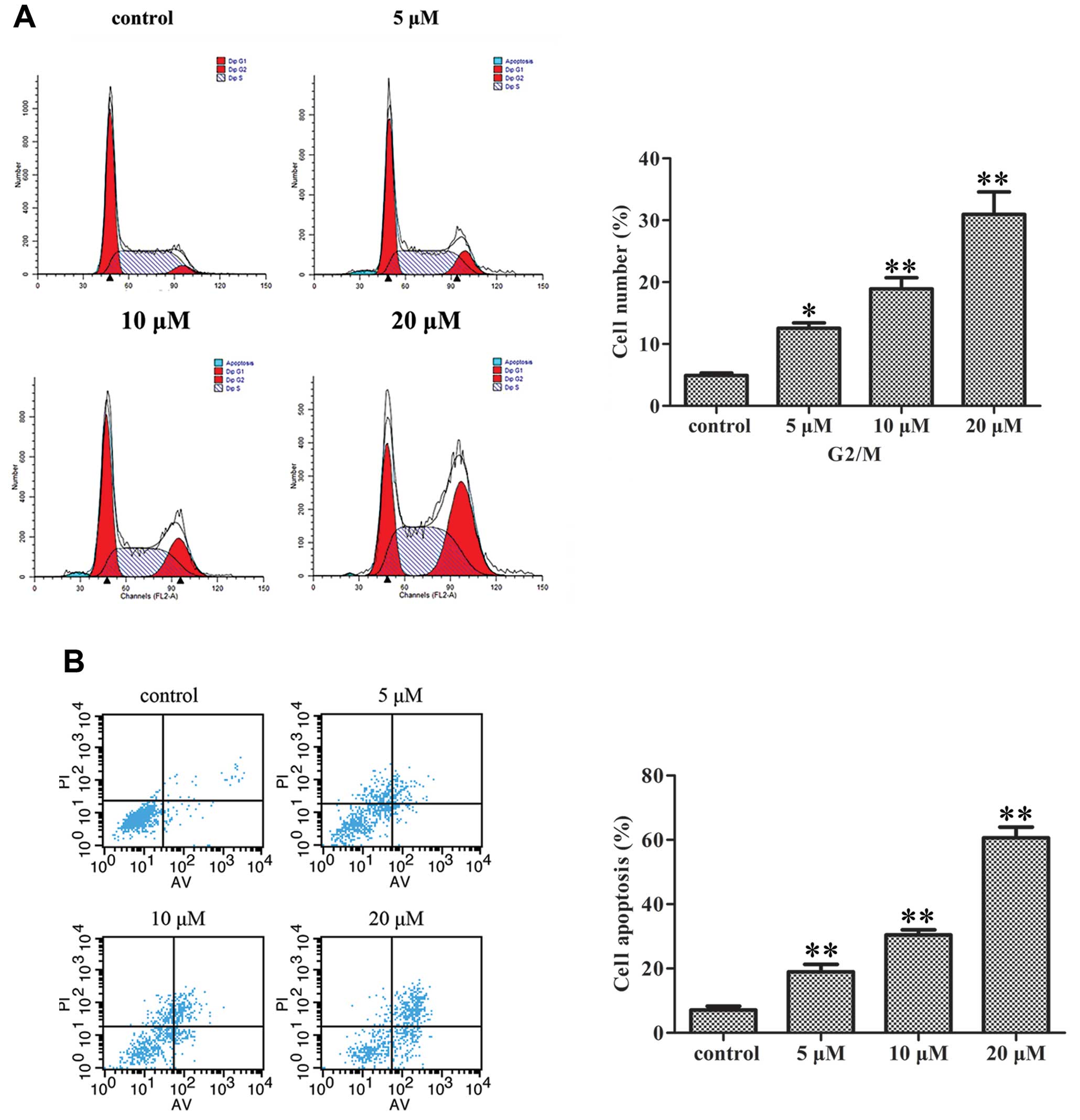 | Figure 2Effect of Gen on MDA-MB-231 cell
apoptosis and cycle. (A) The cell death assay was used to measure
apoptosis induced by Gen. The representative dual-parameter
fluorescence histograms were derived from cell cultures exposed to
various Gen concentrations, 0, 5, 10 and 20 μM for 72 h.
Cells were incubated with Annexin V-FITC and PI. Results are
reported as an apoptotic index. Bars, mean ± SD,
**P<0.01, significantly different from control. (B)
Cell cycle analyses for measuring cell cycle distribution of
MDA-MB-231 cells treated with Gen. Cells were treated with 0, 5, 10
and 20 μM Gen for 72 h. The percentage of cells in each cell
cycle phase (G1, S and G2/M) was determined by flow cytometry.
Bars, mean ± SD, *P<0.05, **P<0.01,
significantly different from control. |
Gen-treated MDA-MB-231 cells arrest in
the G2/M phase of the cell cycle
MDA-MB-231 cells were treated with 0, 5, 10 or 20
μM concentrations of Gen for 72 h, and the cell cycle phase
was determined by flow cytometry. Gen induced a significant
accumulation of cells in the G2/M phase of the cell cycle in a
dose-dependent manner (Fig. 2B).
The percentages of cells in the G2/M phase of the cell cycle after
treatment with 0, 5, 10 or 20 μM were 4.93, 12.54, 18.93 and
30.95%, respectively.
Gen inhibits the NF-κB pathway through
the Notch-1 pathway in MDA-MB-231 cellsGen inhibits Notch-1
expression
To investigate whether the Notch-1 pathway is
involved in the action of Gen, western blot analysis was used to
determine the status of Notch-1 in MDA-MB-231 cells treated with
different concentrations of Gen (0, 5, 10 and 20 μM). We
found that the protein level of Notch-1 was downregulated in
MDA-MB-231 cells in a dose-dependent manner (Fig. 3A).
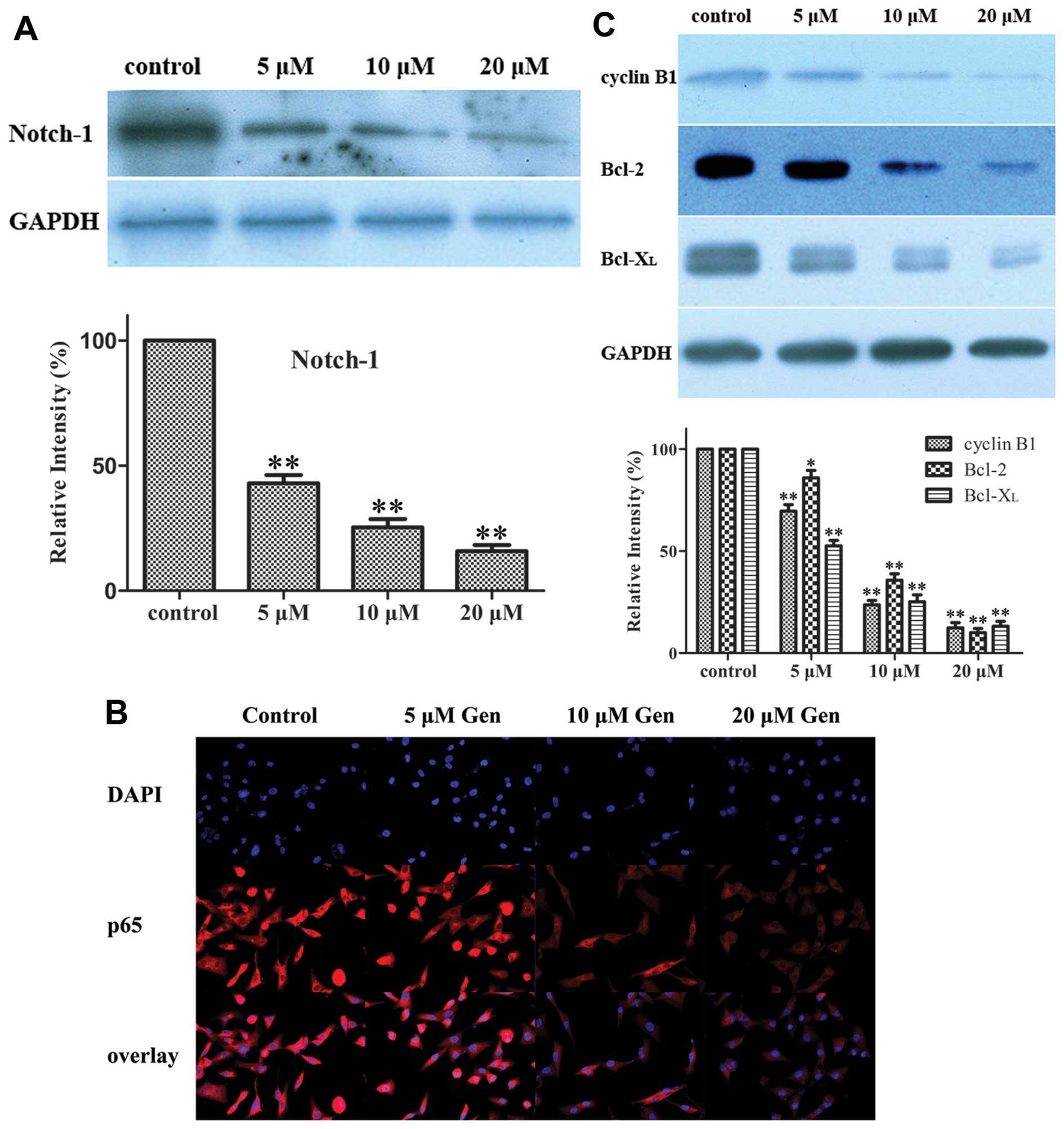 | Figure 3Effect of Gen on the expression of
the Notch-1 protein, NF-κB targeted proteins and NF-κB activation.
(A) Effect of Gen on the expression of Notch-1. MDA-MB-231 cells
were treated with 0, 5, 10, 20 μM Gen for 72 h. Western blot
analyses for the expression of Notch-1. GAPDH was used to normalize
for protein loading. Histograms represent densitometric measurement
of bands. Bars, mean ± SD, *P<0.05,
**P<0.01, significantly different from the control.
(B) Effect of Gen on NF-κB activation. MDA-MB-231 cells were seeded
in a 6-well culture plate, and subsequently treated with 0, 5, 10,
20 μM Gen for 72 h. After treatment, cells were incubated
with p65 antibody and Cy3 fluorescein-conjugated secondary
antibody, and nuclei were stained with DAPI. The images were
obtained by confocal laser microscopy and overlay. The pink
fluorescence indicates location of p65 protein in nuclei. (C)
Effect of Gen on expression of NF-κB targeted proteins. MDA-MB-231
cells were treated with 0, 5, 10, 20 μM Gen for 72 h.
Western blot analyses for the expressions of cyclin B1, Bcl-2 and
Bcl-xL were presented. GAPDH was used to normalize for protein
loading. Histograms represent densitometric measurement of bands.
Bars, mean ± SD, *P<0.05, **P<0.01,
significantly different from control. |
Gen inhibits NF-κB activation and NF-κB
targeted proteins
The nuclear translocation assay kit was used to
determine NF-κB activation. The results showed that various
concentrations of Gen can downregulate NF-κB activation in
MDA-MB-231 cells in a dose-dependent manner (Fig. 3B). Western blot analysis showed
that the levels of NF-κB targeted proteins, cyclin B1, Bcl-2,
Bcl-xL, were downregulated in MDA-MB-231 cells treated by Gen
compared with the control cells (Fig.
3C).
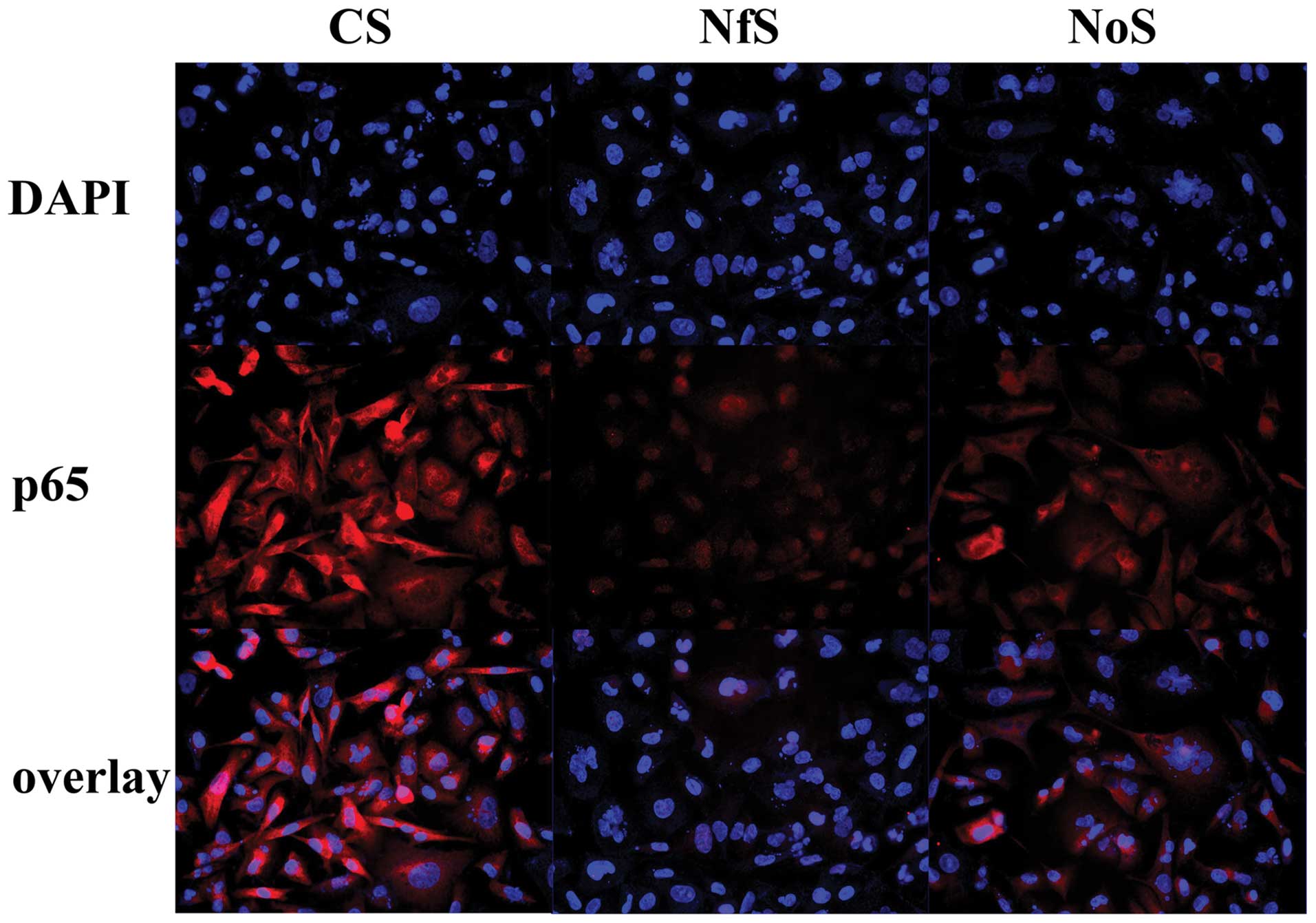
NF-κB inactivation is mediated via the
Notch-1 pathway
Both Notch-1 and NF-κB were downregulated by siRNA
(Fig. 4). The transfection of
Notch-1 siRNA into MDA-MB-231 cells inhibited the translocation of
NF-κB into the nucleus (Fig. 5).
The downregulation of Notch-1 and NF-κB expression by siRNA both
inhibited the expression of cyclin B1, Bcl-2 and Bcl-xL (Fig. 4). These results suggest that NF-κB
inactivation was mediated via the Notch-1 pathway.
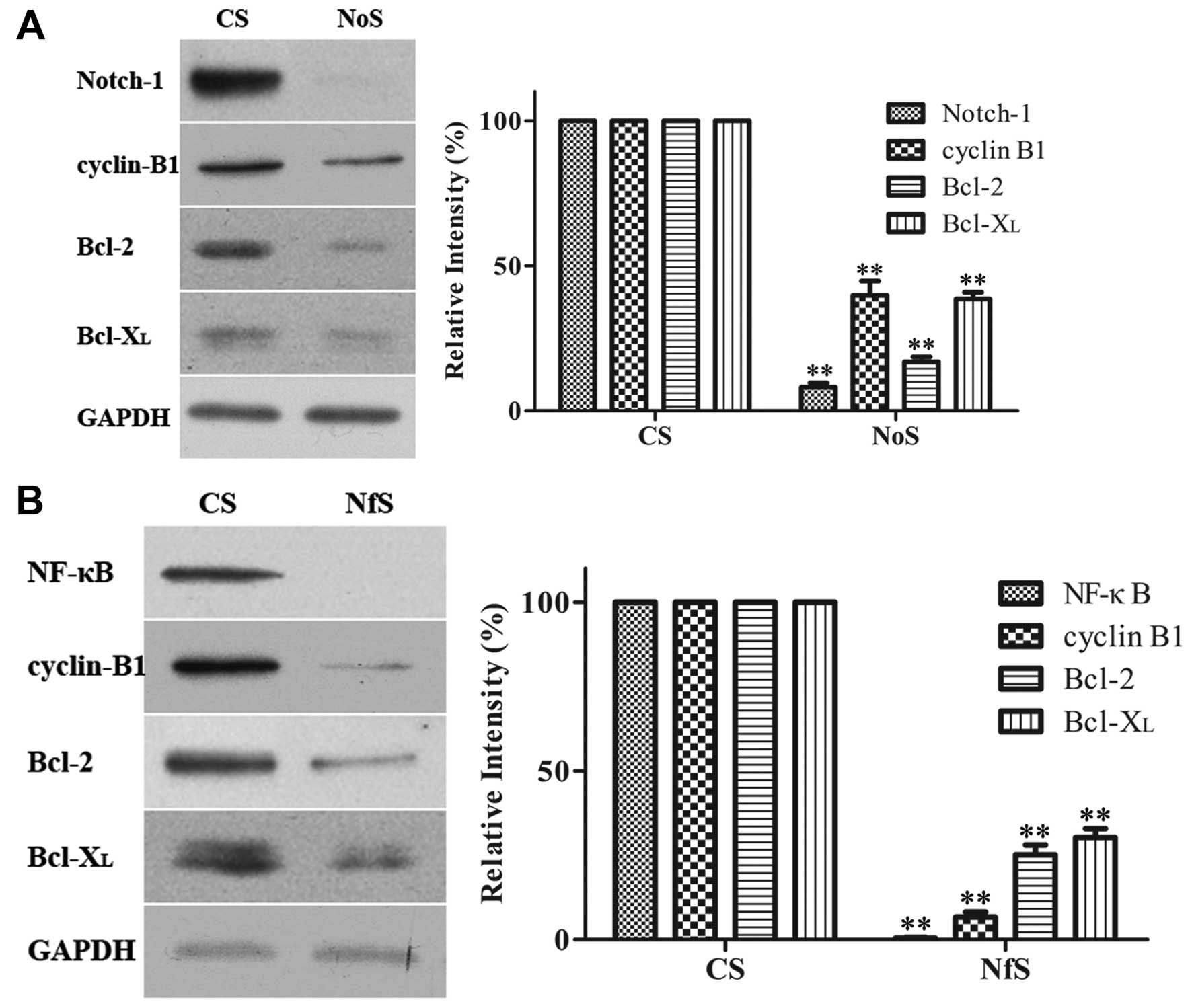 | Figure 4Effect of siRNA on the expression of
several proteins. MDA-MB-231 cells were treated with Notch-1 siRNA,
NF-κB siRNA, and siRNA control for 72 h. Western blot analyses for
the expression of proteins were presented. GAPDH was used to
normalize for protein loading. Histograms represent densitometric
measurement of specific bands using GAPDH levels as the control.
Bars, mean ± SD, *P<0.05, **P<0.01,
significantly different from the control. (A) Downregulation of
NF-κB and its target gene cyclin B1, Bcl-2 and Bcl-xL by siRNA in
MDA-MB-231 cells. NfS, NF-κB siRNA; CS, control siRNA. (B)
Downregulation of Notch-1 and its target gene cyclin B1, Bcl-2 and
Bcl-xL by siRNA in MDA-MB-231 cells. NoS, Notch-1 siRNA; CS,
control siRNA. Inhibition of protein expression by Gen or siRNA in
MDA-MB-231 cells. |
Discussion
Our study reveals that Gen elicits a dramatic effect
on cell growth inhibition in a dose-dependent and time-dependent
manner. Gen induced MDA-MB-231 cell apoptosis and induced cell
cycle arrested in the G2/M phase. On the other hand, we examined
the mechanisms of the action of Gen on MDA-MB-231 cells. We
demonstrated for the first time, to our knowledge, that in TNBC
cells, inhibition of the activity of NF-κB via the Notch-1 pathway
may be an important mechanism for growth inhibition by Gen. What’s
more, we found that Gen downregulated the expression of cyclin B1,
Bcl-2 and Bcl-xL in MDA-MB-231 cells, possibly mediated by NF-κB
activation via the Notch-1 signaling pathway. Based on the results,
we speculate that Gen is a potential therapeutic agent for
TNBC.
Gen, as one of the major soy isoflavones in soy
products, is a relatively nontoxic natural agent. Apart from its
efficiency, attention needs to be paid to its safety. Because of
its structural similarity with 17β-estradiol, Gen has the capacity
to bind to ERα and induce hormone-like effects. Thus, at lower dose
(<10 µM) Gen may stimulate ERα+ cell growth
and entry into the cell cycle. However, there is no need to worry
about these effects on TNBC cells, like MDA-MB-231 cells, in the
present study. Notably, numerous epidemiological studies support a
significant inverse association between soy isoflavones intake and
risk of breast cancer incidence and recurrence. Gen may be a
potential attractive therapeutic agent for TNBC.
The Notch pathway is an important transduction
pathway in cancer cells, which is always aberrantly activated in
breast cancer (19). Notch
signaling is normally activated by ligand-receptor binding between
two neighboring cells (20). It
consists of Notch receptors (Notch-1–4), Notch ligands
(Delta-like-1, −3, −4, and Jagged-1, −2) and CSL (DNA binding
protein) (21). High-level
co-expression of Notch-1 and Jagged-1 has been associated with poor
overall survival (22–24). Based on previous studies, Notch
signaling pathway plays a critical role in controlling breast
cancer cell growth and apoptosis (25). Considering the CBF-1
transcriptional activity, reported by Rizzo et al (26), it is suggested that factors other
than expression levels of Notch-1 regulate Notch-dependent reporter
activity. A previous study demonstrated that the TNBC cell line
MDA-MB-231 had much higher Notch-dependent reporter activity than
the ERα+ lines (MCF-7 and T47D) and Her2/Neu
overexpression line SKBR3. Importantly, the Notch-1 signaling
pathway has been demonstrated to play a critical role in TNBC
(14). It was reported that
MDA-MB-231 xenografts were highly sensitive to the treatment of
GSI, a Notch inhibitor (26).
Therefore, the Notch-1 pathway is a rational target of breast
cancer, especially for TNBC. We demonstrated for the first time, to
our knowledge, that Gen inhibits the Notch-1 signaling pathway in
MDA-MB-231 cells.
Additionally, NF-κB is a putative target gene of
activated Notch-1 (18). Numerous
studies have documented elevated NF-κB DNA-binding activity in both
mammary carcinoma cell lines and primary human breast cancer
tissues (27–29). NF-κB controls breast cancer
initiation, progression, metastasis, and resistance to chemotherapy
(30). Our study reveals that
various concentrations of Gen can downregulate NF-κB activation in
a dose-dependent manner.
Translocation of NF-κB into the nucleus can activate
transcription of genes (31). We
found that Gen downregulated expression of cyclin B1, Bcl-2 and
Bcl-xL in MDA-MB-231 cells, possibly mediated by NF-κB, via the
Notch-1 signaling pathway. Furthermore, our data suggests that
knockdown of Notch-1 or NF-κB by siRNA leads to downregulation of
cyclin B1, Bcl-2 and Bcl-xL in MDA-MB-231 cells. Cyclin B1 is a
cell cycle regulatory protein, which is overexpressed in breast
cancer (32). Decreasing cyclin
B1 expression inhibits proliferation and induces apoptosis in tumor
cells (33). Bcl-2 protein is a
member of the Bcl-2 family that regulates apoptosis, which is a
potent inhibitor of apoptosis (34). As far as breast cancer is
concerned, Bcl-2 protein is generally expressed in 60–80% of
invasive breast carcinoma (35,36). To date, Bcl-xL, another member of
the Bcl-2 family, has been shown to correlate with chemotherapy
resistance of mammary tumors in a mouse model (37).
In conclusion, the results of our study demonstrate
that Gen inhibits triple-negative breast cancer MDA-MB-231 cell
growth by inhibiting NF-κB activity via the Nocth-1 signaling
pathway. Our study gives strong evidence that Notch-1 is an
attractive molecular target for the treatment of TNBC. Gen may be
used alone or in combination, to achieve better treatment outcome
of TNBC. Further preclinical and clinical studies should be
performed to confirm the hypothesis to apply the use of Gen in
patients with TNBC.
Acknowledgements
This study was mainly supported by the
Foundation from Jiangsu provincial administration of traditional
Chinese Medicine (LZ11079). This study was also supported in part
by the National Natural Science Foundation of China (81071753 and
81172502), the Wu Jie-Ping Foundation (320.670010009), the Six
Kinds of Outstanding Talent Foundation of Jiangsu Province
(2010-ws-079 to W.H. and 2009 to Q.D.), the Science and Education
for Health Foundation of Jiangsu Province (RC2007054), the Natural
Science Foundation of Jiangsu Province (BK2009438, BK2010581,
BK2011853 and BK2011855), the Program for Development of Innovative
Research Team in the First Affiliated Hospital of NJMU (IRT-008),
and a project funded by the Priority Academic Program Development
of Jiangsu higher Education Institutions (PAPD).
References
|
1.
|
A JemalR SeigeJ XuE WardCancer statistics,
2010CA Cancer J Clin60277300201010.3322/caac.20073
|
|
2.
|
RA SmithV CokkinidesD BrooksD SaslowM
ShahOW BrawleyCancer screening in the United States, 2011: a review
of current American Cancer Society guidelines and issues in cancer
screeningCA Cancer J Clin61830201110.3322/caac.2009621205832
|
|
3.
|
F Lara-MedinaV Perez-SanchezD
Saavedra-PerezTriple negative breast cancer in Hispanic patients:
high prevalence, poor prognosis, and association with menopausal
status, body mass index, and
parityCancer11736583669201110.1002/cncr.2596121387260
|
|
4.
|
LA CareyCM PerouCA LivasyRace, breast
cancer subtypes, and survival in the Carolina Breast Cancer
StudyJAMA29524922502200610.1001/jama.295.21.249216757721
|
|
5.
|
R DentM TrudeauKI PritchardTriple-negative
breast cancer: clinical features and patterns of recurrenceClin
Cancer Res1344294434200710.1158/1078-0432.CCR-06-304517671126
|
|
6.
|
BG HafftyQ YangM ReissLocoregional relapse
and distant metastasis in conservatively managed triple negative
early-stage breast cancerJ Clin
Oncol2456525657200610.1200/JCO.2006.06.566417116942
|
|
7.
|
EA RakhaME EI-SayedAR GreenAH LeeJF
RobertsonIO EllisPrognostic markers in triple-negative breast
cancerCancer1092532200710.1002/cncr.2238117146782
|
|
8.
|
M TischkowitzJS BrunetLR BeginUse of
immunohistochemical markers can refine prognosis in triple negative
breast cancerBMC Cancer7134200710.1186/1471-2407-7-13417650314
|
|
9.
|
AH WuMC YuCC TsengMC PikeEpidemiology of
soy exposures and breast cancer riskBr J
Cancer98914200810.1038/sj.bjc.660414518182974
|
|
10.
|
AH WuWP KohR WangHP LeeMC YuSoy intake and
breast cancer risk in Singapore Chinese Health StudyBr J
Cancer99196200200810.1038/sj.bjc.660444818594543
|
|
11.
|
SA LeeXO ShuH LiAdolescent and adult soy
food intake and breast cancer risk: results from the Shanghai
Women’s Health StudyAm J Clin Nutr8919201926200919403632
|
|
12.
|
XO ShuY ZhengH CaiK GuZ ChenW ZhengW LuSoy
food intake and breast cancer
survivalJAMA30224372443200910.1001/jama.2009.178319996398
|
|
13.
|
JY DongLQ QinSoy isoflavones consumption
and risk of breast cancer incidence or recurrence: a meta-analysis
of prospective studiesBreast Cancer Res
Treat125315323201110.1007/s10549-010-1270-821113655
|
|
14.
|
Z LiJ LiB MoGenistein induces cell
apoptosis in MDA-MB-231 breast cancer cells via the
mitogen-activated protein kinase pathwayToxicol in
Vitro2217491753200810.1016/j.tiv.2008.08.00118761399
|
|
15.
|
Z LiJ LiB MoGenistein induces G2/M cell
cycle arrest via stable activation of ERK1/2 pathway in MDA-MB-231
breast cancer cellsCell Biol
Toxicol24401409200810.1007/s10565-008-9054-118224451
|
|
16.
|
J SpeiserK ForemanE DrinkaNotch-1 and
Notch-4 biomarker expression in triple-negative breast cancerInt J
Surg PatholNov132011(Epub ahead of print).
|
|
17.
|
IeM ShihTL WangNotch signaling,
gamma-secretase inhibitors, and cancer therapyCancer
Res6718791882200710.1158/0008-5472.CAN-06-395817332312
|
|
18.
|
F OswaldS LiptayG AdlerRM SchmidNF-kappaB2
is a putative target gene of activated Notch-l via RBP-JkappaMol
Cell Biol182077208819989528780
|
|
19.
|
S StylianouRB ClarkeK BrennanAberrant
activation of notch signaling in human breast cancerCancer
Res6615171525200610.1158/0008-5472.CAN-05-305416452208
|
|
20.
|
GE GrayRS MannE MitsiadisHuman ligands of
the Notch receptorAm J
Pathol154785794199910.1016/S0002-9440(10)65325-410079256
|
|
21.
|
B GidzinskaNotch1 receptor: structure and
role during T-cell developmentPostepy Hig Med Dosw573693762003(In
Polish).
|
|
22.
|
M ReedijkS OdorcicL ChangHigh-level
coexpression of JAG1 and NOTCH1 is observed in human breast cancer
and is associated with poor overall survivalCancer
Res6585308537200510.1158/0008-5472.CAN-05-106916166334
|
|
23.
|
M ReedijkD PinnaduwageBC DicksonJAG1
expression is associated with a basal phenotype and recurrence in
lymph node-negative breast cancerBreast Cancer Res
Treat111439448200810.1007/s10549-007-9805-317990101
|
|
24.
|
BC DicksonAM MulliganH ZhangG LockwoodFP
O’MalleySE EganM ReedijkHigh-level JAG1 mRNA and protein predict
poor outcome in breast cancerMod
Pathol20685693200710.1038/modpathol.380078517507991
|
|
25.
|
L MieleRational targeting of Notch
signaling in breast cancerExpert Rev Anticancer
Ther811971202200810.1586/14737140.8.8.119718699758
|
|
26.
|
P RizzoH MiaoG D’SouzaCross-talk between
notch and the estrogen receptor in breast cancer suggests novel
therapeutic approachesCancer
Res6852265235200810.1158/0008-5472.CAN-07-574418593923
|
|
27.
|
PC CogswellDC GuttridgeWK FunkhouserAS
Baldwin JrSelective activation of NF-kappa B subunits in human
breast cancer: potential roles for NF-kappa B2/p52 and for
Bcl-3Oncogene1911231131200010.1038/sj.onc.120341210713699
|
|
28.
|
H NakshatriP Bhat-NakshatriDA MartinRJ
Goulet JrGW Sledge JrConstitutive activation of NF-kappaB during
progression of breast cancer to hormone-independent growthMol Cell
Biol173629363919979199297
|
|
29.
|
MA SovakRE BellasDW KimAberrant nuclear
factor-kappaB/Rel expression and the pathogenesis of breast cancerJ
Clin Invest10029522960199710.1172/JCI1198489399940
|
|
30.
|
H NakshatriRJ Goulet JrNF-kappaB and
breast cancerCurr Probl
Cancer26282309200210.1067/mcn.2002.12997712429950
|
|
31.
|
LF ChenW FischleE VerdinWC GreeneDuration
of nuclear NF-kappaB action regulated by reversible
acetylationScience29316531657200110.1126/science.106237411533489
|
|
32.
|
H KawamotoH KoizumiT UchikoshiExpression
of the G2-M checkpoint regulators cyclin B1 and cdc2 in
nonmalignant and malignant human breast lesions: immunocytochemical
and quantitative image analysesAm J Pathol150152319979006317
|
|
33.
|
J YuanR YanA KrämerF EckerdtM RollerM
KaufmannK StrebhardtCyclin B1 depletion inhibits proliferation and
induces apoptosis in human tumor
cellsOncogene2358435852200410.1038/sj.onc.120775715208674
|
|
34.
|
P ViatourM Bentires-AljA ChariotV
DeregowskiL de LevalMP MervilleV BoursNF-kappa B2/p100 induces
Bcl-2
expressionLeukemia1713491356200310.1038/sj.leu.240298212835724
|
|
35.
|
S Luna-MoréS CasqueroA Pérez-MelladoF
RiusB WeillI GornemannImportance of estrogen receptors for the
behavior of invasive micropapillary carcinoma of the breast. Review
of 68 cases with follow-up of 54Pathol Res
Pract1963539200010674270
|
|
36.
|
S WangD YangME LippmanTargeting Bcl-2 and
Bcl-xL with nonpeptidic small-molecule antagonistsSemin
Oncol30133142200310.1053/j.seminoncol.2003.08.01514613034
|
|
37.
|
R LiuC PageDR BeidlerMS WichaG
NúñezOver-expression of Bcl-x(L) promotes chemotherapy resistance
of mammary tumors in a syngeneic mouse modelAm J
Pathol15518611867199910.1016/S0002-9440(10)65505-810595916
|