Introduction
Aging is a physiological phenomenon, which commonly
occurs in various organs and tissues (1). Age-dependent morphological and cell
kinetic changes in the organs and tissues are associated with the
development of various diseases in the elderly. A decline in the
function of organs and tissues is a normal phenomenon associated
with aging, and is also considered to reduce the quality of life
(1).
Several reports are available regarding the
relationship between aging and periodontitis (2–5).
The aging of the periodontal tissue is involved in the development
of periodontitis in elderly individuals (6).
The age-dependent morphological and cell kinetic
changes of the gingival tissue were delineated, using both the
5-bromo-2′-deoxyuridine (BrdU) incorporation and the terminal
deoxynucleotidyl transferase-mediated deoxyuridine-5′-triphosphate
(dUTP)-biotin nick end-labeling (TUNEL) methods (7,8).
Those studies demonstrated that with aging there is a significant
apoptosis-induced decrease in the cellular component of the
subepithelial connective tissue of both the gingival and junctional
epithelial layer. Furthermore, an age-dependent increase in the
number of TUNEL-positive cells occurred only in the subepithelial
connective tissue, although gingival tissue, buccal mucosa, tongue
dorsal, ventral mucosae and skin have similar histological
structures (9).
Oxidative stress is one of the most important
causative factors for the induction of cell apoptosis (10,11). Incubation-induced subcytotoxic
stress with potentially harmful molecules, such as hydrogen
peroxide (H2O2) brought the cells into a
state similar to senescence, termed stress-induced premature
senescence (SIPS) (12–14). Therefore, in this study, the
age-dependent changes in the cell number in cultured mouse gingival
fibroblasts (MGFs), as well as the changes in the biological
behavior subsequent to treatment with H2O2 in
the MGFs were investigated.
Materials and methods
Animals
BALB/c mice were used to investigate the age-related
changes in the cell number in MGFs in 10-, 15-, 30- and 52-week-old
mice (n=3 per group). In addition, 10-week-old mice were used for
the oxidative stress experiment (n=3 per group). The animals were
bred in a strictly monitored air-conditioned clean room and were
fed standard laboratory pellets and water ad libitum. The
experiment was performed according to the guidelines of the Animal
Center at the Kyushu University.
Gingival fibroblast cultures
The animals were sacrificed using an excessive
amount of ether. The gingival tissues were removed, immediately
washed in phosphate-buffered saline (PBS) with gentamicin (10
μg/ml; Invitrogen, Carlsbad, CA, USA) and transferred into a
culture dish. The gingival fibroblasts were grown in α-MEM,
supplemented with 10% fetal bovine serum (FBS), 100 IU/ml
penicillin and 100 μg/ml streptomycin in a humidified atmosphere
with 5% CO2 at 37°C. The medium was changed every other
day. The cells were grown to semi-confluence, harvested by
trypsinization at 37°C for 5 min, then subcultivated with culture
medium in a new dish. The experiments were performed using
early-passage fibroblasts before the fourth passage.
Cell growth assay
MGFs (1.0×104 cells/well) were seeded
onto 12-well plates in the α-MEM with serum. The cell numbers in
three-wells of each group were counted chronologically at 2, 4, 6
and 8 days after cultivation. The results were expressed as the
mean ± SD.
Oxidative stress
At 48 h after seeding, MGFs were exposed to
oxidative stress for 2 h. Various concentrations of
H2O2 were diluted in α-MEM with 10% FBS.
Subsequent to treatment with H2O2, the
cultures were rinsed twice with PBS and incubated in α-MEM with 10%
FBS. Some samples were stained with nuclear fast red to observe
cellular and nuclear morphological changes in the MGFs after the
oxidative stress.
Senescence-associated β-galactosidase
(SA-β-Gal) activity
MGFs were cultured for 14 days subsequent to
treatment with H2O2. The SA-β-Gal-positive
cell ratios were determined in three wells, using a senescence
detection kit, according to the manufacturer’s instructions. To
avoid any non-specific staining due to confluence, SA-β-Gal
cytochemical staining was performed on non-confluent cells.
Semi-quantitative reverse transcriptase
polymerase chain reaction (RT-PCR)
A semi-quantitative RT-PCR analysis was carried out.
Total-RNA extracted from cultured mouse gingival cells was isolated
using the SV total-RNA isolation system, according to the
manufacturer’s instructions. cDNA was generated from isolated
total-RNA by reverse transcription (RT) with SuperScript III and
subjected to PCR amplification with the specific primer sets
(Table I).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the
internal RNA control for the comparison of RNA levels in each
sample. The PCR products were separated by electrophoresis on
agarose gels and then stained with ethidium bromide.
 | Table I.Primer sets used for
semi-quantitative RT-PCR. |
Table I.
Primer sets used for
semi-quantitative RT-PCR.
| Target | Sequence |
|---|
| p53 | F: 5′-GGA AAT TTG
TAT CCC GAG TAT CTG-3′ |
| R: 5′-GTC TTC CAG
TGT GAT GAT GGT AA-3′ |
| p21 | F: 5′-TGT CCA ATC
CTG GTG ATG TC-3′ |
| R: 5′-TCT CTT GCA
GAA GAC CAA TCT G-3′ |
| Mdm2 | F: 5′-CCA GGC CAA
TGT GCA ATA C-3′ |
| R: 5′-GTG AGC AGG
TCA GCT AGT TGA A-3′ |
| GAPDH | F: 5′-ACC ACA GTC
CAT GCC ATC AC-3′ |
| R: 5′-TCC ACC ACC
CTG TTG CTG TA-3′ |
Immunoblotting for the p53
phosphorylation status
An immunoblot analysis for p53 was carried out using
the proteins isolated from the cultured MGFs after the treatment
with 20 μM H2O2 for 2 h. These cells were
lysed in RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% Triton
X-100, 1 mM EDTA pH 8.0, 0.1% SDS), supplemented with protease
inhibitor cocktail (50 μM), lactacystin (20 μM) and PMSF. The
protein samples were separated using SDS-PAGE, transferred to an
Immun-Blot® PVDF Membrane, and immunoblotted with
anti-p53 antibody (sc-6243; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and anti-phospho-p53 antibody (S15; R&D Systems,
Inc., Minneapolis, MN, USA). The membrane was incubated with
suitable secondary antibody conjugated with horseradish peroxidase.
Immunoreactive proteins were visualized using an ECL detection
system. Any emitted light was detected using a cooled CCD-camera
(LAS-1000; Fujifilm, Tokyo, Japan).
Statistical analysis
The experiments were repeated three times for the
independent MGF samples. A statistical analysis was performed
combining one-way ANOVA with the Tukey-Kramer comparison test or
Student’s t-test. P-values <0.05 or 0.01 were considered to
indicate statistically significant differences.
Results
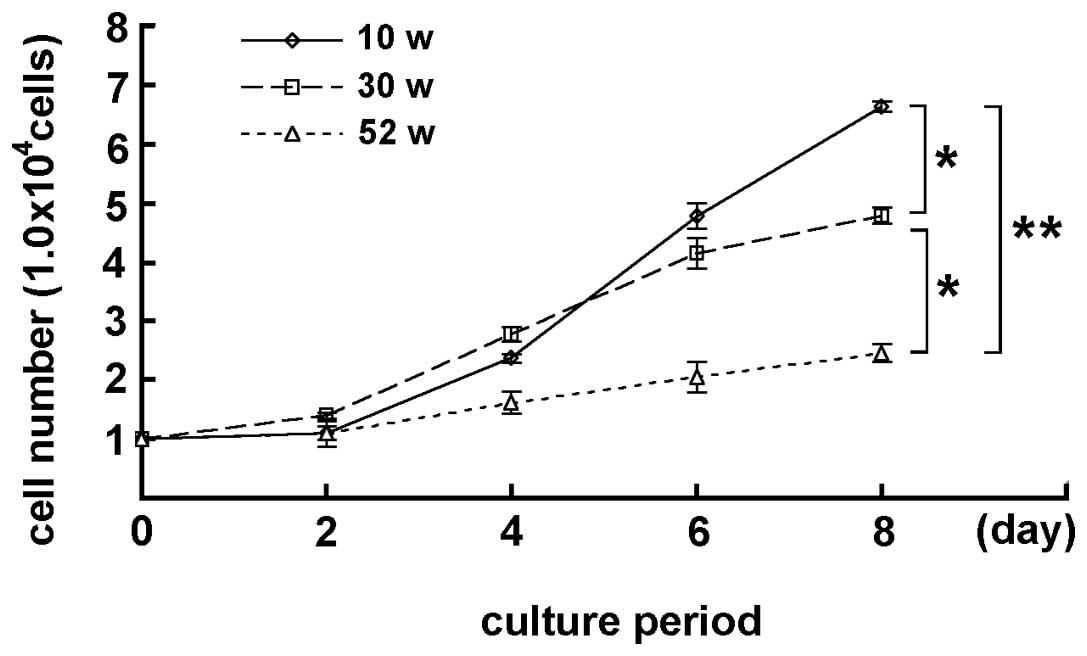
Age-dependent change in the cell number
in MGFs
MGFs isolated from 10-, 30- and 52-week-old mice
were cultured for 2, 4, 6 and 8 days. Although the MGFs showed cell
proliferation while cultured, the number of MGFs was different on
each culture day, depending on the age of the mouse, from which the
sample was derived. Marked differences were detected in the cell
number in 10- and 30-week-old, and 30- and 52-week-old mice
(P<0.05) at day 8 of the culturing. A statistically significant
difference was also observed between 10- and 52-week-old mice
(P<0.01), indicating that the increase in the number of cultured
MGFs was apparently lower in samples from older mice (Fig. 1). In the subsequent oxidative
stress experiments MGFs derived from 10-week-old mice were
used.
Effects of oxidative stress on the
morphological change in MGFs subsequent to
H2O2 treatment
MGFs were treated with various concentrations of
H2O2. The MGFs were observed under the
microscope after 24 h. Non-treated MGFs showed a fibroblastic
spindle shape. As seen in previously-reported SIPS-like changes,
the H2O2-treated cells had a round shape with
enlarged nucleus and expanded cytoplasm (Fig. 2) (13,14). The detachment of the cells from
the culture dish also increased in an H2O2
concentration-dependent manner, indicating that cell death occurred
in the MGFs.
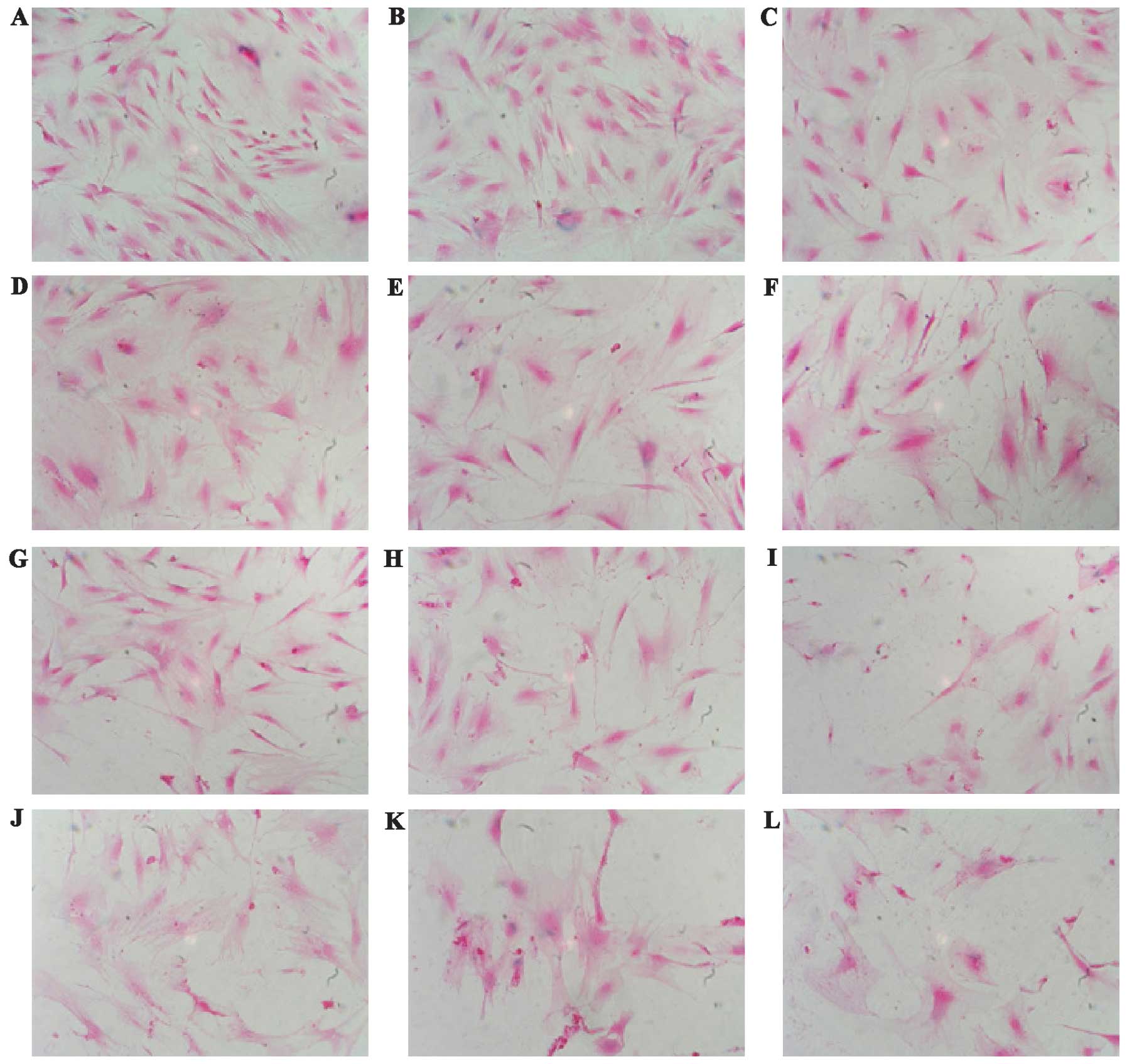 | Figure 2.Morphological change in MGFs
subsequent to treatment with H2O2 is shown.
MGFs were (A) non-treated and (B–L) treated with various
concentrations of H2O2.
H2O2-treated cells showed a round shape with
enlarged nuclei and expanded cytoplasm. A decrease in the cell
number was evident in the H2O2-treated
samples. (A) Non-treated, (B) 50 μM, (C) 100 μM, (D) 150 μM, (E)
200 μM, (F) 250 μM, (G) 300 μM, (H) 350 μM, (I) 400 μM, (J) 450 μM,
(K) 500 μM and (L) 550 μM. |
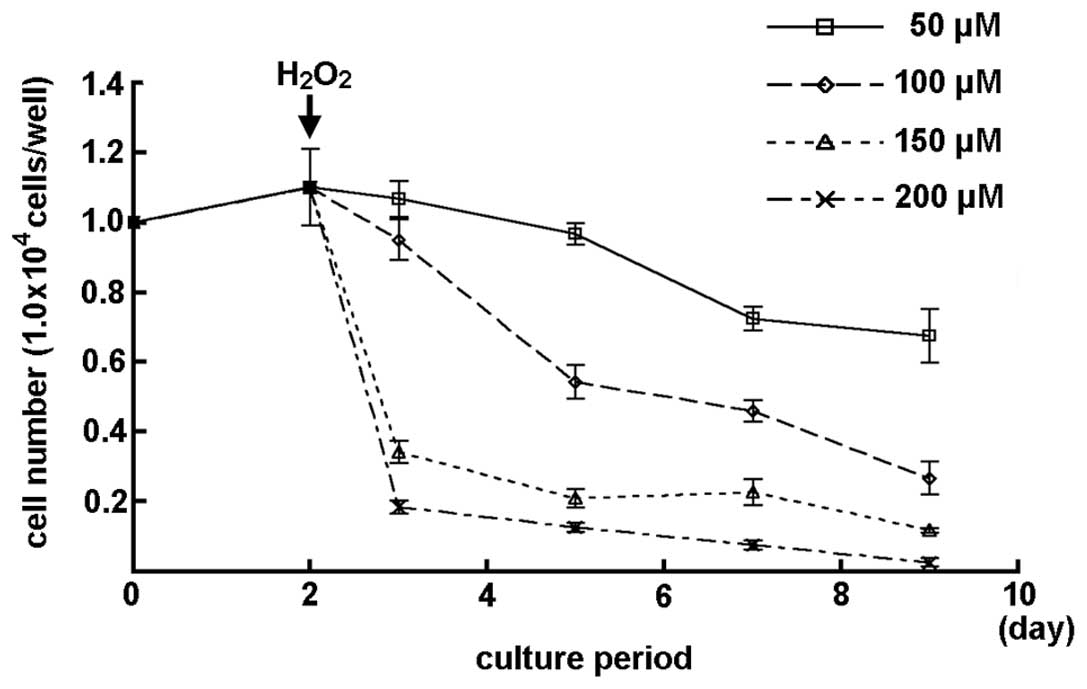
Effects of oxidative stress on the cell
number of MGFs subsequent to H2O2
treatment
The number of MGFs subsequent to treatment with
various concentrations of H2O2 is shown in
Fig. 3. A slight decrease in the
cell number was observed in the MGFs treated with 50 μM
H2O2. A marked decrease in the cell number of
MGFs was detected subsequent to treatment with 100 μM or higher
concentrations of H2O2 (Fig. 3).
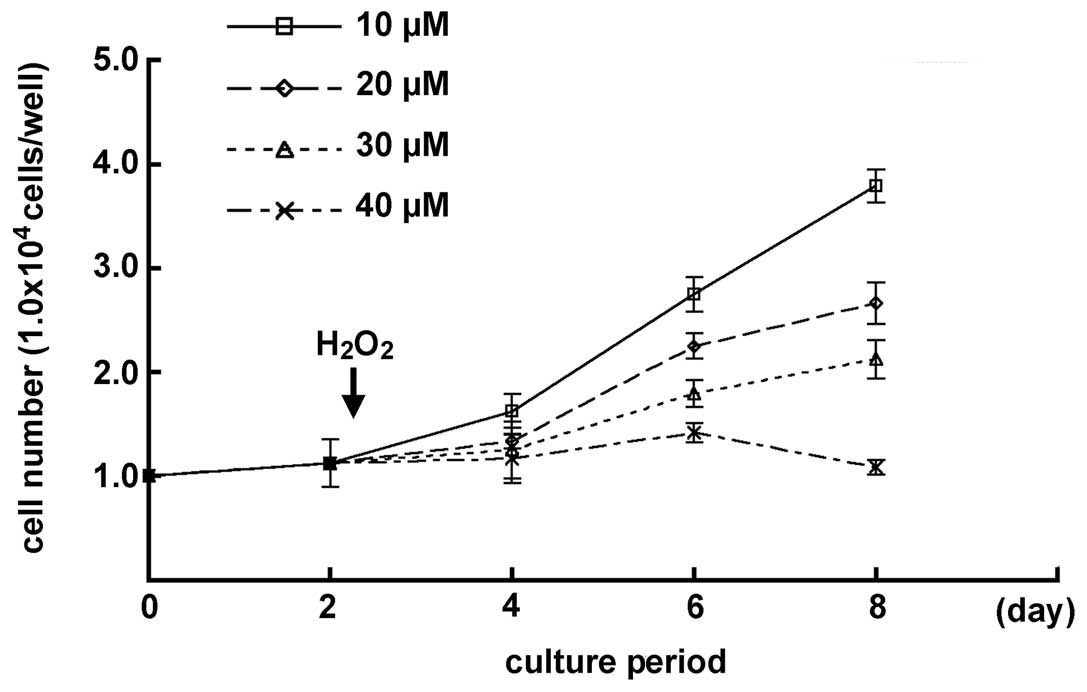
Effect of lower concentrations of
H2O2 on the cell growth in MGFs
The effects of low H2O2
concentrations on the MGFs were examined, since the cell number of
MGFs decreased gradually when treated with 50 μM
H2O2. A chronological increase in the cell
number was demonstrated in samples with 10, 20 and 30 μM
H2O2-treatment. MGFs treated with 20 μM
H2O2 showed a chronological cell growth
curve, similar to the one seen in the 52-week-old mice. By
contrast, no significant change was detected in the cell number of
the samples subsequent to treatment with 40 μM
H2O2 by day 6 of culturing, while a decreased
number of cells was observed at day 8. Statistically significant
differences were demonstrated in each sample at day 8 of culturing
(P<0.05) (Fig. 4).
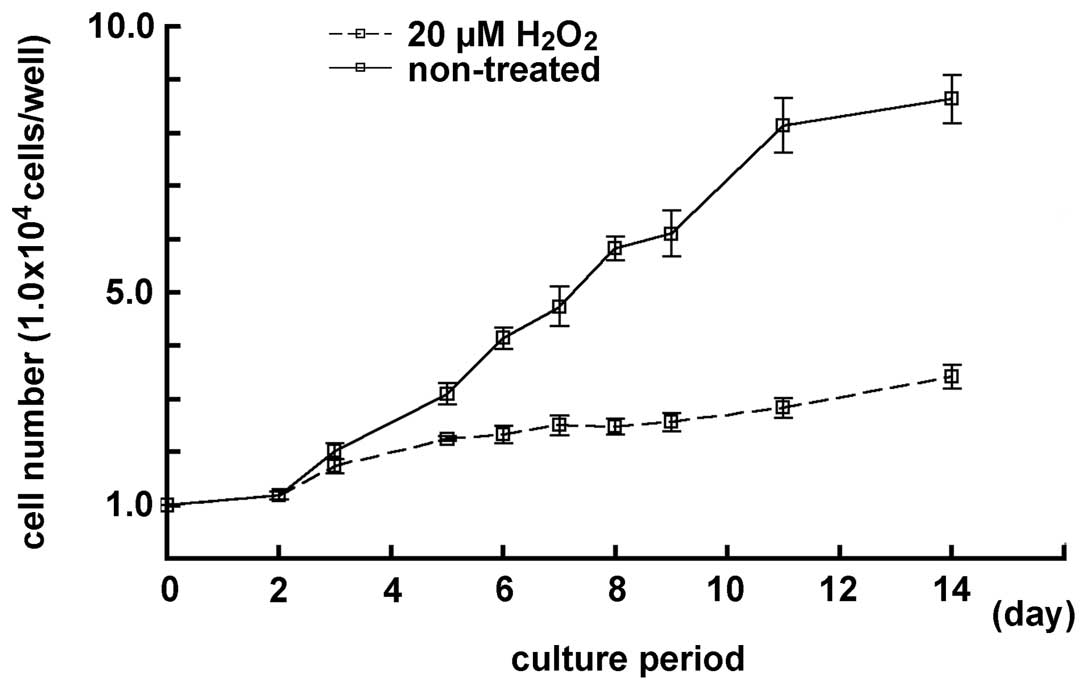
Effect of 20 μM concentration of
H2O2 treatment on the cell growth in MGFs in
a prolonged culturing
The cell number in the samples treated with 20 μM
H2O2 was examined and compared with the cell
number in the non-treated samples, for a period prolonged by 14
days. After 8 days of culturing, the cell number in samples treated
with a lower concentration of H2O2 decreased
in the cultured MGFs, and a low cell number increase-ratio was
observed. There was a notable difference between the non-treated
and the treated samples (P<0.05) (Fig. 5). Furthermore,
H2O2-treated cells had a round shape with
enlarged nucleus and expanded cytoplasm, as observed in the
senescent fibroblasts. Therefore, MGFs treated with 20 μM
H2O2 seemed to mimic the age-dependent
decrease of the proliferative activity in the 52-week-old mice.
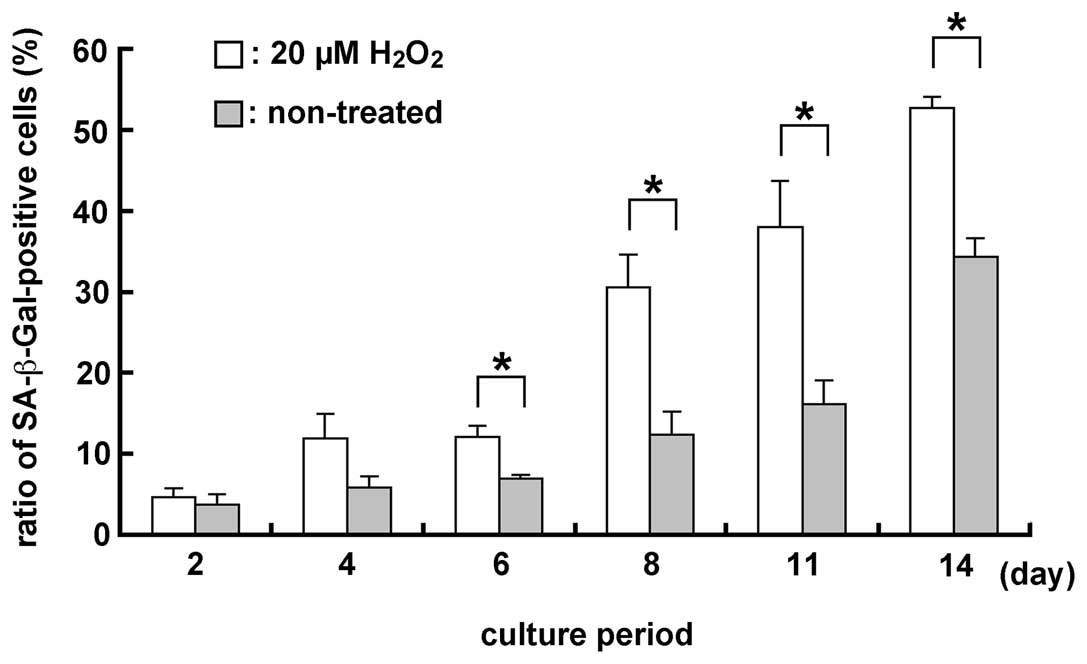
Detection of SA-β-Gal in the MGFs using a
20 μM H2O2 treatment
The ratio of SA-β-Gal (a senescence marker)-positive
cells was examined in H2O2-treated and
non-treated samples, since oxidative stress after
H2O2 treatment induced senescence-like
morphological and functional changes. Two days after the treatment,
the ratio of SA-β-Gal-positive cells was higher in
H2O2-treated compared to non-treated samples
(Fig. 6). Significant differences
were noted in the number of SA-β-Gal-positive cells in 20 μM
H2O2-treated and non-treated samples
(P<0.05), subsequent to culture for 6, 8, 11 and 14 days.
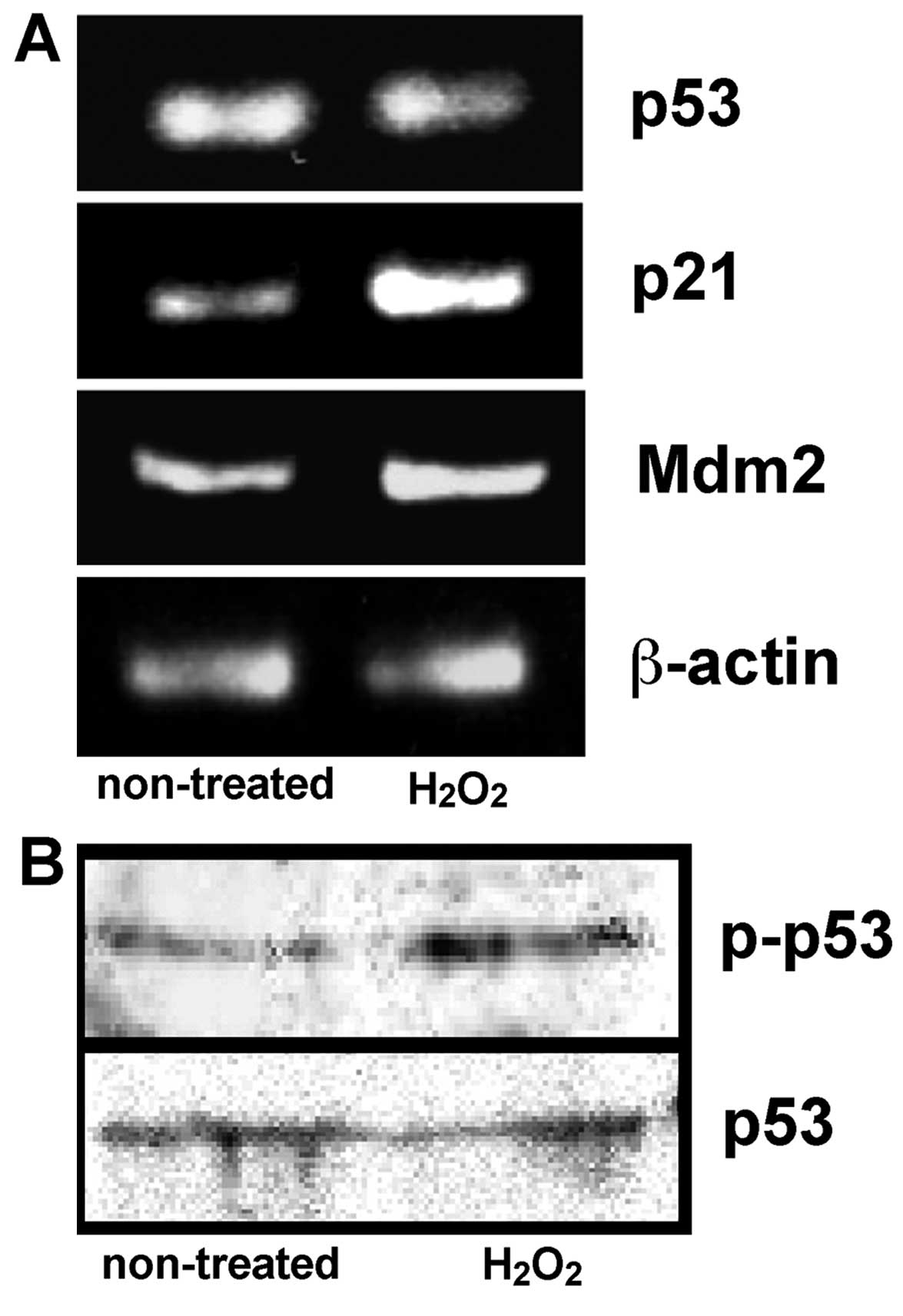
Expression of p53, p21 and Mdm2 mRNA, and
the phosphorylation of p53 protein in the culture MGFs
Oxidative stress induced senescence-like changes in
the MGFs, and therefore the expression of p53 having the potential
to initiate cell cycle arrest or cell death (15,16) was further examined. In addition,
we examined the expression of p53 downstream genes,
cyclin-dependent kinase inhibitor p21 and Mdm2 (17) was examined. The phosphorylated p53
protein was detected using an antibody specifically recognizing
human, monkey and rat p53 phosphorylated at the serine-15 and the
comparable phosphorylated site in mouse p53 (serine-18). By
contrast, unphosphorylated p53 is not detected by the antibody. No
significant change regarding the mRNA and protein expression of p53
was detected between the non-treated MGFs and MGFs treated with 20
μM H2O2, whereas an increased expression of
phosphorylated p53 protein was observed in the samples treated with
20 μM H2O2. An increased expression of p21
mRNA was demonstrated in the same sample, thus indicating the
induction of increased p21 expression by phosphorylated p53
protein. The Mdm2 mRNA was also increased in the cultured MGFs
treated with 20 μM H2O2 (Fig. 7).
Discussion
The present study demonstrated that oxidative stress
generated by treatment with a lower concentration of
H2O2 induced a decrease in the cell number in
the cultured MGFs with an increased ratio of SA-β-Gal-positive
cells, a marker of cellular senescence. Oxidative stress generated
by externally added H2O2 induces SIPS in
variety of cell types (13,14,18). This study demonstrated, for the
first time, that SIPS in gingival fibroblasts was induced by a
lower-concentration H2O2-treatment than
previously reported. Previous reports have proven a low
concentration of H2O2 treatment to induce a
higher proliferative activity in human dorsal fibroblasts, rabbit
lens epithelial cells, baby hamster kidney fibroblasts and
embryonic Chinese hamster ovary fibroblasts (19–21). Therefore, it is reasonable to
assume that the gingival fibroblast is more sensitive to
H2O2 oxidative stress.
Oxidative stress is one of the most important
causative factors for the induction of pathological states,
including periodontitis (22–26). Periodontal tissue tends to
deteriorate under persistent oxidative stress induced by
inflammatory reactions in the microflora of the oral cavity
(27). A previous report
demonstrated that gingival fibroblast and dorsal fibroblast had
different cellular properties, such as the expression of integrin
and extracellular matrix receptors. Consequently, experiments using
dorsal fibroblasts are not necessarily valid for oral tissues as
well (28). These different
cellular properties appear to be correlated with higher sensitivity
to oxidative stress, thus resulting in the induction of SIPS.
The p53 gene expression was examined since gingival
fibroblasts appeared to be more sensitive to oxidative stress. p53
activation is closely correlated with cell cycle arrest and
apoptosis when cells deteriorate due to pathogenic stress. There
was no change in the level of p53 mRNA and protein expression shown
by the semi-quantitative RT-PCR analysis and western blotting,
respectively. However, western blotting demonstrated an increase of
phosphorylated p53 protein. Therefore, an increased p21 expression
level, suppressing the CDK4- and CDK6-activation, is considered to
be induced by the increase of phosphorylated p53 protein, resulting
in a decrease of cell number in the MGFs treated with a lower
concentration of H2O2. The Mdm2 expression
was also activated at the transcription level. These results
suggest that Mdm2 functions as a negative feedback regulator to
maintain p53 at a low level under oxidative stress, since the major
function of Mdm2 is to interact with p53 and thereby induce the
ubiquitination and degradation of p53 (29).
In this study, H2O2-induced
oxidative stress generated an increase in the phosphorylated p53
protein-level at serine-18 in the MGFs. The phosphorylation at this
site in mouse p53 is comparable to that of human p53 at serine-15.
Phosphorylation of human p53 at serine-15 has a significant role in
its reduced interaction with the Mdm2, its negative regulator, and
is involved in the impairment of the Mdm2 function by inhibiting
p53-dependent transactivation (30). Therefore, the serine-18 of mouse
p53 protein may be one of the critical phosphorylation sites that
lead to cell cycle arrest and SIPS under oxidative stress. However,
the H2O2-mediated protein kinases involved in
p53 phosphorylation remain unknown. Nevertheless, these results do
not exclude the possibility that phosphorylation at other sites on
p53 may be associated with the occurrence of these phenomena.
Therefore, gingival fibroblasts are more sensitive
to oxidative stress, resulting in cell cycle arrest due to an
increase of phosphorylated p53 protein. This characteristic of the
gingival fibroblasts may be associated with the marked
age-dependent decrease of cellular components in the periodontal
tissue described in previous studies (7–9),
as well as with the development of periodontal diseases. In order
to develop new preventive methods against various periodontal
diseases, additional investigations regarding the molecular
interactions within the gingival fibroblasts in association with
oxidative stress are required.
Acknowledgements
This study was funded in part by
Grant-in-Aid from the Ministry of Education, Culture, Sports,
Science and Technology of Japan nos. 20390466 and 23659880 to H.S.
and no. 23659859 to T.K.
References
|
1.
|
AM JoaquinS GollapudiFunctional decline in
aging and disease: a role for apoptosisJ Am Geriatr
Soc4912341240200110.1046/j.1532-5415.2001.04990.x11559385
|
|
2.
|
U van der VeldenThe onset age of
periodontal destructionJ Clin Periodontol1838038319911890216
|
|
3.
|
PN PapapanouJ LindheJD SterrettL
EnerothConsiderations on the contribution of aging to loss of
periodontal tissue supportJ Clin
Periodontol18611615199110.1111/j.1600-051X.1991.tb00098.x1795058
|
|
4.
|
BA BurtEpidemiology of dental diseases in
the elderlyClin Geriatr Med844745919921504937
|
|
5.
|
BA BurtPeriodontitis and aging: reviewing
recent evidenceJ Am Dent
Assoc125273279199410.14219/jada.archive.1994.00348157839
|
|
6.
|
H OkamuraM YamaguchiY AbikoEnhancement of
lipopolysaccharide-stimulated PGE2 and IL-1beta production in
gingival fibroblast cells from old ratsExp
Gerontol34379392199910.1016/S0531-5565(99)00006-610433392
|
|
7.
|
T SakaiY OhsakiM KidoM GotoY TeradaH
SakaiThe distribution of fibronectin and laminin in the murine
periodontal membrane, indicating possible functional roles in the
apical migration of the junctional epitheliumArch Oral
Biol41885891199610.1016/S0003-9969(96)00014-3
|
|
8.
|
T SakaiT KiyoshimaI KobayashiR MoroiT
IbukiM NagadomeY TeradaH SakaiAge-dependent changes in the
distribution of BrdU- and TUNEL-positive cells in the murine
gingival tissueJ
Periodontol70973981199910.1902/jop.1999.70.9.97310505799
|
|
9.
|
N EnokiT KiyoshimaT SakaiI KobayashiK
TakahashiY TeradaH SakaiAge-dependent changes in cell proliferation
and cell death in the periodontal tissue and the submandibular
gland in mice: a comparison with other tissues and organsJ Mol
Histol38321332200710.1007/s10735-007-9105-617578672
|
|
10.
|
NJ HolbrookS IkeyamaAge-related decline in
cellular response to oxidative stress: links to growth factor
signaling pathways with common defectsBiochem
Pharmacol649991005200210.1016/S0006-2952(02)01169-312213598
|
|
11.
|
N MiyoshiH OubrahimPB ChockER
StadtmanAge-dependent cell death and the role of ATP in hydrogen
peroxide-induced apoptosis and necrosisProc Natl Acad Sci
USA10317271731200610.1073/pnas.051034610316443681
|
|
12.
|
O ToussaintP DumontJF DierickT PascalC
FrippiatF ChainiauxJP MagalhaesF EliaersJ RemacleStress-induced
premature senescence as alternative toxicological method for
testing the long-term effects of molecules under development in the
industryBiogerontology1179183200010.1023/A:1010035712199
|
|
13.
|
C FrippiatQM ChenS ZdanovJP MagalhaesJ
RemacleO ToussaintSubcytotoxic H2O2 stress
triggers a release of transforming growth factor-beta 1, which
induces biomarkers of cellular senescence of human diploid
fibroblastsJ Biol Chem27625312537200111060295
|
|
14.
|
C FrippiatJ DewelleJ RemacleO
ToussaintSignal transduction in H2O2-induced
senescence-like phenotype in human diploid fibroblastsFree Radic
Biol Med3313341346200212419465
|
|
15.
|
KH Vousdenp53: death
starCell103691694200010.1016/S0092-8674(00)00171-911114324
|
|
16.
|
S HauptM BergerZ GoldbergY HauptApoptosis
- the p53 networkJ Cell
Sci11640774085200310.1242/jcs.0073912972501
|
|
17.
|
Y BarakT JuvenR HaffnerM OrenMdm2
expression is induced by wild type p53 activityEMBO
J1246146819938440237
|
|
18.
|
GP DimriX LeeG BasileM AcostaG ScottC
RoskelleyEE MedranoM LinskensI RubeljO Pereira-SmithA biomarker
that identifies senescent human cells in culture and in aging skin
in vivoProc Natl Acad Sci
USA9293639367199510.1073/pnas.92.20.93637568133
|
|
19.
|
RH BurdonD AllianganaV GillHydrogen
peroxide and the proliferation of BHK-21 cellsFree Radic
Res23471486199510.3109/107157695090652687581830
|
|
20.
|
RH BurdonV GillD AllianganaHydrogen
peroxide in relation to proliferation and apoptosis in BHK-21
hamster fibroblastsFree Radic
Res248193199610.3109/107157696090880048845916
|
|
21.
|
AG WieseRE PacificiKJ DaviesTransient
adaptation of oxidative stress in mammalian cellsArch Biochem
Biophys318231240199510.1006/abbi.1995.12257726566
|
|
22.
|
S KimuraT YonemuraH KayaIncreased
oxidative product formation by peripheral blood polymorphonuclear
leukocytes in human periodontal diseasesJ Periodontal
Res28197203199310.1111/j.1600-0765.1993.tb01069.x
|
|
23.
|
SJ WeissTissue destruction by neutrophilsN
Engl J Med320365367198910.1056/NEJM1989020932006062536474
|
|
24.
|
C GuarnieriG ZucchelliF BernardiM SchedaAF
ValentiniM CalandrielloEnhanced superoxide production with no
change of the antioxidant activity in gingival fluid of patients
with chronic adult periodontitisFree Radic Res
Commun151116199110.3109/107157691090491201663065
|
|
25.
|
CC TsaiYP HoCC ChenLevels of interleukin-1
beta and interleukin-8 in gingival crevicular fluids in adult
periodontitisJ
Periodontol66852859199510.1902/jop.1995.66.10.8528537867
|
|
26.
|
Y RenJC MalthaMA Van’t HofJW Von Den
HoffAM Kuijpers-JagtmanD ZhangCytokine levels in crevicular fluid
are less responsive to orthodontic force in adults than in
juvenilesJ Clin
Periodontol29757762200210.1034/j.1600-051X.2002.290813.x12390573
|
|
27.
|
DV SculleySC Langley-EvansSalivary
antioxidants and periodontal disease statusProc Nutr
Soc61137143200210.1079/PNS200114112002788
|
|
28.
|
AA PalaiologouRA YuknaR MosesTE
LallierGingival, dermal, and periodontal ligament fibroblasts
express different extracellular matrix receptorsJ
Periodontol72798807200110.1902/jop.2001.72.6.798
|
|
29.
|
SY FuchsV AdlerT BuschmannX WuZ RonaiMdm2
association with p53 targets its
ubiquitinationOncogene1725432547198810.1038/sj.onc.12022009824166
|
|
30.
|
SY ShiehM IkedaY TayaC PrivesDNA
damage-induced phosphorylation of p53 alleviates inhibition by
MDM2Cell91325334199710.1016/S0092-8674(00)80416-X9363941
|