Introduction
The extracellular matrix (ECM) of bone, which
functions largely to provide biomechanical strength, is a complex
network composed of heterogeneous macromolecules, including a
number of small leucine-rich proteoglycans (SLRPs) (1). Biglycan (BGN), a member of the SLRP
family, consists of a 45 kDa protein core and 2 glycosaminoglycan
(GAG) chains, chondroitin sulfate (CS) and dermatan sulfate (DS),
which are covalently linked to the protein core (2). The CS/DS chains are attached at
amino acids 5 and 10 in the human BGN core protein (3). BGN is highly expressed in the ECM of
bone and is localized on the surface of osteoblasts (4).
BGN is multifunctional and is widely involved in
many biological processes. A noteble discovery is that BGN promotes
osteoblast differentiation. BGN knockout (KO) mice have an
age-dependent osteoporosis-like phenotype including a reduced
growth rate, lower bone mass due to decreased bone formation and
significantly shortened femurs (5–7).
BGN modulates osteoblast differentiation by regulating bone
morphogenetic protein-4 (BMP-4) signaling. Chen et al
(8) were the first to propose
that BGN modulates BMP-4-induced signaling to control osteoblast
differentiation. Their results showed that BGN deficiency affected
BMP-4 signal transduction, thus reducing core-binding factor α1
(Cbfa1) expression and in turn causing defective osteoblast
differentiation. BGN plays an important role as a reservoir for
balancing the growth factor activity of BMP-4 to control osteoblast
differentiation. The absence of BGN caused less BMP-4 binding and
subsequently reduced the sensitivity of osteoblasts to BMP-4
stimulation, which ultimately led to a defect in the
differentiation of osteoblasts (8). This function could be attributed to
the ability of BGN to bind BMP-4 and other molecules (8,9).
However, the domains of BGN that are involved in its positive
modulation of BMP-4 function remain unknown.
Recent research has focused on the GAG chains of
BGN. GAGs are highly negatively charged polysaccharides that
maintain the structural integrity and viscosity of the
extracellular environment (10,11). GAGs have been reported to be
associated with a variety of amyloid deposits (12,13). The DS GAGs of decorin are involved
in the organization of the collagen fibrils (14). Furthermore, GAGs affect cell
growth in both malignant and normal cells of the osteoblastic
lineage in a concentration-dependent manner (15). GAGs contribute to numerous
regulatory molecular networks through binding a variety of
signaling molecules, including cytokines, chemokines, growth
factors and differentiation factors (16). The present study investigated the
role of the GAGs of BGN in BGN-assisted BMP-4 function using BGN-KO
calvarial osteoblastic cells.
Materials and methods
Animals
Wild-type (WT) and biglycan knockout (BGN-KO) male
mice (C57B6/129) were purchased from the SLAC Laboratory Animal
Center (Shanghai, China). All animal care and experimental
procedures were approved by the Ethics Committee of Tongji Medical
College, Huazhong University of Science and Technology. Mice were
housed in polypropylene cages (32×40×18 cm) under controlled
temperature (22–24°C) and humidity with a 12-h light/dark cycle and
free access to food and water. All experiments were performed using
WT and BGN-KO mice (1–5 days old). The genotypes of the WT and
BGN-KO mice were confirmed by polymerase chain reaction (PCR) as
previously described (7).
Reagents
Human recombinant BMP-4 (hrBMP-4) was purchased from
R&D Systems, Inc. (Minneapolis, MN, USA). The bicinchoninic
acid (BCA) protein detection kit, chemiluminescent substrate kit
and FITC-conjugated goat anti-rabbit secondary antibody were
purchased from Pierce Chemical Co. (Thermo Fisher Scientific,
Waltham, MA, USA). The alkaline phosphatase (ALP) kit was purchased
from Sigma Chemical Co. (Sigma-Aldrich, USA). CLS-2 bacterial
collagenase was purchased from Worthington (Lakewood, USA).
Culture medium
The complete medium consisted of α-modified minimum
essential medium (Gibco-BRL, USA) supplemented with glutamine (2
mM), penicillin (100 U/ml), streptomycin (100 μg/ml) (Sigma),
2-mercaptoethanol (0.1 mM) and 10% fetal bovine serum (Gibco-BRL).
The differentiation medium consisted of the complete medium
supplemented with 2 mM β-glycerophosphate and 0.1 mM L-ascorbic
acid phosphate magnesium (Sigma).
Preparation of murine calvarial
cells
Neonatal murine calvarial cells were prepared as
previously described (17).
Briefly, calvariae harvested from WT or BGN-KO mice (1–5 days old)
were pretreated with 4 mM EDTA in phosphate-buffered saline (PBS)
for 2–10 min. The calvariae were digested with CLS-2 bacterial
collagenase at 200 U/ml in PBS for 5–10 min. Cells from the last 3
digestions were collected and served as the starting population of
highly enriched osteoblastic cells (18). The cells were washed twice with
complete culture medium, seeded into 6-well plates at a density of
1,000–2,000 cells/cm2 and cultured at 37°C in a
humidified atmosphere containing 5% CO2 in the presence
or absence of BMP-4 (30 ng/ml). Cultures were fed with the
differentiation medium twice a week once they reached confluence
(∼7 days). The cells were then collected from cultures at intervals
indicated in the following experiments.
Transfection of BGN-KO cells
Adenovirus strains without the BGN gene (Adv-Emp),
overexpressing wild-type BGN (Adv-BGN), or expressing GAG-mutant
BGN (Adv-BGNS5AS10A or Adv-BGNm) were purchased from GeneChem
(Shanghai, China). The BGN-KO cells were plated on 12-well plates
in triplicate at a density of 2×104 cells/well in
complete medium, cultured to 30% confluence and transfected either
with 3.75×107 PFU/ml of Adv-BGN, 3.75×107
PFU/ml of Adv-BGNm or 3.75×107 PFU/ml of recombinant adenovirus
without the BGN gene/cDNA (Adv-Emp) for 72 h. Then, the transfected
cells were tested as specified in the text herein.
Western blot analysis
For western blotting, confluent cells were cultured
in medium with 2% serum overnight and then treated with BMP-4 (30
ng/ml) for the duration specified. The cells were washed with PBS
and lysed in an extraction buffer (25 mM Tris-Cl, pH 7.2, 1% Triton
X-100, 0.1% SDS, 1% sodium deoxycholate, 0.1 M NaCl and 1 mM EDTA)
containing a protease inhibitor cocktail. Then, the lysate was
mixed with sample buffer containing 50 mM Tris/HCl (pH 7.6), 2%
SDS, 10% glycerol, 10 mM dithiothreitol and 0.2% bromophenol blue
and boiled for 5 min. The protein concentration in the supernatant
was determined using the BCA kit according to the manufacturer’s
instructions. The proteins were separated by SDS-PAGE (10% gel),
transferred to a nitrocellulose membrane and incubated with primary
antibody at 4°C overnight. Immune complexes were detected with the
appropriate secondary antibodies and enhanced chemiluminescence
(ECL) and quantitatively analyzed using Kodak Digital Science 1D
software (Eastman Kodak Company). The relative intensity was
expressed as the total optical density.
Measurement of alkaline phosphatase
activity
Confluent cells were incubated for 48 h in the
presence or absence (control) of 30 ng/ml BMP-4 in serum-free
medium and lysed in lysis buffer (20 mM Tris, 0.5 mM
MgCl2, 0.1 mM ZnCl2 and 0.1% Triton X-100).
The alkaline phosphatase (ALP) levels in the lysates were
determined using an ALP kit and the production of p-nitrophenol was
measured by spectrophoto-metric absorbance at 405 nm. The ALP value
was calculated using standards and expressed as Sigma U/mg of
protein lysate. One Sigma unit is equal to 1 μM
p-nitrophenol/h.
Immunofluorescence
Well-cut slides were placed into 12-well plates.
Cells were then seeded into each well at 1×104
cells/well in complete medium and cultured until confluence. To
detect BMP-4 binding, the cells were preincubated with BMP-4 (10
μg/ml) for 2 h at room temperature and washed with PBS 3 times. The
slides were removed from the plates, and the cells were fixed in 4%
phosphate-buffered formaldehyde for 30 min, then washed and blocked
in PBS with 0.1% BSA and 5% normal goat serum (blocking solution)
for 30 min at room temperature. To identify BMP-4-positive cells,
the slides were incubated with anti-BMP-4 antibody at 4°C
overnight, then washed and incubated with FITC-conjugated goat
anti-rabbit secondary antibody for 2 h at room temperature. The
cells were subsequently washed and incubated with Hoechst to stain
the nuclei. Slides were then imaged using a fluorescence microscope
(Olympus BX51; Tokyo, Japan), and the results were analyzed
quantitatively with Image-Pro® Plus (IPP) software.
Statistical analysis
Results are expressed as the mean ± standard
deviation (SD), calculated from 3 independent experiments. The
results were analyzed with SPSS 13.0 statistical software. A
one-way ANOVA procedure followed by least significant difference
post hoc tests was used to determine the statistical significance
of differences of the means. P<0.05 was considered to indicate
statistically significant differences.
Results
Characterization of adenovirus-mediated
expression of wild-type and mutant BGN in BGN-KO osteoblastic
cells
To test the adenovirus transfection efficiency and
the BGN expression in the osteoblasts, we measured the quantity of
BGN produced by osteoblasts in complete medium following adenoviral
transfection. To confirm that the BGN expression persisted for at
least 2 weeks, BGN synthesis was analyzed at Days 3, 6 and 14 using
western blot analysis with specific antibodies (Fig. 1A).
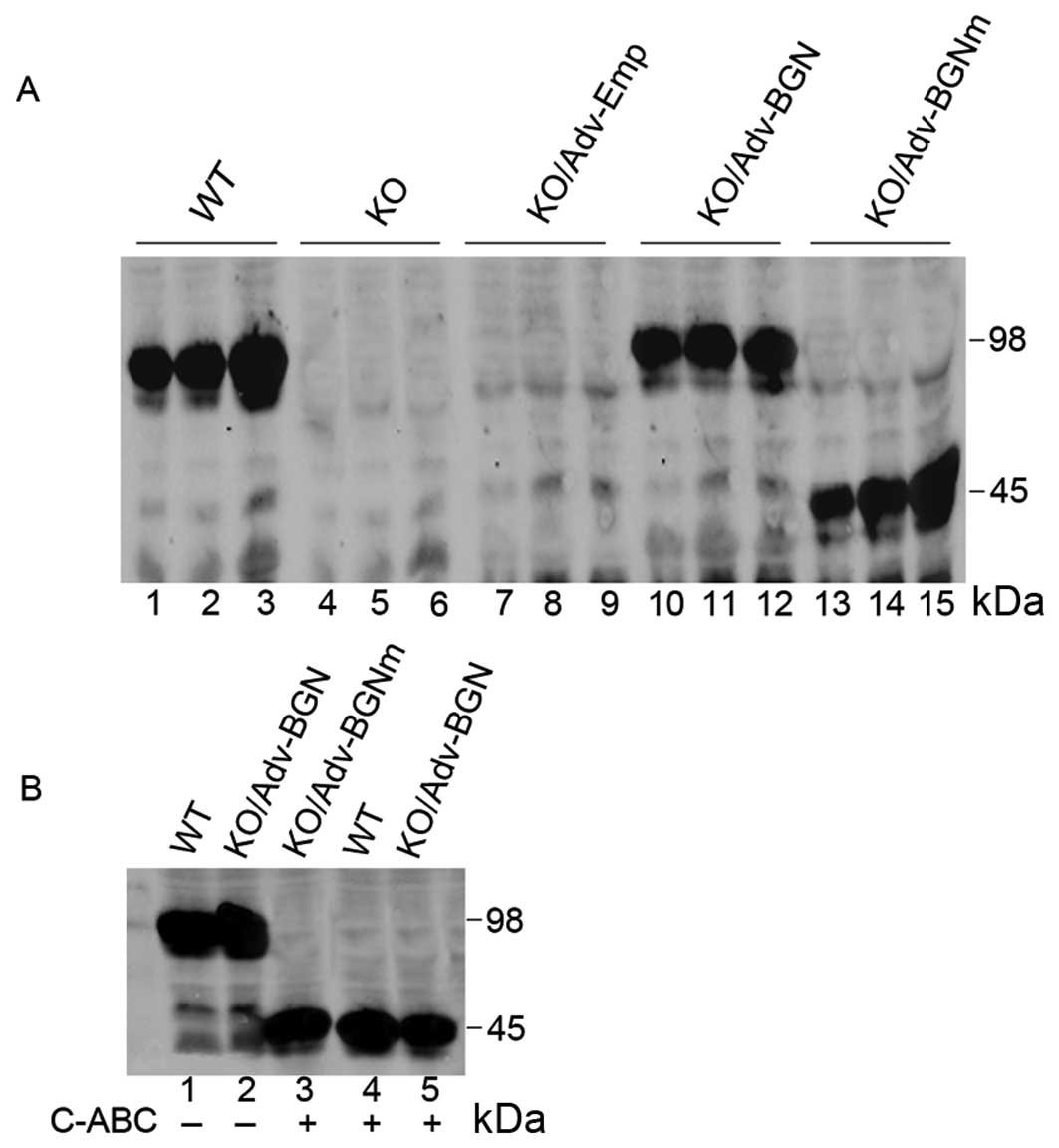 | Figure 1.Expression of wild-type BGN and
mutant BGN in Adv-BGN and Adv-BGNm-transfected BGN-KO osteoblastic
cells was evaluated using SDS-PAGE. (A) Lanes 1–3, positive
control: BGN expression in wild-type osteoblasts (Days 3, 6 and
14); lanes 4–6, blank control: BGN expression in BGN-KO osteoblasts
(Days 3, 6 and 14); lanes 7–9, negative control: BGN expression in
BGN-KO osteoblasts transfected with Adv-Emp (Days 3, 6 and 14);
lanes 10–12, BGN expression in BGN-KO osteoblasts transfected with
Adv-BGN (Days 3, 6 and 14); and lanes 13–15, BGN expression in
BGN-KO osteoblasts transfected with Adv-BGNm (Days 3, 6 and 14).
(B) Lane 1, BGN expression in wild-type osteoblasts (Day 6); lane
2, BGN expression in BGN-KO osteoblasts transfected with Adv-BGN
(Day 6); lane 3, BGN derived from Adv-BGNm transfected BGN-KO
osteoblasts (Day 6) treated with C-ABC lyase; lane 4, BGN derived
from wild-type osteoblasts (Day 6) treated with C-ABC lyase; and
lane 5, BGN derived from Adv-BGN-transfected BGN-KO osteoblasts
(Day 6) treated with C-ABC lyase. |
The expression level of BGN in BGN-KO osteoblastic
cells transfected with Adv-BGN was highest at Day 6 and began to
decrease at Day 14. However, the synthesis of mutant BGN in BGN-KO
osteoblastic cells transfected with Adv-BGNm increased continually
from Day 3 to 14. Furthermore, as judged by electrophoretic
mobility on SDS-PAGE, BGN produced from Adv-BGN-transfected BGN-KO
osteoblastic cells and endogenous BGN produced from WT osteoblasts
were processed similarly, suggesting that WT BGN was correctly
expressed in Adv-BGN-transfected BGN-KO osteoblastic cells.
Chondroitinase-ABC (C-ABC) lysates (0.3 U/100 μg)
were used to generate deglycated BGN. Following digestion with
C-ABC lyases, the BGN produced by Adv-BGN- and Adv-BGNm-transfected
BGN-KO cells or WT osteoblasts migrated similar distances on
SDS-PAGE (Fig. 1B), which
suggested that the mutant BGN lacking GAG chains was correctly
expressed in BGN-KO osteoblastic cells.
The expression of mutant BGN in BGN-KO
calvarial osteoblastic cells cannot rescue its differentiation
deficiency as efficiently as wild-type BGN
Several studies have indicated that Cbfa1 is an
osteoblast-specific transcription factor and a regulator of
osteoblast differentiation, controlling the expression of specific
extracellular matrix proteins and intra-cellular proteins,
including osteopontin, bone sialoprotein (BSP) and osteocalcin
(19–21). The expression of Cbfal is
downregulated in BGN-KO cells (22). In the present study, we measured
Cbfa1 expression in BGN-KO osteoblastic cells at the protein level
by western blot analysis and found that both Adv-BGN and Adv-BGNm
transfection enhanced Cbfal protein expression in BGN-KO
osteoblastic cells compared with the Adv-Emp control, either with
or without BMP-4 treatment. Notably, Cbfal expression in
Adv-BGN-transfected osteoblastic cells was significantly
upregulated compared to Adv-BGNm-transfected osteoblastic cells
after treatment with BMP-4. This result suggested that BGN,
particularly its GAG chains, is essential for BMP-4-induced Cbfal
expression (Fig. 2A and B).
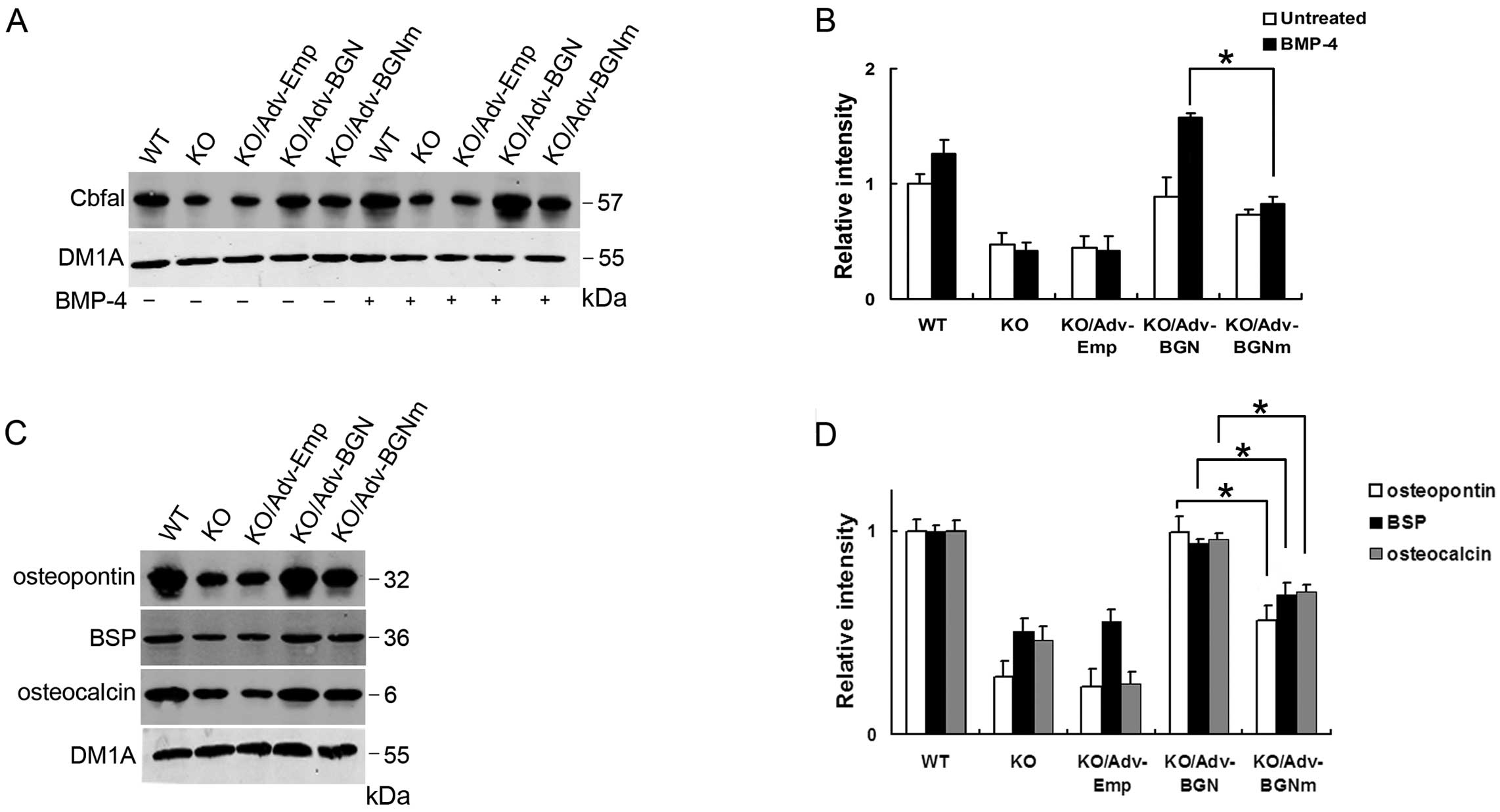 | Figure 2.Cbfal and related osteoblastic
markers were significantly upregulated in Adv-BGN-transfected
osteoblasts. (A and B) Lanes 1–5, Cbfal expression in WT, BGN-KO,
Adv-Emp-transfected, Adv-BGN-transfected and Adv-BGNm-transfected
osteoblasts without BMP-4 treatment; lanes 6–10, Cbfal expression
in WT, BGN-KO, Adv-Emp-transfected, Adv-BGN-transfected and
Adv-BGNm-transfected osteoblasts after BMP-4 treatment. Equal
protein loading is demonstrated by probing the same blot with a
monoclonal antibody against DM1A. The Cbfal expression level in the
Adv-BGN-transfected cells is significantly higher than that in the
Adv-BGNm-transfected cells (*P<0.05,
Adv-BGN/Adv-BGNm). (C and D) The expression of osteopontin, BSP and
osteocalcin were detected in WT, BGN-KO, Adv-Emp-transfected,
Adv-BGN-transfected and Adv-BGNm-transfected osteoblasts (lanes
1–5) after BMP-4 treatment. Equal protein loading is demonstrated
by probing the same blot with a monoclonal antibody against DM1A.
The osteopontin, BSP and osteocalcin expression is significantly
higher in Adv-BGN-transfected cells than in Adv-BGNm-transfected
cells *P<0.05, Adv-BGN/Adv-BGNm. |
We also evaluated the expression of osteoblastic
proteins in WT and BGN-KO osteoblastic cells transfected with
Adv-Emp, Adv-BGN and Adv-BGNm. Osteopontin, BSP and osteocalcin
expression were measured by western blot analysis. These results
showed that the expression of osteopontin, BSP and osteocalcin were
all downregulated in BGN-KO cells and Adv-Emp-transfected BGN-KO
cells compared with WT osteoblastic cells. Both Adv-BGN and
Adv-BGNm transfection can rescue the deficiency of these 3 specific
proteins in BGN-KO cells. However, the expression of these proteins
in the Adv-BGN-transfected group is significantly higher than that
in the Adv-BGNm-transfected group (Fig. 2C and D). DM1A was selected as the
internal control.
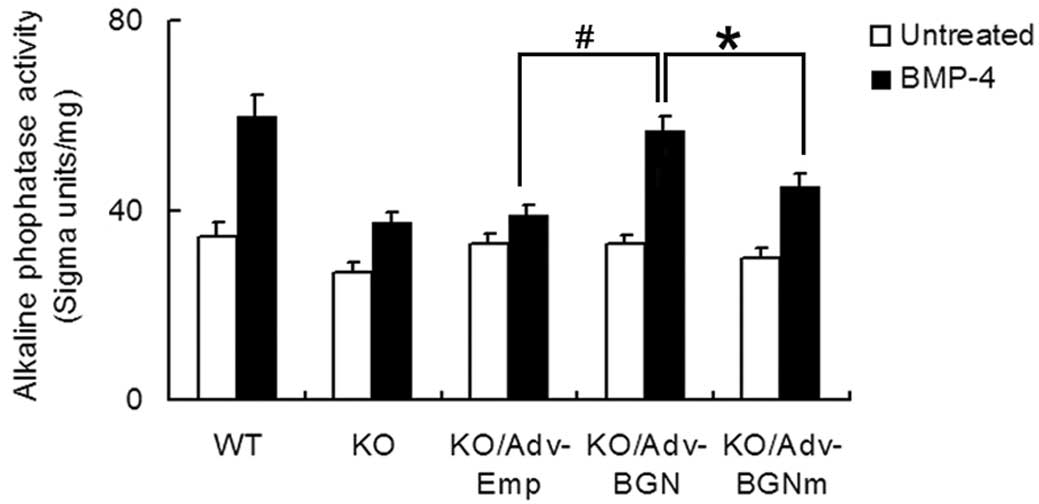
As an early osteogenic differentiation marker, ALP
activity was also analyzed using an ALP activity assay (Fig. 3). The ALP level of
Adv-BGN-transfected BGN-KO osteoblastic cells treated with BMP-4
increased 1.7-fold (from 33±1.7 to 57±3.0, P<0.01). The ALP
level in Adv-BGNmtransfected BGN-KO osteoblastic cells treated with
BMP-4 also increased 1.5-fold (from 30±2.0 to 45±2.6, P<0.01).
The ALP level before and after treatment with BMP-4 was highest in
WT osteoblastic cells than in all other groups. Collectively, these
data suggest that BMP-4-induced ALP expression in BGN-KO
osteoblastic cells could be partially rescued by Adv-BGN
transfection.
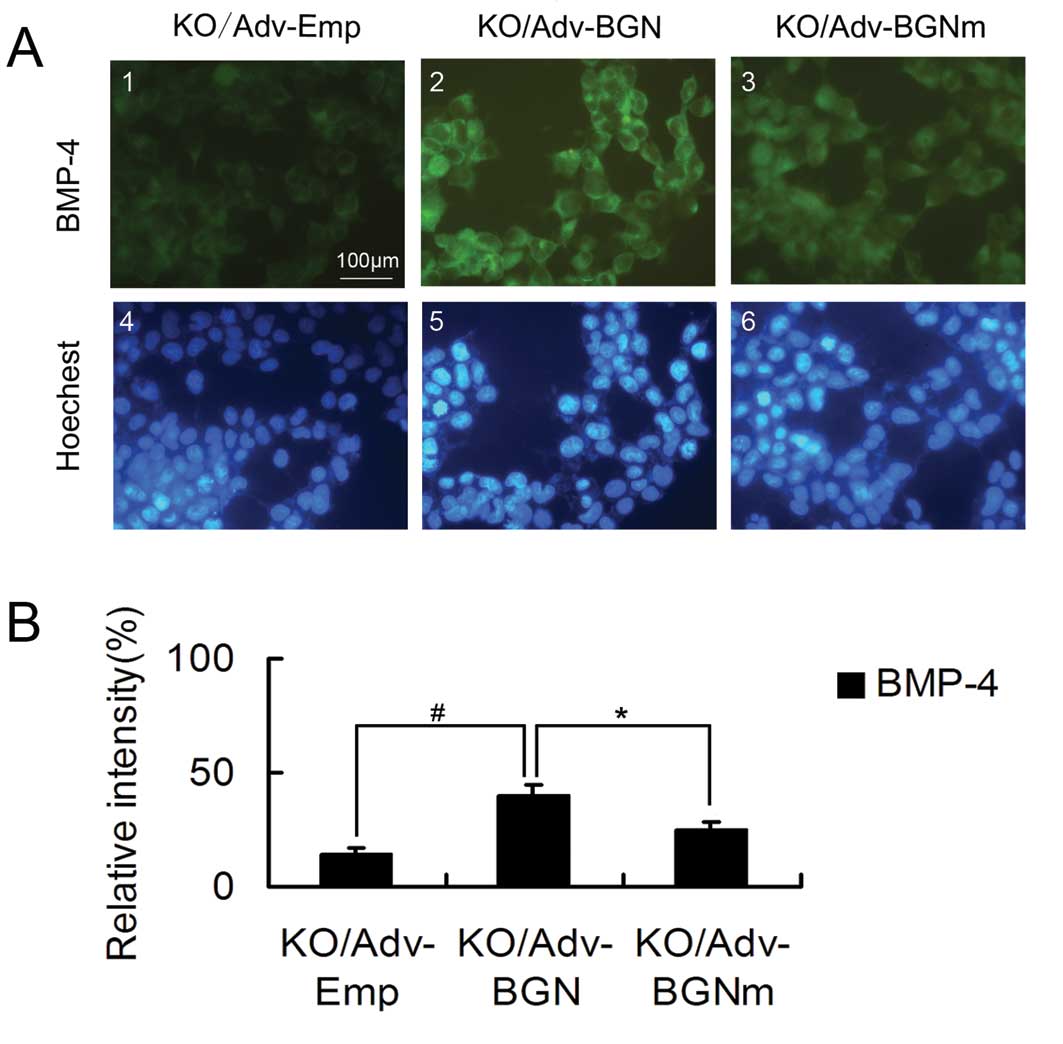
BGN-KO osteoblastic cells transfected
with Adv-BGN exhibit significantly greater BMP-4 binding than
BGN-KO cells transfected with Adv-BGNm
BGN-KO osteoblastic cells have a low affinity for
BMP-4 binding. Therefore, we investigated whether the ectopic
expression of BGN or mutant BGN could upregulate the binding of
BGN-KO cells to BMP-4. BMP-4-positive cells were stained using
immunofluorescence.
Specifically, cells were incubated with BMP-4 for 2
h. After washing with PBS, cells were stained with a fluorescently
labeled anti-BMP-4 antibody. The BGN-KO osteoblastic cells
transfected with Adv-BGN or Adv-BGNm had higher fluorescence
intensity than the BGN-KO cells transfected with Adv-Emp (Fig. 4).
However, transfection with Adv-BGNm did not enhance
the binding affinity of BGN-KO cells to BMP-4 as effectively as
transfection with Adv-BGN. Quantitative analysis showed that the
Adv-BGNm-transfected BGN-KO osteoblastic cells had less overall
fluorescence intensity (25 vs. 40%, P<0.05) than
Adv-BGN-transfected cells (Fig.
4). Endogenous BMP-4 was measured prior to the preincubation
with BMP-4 and only <1% of the untreated BGN-KO cells were
BMP-4-positive (data not shown). These results show that the GAG
chains of BGN play an important role in the binding of osteoblastic
cells to BMP-4 at the cellular level, which may explain the reason
why expression of mutant BGN in BGN-KO calvarial osteoblastic cells
could not rescue its differentiation deficiency as efficiently as
WT BGN.
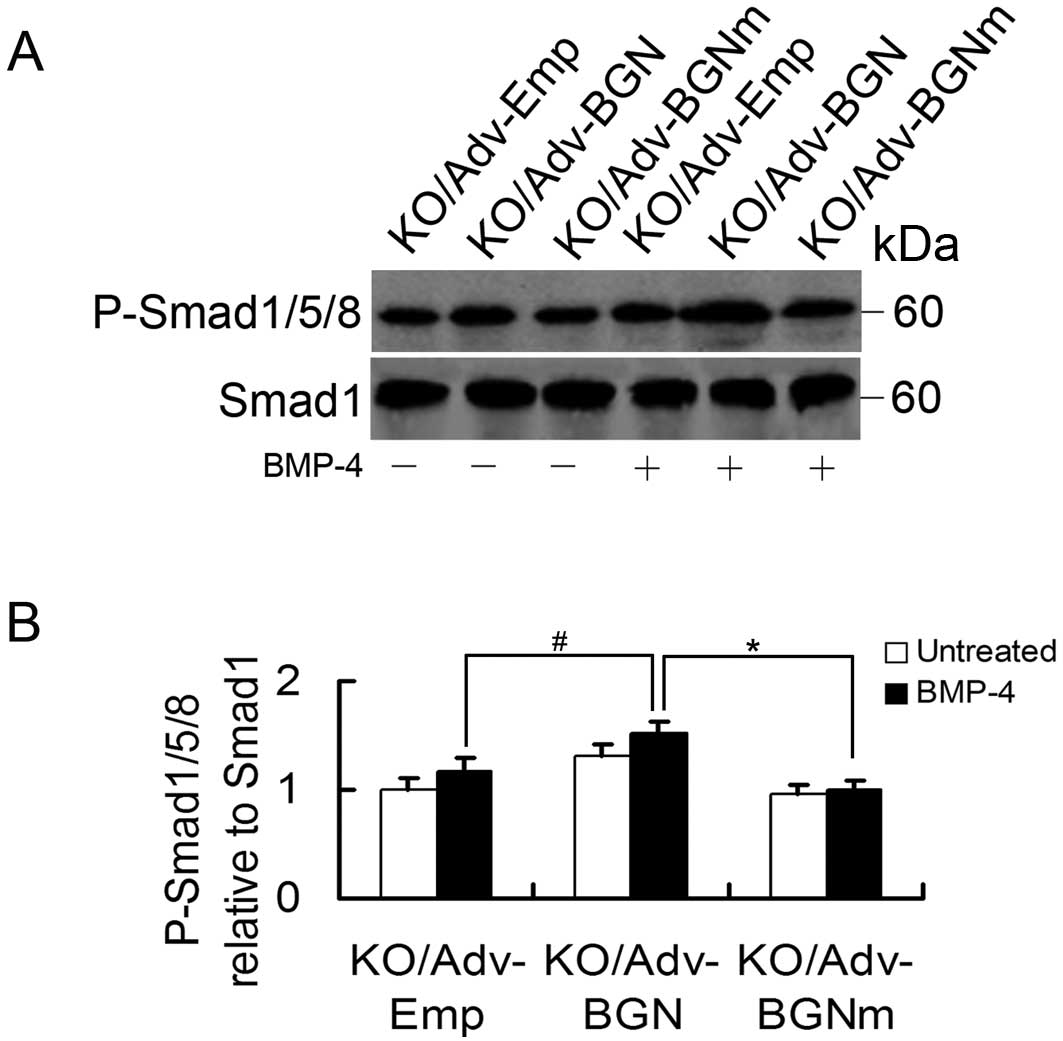
Effects of Adv-BGN on BMP-4-induced
signal transduction in BGN-KO osteoblastic cells
Finally, we examined BMP-4 signal transduction in
BGN-KO osteoblastic cells by measuring phosphorylated Smad1/5/8
(P-Smad1/5/8). To determine whether the level of P-Smad1/5/8 in
BGN-KO cells could be enhanced by the overexpression of BGN, BGN-KO
osteoblastic cells were transfected with Adv-Emp, Adv-BGN and
Adv-BGNm. Once the cells reached confluence, they were treated with
BMP-4 (30 ng/ml) for 30 min and P-Smad1/5/8 levels were measured by
western blot analysis. We found that the P-Smad1/5/8 level was
significantly increased in Adv-BGN-transfected BGN-KO osteoblastic
cells treated with BMP-4 compared to Adv-BGNm- or
Adv-Emp-transfected BGN-KO osteoblastic cells (Fig. 5).
Discussion
Although the ability of BGN to regulate osteoblast
differentiation has been known for many years, the details of the
underlying mechanism have not been fully elucidated. In the present
report, we studied the effects of GAG chains of BGN on the
BMP-4-induced osteoblast differentiation. Our results suggest that
the GAG chains of BGN act as a positive modulator of BGN activity
and promote BMP-4-induced osteoblast differentiation. In
particular, immunofluorescence revealed that the expression of BGN
in BGN-KO osteoblasts enhanced the cellular affinity to BMP-4
compared to cells expressing non-glycanated BGN (Adv-BGNm),
indicating the importance of GAG chains in promoting binding of
osteoblastic cells to BMP-4 and osteoblastic differentiation. In
vitro ALP activity and Smad1/5/8 phosphorylation demonstrated
that Adv-BGNm could not rescue BGN-assisted BMP-4 function and
signaling as efficiently as the glycanated BGN (Adv-BGN). It is
conceivable that the 2 long GAG chains accommodate more interaction
sites for the basic protein BMP-4, thus enhancing the interaction
between BGN and BMP-4. Notably, Adv-BGNm transfection of BGN-KO
osteoblastic cells treated with BMP-4 significantly enhanced the
ALP activity and binding affinity to BMP-4 compared with Adv-Emp
transfection, which suggests that other BGN components, such as
protein core, may also function to promote BMP-4-induced osteoblast
differentiation.
Previous reports support our findings; Miyazaki
et al (23) reported that
hypersulfated chondroitin sulfate (CS)-E binds to BMP-4 and
enhances osteoblast differentiation by increasing the level of
exogenous sulfated GAGs to MC3T3-E1 osteoblastic cells. It has also
been reported that collagen chondroitin sulfate promotes the in
vitro mineralization of 3-dimensional collagen matrices seeded
with bone-derived cells (24).
However, contrary to our present results, a recent report suggested
that the GAG component of BGN suppresses the BGN-assisted BMP-2
function in mouse C2C12 myoblastic cells (25). The apparently different functions
of GAGs in C2C12 myoblastic cells and BGN-KO osteoblastic cells
could be due to the fact that C2C12 myoblastic cells and BGN-KO
osteoblastic cells are at different differentiation stages. Another
possible explanation is that GAGs may have different effects on
different BMP members. The differences in testing methods and the
biological differences between these 2 cell lines should also be
considered as explanations for the discrepancies between these
results.
It is well known that the Smad pathway is active
during osteoblast differentiation. When BGN-KO osteoblastic cells
were transfected with Adv-BGN, Smad1/5/8 phosphorylation, ALP
activity, and the expression of the transcription factor Cbfa1 and
related proteins were all upregulated, indicating that GAGs may
facilitate osteoblast differentiation through Smad1/5/8
phosphorylation and Cbfa1 activity. This finding indicates that the
GAGs are essential for BGN to regulate Cbfa1 transcriptional
activity. A related report showed that glycanated BGN is able to
increase the phosphorylation of Smad1/5/8 (26). Markedly, it was reported that the
levels of non-glycanated forms of BGN increase with age in human
articular cartilage (27). A
similar trend was observed in both articular cartilage and
intervertebral discs. Therefore, we hypothesize that in early
development (i.e., infancy, childhood and adolescence), osteoblast
differentiation is quite active and that the body needs more
glycanated forms of BGN to enable BMP-4 to execute its normal
biological functions, which require the GAG chains of BGN.
Furthermore, we hypothesize that in adulthood, the body is fully
developed and osteoblast differentiation becomes relatively
inactive. Therefore, the GAG chains of BGN may be less essential
during adulthood than during childhood, and this could be reflected
by the increase in the levels of non-glycanated BGN.
In conclusion, our study demonstrated that GAG
chains increase BGN-assisted BMP-4 signaling and osteoblast
differentiation in BGN-KO osteoblastic cells, demonstrating the
positive effect of GAG chains of BGN on BMP-4-induced osteoblast
differentiation. This finding represents an important step towards
the development of new treatments for bone diseases and BGN-related
disorders.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (nos. 30650006 and
31070831) and the PhD Program Foundation of the Ministry of
Education of China (no. 20050487061).
References
|
1.
|
RV IozzoThe biology of the small
leucine-rich proteoglycansFunctional network of interactive
proteins J Biol Chem2741884318846199910383378
|
|
2.
|
L AmeyeMF YoungMice deficient in small
leucine-rich proteoglycans: novel in vivo models for osteoporosis,
osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and
corneal diseasesGlycobiology12107R116R200210.1093/glycob/cwf065
|
|
3.
|
PJ RoughleyRJ WhiteDermatan sulphate
proteoglycans of human articular cartilage. The properties of
dermatan sulphate proteoglycans I and IIBiochem
J26282382719892590169
|
|
4.
|
P BiancoLW FisherMF YoungJD TerminePG
RobeyExpression and localization of the two small proteoglycans
biglycan and decorin in developing human skeletal and non-skeletal
tissuesJ Histochem
Cytochem3815491563199010.1177/38.11.22126162212616
|
|
5.
|
MF YoungY BiL AmeyeXD ChenBiglycan
knockout mice: new models for musculoskeletal diseasesGlycoconj
J19257262200210.1023/A:102533611435212975603
|
|
6.
|
T XuP BiancoLW FisherTargeted disruption
of the biglycan gene leads to an osteoporosis-like phenotype in
miceNat Genet207882199810.1038/17469731537
|
|
7.
|
XD ChenS ShiT XuPG RobeyMF
YoungAge-related osteoporosis in biglycan-deficient mice is related
to defects in bone marrow stromal cellsJ Bone Miner
Res17331340200210.1359/jbmr.2002.17.2.33111811564
|
|
8.
|
XD ChenLW FisherPG RobeyMF YoungThe small
leucine-rich proteoglycan biglycan modulates BMP-4-induced
osteoblast differentiationFASEB
J18948958200410.1096/fj.03-0899com15173106
|
|
9.
|
M MorenoR MunozF ArocaM LabarcaE BrandanJ
LarrainBiglycan is a new extracellular component of the
Chordin-BMP4 signaling pathwayEMBO
J2413971405200510.1038/sj.emboj.760061515775969
|
|
10.
|
M BernfieldM GottePW ParkFunctions of cell
surface heparan sulfate proteoglycansAnnu Rev
Biochem68729777199910.1146/annurev.biochem.68.1.72910872465
|
|
11.
|
B CasuU LindahlStructure and biological
interactions of heparin and heparan sulfateAdv Carbohydr Chem
Biochem57159206200110.1016/S0065-2318(01)57017-111836942
|
|
12.
|
DG SeidlerR DreierDecorin and its
galactosaminoglycan chain: extracellular regulator of cellular
function?IUBMB Life60729733200810.1002/iub.11518800386
|
|
13.
|
NM TimmerHB KuiperijRM de WaalMM VerbeekDo
amyloid beta-associated factors co-deposit with Abeta in mouse
models for Alzheimer’s disease?J Alzheimers
Dis22345355201020847441
|
|
14.
|
JE ScottSupramolecular organization of
extracellular matrix glycosaminoglycans, in vitro and in the
tissuesFASEB J62639264519921612287
|
|
15.
|
D NikitovicA ZafiropoulosGN TzanakakisNK
KaramanosAM TsatsakisEffects of glycosaminoglycans on cell
proliferation of normal osteoblasts and human osteosarcoma cells
depend on their type and fine chemical compositionsAnticancer
Res2528512856200516080537
|
|
16.
|
M BouvierML CoubleDJ HartmannJP GauthierH
MagloireUltrastructural and immunocytochemical study of
bone-derived cells cultured in three-dimensional matrices: inf
luence of chondroitin-4 sulfate on
mineralizationDifferentiation45128137199010.1111/j.1432-0436.1990.tb00466.x2129117
|
|
17.
|
XD ChenHY QianL NeffK SatomuraMC
HorowitzThy-1 antigen expression by cells in the osteoblast
lineageJ Bone Miner
Res14362375199910.1359/jbmr.1999.14.3.36210027901
|
|
18.
|
TL McCarthyM CentrellaE CanalisFurther
biochemical and molecular characterization of primary rat parietal
bone cell culturesJ Bone Miner
Res3401408198810.1002/jbmr.56500304063265577
|
|
19.
|
T KomoriH YagiS NomuraTargeted disruption
of Cbfa1 results in a complete lack of bone formation owing to
maturational arrest of
osteoblastsCell89755764199710.1016/S0092-8674(00)80258-59182763
|
|
20.
|
P DucyR ZhangV GeoffroyAL RidallG
KarsentyOsf2/Cbfa1: a transcriptional activator of osteoblast
differentiationCell89747754199710.1016/S0092-8674(00)80257-39182762
|
|
21.
|
G KarsentyThe genetic transformation of
bone biologyGenes
Dev1330373051199910.1101/gad.13.23.303710601030
|
|
22.
|
D ParisuthimanY MochidaWR DuarteM
YamauchiBiglycan modulates osteoblast differentiation and matrix
mineralizationJ Bone Miner
Res2018781886200510.1359/JBMR.05061216160746
|
|
23.
|
T MiyazakiS MiyauchiA TawadaT AnadaS
MatsuzakaO SuzukiOversulfated chondroitin sulfate-E binds to BMP-4
and enhances osteoblast differentiationJ Cell
Physiol217769777200810.1002/jcp.2155718720384
|
|
24.
|
E SchonherrP Witsch-PrehmB HarrachH
RobenekJ RauterbergH KresseInteraction of biglycan with type I
collagenJ Biol Chem27027762783199510.1074/jbc.270.6.27767852349
|
|
25.
|
PA MiguezM TerajimaH NagaokaY MochidaM
YamauchiRole of glycosaminoglycans of biglycan in BMP-2
signalingBiochem Biophys Res
Commun405262266201110.1016/j.bbrc.2011.01.02221219861
|
|
26.
|
X WangK HarimotoS XieH ChengJ LiuZ
WangMatrix protein biglycan induces osteoblast differentiation
through extracellular signal-regulated kinase and Smad pathwaysBiol
Pharm Bull3318911897201010.1248/bpb.33.1891
|
|
27.
|
PJ RoughleyRJ WhiteMC MagnyJ LiuRH
PearceJS MortNon-proteoglycan forms of biglycan increase with age
in human articular cartilageBiochem J29542142619938240239
|