Introduction
Rapid and sensitive detection of viruses in blood is
critically important in order to reduce the spread of disease as a
result of transfusion (1). Human
immunodeficiency virus type-1 (HIV-1) is a major virus linked to
transfusion-associated transmission of disease. Although
serological detection of antibodies against HIV-1 can reduce the
risk of disease transmission, a better procedure is urgently
required (2–8). For example, during the
pre-seroconversion window period the quantity of antibody against
the virus is low despite the a high load of HIV-1 present in the
blood. Infection with immunovariant viruses and immunosilent
carriage cause a similar condition. Recent developments in
enzyme-linked immunosorbent assay (ELISA), polymerase chain
reaction (PCR) and immunochromatography facilitate the detection of
HIV-1 in biological samples (9–12).
Nonetheless, the sensitivity of these procedures is insufficient to
eliminate the risk of viral transmission.
There are two major limiting factors in the
development of a protocol to concentrate HIV-1: i) compatibility
with current methods of detection and ii) requirement for a
straightforward procedure. Several approaches have been used to
increase the concentration of viruses in order to enhance the
sensitivity of detection (13–15). For example, ultracentrifugation
and polyethylene glycol (PEG) mediated precipitation have been used
to concentrate a number of different viruses including HIV-1.
Ultracentrifugation is a well-known procedure, but is
time-consuming and can increase the false-positive rate when
combined with PCR (12,16). Although PEG precipitation is
simple and easy to perform, the PEG sometimes interferes with the
subsequent PCR (17). One
alternative approach is to use magnetic beads coated with molecules
that efficiently bind viral particles. Indeed, we and other groups
have reported that an anionic polymer, poly(methyl vinyl
ether-maleic anhydrate) [poly(MVE-MA)] can be used to capture
different viruses.
Here, we report that magnetic beads coated with
poly(MVE-MA) are useful for the capture of various subtypes and
circulating recombinant form (CRF) of HIV-1. The potential of this
method and the mechanisms by which the beads bind HIV-1 are being
discussed.
Materials and methods
Reagents
Unless otherwise specified, chemical reagents were
obtained from Sigma-Aldrich (St. Louis, MO) or Wako Pure Chemical
Industries, Ltd. (Osaka, Japan). The 300-nm-diameter magnetic
particles (reducing sedimentation and offering a broad binding
surface) with a high ferrite content (allowing separation under a
magnetic field) were prepared by grafting of poly(MVE-MA) in
dimethyl sulfoxide/phosphate buffer 5/95 solution for 3 h at 37°C
(Flavigny et al, American Society for Microbiology
104th General Meeting, 166, 2004). The anionic magnetic
beads, Viro-adembeads, were obtained from Ademtech (Pessac,
France).
Samples
For analysis, we used either cell culture medium of
HIV-1 (LAI or L2)-infected MT4 cells (NIH AIDS Research and Reagent
Program) or 293T cells (American Type Culture Collection CRL-11268)
transfected with HIV-1 molecular clones (pNL4-3, pBal, pIndie-C1,
pL2 and 95TNIH022). In addition, plasma from 4 HIV-1-infected
individuals was also used. The plasma from HIV-1-infected
individuals at a very early stage of infection was purchased from
Alpha Therapeutic Corporation (Calexico, CA). The plasma samples
were tested for the following: human hepatitis B virus (HBV)
surface antigen (−), anti-HIV-1/HIV-2 (−), HIV-1 by PCR (+),
anti-human hepatitis virus (HCV) (−), HCV by PCR (−), HBV by PCR
(−), human hepatitis A virus by PCR (−) and parvo-virus by PCR (−).
These results were reported in the Final Viral Marker Report
(Repeat Donors) of Alpha Therapeutic Corporation Consolidated Test
Results from Memphis Lab and PCR pooling Lab (Finalized date:
14-Apr-2003).
HIV-1 capture
Viral capture was performed according to the
manufacturer’s instructions (Ademtech). Briefly, after 2 washes
with binding buffer, anionic magnetic beads (50 μl) were further
washed twice with phosphate-buffered saline (PBS). Then, 50 μl of
cell culture medium or plasma diluted with 450 μl of PBS was added
to the washed beads and incubated for 20 min at room temperature. A
magnetic field was then applied to the tubes containing the
magnetic beads. The supernatant was discarded and the beads were
thoroughly washed 3 times with PBS. The washed beads were
resuspended with PBS and subjected to viral RNA extraction, western
blotting or ELISA. After separation, 4 fractions were obtained as
follows: i) bead fraction (BD), ii) sample before incubation with
the beads (BF), iii) supernatant after incubation (SP) and iv)
total sample containing the same quantity (50 μl) of cell culture
medium or plasma as BD (TL). The viral capture procedure was
typically completed within 30 min.
Capture inhibition by anti-HIV
antibody
In order to verify the mechanism of viral capture,
HIV-1-infected cell culture media were incubated with anti-HIV-1
Env gp41 antibody, 4E10 (Polymun Scientific Immunbiologische
Forschung GmbH) or anti-α-tubulin, B-5-1-2 (Sigma-Aldrich) for 30
min at 37°C prior to addition of the magnetic beads. The samples
were then subjected to bead incubation and magnetic separation as
described before.
Western blotting
Each fraction was solubilized in an equal volume of
2X sodium dodecyl sulfate (SDS) gel-loading buffer [90 mM Tris-HCl
(pH 6.8), 10% mercaptoethanol, 2% SDS, 0.02% bromophenol blue and
20% glycerol], boiled for 5 min and separated on an SDS-12%
polyacrylamide gel electrophoresis (PAGE) before being
electroblotted onto a polyvinylidene difluoride (PVDF) membrane
(Hybond-P; Amersham Pharmacia Biotech, Piscataway, NJ) for 60 min
at 15 V. Blots were treated with 5% skimmed milk for 1 h at room
temperature and then incubated with anti-HIV-1 p24 antibody
(03-HIV-18; Biomarket, Ltd.), Nef antibody (clone 2A3, 03-HIV-3;
Biomarket, Ltd.), envelope (Env) antibody (SF2 gp120#387, NIH AIDS
Research and Reference Program) and acquired immunodeficiency
syndrome (AIDS) patient serum in PBS containing 0.1% Tween-20
(PBS-T) and 0.5% skimmed milk for 1 h at room temperature. After 3
washes with PBS-T, the membrane was incubated in horseradish
peroxidase (HRP)-conjugated anti-mouse IgG or anti-human IgG
(Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) in
PBS-T and 0.5% skimmed milk for 1 h at room temperature. After 3
washes with PBS-T, the probed proteins were detected using an
enhanced chemiluminescence detection kit (Amersham Pharmacia
Biotech).
ELISA
ELISA for HIV-1 p24 was performed using HIV-1 p24
ELISA kit (BioAcademia, Osaka, Japan). Absorbance at 450 nm was
measured to quantify the level of HIV-1 by microplate reader
(Labsystems Multiskan MS; Dainippon Sumitomo Pharma Co., Ltd.,
Osaka, Japan).
Reverse transcription (RT)-PCR
Viral RNA from beads or an aliquot of each sample
was extracted with the QIAamp viral RNA mini kit (Qiagen, Hilden,
Germany) according to the manufacturer’s instructions. RNA was
extracted from the magnetic beads by adding lysis buffer prior to
removing the beads. RNA was then eluted in 60 μl of nuclease-free
water. For the RT-reaction, random primers were added and after
incubation at 25°C for 10 min, the RNA was reverse-transcribed at
65°C for 50 min followed by denaturation of the enzyme at 85°C for
5 min. The diluted cDNA was amplified in a reaction mixture
containing primers, Ex Taq (Takara Bio, Inc., Otsu, Japan) and Ex
Taq buffer under conditions of 30 cycles of 94°C for 1 min, 60°C
for 1 min and 72°C for 1 min. PCR was carried out using the
following primers for the HIV-1 Gag gene: HIV Gag RTPCR AE B common
F, 5′-ggggaagtgacatagcagga-3′ and R, 5′-ctgttggctctggtctgctc-3′;
for the HIV-1 Env gene: HIV Env RTPCR AE B common F:
5′-gacggtacaggccag acaat-3′ and R, 5′-tcccagaagttccacaatcc-3′; for
the HIV-1 5′long terminal repeat (LTR): HIV 5′LTR RTPCR AE B common
F, 5′-ccctgattggcagaactacac-3′ and R, 5′-agcactcaaggcaagcttta-3′.
The amplified products were purified and cloned in pT7Blue T-vector
(Novagen, Madison, WI). DNA sequencing (ABI PRISM3100 Genetic
Analyzer; Applied Biosystems, Foster City, CA) with the R-20mer
primer and U-19mer primer (Novagen) was used to verify the product
sequence.
Real-time RT-PCR
The cDNAs produced in the RT reactions above were
also analyzed by real-time PCR (Q-PCR, quantitative PCR). For
real-time PCR, a Brilliant SYBR-Green Q-PCR mastermix was used
according to the manufacturer′s instructions (Stratagene, La Jolla,
CA). Briefly, the Q-PCR components included Brilliant Q-PCR
mastermix, reverse-transcribed cDNA, and the forward and reverse
target gene primers: realHIVgag F, 5′-caagcagggagctagaacga-3′ and
R, 5′-ttgtctacagccttctgatgtctc-3′; realHIVpol F,
5′-aaattcaaaattttcgggtttattac-3′ and R, 5′-aggagctttgctggtccttt-3′.
The Q-PCR program used in a Mx3000P™ Real-time Q-PCR System
(Stratagene) was: denaturation (at 95°C for 10 min) and then 40
cycles of denaturation (95°C for 30 sec), annealing (58°C for 60
sec) and extension (72°C for 30 sec). Each reaction was done in
triplicate. The results were analyzed using the Mx3000P™ system
software. The relative expression ratio of each sample was
calculated using a mathematical model based on the amplification
efficiency. PCR specificity was verified by dissociation curve
analysis of the amplified DNA fragments.
Results
To investigate whether Viro-Adembeads could be used
to capture HIV-1, cell culture medium from HIV-1 (LAI)-infected MT4
cells was mixed with the anionic polymer-coated magnetic beads. The
mixture was then magnetically separated and bead (BD), supernatant
(SP) and total (TL) fractions were prepared. Cell culture medium
from mock-infected MT4 cells was also used to prepare control
fractions. The fractions were then analyzed by RT-PCR, real-time
PCR, western blotting and ELISA to determine the extent of HIV-1
capture by the beads.
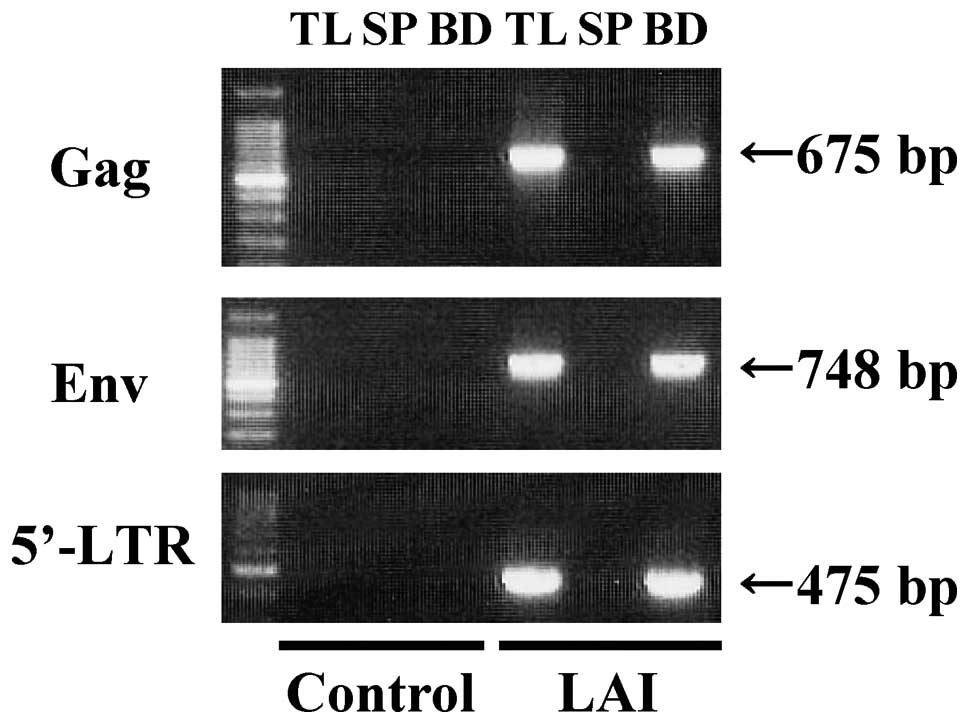
Firstly, RT-PCR was performed to detect HIV-1
genomic RNA in order to examine the capacity of the beads to
capture HIV-1 (Fig. 1). RT-PCR
analysis gave a single band of 675 bp for Gag, 748 bp for Env and
475 bp for 5′-LTR in the bead fraction (BD) and samples containing
the same quantity of cell culture medium as BD (TL) using cell
culture medium from HIV-1 (LAI)-infected MT-4 cells. No signal was
detected in the supernatant after incubation (SP). In contrast,
RT-PCR analysis of BD, SP and TL using cell culture medium obtained
from Mock-infected MT4 cells (control) did not give any signal. The
675-, 748- and 475-bp bands were confirmed to be Gag, Env and
5′-LTR gene of HIV-1, respectively by DNA sequencing (identity to
Genebank accession number AF324493 was 96, 98 and 99%,
respectively). Therefore, these results confirm that the bead
fraction includes the HIV-1 genomic RNA.
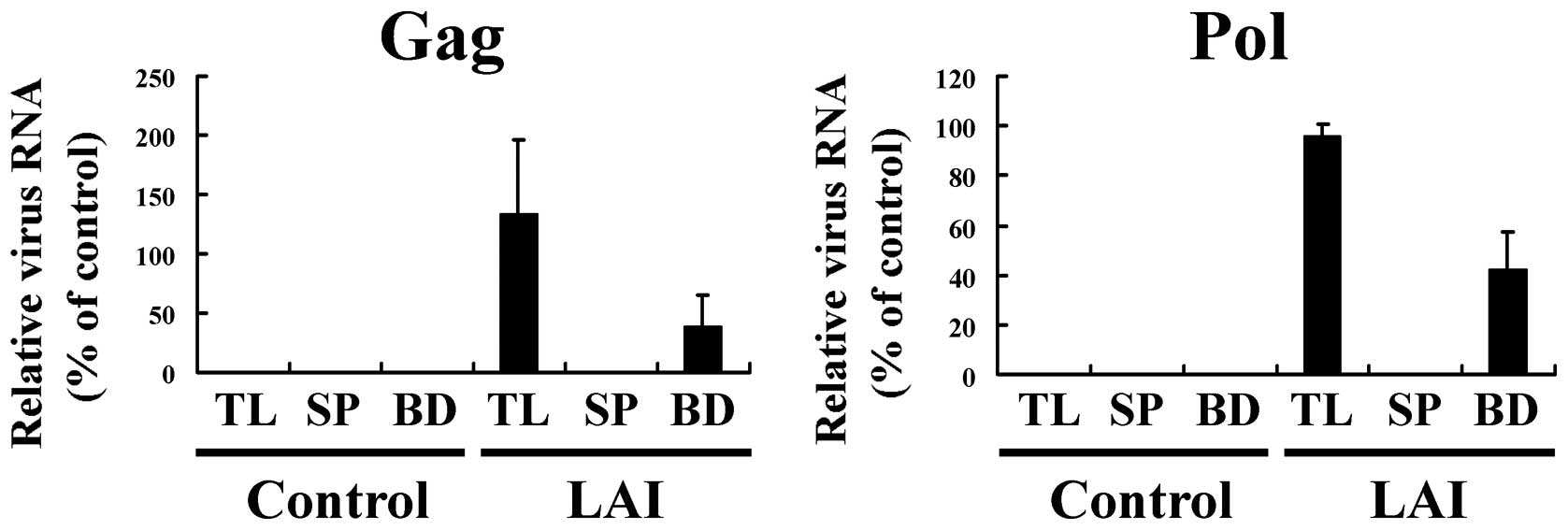
The amount of HIV-1 genomic RNA in the BD, SP and TL
fractions was measured by real-time RT-PCR, relative to that in a
control (a TL sample with the highest value was taken as 100 [%]).
For HIV-1 (LAI)-infected cell culture medium, the amount of viral
RNA in the BD fraction was ∼33 and 50% that in the TL from
real-time PCR of Gag and Pol genes, respectively (Fig. 2). In contrast, cell culture medium
from mock-infected MT4 cells (control) showed no amplification of
genomic RNA in BD, SP and TL. The specificity of these PCR
reactions was confirmed by dissociation curve analysis of the
reaction products. These results showed that HIV-1 in cell culture
medium could be captured by the magnetic beads, but that a fraction
of HIV-1 was lost during the procedure. A significant fraction of
HIV-1 in the culture medium of virus-infected cells was lost during
the capture procedure using anionic polymer-coated magnetic beads.
The loss of HIV-1 may have been due to serum components in the cell
culture medium, such as albumin, binding to the magnetic beads and
thereby hindering viral capture (18).
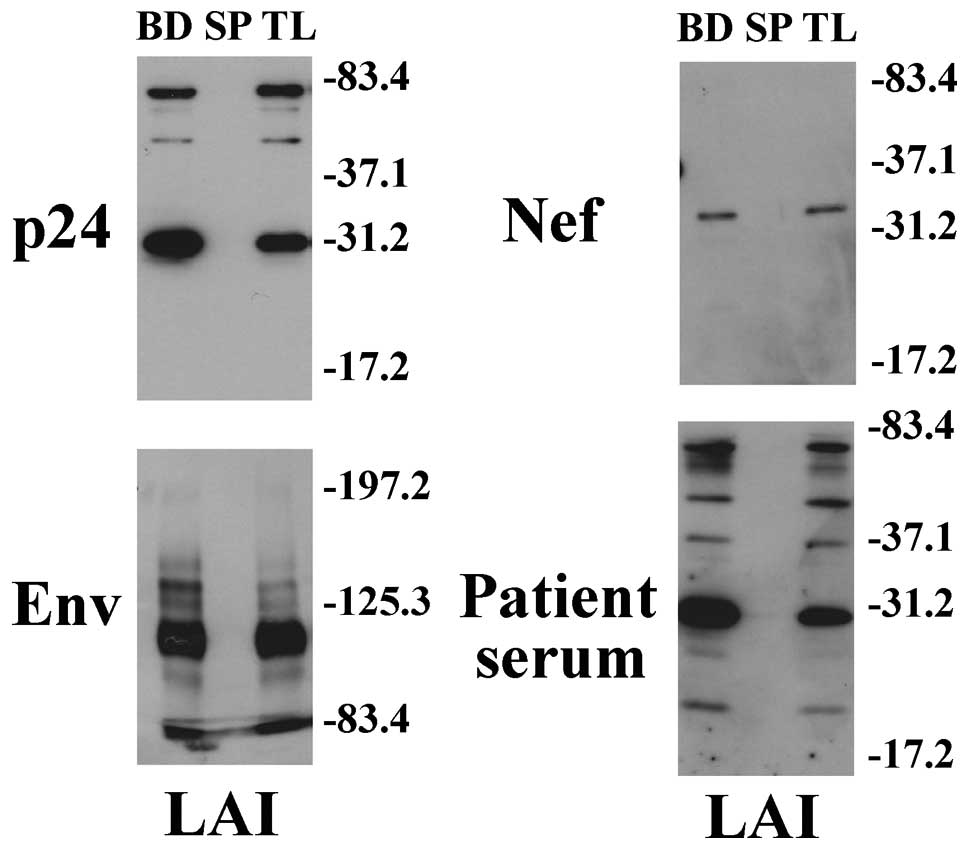
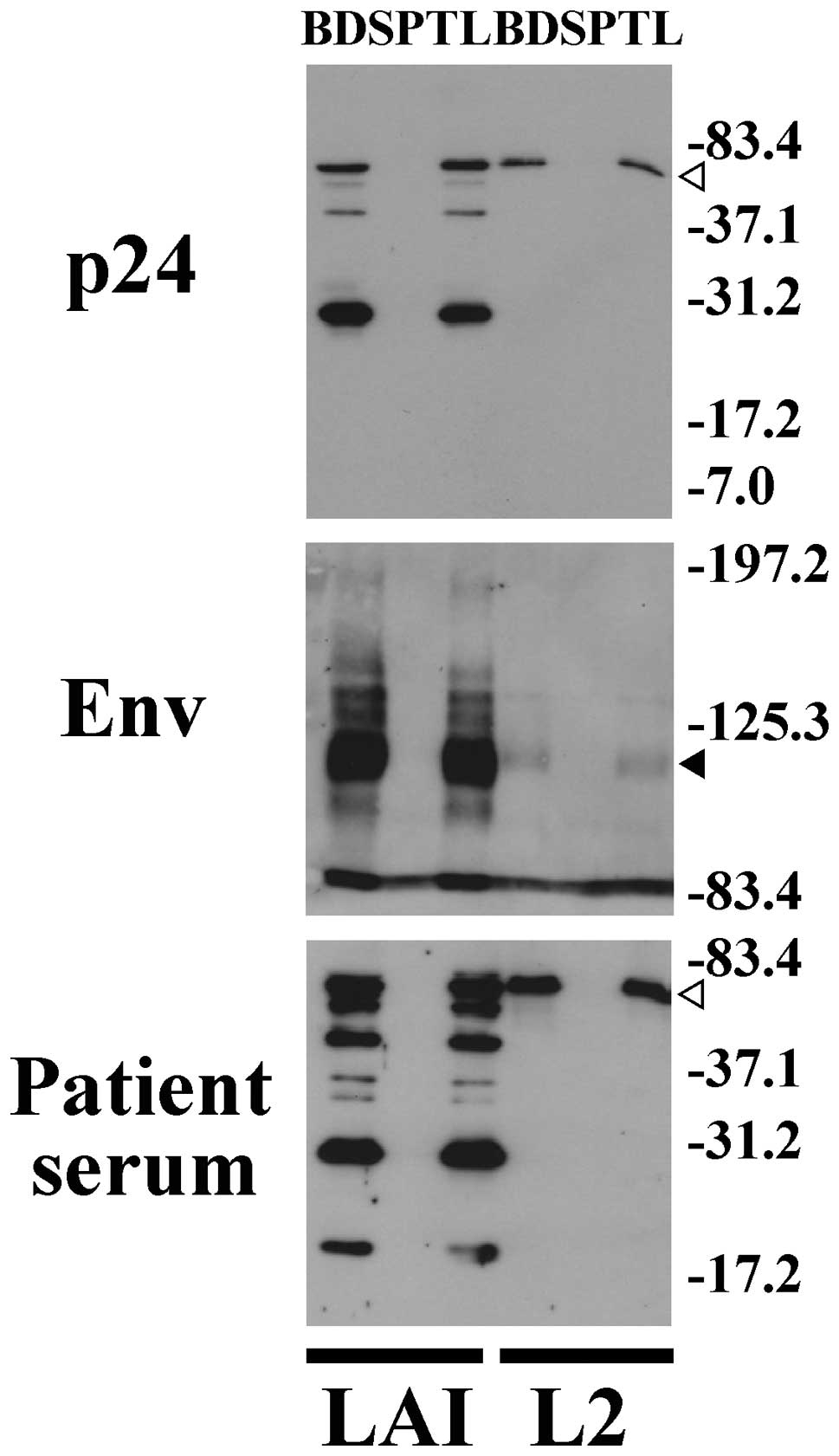
Western blotting demonstrated that the total sample
fraction (TL) and bead fraction (BD), but not the supernatant
fraction (SP), in cell culture medium of HIV-1 (LAI)-infected MT4
cells had a major band of 30, 34 and 110 kDa for p24, Nef and Env
proteins, respectively (Fig. 3).
These bands correspond to the respective deduced mass of HIV-1 p24,
Nef and Env protein based on their amino acid sequences. Thus,
HIV-1 was detected at similar levels in the total sample fraction
(TL) and in the bead fraction (BD), but not at all in the
supernatant fraction (SP). In addition, the corresponding bands
were detected using serum from an AIDS patient. These results
support the idea that HIV-1 is efficiently captured by anionic
magnetic beads.
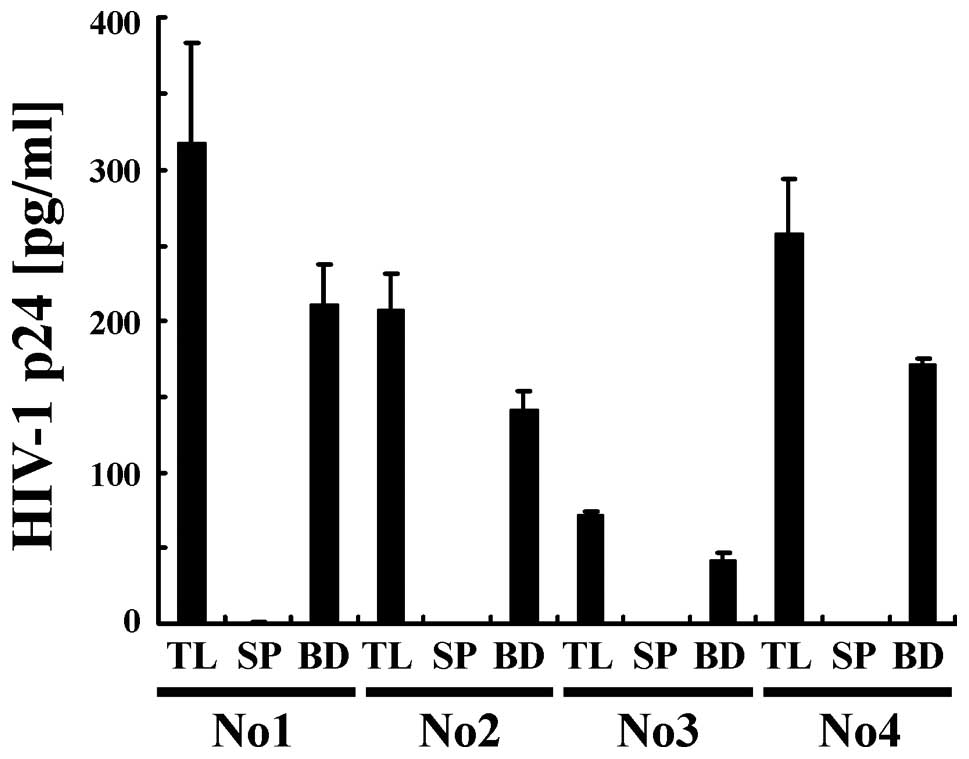
Next, we examined the efficiency with which HIV-1
was captured from plasma by conducting a quantitative analysis
using ELISA (Fig. 4). HIV-1 in
plasma from 4 HIV-1-infected individuals (No. 1-4) was recovered
using anionic magnetic beads (BD) at a level of 65–80% that from
samples containing the same quantity of plasma as BD (TL). In
contrast, HIV-1 was below the detection limit in the supernatant
after incubation (SP). These findings suggest that most of the
HIV-1 was efficiently captured from plasma by the magnetic
beads.
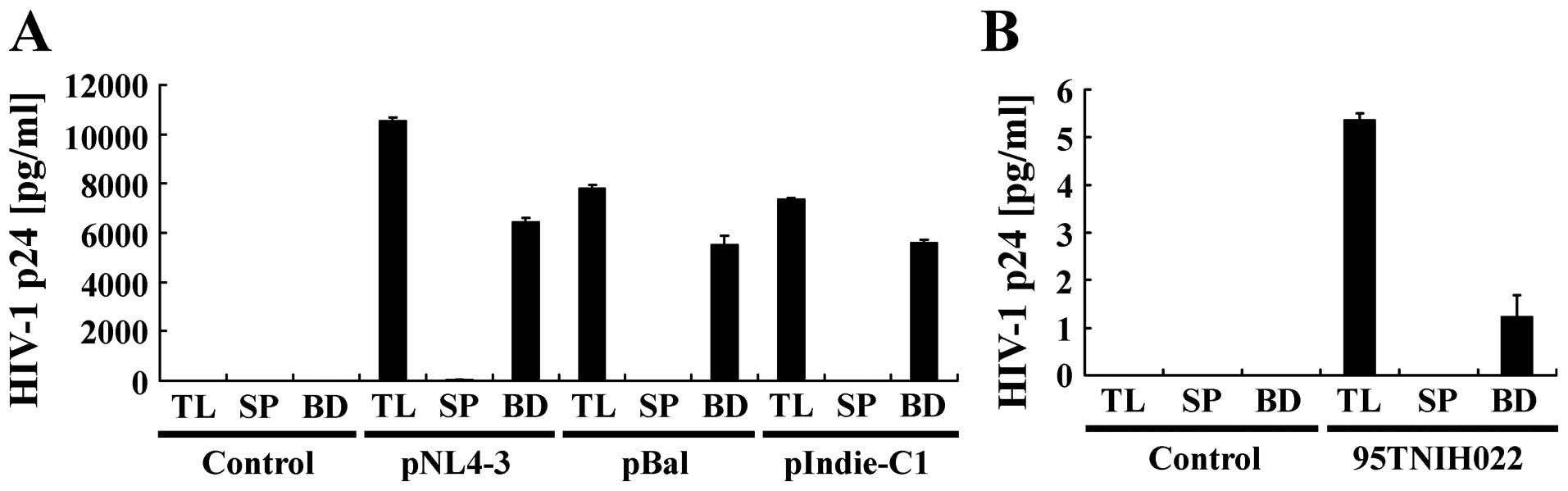
To further examine whether this magnetic capture
method can be applied to broad subtypes of HIV-1, cell culture
medium of 293T cells transfected with various types of HIV-1
molecular clones, such as pNL4-3 (subtype B), pBal (subtype B),
pIndie-C1 (subtype C) and 95TNIH022 (CRF01_AE), were subjected to
magnetic capture (Fig. 5). ELISA
showed that HIV-1 from cell culture media of 293T cells transfected
with HIV-1 pNL4-3, pBal or pIndie-C1 could be captured by magnetic
beads at a similar level of capture efficiently (60–80%). However,
cell culture medium of 293T cells transfected with 95TNIH022,
showed a lower efficiency of HIV-1 capture compared to the
molecular clones of subtype B and C. This may be due to the overall
lower concentration (about 100-fold lower) of HIV-1 in cell culture
of 293T cells transfected with 95TNIH022 compared to the other
molecular clones.
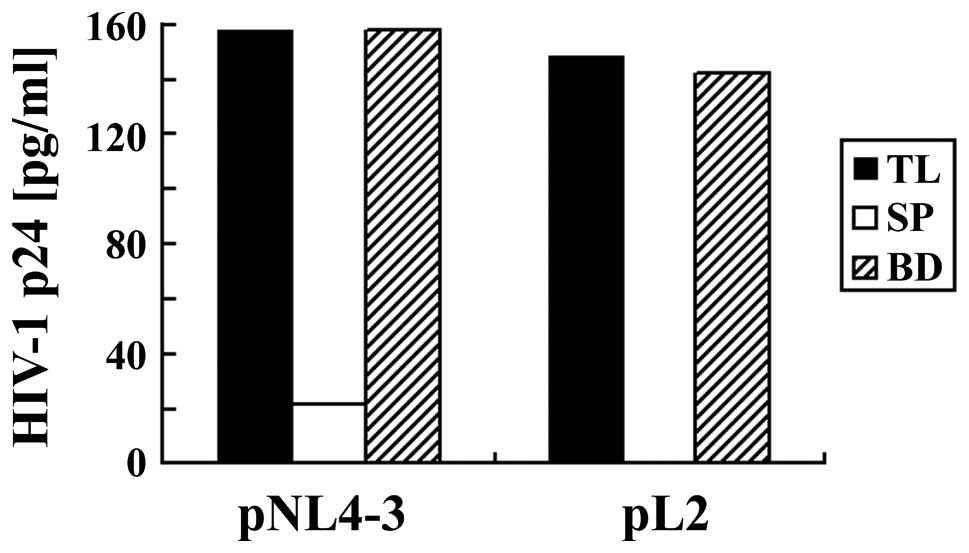
Next, we used gp120-containing, protease-deficient
clone (L2), which is derived from LAI and generate immature and
defective doughnut-shaped particles (19) (Fig.
6 and 7). ELISA (Fig. 6) and western blotting (Fig. 7) showed that HIV-1 produced by
transfection of pL2 into 293T cells could be efficiently captured
by magnetic beads. Although L2 expresses an immature form of
polymerase and decreased levels of Env compared to wild type LAI,
the L2 polymerase and Env were efficiently captured by anionic
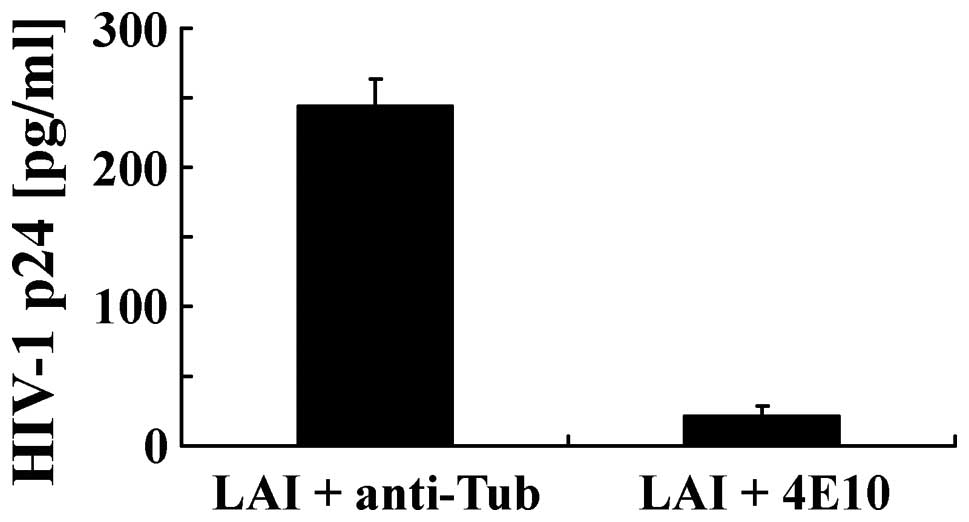
beads. Finally, we investigated the mechanisms by which
poly(MVE-MA) binds HIV-1. HIV-1 LAI in cell culture medium was
preincubated with anti-HIV-1 Env gp41 neutralizing antibody 4E10
before incubation with the magnetic beads (Fig. 8). Our results revealed that
anti-HIV-1 Env antibody 4E10 inhibited the binding of HIV-1 to the
magnetic beads, whereas the anti-tubulin antibody, which was used
as a negative control, showed no such inhibition.
Discussion
The magnetic bead-mediated capture method for HIV-1
is a simple and quick procedure that significantly reduces problems
associated with loss of signal and possible cross-contamination
commonly observed in multi-step protocols. Moreover, the magnetic
bead-mediated capture method can be applied to a range of sample
types, such as cell culture and plasma as well as other body
fluids, and is compatible with conventional detection methods, such
as PCR, ELISA and western blotting. Indeed, previously described
methods for concentrating viruses are often incompatible with
conventional detection procedures (12,16,17). For example, the
ultracentrifugation procedure requires expensive specialist
equipment and is relatively time-consuming compared to the magnetic
bead-mediated capture method. PEG precipitation is sometimes
incompatible with PCR due to PEG mediated inhibition of the DNA
polymerase. Therefore, capture by magnetic beads is a promising
approach compared to previous virus concentrating methods. There
are several methods for concentrating a virus using magnetic beads
coated with an antibody for a specific virus (16,20,21) and polymers such as
polyethyleneimine (PEI) for simian virus 40 (SV40) (22), herpes simplex virus type 1 (HSV-1)
(22), Sindbis virus (22), vesicular stomatitis virus (VSV)
(22), amphotropic murine
leukemia virus (23), poliovirus
(24), hepatitis A virus (HAV)
(24), hepatitis B virus (HBV)
(24), hepatitis C virus (HCV)
(24), and cytomegalovirus (CMV)
(25) or sulfonated (SO) magnetic
beads in the presence of divalent cations for cytomegalovirus
(25), Sindbis virus (25), poliovirus (25) and porcine parvovirus (25). Moreover, poly(MVE-MA)-coated
magnetic beads can be used for efficient capture of avian and human
influenza virus (18,26), respiratory syncytial virus
(27), Borna disease virus
(28), dengue virus (29), HIV-1 subtype B (30), CMV (30), Rota virus (30), herpesvirus (31) and vaccinia virus (31).
The present study clearly shows that
poly(MVE-MA)-coated magnetic beads can be used to capture HIV-1
with various subtypes and CRF. Although the mechanism by which the
magnetic beads bind to HIV-1 remains unclear, Env may be involved
in this process because preincubation of the beads with
neutralizing antibody against HIV-1 Env prevented capture of HIV-1.
Electrostatic, hydrophilic, hydrophobic and steric organization of
poly(MVE-MA) may also contribute to the binding (18). Because poly(MVE-MA) is negatively
charged, modification of the spatial organization of the polymers
may change binding efficiency and capacity of HIV-1 (32). Therefore, charge density and
steric spatial organization may provide some information on the
binding mechanism. However, we also show that the immature form of
HIV-1 L2 with low production of Env can also be captured,
suggesting that the beads can bind to HIV-1 by a mechanism
independent of the Env expression level. The 20–35% loss of HIV-1
during bead capture from HIV-1-infected plasma may be due to
non-specific binding of albumin and immunoglobulin as previously
shown (18). However,
modification of charge density and surface organization of polymer
may reduce the non-specific binding.
In conclusion, we demonstrated that magnetic beads
coated with an anionic polymer are useful for the capture and
concentration of HIV-1. In the captured HIV-1, the presence of a
viral genome, Env, polymerase and Nef were confirmed by RT-PCR,
real-time PCR, ELISA and western blotting. Therefore, this method
can be used in combination with conventional means of detection.
The applicability of this method to different types of viruses is
currently being studied.
Acknowledgements
The authors thank Mr. Takanori
Kobayashi for technical assistance as well as Ms. Etsuko Kita and
Yurie Shimada for their administrative support. This work was
supported by Grant-in-Aid for Promotion of Basic Research
Activities for Innovative Biosciences from Bio-oriented Technology
Research Advancement Institution (BRAIN) and for Scientific
Research on Innovative Areas from Japan Society for the Promotion
of Science.
References
|
1.
|
GB SchreiberMP BuschSH KleinmanJJ
KorelitzThe risk of transfusion-transmitted viral infections. The
Retrovirus Epidemiology Donor StudyN Engl J
Med33416851690199610.1056/NEJM199606273342601
|
|
2.
|
D CandottiY Adu-SarkodieF DaviesAIDS in an
HIV-seronegative Ghanaian woman with intersubtype A/G recombinant
HIV-1 infectionJ Med
Virol6218200010.1002/1096-9071(200009)62:1%3C1::AID-JMV1%3E3.0.CO;2-310935981
|
|
3.
|
M GermainS GelinasG DelageEstimates of
risk of window-period transmission of blood-borne viral diseases in
QuebecCMAJ17010771078200410.1503/cmaj.103193615051677
|
|
4.
|
I Loussert-AjakaTD LyML ChaixHIV-1/HIV-2
seronegativity in HIV-1 subtype O infected
patientsLancet34313931394199410.1016/S0140-6736(94)92524-07910884
|
|
5.
|
R PhelpsK RobbinsT LibertiWindow-period
human immunodeficiency virus transmission to two recipients by an
adolescent blood
donorTransfusion44929933200410.1111/j.1537-2995.2004.03364.x15157262
|
|
6.
|
K SoldanJA BarbaraME RamsayAJ
HallEstimation of the risk of hepatitis B virus, hepatitis C virus
and human immunodeficiency virus infectious donations entering the
blood supply in England, 1993–2001Vox Sang84274286200312757501
|
|
7.
|
TC GranadeS WorkmanSK WellsRapid detection
and differentiation of antibodies to HIV-1 and HIV-2 using
multiva-lent antigens and magnetic immunochromatography testingClin
Vaccine Immunol1710341039201010.1128/CVI.00029-1020410326
|
|
8.
|
W SchrammGB AnguloPC TorresA
Burgess-CasslerA simple saliva-based test for detecting antibodies
to human immunodeficiency virusClin Diagn Lab
Immunol6577580199910391866
|
|
9.
|
BR JacksonMP BuschSL StramerJP AuBuchonThe
cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood
donationsTransfusion43721729200310.1046/j.1537-2995.2003.00392.x12757522
|
|
10.
|
SA KleinS KarstenB RusterComparison of
TaqMan real-time PCR and p24 ELISA for quantification of in vitro
HIV-1 replicationJ Virol
Methods107169175200310.1016/S0166-0934(02)00230-612505631
|
|
11.
|
P CunninghamD MarriottC HarrisFalse
negative HIV-1 proviral DNA polymerase chain reaction in a patient
with primary infection acquired in ThailandJ Clin
Virol26163169200310.1016/S1386-6532(02)00115-412600648
|
|
12.
|
WK RothM WeberE SeifriedFeasibility and
efficacy of routine PCR screening of blood donations for hepatitis
C virus, hepatitis B virus, and HIV-1 in a blood-bank
settingLancet353359363199910.1016/S0140-6736(98)06318-19950441
|
|
13.
|
L KittigulP KhamounD SujiraratF
UtrarachkijK ChitpiromN ChaichantanakitK VathanophasAn improved
method for concentrating rotavirus from water samplesMem Inst
Oswaldo Cruz96815821200110.1590/S0074-0276200100060001311562708
|
|
14.
|
D SanyalG KudesiaG CorbittComparison of
ultracentrifugation and polyethylene glycol precipitation for
concentration of hepatitis B virus (HBV) DNA for molecular
hybridisation tests and the relationship of HBV-DNA to HBe antigen
and anti-HBe statusJ Med
Microbiol35291293199110.1099/00222615-35-5-291
|
|
15.
|
P TrepanierP PaymentM TrudelConcentration
of human respiratory syncytial virus using ammonium sulfate,
polyethylene glycol or hollow fiber ultrafiltrationJ Virol
Methods3201211198110.1016/0166-0934(81)90071-9
|
|
16.
|
S KobayashiK NatoriN TakedaK
SakaeImmunomagnetic capture rt-PCR for detection of norovirus from
foods implicated in a foodborne outbreakMicrobiol
Immunol48201204200410.1111/j.1348-0421.2004.tb03506.x15031533
|
|
17.
|
J NovotnýJ SvobodováLA RansnäsK KubistováA
method for the preparation of purified antigens of coxsackievirus
B3 from a large volume of cell culture supernatantActa
Virol3648348719921364026
|
|
18.
|
A SakudoK BabaM TsukamotoAnionic polymer,
poly(methyl vinyl ether-maleic anhydride)-coated beads-based
capture of human influenza A and B virusBioorg Med
Chem17752757200910.1016/j.bmc.2008.11.04619081256
|
|
19.
|
K OhkiY KishiY NishinoNoninfectious
doughnut-shaped human immunodeficiency virus type 1 can induce
syncytia mediated by fusion of the particles with CD4-positive
cellsJ Acquir Immune Defic Syndr4123312401991
|
|
20.
|
CR ClavetAB MargolinPM ReganHerpes simplex
virus type-2 specific glycoprotein G-2 immunomagnetically captured
from HEp-2 infected tissue culture extractsJ Virol
Methods119121128200410.1016/j.jviromet.2004.03.00815158593
|
|
21.
|
N JothikumarDO CliverTW
MariamImmunomagnetic capture PCR for rapid concentration and
detection of hepatitis A virus from environmental samplesAppl
Environ Microbiol6450450819989464385
|
|
22.
|
K SatohA IwataM MurataVirus concentration
using polyethyleneimine-conjugated magnetic beads for improving the
sensitivity of nucleic acid amplification testsJ Virol
Methods1141119200310.1016/j.jviromet.2003.08.002
|
|
23.
|
E UchidaK SatoA IwataAn improved method
for detection of replication-competent retrovirus in retrovirus
vector
productsBiologicals32139146200410.1016/j.biologicals.2004.08.00215536044
|
|
24.
|
E UchidaM KogiT OshizawaOptimization of
the virus concentration method using polyethyleneimine-conjugated
magnetic beads and its application to the detection of human
hepatitis A, B and C virusesJ Virol
Methods14395103200710.1016/j.jviromet.2007.02.014
|
|
25.
|
A IwataK SatohM MurataVirus concentration
using sulfonated magnetic beads to improve sensitivity in nucleic
acid amplification testsBiol Pharm
Bull2610651069200310.1248/bpb.26.106512913251
|
|
26.
|
A SakudoK IkutaEfficient capture of
infectious H5 avian influenza virus utilizing magnetic beads coated
with anionic polymerBiochem Biophys Res
Commun3778588200810.1016/j.bbrc.2008.09.08318823941
|
|
27.
|
A SakudoK BabaM TsukamotoK IkutaUse of
anionic polymer, poly(methyl vinyl ether-maleic anhydride)-coated
beads for capture of respiratory syncytial virusBioorg Med Chem
Lett1944884491200910.1016/j.bmcl.2009.05.12719546003
|
|
28.
|
A SakudoY TanakaK IkutaCapture of
infectious borna disease virus using anionic polymer-coated
magnetic beadsNeurosci
Lett494237239201110.1016/j.neulet.2011.03.02321406215
|
|
29.
|
A SakudoP MasrinoulY TanakaK IkutaCapture
of dengue virus type 3 using anionic polymer-coated magnetic
beadsInt J Mol Med28625628201121720701
|
|
30.
|
Ademtech: Viro-Adembeads: virus capture
and culture. http://www.ademtech.com/images/viro-adembeads.pdf.
Accessed January 4, 2012
|
|
31.
|
B HatanoA KojimaT SataH KatanoVirus
detection using Viro-Adembeads, a rapid capture system for viruses,
and plaque assay in intentionally virus-contaminated beveragesJpn J
Infect Dis635254201020093763
|
|
32.
|
A SakudoT OnoderaVirus capture using
anionic polymer-coated magnetic beads (Review)Int J Mol
Med3037201222505019
|