Introduction
Chronic periodontal disease is an inflammatory
condition characterized by a shift in the microbial ecology of
subgingival plaque biofilms and the progressive host-mediated
destruction of tooth supporting structures (1). It can affect up to 90% of the world
wide population: there are close connections between periodontal
inflammation and major chronic condition, such as diabetes, heart
disease and chronic autoimmune disease (2). The underlying mechanisms causing
these pathological conditions are still unclear. Two mechanisms
were proposed to explain this progression: bacteria acquire the
ability to penetrate the deeper tissues (3), and/or the host response is degraded
(4). In particular resident
periodontal ligament and gingival fibroblasts have been reported to
secrete matrix metalloproteinases (MMPs) and chemoattractants for
epithelial cells (5). An
important role in the inflammation during periodontal disease seems
to also be played from different proteoglycans and
glycosaminoglycans, such as syndecan 1 (6,7).
The progression of inflammation and periodontal destruction
requires a response to a bacterial insult that includes resident
cells that sustain signals to trigger the immune response.
Host-derived cytokines released upon microbial challenge have
significant effects on the immune and inflammatory responses in
periodontal disease (8,9); indeed, many cross-sectional studies
have explored the production of Th1 and Th2 cytokines in human
periodontitis (10,11). A prominent factor in connective
tissue remodeling, and inflammatory diseases, is transforming
growth factor-β (TGF-β): one of these isoforms, TGF-β1 is a
multifunctional cytokine that regulates cell growth,
differentiation and matrix production (12). It has potent immunosuppressive
activity and downregulates the transcription of other
pro-inflammatory cytokines, including interleukin-1, tumor necrosis
factor-α and several metalloproteinases (13). TGF-β is a key mediator of tissue
fibrosis and may lead to ECM accumulation in pathological states
(14), moreover it mediates
fibroblast activation, proliferation and signaling in cells
cultures (15). The gene
polymorphisms of this cytokine have been associated with risk for
systemic diseases, including cardiovascular diseases and rheumatoid
arthritis which are related to periodontitis (16).
In the development of chronic inflammatory disease,
an important role is also played by the vascular endothelial growth
factor (VEGF), a 45-kDa a homodimeric pro-inflammatory glycoprotein
that potently increases microvascular permeability, promotes
angiogenesis, and stimulates endothelial cell proliferation,
migration, and survival (17);
this protein seems to be involved in the onset and progression of
gingivitis and periodontitis, mainly promoting the vascular network
expansion generally observed in inflammation (18). Studies intended to associate the
action of VEGF with the pathogenesis of periodontal disease have
reported controversial findings. Nevertheless, VEGF expression is
more strongly related to the healing stage of periodontal disease
than to the destruction stage of the lesion (19).
In recent years, it has become apparent that
patients with rheumatic diseases and periodontitis share common
pathogenetic characteristics, such as a pro-inflammatory trait
(20). Many reports have shown
that periodontal disease is more common in rheumatoid arthritis
(21), and that periodontal
therapy reduces the severity of rheumatoid arthritis (22).
Systemic sclerosis (SSc) or scleroderma is a
rheumatic acquired disorder that typically results in the fibrosis
of the skin and internal organs (23). Previous findings indicate that the
pathogenesis of this disorder includes inflammation, autoimmune
attack, and vascular damage, leading to fibroblast activation
(24,25). However, cultured SSc fibroblasts,
which are free from such environmental factors, continue to produce
the excessive amount of extracellular matrix (ECM) proteins,
suggesting that SSc fibroblasts establish a constitutive
self-activation system once activated (26).
The etiology of SSc remains uncertain, but one of
the major cytokines that may be involved in this process is TGF-β1
(27), and the principal effect
of this cytokine on mesenchymal cells is the stimulation of ECM
deposition and angiogenesis alterations. We have already studied
the distribution, by immunohistochemical analysis, of the major
integrin and sarcoglycans subcomplex in bisphosphonate-induced
osteonecrosis of the jaw (28),
and the distribution of collagen I and IV into the periodontal
ligament during orthodontic tooth movement (29) and for the first time, in the
literature, we investigated the role of TGF-β1 and VEGF in the
gingival tissues in the pathogenesis of rheumatic disease, SSc.
Therefore, the objective of the present study was to
immunohistochemically determine the expression and distribution of
TGF-β1 and VEGF in the gingival tissues and periodontal ligament of
patients with chronic periodontitis (CP) and SSc compared to the
healthy control group (CO).
Materials and methods
Patient selection
Ninety patients were enrolled in this crosssectional
study performed from August 2010 and July 2011 at the University of
Messina, Messina, Italy.
Thirty patients (5 male, 25 female, mean age 52.4,
SD ±8.5), as defined by the American College of Rheumatology
classification criteria for SSc were classified as diffuse SSc or
limited SSc based on the extent of skin involvement (30). Disease onset was determined by
patients’ recall of the first non-Raynaud symptom clearly
attributable to scleroderma (31). Thirty patients with chronic adult
periodontitis (11 male, 19 female, mean age 54, SD ±9.2) based on
the criteria defined by the American Academy of Periodontology
(32) and 30 healthy control
subjects (16 male, 14 female, mean age 48.9, SD ±8.2) were
enrolled. Therefore, the patients were divided in: CP, SSc and CO
groups.
The protocol was approved by the Ethics and Research
Committee of University of Messina, and ethical approval was
obtained for the experimental procedures applied in humans, in
accordance with the provisions of the World Medical Association’s
Declaration of Helsinki of 1975, as revised in 2000. All patients
included in the study signed an informed consent form. Patients
with: diabetes mellitus, liver, kidney, or salivary gland
dysfunction, history of alcoholism, a recent history or the
presence of other acute or chronic infection, systemic antibiotic
treatment or immunosuppressant medication (non SSc groups only)
within previous three months, pregnancy and intense physical
activity, smoking history or the presence of an oral mucosal
inflammatory condition, were excluded from the study. Inclusion
criteria included: ≥18 years of age who were in good general health
(excluding the case definition) and had ≥18 erupted teeth.
The CP group had >30% of sites with bleeding on
probing (BOP), >20% of sites with probing depth (PD) >4 mm,
>10% of sites with interproximal clinical attachment level (CAL)
>2 mm. The healthy control subjects had <10% sites with BOP
<2% of sites with PD >5 mm, no sites with PD >6 mm, <1%
of sites with CAL >2 mm and no radiographic bone loss (evident
in posterior vertical bitewings films).
Clinical measurements
On each subject, plaque index (PI) (33), PD, clinical attachment level
(CAL), community periodontal index of treatment needs (CPITN)
(34) and presence of BOP were
measured at 6 sites and recorded on each tooth. Every clinical
periodontal measurement was performed by one calibrated
examiner.
Gingival tissue biopsies
Gingival tissue and periodontal samples were carried
out during routine erupted third molar extractions, advanced caries
and orthodontic indications for the PD, SSc and healthy control
groups. The collection of tissues of 2x2 mm in size were washed
with saline solution and fixed in 10% neutral-buffered formalin,
transported at 4°C and processed for immunohistochemistry.
Immunohistochemistry
From each biopsy, 25 sections were prepared. The
samples were snap-frozen in liquid nitrogen and 20 μm sections were
prepared in a cryostat for their use in a protocol to perform
immunofluorescence. Finally, the sections were incubated with
primary antibodies. The following primary antibodies were used:
anti-TGF-β1 diluted 1:50 (sc146, Santa Cruz Biotechnology, Inc.,
Heidelberg, Germany), anti-VEGF diluted 1:50 (VG1, Novus
Biologicals, Littleton, CO, USA). Primary antibodies were detected
using Texas Red-conjugated IgG (Jackson ImmunoResearch
Laboratories, Inc.). Slides were finally washed in PBS and sealed
with mounting medium.
In the analysis, each specimen was divided into the
following three areas to allow quantification of the distribution
of cluster designation (CD) marked cells: i) the sulcular
epithelium, ii) the middle area (lamina propria), and iii)
the oral gingival epithelium. The investigations were conduced on
2,250 images by a blinded pathologist who performed the analysis in
a blinded manner. The sections were then analyzed and images were
acquired using a Zeiss LSM 5 DUO confocal laser scanning microscope
(Carl Zeiss MicroImaging GmbH, Jena, Germany). All images were
digitalized at a resolution of 8 bits into an array of 2,048x2,048
pixels. Optical sections of fluorescent specimens were obtained
using a HeNe laser (wave-length, 543 nm) and an Argon laser
(wavelength, 458 nm) at a 1-min 2-sec scanning speed with up to 8
averages; 1.50-μm sections were obtained using a pinhole of 250.
Each image was acquired within 62 sec, in order to minimize
photodegradation (Adobe Photoshop 7.0; Adobe Systems, Palo Alto,
CA, USA).
Statistical analysis
Frequency distributions, median and standard
deviation (SD) values were determined at baseline in each group to
describe the clinical parameters (CAL, PD, CPITN, PI and BOP). The
Kruskal-Wallis and the Mann-Whitney U tests were carried out when
comparing the clinical parameters (CAL, PD and CPITN) in the three
independent groups and the Wilcoxon singed rank test was used when
comparing three matched-pair groups. The differences were
considered statistically significant when P<0.05 (or 5%). The
data were analyzed with software Prism (Graphpad Instat, version
5.00; GraphPad Software, San Diego, CA, USA).
Results
A higher CAL (≥4 mm), PD site (≥5 mm) and PD values
(<1), was observed in the SSc group compared with the controls
(P<0.05); additionally, the BOP values (<1) were
significantly higher in the SSc group than the healthy controls
(P<0.05) (Fig. 1). Results are
presented as the medians, confidence levels, lower and upper (L-U)
quartiles (Table I). Higher
levels of PI were observed in the CP compared to the CO group. BOP,
as a measure of acute periodontal inflammation, was elevated in
patients with CP compared to the CO group.
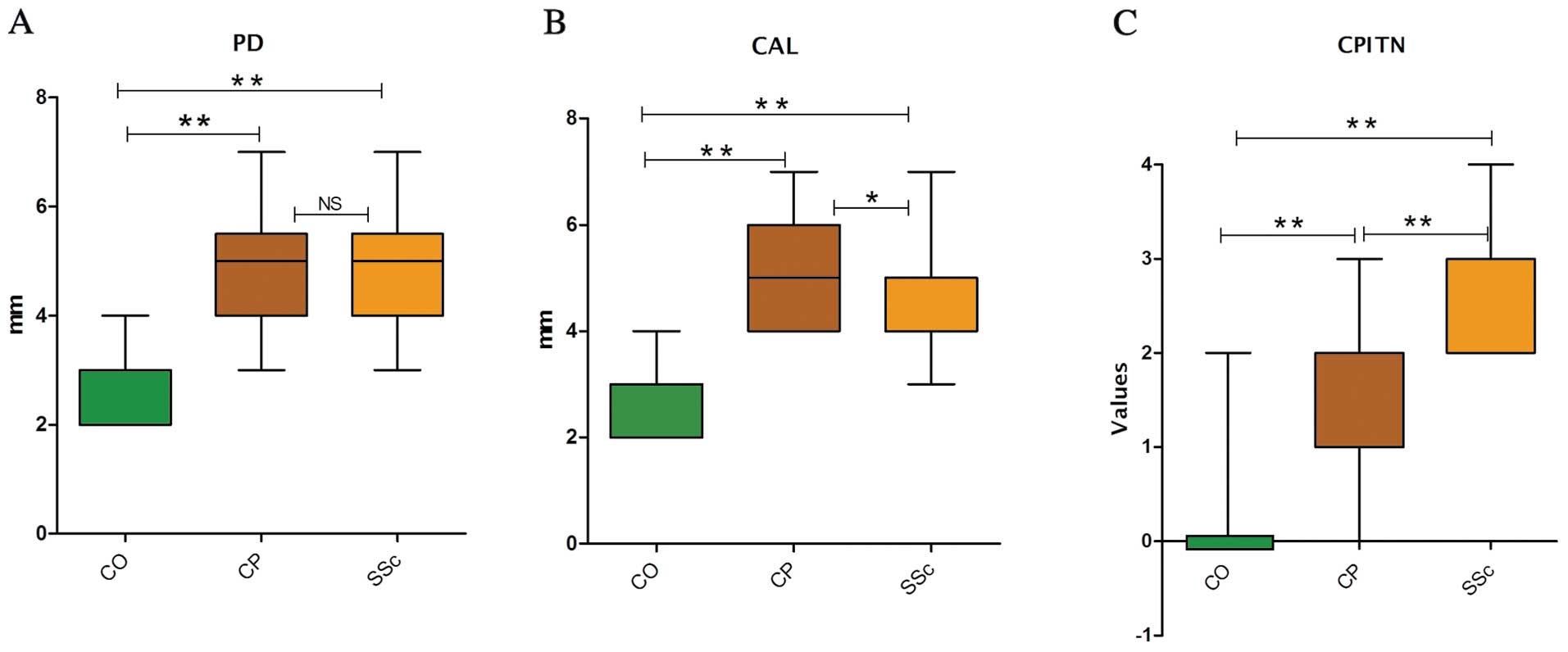 | Figure 1.Comparison of clinical parameters
between three groups (n=90 patients). Results of (A) probing depth
(PD) and (B) clinical attachment level (CAL) are shown as box
plots, with median, upper and lower quartiles and max and min
values. (C) Community Periodontal Index of Treatment Needs (CPITN)
shown as bars with standard deviation (SD) of the values. CO,
healthy control group (n=30); CP, chronic periodontitis group
(n=30); SSc, scleroderma group (n=30). The medians for PD were 3, 5
and 5 for the CO, CP and SSc groups, respectively. The medians for
CAL were 3, 5 and 4 and those for CPITN were 0, 1 and 3 for the CO,
CP and SSc groups, respectively. Comparison between the three
groups test: *P≤0.05 (two-sided) significant;
**P≤0.001 (two-sided) highly significant; NS, not
significant. Multiple comparisons: significance is obtained between
CP and controls, SSc and controls and CP and SSc. PD,
**CP vs. CO; **SSc vs. CO; NS, CP vs. SSc.
CAL, **CP vs. CO; **SSc vs. CO;
*CP vs. SSc. CPITN, **CP vs. CO;
**SSc vs. CO; **CP vs. SSc. |
 | Table I.Clinical characteristics of the
patients at baseline (90 patients). |
Table I.
Clinical characteristics of the
patients at baseline (90 patients).
| Healthy control
| Chronic
periodontitis
| Scleroderma
|
|---|
| Clinical
measurement | n | % | n | % | n | % |
|---|
| CAL (mm) | | | | | | |
| <2.5 | 24 | 80.0 | - | - | 4 | 13.3 |
| >2.5 to
<3.5 | 6 | 20.0 | 2 | 6.6 | 10 | 33.3 |
| ≥3.5 to
<4.5 | - | - | 18 | 60.0 | 12 | 40.0 |
| ≥4.5 | - | - | 10 | 33.3 | 4 | 13.3 |
| Median | 3 | | 5 | | 4 | |
| CI (95%) | 21 | | 44 | | 55 | |
| L-U | 2.83–2.91 | | 4.83–5.26 | | 4.25–4.78 | |
| PD (mm) | | | | | | |
| <2.5 | 22 | 73.3 | - | - | 5 | 16.6 |
| ≥2.5 to
<3.5 | 8 | 26.6 | 5 | 16.6 | 9 | 30.0 |
| ≥3.5 to
<4.5 | - | - | 16 | 53.3 | 11 | 36.6 |
| ≥4.5 | - | - | 9 | 30.0 | 5 | 16.6 |
| Median | 3 | | 5 | | 5 | |
| CI (95%) | 22 | | 5 | | 47 | |
| (L-U) | 2.69–2.91 | | 4.7–5.19 | | 4.53–4.98 | |
| PI (value) | | | | | | |
| <1 | 25 | 83.3 | - | - | 5 | 16.6 |
| >1 to
<2 | 4 | 13.3 | 21 | 70.0 | 15 | 50.0 |
| ≥2 to <3 | - | | 9 | 30.0 | 5 | 16.6 |
| Median | 0 | | 1 | | 2 | |
| CI (95%) | 20 | | 37 | | 27 | |
| (L-U) | 0.28–0.48 | | 1.01–0.36 | | 1.44–1.7 | |
| BOP (value) | | | | | | |
| <1 | 25 | 83.3 | 1 | 3.7 | 7 | 23.3 |
| >1 to
<2 | 5 | 16.6 | 21 | 70.0 | 17 | 56.6 |
| ≥2 to <3 | - | - | 7 | 26.3 | 3 | 10.0 |
| Median | 0 | | 1 | | 1 | |
| CI (95%) | 19 | | 27 | | 37 | |
| L-U | 0.25–0.44 | | 1.39–1.65 | | 0.91–1.27 | |
| CPITN (value) | | | | | | |
| <1 | 29 | 96.6 | 4 | 13.3 | 6 | 20.0 |
| >1 to
<2 | 1 | 3.3 | 11 | 36.6 | 17 | 56.6 |
| ≥2 to <3 | - | - | 12 | 40.0 | 4 | 13.3 |
| ≥3 to <4 | - | - | 3 | 10.0 | 3 | 10.0 |
| Median | 0 | | 1 | | 3 | |
| CI (95%) | 18 | | 33 | | 29 | |
| L-U | 0.06–0.24 | | 1.21–1.54 | | 2.57–2.85 | |
In order to investigate the relationship between
gingival biomarker levels and clinical parameters of periodontal
disease, we performed immunofluorescence reactions on gingival and
periodontal ligament samples obtained from CO, CP and SSc, using
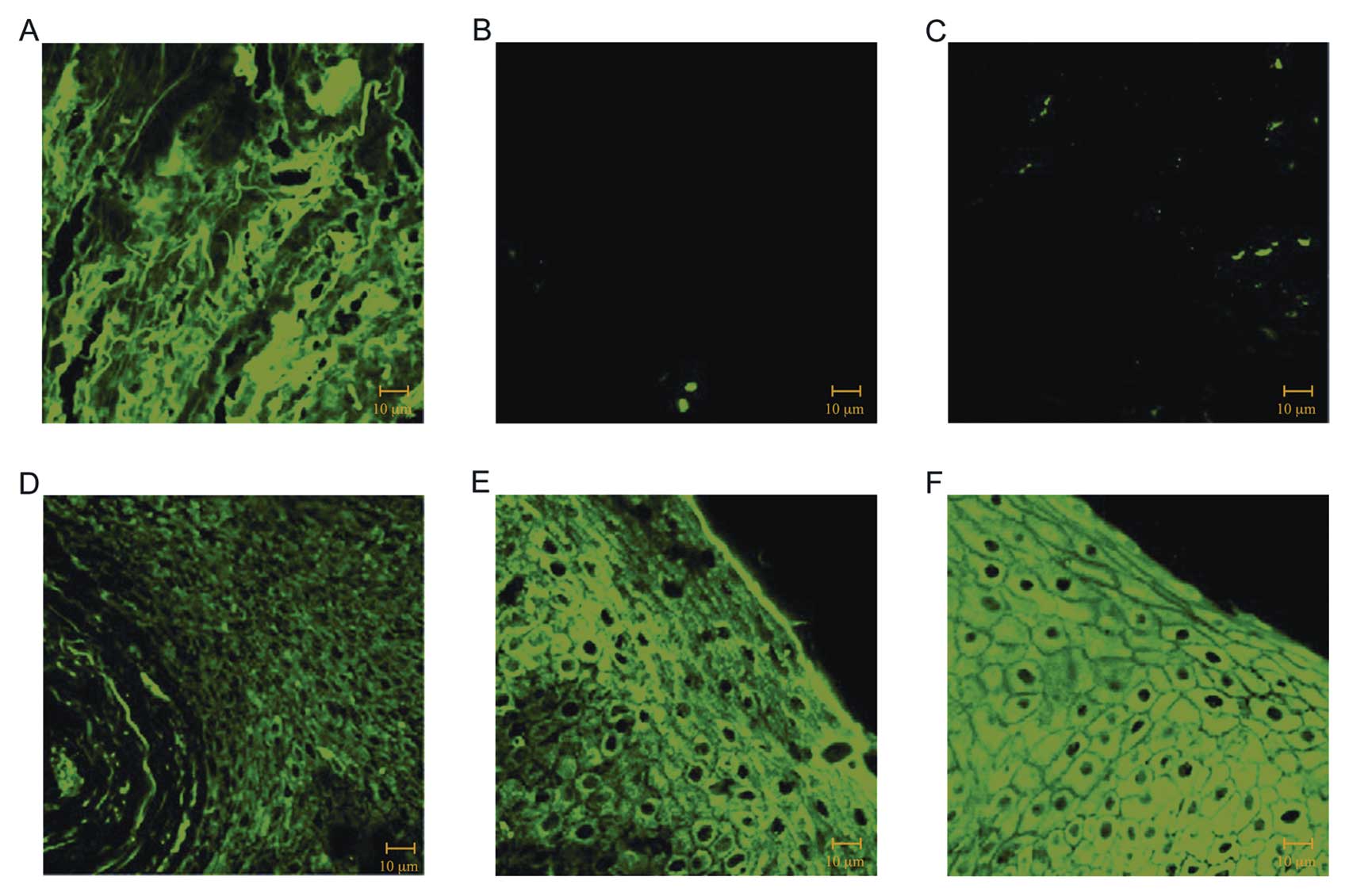
antibodies against TGF-β1 and VEGF. First of all, we performed a
negative control both on gingival samples with TGF-β1 (Fig. 2A) and VEGF (Fig. 2B) and periodontal ligament samples
with TGF-β1 (Fig. 2C) and VEGF
(Fig. 2D) using the secondary
antibody only. Our results on gingival samples clearly showed a
normal staining pattern for TGF-β1 in CO (Fig. 3A), whereas the staining was
severely reduced in samples of patients with CP (Fig. 3B) and SSc (Fig. 3C). In contrast in
immunofluorescence reactions performed using VEGF antibodies,
staining patterns showed a higher intensity in CP (Fig. 3E) and SSc (Fig. 3F) than that observed in CO
(Fig. 3D).
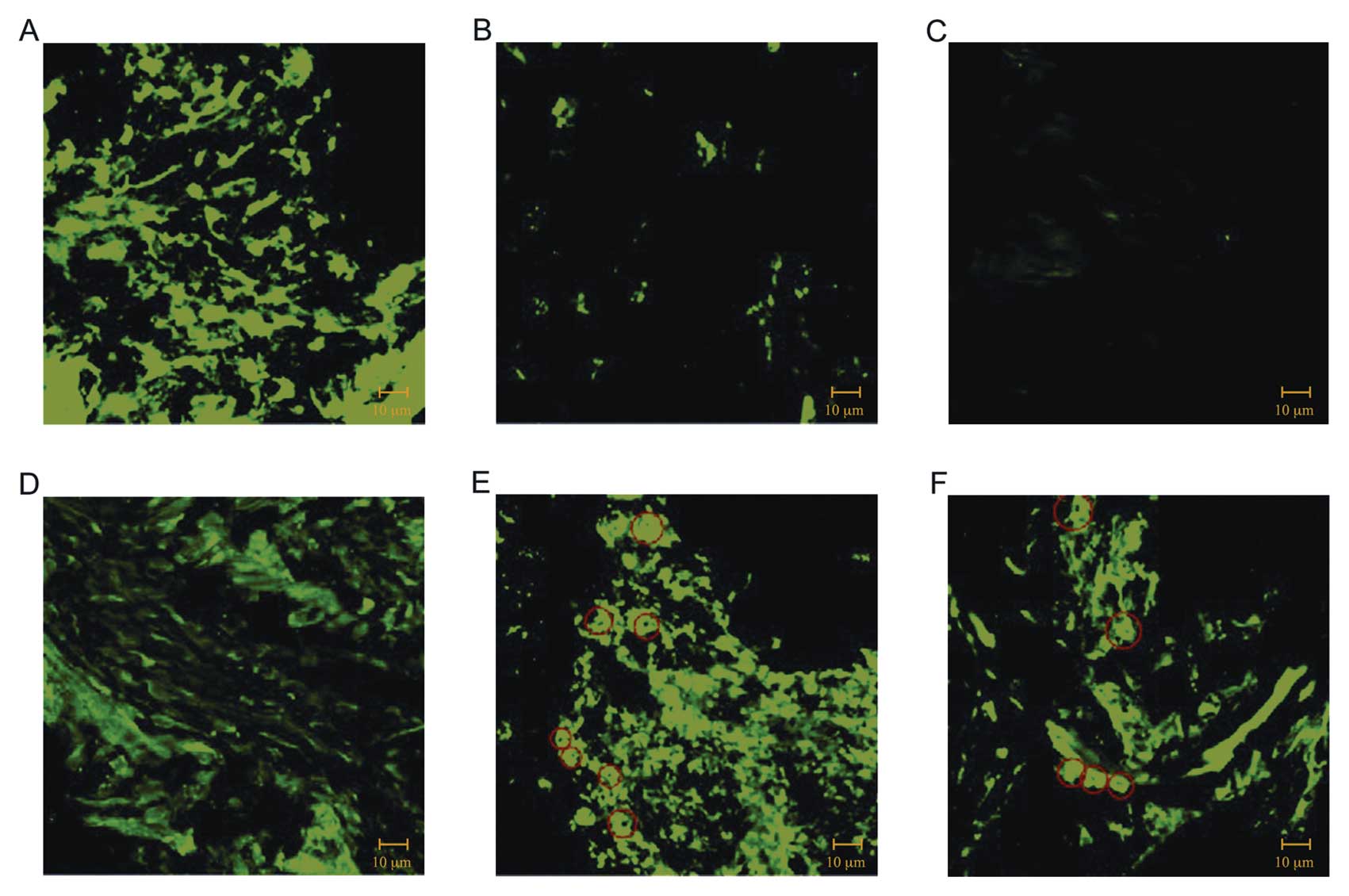
Similar results were obtained by immunof luorescence
analysis of periodontal ligament (PDL) samples. Immunofluorescence
reactions performed with TGF-β1, demonstrated that the staining
signal was nearly absent in PDL obtained from patients affected by
CP (Fig. 4B), and SSc (Fig. 4C) compared to PDL obtained from CO
(Fig. 4A). Moreover
immunofluorescence performed using the VEGF antibody revealed a
higher staining pattern for VEGF in CP (Fig. 4E) and SSc (Fig. 4F) than that observed in CO
(Fig. 4D). Furthermore, in
periodontal diseases an increase of fluorescence was detected
around the blood vessels (red circles in Fig. 4E and F).
Finally comparison of the clinical parameters
between the studied groups, by statistical analysis, showed
significantly higher differences in PD, CAL and CPITN, between
patients with CP and SSc compared to CO.
There was a higher statistically significant
difference (P<0.001) in the percentage of sites with CAL among
the CP and SSc group and the control group (Fig. 1). There was no statistically
significant difference at the PD sites between the CP and SSc
group, but a statistically difference (P<0.05) in CAL between
the CP and SSc group.
Discussion
To study the role and the production of autocrine
TGF-β1 and VEGF signaling, the production of these growth factors
in CO, CP, SSc groups were examined, analyzing gingival and PDL
biopsies obtained from each patient. Contrast and brightness were
established by examining the most brightly labeled pixels and
choosing the settings that allowed clear visualization of the
structural details while keeping the pixel intensity at its highest
(∼200).
This study showed that patients with CP and SSc had
major severe periodontal disease in respect to the control group
(Fig. 1). These studies also
showed a particular relationship between periodontal disease and
other chronic inflammatory diseases, such as SSc. These biomarkers
contribute to sustaining the destructive inflammatory cascades seen
in chronic periodontitis.
Transforming growth factor-β1 (TGF-β1) is known as
one of the major anti-inflammatory cytokines (35). The large number of previous
reports examining these polymorphisms of the TGF-β1 gene in various
diseases reflects the interest in the role of this gene in chronic
inflammatory diseases. Skaleric et al (36) found elevated TGF-β1 levels in
gingival crevicular fluid samples from sites with deeper
periodontal pockets.
Our results suggest that high TGF-β1 production may
be a protective factor for periodontitis: potentially, this growth
factor also accelerates connective tissue remodeling and
angiogenesis (37): its
biological activities result in insufficient remodeling and
perfusion of tooth-supporting tissues contributing to periodontal
destruction. However, previous reports rendered contradictory
findings about the role of this growth factor (38).
Upregulation of TGF-β1 cytokine in patients with
adult periodontitis may counterbalance the destructive gingival
inflammatory responses in the acute phase of periodontitis
(39). On the other hand, TGF-β1
was shown to induce chemotaxis for neutrophils, monocytes, mast
cells and lymphocytes and is also an important mediator of the
T-lymphocyte population (40).
Research to date has shown that TGF-β1 is mainly
important in the early development stage of SSc (41). The main role in the fibrosis
processes could be played by this growth factor, produced in excess
by peripheral blood mononuclear cells (PBMC) (42).
TGF-β1 seems to be more significant in the fibrosis
process than during the angiokinetic changes. TGF-β1 strongly slows
down the PBMC adhesion to the endothelium and the generation of
free radicals but can stimulate chemotaxis. Therefore, TGF-β1 may
intensify inflammatory infiltrations around the vessels correlating
with the beginning of the fibrosis process (43).
In addition, the present study clearly showed that
VEGF expression was increased significantly in the destruction
stage of the lesion, in contrast with a previous study that showed
higher level of VEGF in the healing stage of the periodontal
disease (19). Our study also
showed that the mean concentrations of VEGF in periodontal tissues
increased progressively from healthy to SSc subjects. This growth
factor, in concert with TGF-β1, acts to stabilize the vascular wall
and its imbalanced expression has been implicated in aberrant
angiogenesis, a crucial factor in the pathogenesis of the SSc
(44). However, angiogenesis in
SSc is somewhat controversial. On one hand, Ozcelik et al
(45) observed lower percentages
of local VEGF expression in gingival biopsies of SSc patients when
compared to the control group. On the other hand, Koch and Distler
(46) showed, in accordance with
our results, an increased production and stimulated
neovascularization of VEGF in the blood vessels in the skin of SSc
patients.
In conclusion, it is important to determine the
exact function of these genetic polymorphisms during periodontitis
in order to better understand their importance during disease
progression. There is no doubt that we now have the technological
basis to generate and analyze large volumes of information in the
pursuit of understanding complex diseases such as periodontal
disease at the molecular genetic level.
Our results suggest that TGF-β1 may contribute both
to inflammatory regulation and remodeling events during periodontal
disease. Therefore, we have documented that TGF-β1 downregulation
in active periodontitis lesions has a key role in tissue
destruction and bone resorption.
The findings presented here make it clear that
biomarkers, such as TGF-β1 and VEGF play a key role in the
evolution of the immune response in the periodontal and scleroderma
disease, which in turn influences the outcome of disease
establishment and evolution. It is important, therefore, to
identify biomarkers that correlate with disease activity, prognosis
and response to therapy allowing physicians to accurately identify
patients early, those likely to respond and to predict prognosis of
the disease.
References
|
1.
|
PD MarshMicrobial ecology of dental plaque
and its significance in health and diseaseAdv Dent
Res826327119947865085
|
|
2.
|
BL PihlstromBS MichalowiczNW
JohnsonPeriodontal
diseaseLancet1918091820200510.1016/S0140-6736(05)67728-8
|
|
3.
|
DT GravesD FineYT TengTE Van DykeG
HajishengallisThe use of rodent models to investigate host-bacteria
interactions related to periodontal diseasesJ Clin
Periodontol3589105200810.1111/j.1600-051X.2007.01172.x18199146
|
|
4.
|
PM BartoldAS NarayananMolecular and cell
biology of healthy and diseased periodontal tissuesPeriodontol
2000402949200610.1111/j.1600-0757.2005.00140.x16398684
|
|
5.
|
WV GiannobileHost-response therapeutics
for periodontal diseasesJ
Periodontol7915921600200810.1902/jop.2008.08017418673015
|
|
6.
|
S KotsovilisS Tseleni-BalafoutaA CharonisI
FourmousisD NikolidakisJA VrotsosSyndecan-1 immunohistochemical
expression in gingival tissues of chronic periodontitis patients
correlated with various putative factorsJ Periodontal
Res455205312010
|
|
7.
|
AN AlexopoulouHA MulthauptJR
CouchmanSyndecans in wound healing, inflammation and vascular
biologyInt J Biochem Cell
Biol39505528200710.1016/j.biocel.2006.10.01417097330
|
|
8.
|
F D'AiutoM ParkarG AndreouJ SuvanPM BrettD
ReadyMS TonettiPeriodontitis and systemic inflammation: control of
the local infection is associated with a reduction in serum
inflammatory markersJ Dent Res83156160200414742655
|
|
9.
|
MA TaubmanT KawaiX HanThe new concept of
periodontal disease pathogenesis requires new and novel therapeutic
strategiesJ Clin
Periodontol34367369200710.1111/j.1600-051X.2007.01065.x17448041
|
|
10.
|
X HanT KawaiJW EastcottMA
TaubmanBacterial-responsive B lymphocytes induce periodontal bone
resorptionJ
Immunol176625631200610.4049/jimmunol.176.1.62516365458
|
|
11.
|
Q JinJA CirelliCH ParkJV SugaiM Taba JrPJ
KostenuikWV GiannobileRANKL inhibition through osteoprotegerin
blocks bone loss in experimental periodontitisJ
Periodontol7813001308200710.1902/jop.2007.07007317608585
|
|
12.
|
AB RobertsMB SpornTransforming growth
factor betaAdv Cancer
Res51107145198810.1016/S0065-230X(08)60221-3
|
|
13.
|
H Birkadel-HansenRole of matrix
metalloproteinases in human periodontal diseasesJ
Periodontol64474484199310.1902/jop.1993.64.5.4748315570
|
|
14.
|
KJ GordonGC BlobeRole of transforming
growth factor-beta superfamily signaling pathways in human
diseaseBiochim Biophys
Acta1782197228200810.1016/j.bbadis.2008.01.00618313409
|
|
15.
|
MC WilkesH MitchellSG PenheiterJJ DoreK
SuzukiM EdensDK SharmaRE PaganoEB LeofTransforming growth factor-b
activation of phosphatidylinositol 3-kinase is independent of Smad2
and Smad3 and regulates fibroblast responses via p21-activated
kinase-2Cancer Res651043110440200510.1158/0008-5472.CAN-05-1522
|
|
16.
|
F MercadoRI MarshallA KlestovPM
BartoldRelationship between rheumatoid arthritis and periodontitisJ
Periodontol72779787200110.1902/jop.2001.72.6.77911453241
|
|
17.
|
F UnlüPG GüneriM HekimgilB YeşilbekH
BoyacioğluExpression of vascular endothelial growth factor in human
periodontal tissues: comparison of healthy and diabetic patientsJ
Periodontol74181187200312666706
|
|
18.
|
RB JohnsonFG SerioX DaiVascular
endothelial growth factors and progression of periodontal diseasesJ
Periodontol70848852199910.1902/jop.1999.70.8.84810476891
|
|
19.
|
BO CetinkayaGC KelesB AyasEE SakalliogluG
AcikgozThe expression of vascular endothelial growth factor in a
rat model at destruction and healing stages of periodontal diseaseJ
Periodontol7811291135200710.1902/jop.2007.06039717539728
|
|
20.
|
PM BartoldRI MarshallDR
HaynesPeriodontitis and rheumatoid arthritis: a reviewJ
Periodontol7620662074200510.1902/jop.2005.76.11-S.206616277578
|
|
21.
|
FB MercadoRI MarshallPM
BartoldInter-relationships between rheumatoid arthritis and
periodontal disease. A reviewJ Clin
Periodontol30761772200310.1034/j.1600-051X.2003.00371.x12956651
|
|
22.
|
MK Al-KatmaNF BissadaJM BordeauxJ SueAD
AskariControl of periodontal infection reduces the severity of
active rheumatoid arthritisJ Clin
Rheumatol13134137200710.1097/RHU.0b013e318069061617551378
|
|
23.
|
EC LeRoySystemic sclerosis
(scleroderma)Cecil's Textbook of MedicineJB WyngaardenLH SmithJC
Bennett19th editionSaunders WBPhiladelphia153015351992
|
|
24.
|
JH KornImmunologic aspects of
sclerodermaCurr Opin
Rheumatol1479484198910.1097/00002281-198901040-000112702049
|
|
25.
|
GA ScardinaME PizzigattiP
MessinaPeriodontal micro-circulatory abnormalities in patients with
systemic sclerosisJ
Periodontol7619911995200510.1902/jop.2005.76.11.199116274320
|
|
26.
|
A JelaskaM ArakawaG BroketaJH
KornHeterogeneity of collagen synthesis in normal and systemic
sclerosis skin fibroblasts: increased proportion of high
collagen-producing cells in systemic sclerosis fibroblastsArthritis
Rheum3913381346199610.1002/art.1780390811
|
|
27.
|
EC LeRoyEA SmithMB KahalehM TrojanowskaRM
SilverA strategy for determining the pathogenesis of systemic
sclerosis: is transforming growth factor the answer?Arthritis
Rheum3281782519892665755
|
|
28.
|
E Nastro SiniscalchiG CutroneoL CatalfamoG
SantoroA AllegraG OteriD CicciùA AlonciG PennaC
MusolinoImmunohistochemial evaluation of sarcoglycans and integrins
in gingival epithelium of multiple myeloma patients with
bisphosphonate-induced osteonecrosis of the jawOncol
Rep24129134201020514453
|
|
29.
|
G AnastasiG CordascoG MatareseG RizzoR
NuceraM MazzaA MilitiM PortelliG CutroneoA FavaloroAn
immunohistochemical, histological, and electron-microscopic study
of the human periodontal ligament during orthodontic treatmentInt J
Mol Med215455542008
|
|
30.
|
AT MasiSubcommittee for Scleroderma
Criteria of the American Rheumatism Association Diagnostic and
Therapeutic Criteria CommitteePreliminary criteria for the
classification of systemic sclerosis (scleroderma)Arthritis
Rheum23581590198010.1002/art.1780230510
|
|
31.
|
B WhiteEA BauerLA GoldsmithGuidelines for
Clinical Trials in Systemic Sclerosis (Scleroderma). I
Disease-modifying interventions. The American College of
Rheumatology Committee on Design and Outcomes in Clinical Trials in
Systemic SclerosisArthritis Rheum383513601995
|
|
32.
|
GC ArmitagePeriodontal diagnoses and
classification of periodontal diseasesPeriodontology
200034921200410.1046/j.0906-6713.2002.003421.x14717852
|
|
33.
|
J SilnessH LoePeriodontal disease in
pregnancy. II Correlation between oral hygiene and periodontal
conditionActa Odontol
Scand22121135196410.3109/0001635640899396814158464
|
|
34.
|
World Health OrganizationOral Health
Surveys - Basic methodsCommunity Periodontal Index of Treatment
Needs (CPITN)3rd edition3132Geneva1987
|
|
35.
|
KJ TraceyPhysiology and immunology of the
cholinergic antiin flammatory pathwayJ Clin
Invest117289296200710.1172/JCI3055517273548
|
|
36.
|
U SkalericB KramarM PetelinZ PavlicaSM
WahlChanges in TGF-β1 levels in gingival, crevicular fluid and
serum associated with periodontal inflammation in humans and
dogsEur J Oral Sci1051361421997
|
|
37.
|
JR MerwinJM AndersonO KocherCM Van
ItallieJA MadriTransforming growth factor beta 1 modulates
extra-cellular matrix organization and cell-cell junctional complex
formation during in vitro angiogenesisJ Cell
Physiol142117128199010.1002/jcp.1041420115
|
|
38.
|
GV McDonnellCW KirkSA HawkinsCA GrahamLack
of association of transforming growth factor (TGF)-beta 1 and beta
2 gene polymorphisms with multiple sclerosis (MS) in Northern
IrelandMult Scler5105109199910335519
|
|
39.
|
S SteinswollTS HalstensenK SchenkExtensive
expression of TGF-β1 in chronically inflamed periodontal tissueJ
Clin Periodontol263663731999
|
|
40.
|
HW MittruckerSH KaufmannMini-review:
regulatory T cells and infection: suppression revisitedEur J of
Immunol34306312200410.1002/eji.20032457814768034
|
|
41.
|
C QuerfeldB EckesC HuerkampT KriegS
SollbergExpression of TGF-beta 1, -beta 2 and -beta 3 in localized
and systemic sclerodermaJ Dermatol
Sci211322199910.1016/S0923-1811(99)00008-010468187
|
|
42.
|
H OtaS KumagaiA MorinobuH YanagidaK
NakaoEnhanced production of transforming growth factor-beta
(TGF-beta) during autologous mixed lymphocyte reaction of systemic
sclerosis patientsClin Exp
Immunol10099103199510.1111/j.1365-2249.1995.tb03609.x7697928
|
|
43.
|
JL MehtaBC YangBS StratesP MehtaRole of
TGF-beta1 in platelet-mediated cardioprotection during
ischemia-reperfusion in isolated rat heartsGrowth
Factors16179190199910.3109/0897719990900212810372959
|
|
44.
|
A ArmulikA AbramssonC
BetsholtzEndothelial/pericyte interactionsCirc
Res97512523200510.1161/01.RES.0000182903.16652.d716166562
|
|
45.
|
O OzcelikMC HaytacM ErginB AntmenG
SeydaogluThe immunohistochemical analysis of vascular endothelial
growth factors A and C and microvessel density in gingival tissues
of systemic sclerosis patients: their possible effects on gingival
inflammationOral Surg Oral Med Oral Pathol Oral Radiol
Endod105481485200810.1016/j.tripleo.2007.07.021
|
|
46.
|
AE KochO DistlerVasculopathy and
disordered angiogenesis in selected rheumatic diseases: rheumatoid
arthritis and systemic sclerosisArthritis Res Ther9Suppl
2S3200710.1186/ar218717767741
|