Introduction
Neuroblastoma is a childhood tumor derived from the
nervous system. Tumorigenesis of NB is a complicated process. In NB
tumor, the changes on chromosome remodeling have been found,
including gaining of chromosome 17q and losses of 1p, 3p and 11q,
histone methylation and acetylation of H3K9 (1). The alteration at molecular level
includes high expression of oncogene MYCN and ALK, silence of tumor
suppressor genes RAS-association domain family 1 isoform A
(RASSF1A) and SF1, and silence of apoptosis related gene caspase-8
(2). DNA methylation regulation
largely contributes to the abnormal expression of these genes.
Especially, hypermethylation at TSG promoter is observed during
tumorigenesis. Decades of investigation on DNA methylation of NB
found that epigenetic silence of caspase-8 and RASSF1A, among the
75 selected methylated candidate genes, were responsible for the
development and progress of the disease (2).
Dietary soy has been regarded as a healthy food for
reducing heart disease and cancer risk. The epigenetic changes with
dietary soy in cynomolgus monkey have been verified (3). Some flavonoid compositions,
including genistein, may transmit these epigenetic changes to the
next generation. Genistein, as one of the soy-derived bioactive
isoflavones, affects tumorigenesis through epigenetic regulations
(4). The major process of DNA
methylaton is that methyl is transferred from SAM to cytosine by
DNMT (5). Genistein may work as a
DNMT inhibitor that can regulate gene expression by erasing DNA
methylation at the promoter. Genistein has been proved to promote
DNA demethylation of SF1 promoter in endometrial stromal cells
(6), and has the capacity of
preventing cancer risk of breast cancer (5). There are no significant changes in
embryo stem cells treated with genistein, but for a set of genes,
regulation after de novo DNA methyaltion in the early embryo
may be sensitive to genistein (7), and the next generation can inherit
this pattern of DNA methylation alteration. In genistein-mediated
differentially methylated regions (DMRs), 95 of 149 DMRs are less
methylated in promoters (7).
Overall, genistein alters the configuration of chromatin, and acts
as a DNMT inhibitor, demethylating the hypermethylated regions at
TSG promoters, such as p16 (8–11).
The tumor suppressor factor CHD5 was uncovered
(12), and it has been verified
that the defect of CHD5 leads to excessive proliferation of tissues
and induces tumorigenesis. CHD5 acts under the downstream pathway
of p53 and is recognized as a TSG. Fujita et al proved that
low expression level of CHD5 also exists in NB (13), which prompted us to examine the
role of CHD5 in NB and our results indicate that CHD5 was
associated with malignancy grade of NB and may be regulated by
genistein under an epigenetic pathway.
Materials and methods
Neuroblastoma cell culture
Human neuroblastoma SK-N-SH cells were purchased
from the Cell Bank of the Chinese Institute of Biochemistry and
Cell Biology (Shanghai, China). For routine maintenance, the
neuroblastoma cell line was cultured in MEM supplemented with 0.1 M
L-glutamine, 10% (v/v) FBS, 100 U/ml of penicillin, 100 U/ml of
streptomycin, and 100 U/ml of kanamycin at 37°C with 5% (v/v)
CO2 in a humidified incubator. For the assessment of
estrogen-like activity, the cells were cultured in phenol-red free
MEM supplemented with 10% (v/v) FBS, which was pretreated with
sulphatase and dextran-coated charcoal (CD-FBS) (14).
Nude mice subcutaneous injection and
diet
BALA/C nude mice, ∼4 weeks of age on arrival, were
purchased from Shanghai Slaccas Co., and maintained in
micro-isolator cages under pathogen-free conditions on a 12-h
light/12-h dark schedule for a week. All animal experiments were
approved by the Institutional Animal Use and Care Committee of
Shanghai Jiao Tong University. After housing for a week, the mice
were inoculated with 3×106 SK-N-SH NB cells in 0.1 ml of
PBS. After injection, the mice were randomly divided into four
groups and treated with 2 mg genistein (Sigma), 2 mg BPA and E2 as
control and olive oil as blank control everyday. After 15 days,
mice were sacrificed, and tumors were excised, weighed, fixed in
10% (v/v) buffered formalin and processed for histology
analysis.
Immunohistochemistry and microvessel
density (MVD)
According to the protocol of Zhao et al
(15), the primary antibody of
factor VIII-related antigen (polyclonal, ZA-0111 Santa Cruz) was
used. The stained sections were screened at magnification x200
under a light microscope to identify the five random regions of the
section. Vessels were counted and the average numbers of
microvessels were recorded by two observers, and the mean value was
used for analysis.
Analysis of CpG methylation by bisulfite
sequencing
DNA extraction from NB transplantable tumors was
treated with DNA samples bisulfite treatment provided by EZ DNA
Methylation-Gold kit (Cat: D5005). Reaction conditions: 98°C for 10
min, 64°C for 2.5 h, stored at 4°C for <20 h. Primers were
designed by methylation analysis software MethPrimer. CHD5M-F:
GGGGTATTATTTGGATTTTTTTTG; CHD5M-R: CTAATTACTATAACAACCCCATCCC. PCR
amplification: We used fidelity Taq enzyme (1X Platinum PCR
MasterMix, Invitrogen), 50 μl reaction system containing 45 μl
Platinum PCR MasterMix, 1.5 μl sense primer, 1.5 μl anti-sense
primer, 2 μl bisulfite treated DNA. Reaction conditions: 95°C for 3
min, first cycle: 95°C for 30 sec, 58°C for 30 sec, 72°C for 40
sec, running 8 cycles as touchdown 1°C of annealing temperature at
each cycle, and then 95°C for 30 sec, 50°C for 30 sec, 72°C for 40
sec, running 40 cycles.
Transformation and cloning
PCR products purified with universal DNA
Purification kit (Tiangen) were ligated into PMD-18T vector
(Takara), at the concentration ratio of 1:3 (PCR products:PMD-18T).
The recombinant T vector was transformed into E. coli. DH5α,
and then cultured on LB solid medium containing ampicillin (1:1000
diluted), 40 μl X-gal and 8 μl IPTG overnight. Ten clones for each
PCR product were picked up to be cultured in liquate LB medium for
6 h.
Real-time reverse transcription
polymerase chain reaction (real-time RT-PCR)
Total RNA was isolated using a homogenizer and
TRIzol™ reagent (Invitrogen) according to the manufacturer’s
instructions. The primers sequences are described in Table I. PCR reaction system: 2X PCR
master mix (Tiangen), conditions: 95°C for 3 min, 95°C for 30 sec,
50°C for 30 sec, 72°C for 40 sec, 35 cycles, then 72°C for 10 min,
storing at 4°C. For real-time PCR, ABI 7900 real-time PCR machine
was used. Conditions: Step 1, 95°C for 30 sec; Step 2, 95°C for 5
sec, 60°C for 40 sec, 40 cycles.
 | Table I.Real-time quantitative RT-PCR
primers. |
Table I.
Real-time quantitative RT-PCR
primers.
| Targets | Primers
|
|---|
| Forward | Reverse |
|---|
| CHD5 |
5′-AAACAAGTGTAAAGGGAAGC-3′ |
5′-CCTCCGAGAACAGGTAGTCC-3′ |
| P53 |
5′-CTGCCTTCCGGGTCACTGCC-3′ |
5′-TTGGGACGGCAAGGGGGACA-3′ |
| DNMT1 |
5′-GGAAGGCTACCTGGCTAAAGTCAAG-3′ |
5′-ACTGAAAGGGTGTCACTGTCCGAC-3′ |
| DNMT3a |
5′-TGGAGAATGGCTGCTGTGTGAC-3′ |
5′-CACTCATCCCGTTTCCGTTTG-3′ |
| DNMT3b |
5′-AGTGACCAGTCCTCAGACACGAAG-3′ |
5′-ATCAGAGCCATTCCCATCATCTAC-3′ |
| GAPDH |
5′-TAAGTATGACTCCACCCACG-3′ |
5′-CTAGCACCTTCCCAACTA-3′ |
Western blotting
Protein were separated by 12% SDS-PAGE and then
transferred to polyvinylidene fluoride (PVDF, Sigma) membrane using
electronic transfer method. Primary rabbit anti-CHD5 polyclonal
antiserum (1:200, Abcam, Cambridge, UK) was applied for
hybridization at 4°C overnight. Then the secondary hybridization
was performed using peroxidaseconjugated goat anti-mouse IgG
(1:4000 diluted) at 37°C for another 1 h. Protein bands were then
developed with enhanced chemiluminescence (ECL) detection reagents
(Amersham Biosciences, Piscataway, NJ).
Statistical analysis
All statistical analyses were performed using the
SPSS17.0 software. The results are presented as mean ± standard
deviation (SD). Differences among the groups were assessed by
analysis of variance (ANOVA). P<0.05 was considered
statistically significant.
Results
Genistein inhibited the NB cells growth
in vivo
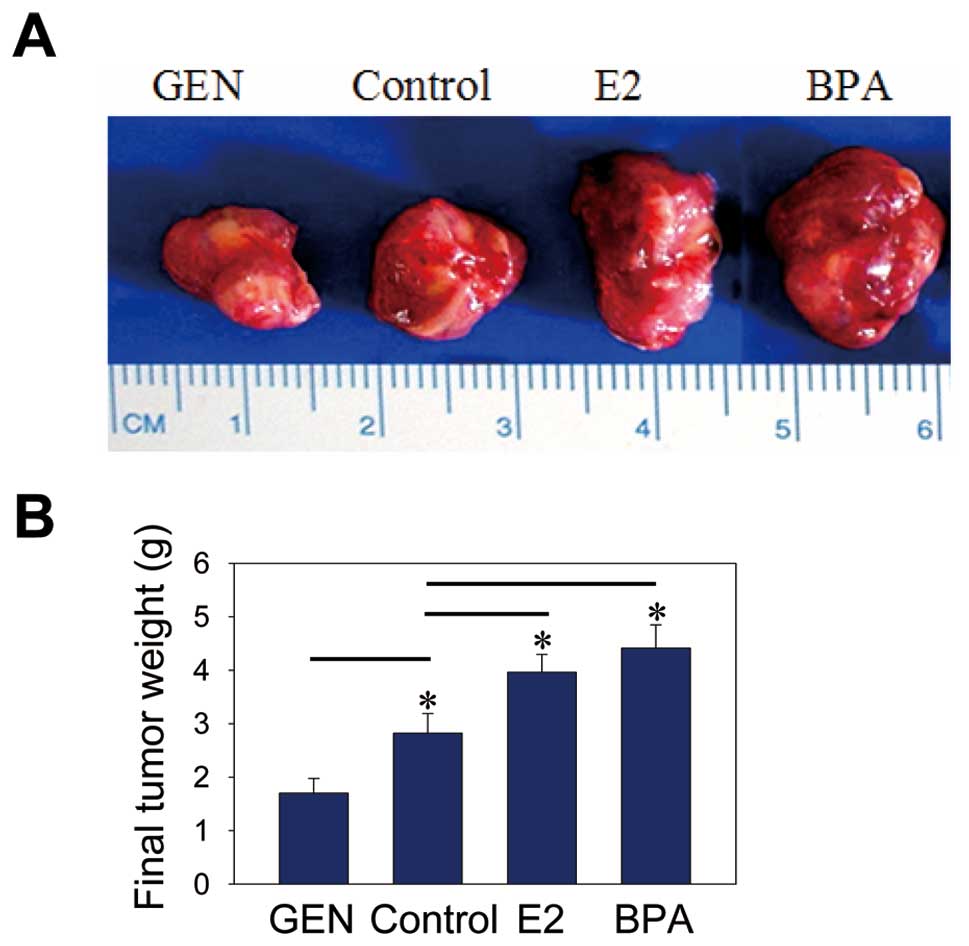
The genistein treatment resulted in smaller tumor
size (Fig. 1A) and significantly
inhibited NB growth (Fig. 1B)
compared to the other three treatments in nude mice. Besides, the
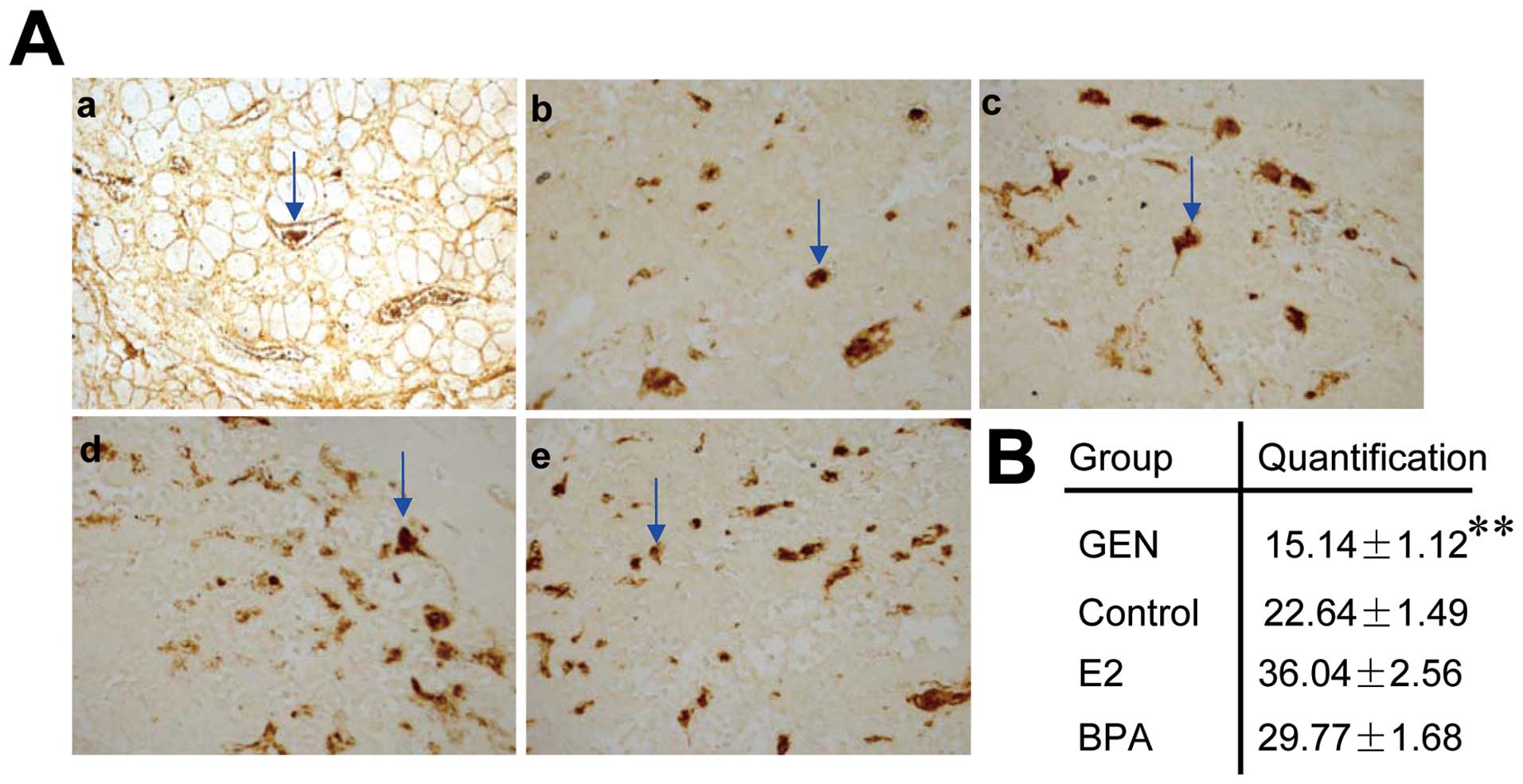
MVD in genistein-treated mice decreased compared with that of other
three groups (Fig. 2, P<0.01).
These results suggested that genistein had antitumor effects.
Genistein demethylated the promoter of
CHD5
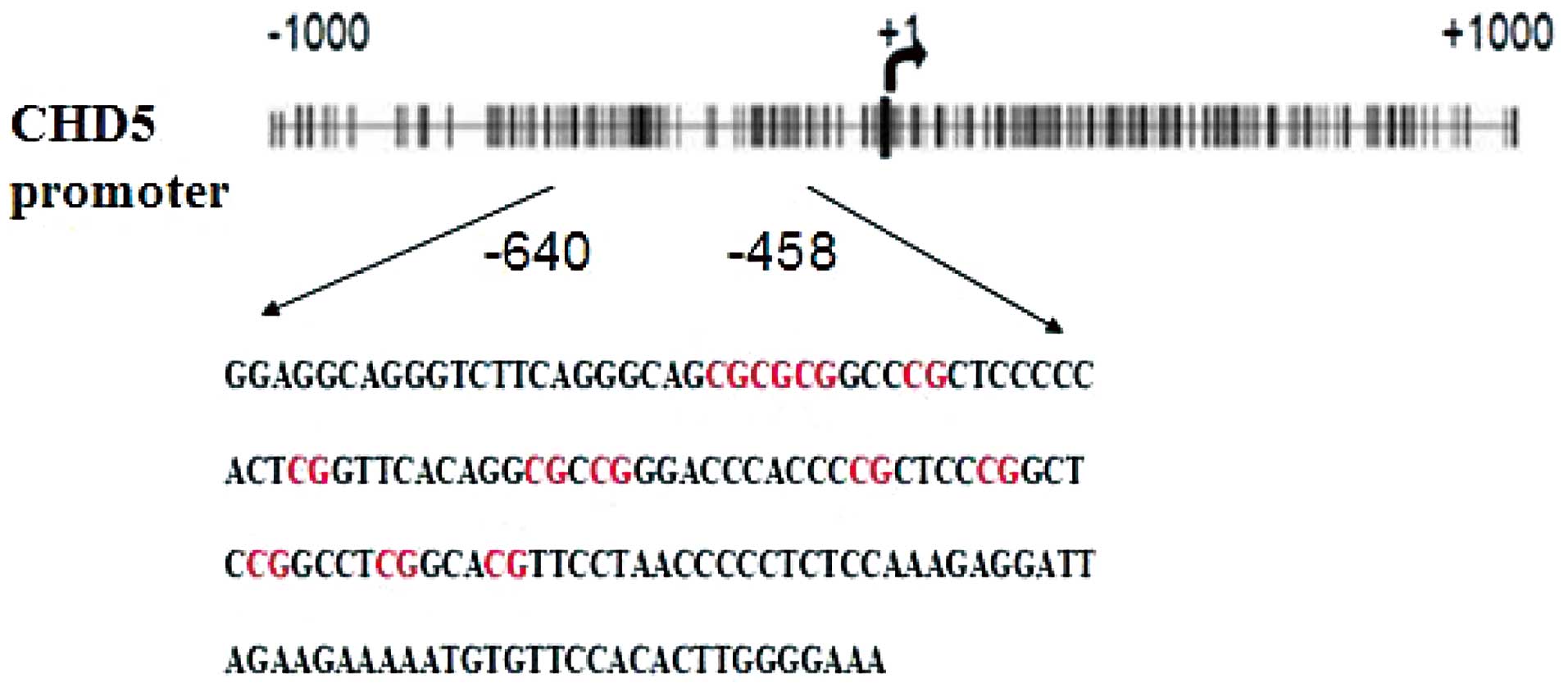
A CpG island near tandem repeats sequence (TRS)
containing 152-nt sequences and 12 CpG dinucleotides, as
highlighted in red in Fig. 3 was
selected for methylation analysis. DNA was extracted from NB and
then treated with EZ DNA Methylation-Gold kit and the PCR
amplification was performed with CHD5 primer pairs. Ten clones were
selected for each PCR product. CpG dinucleotides were indicated as
ellipses (Fig. 4): black, fully
methylated (>60%); gray, partially methylated (40–60%) and
white, unmethylated (<40%). The results showed that the
methylation level at promoter of CHD5 was reduced by genistein
treatment (<60%), and almost no significant changes were found
in BPA and E2 group (>80%) compared with control group
suggesting that tumor suppressor gene CHD5 may be epigenetically
regulated by genistein.
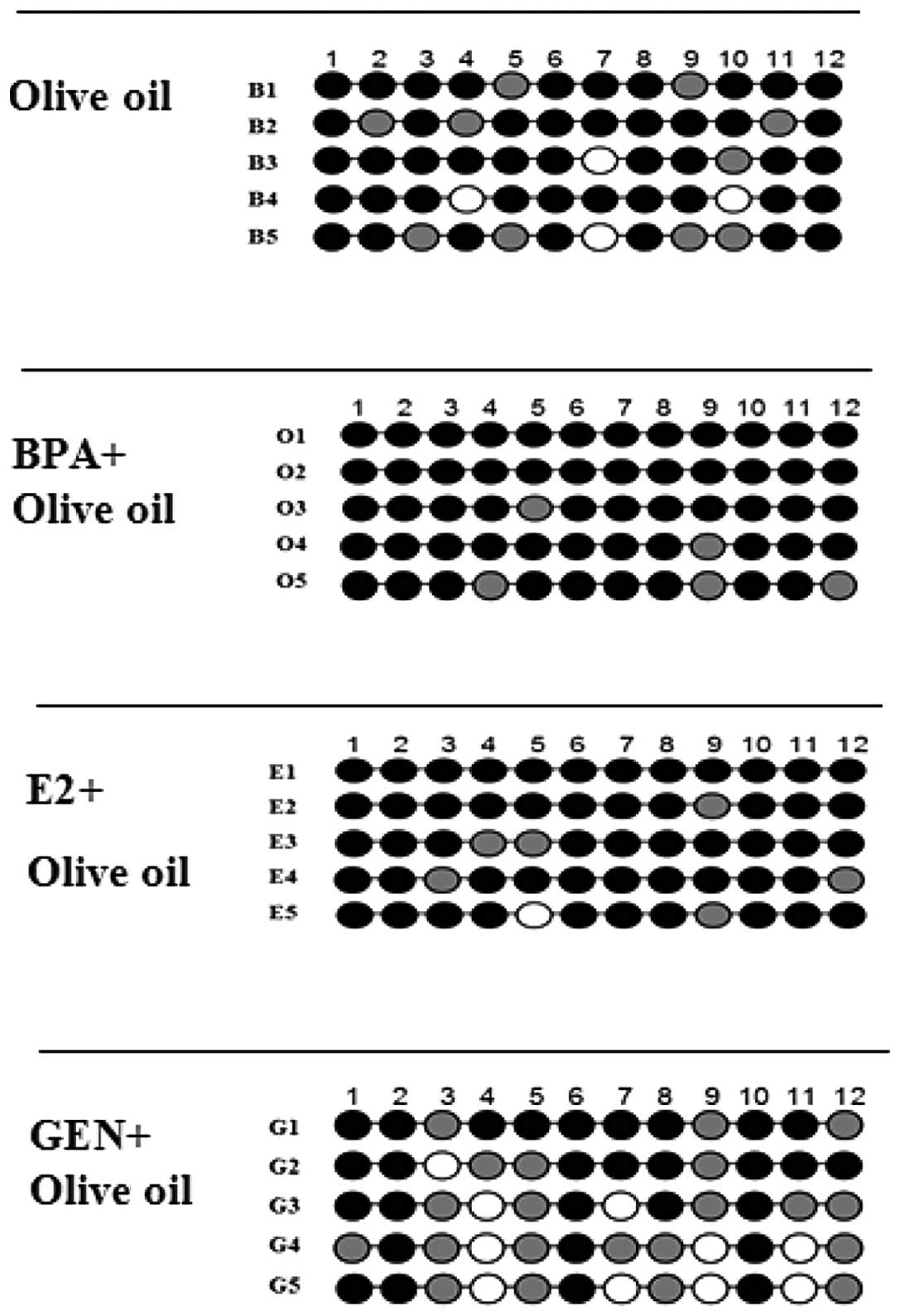 | Figure 4.The methylation levels detected from
relative groups. Five NBs from GEN+olive oil fed mice are marked as
G1, G2, G3, G4 and G5; five NBs from E2+olive oil fed mice are
marked as E1, E2, E3, E4 and E5; five NBs from BPA+olive oil fed
mice are marked as O1, O2, O3, O4 and O5; five NBs from olive oil
fed mice are marked as B1, B2, B3, B4 and B5. |
Effect of genistein on CHD5
expression
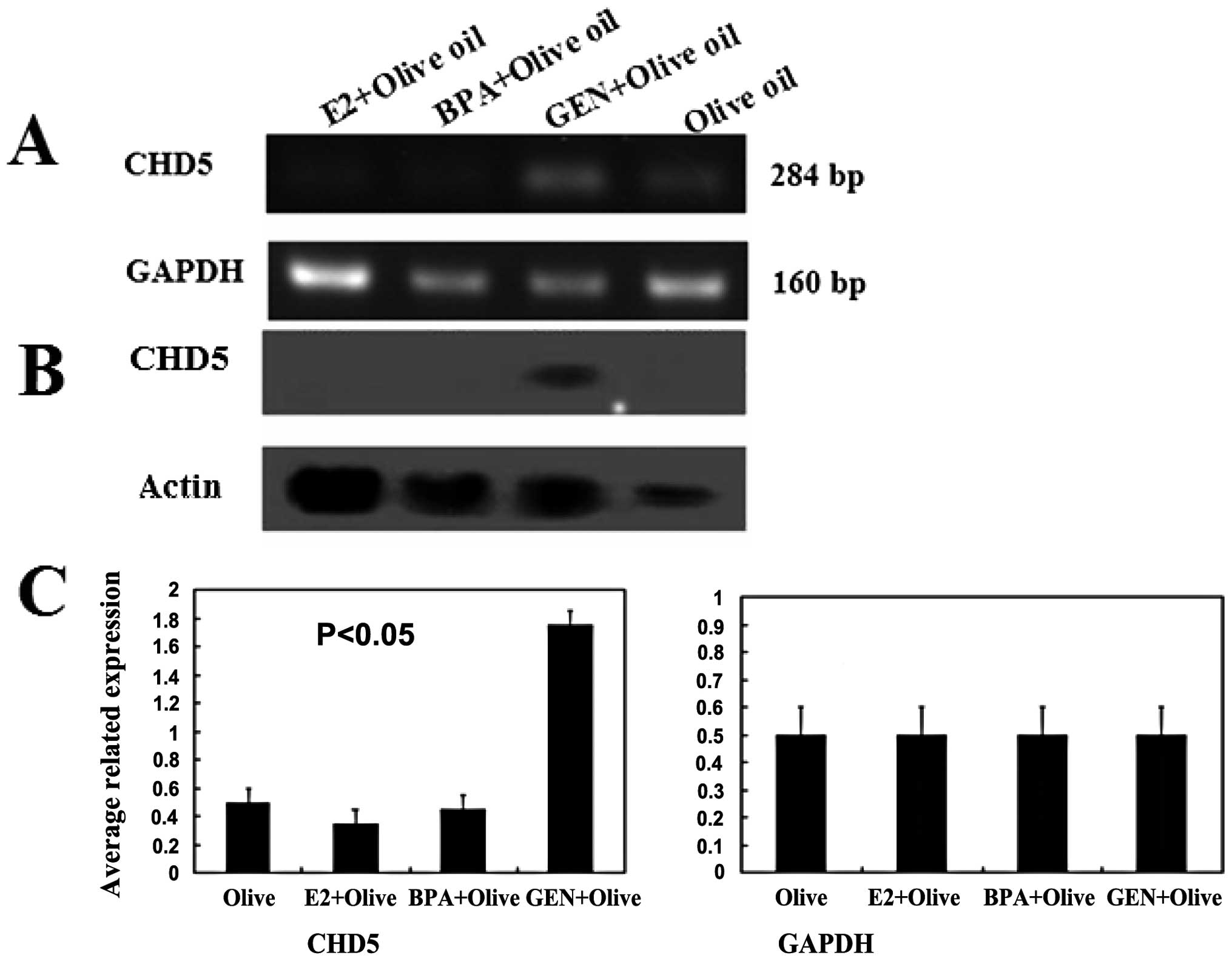
The expression of CHD5 at mRNA level was increased
in genistein treatment group compared with that of control groups,
while BPA, E2 treatments displayed weak expression of CHD5
(Fig. 5A and C). Only the
expression of CHD5 protein in genistein-treated group was obviously
observed (Fig. 5B). The
expression level of CHD5 was associated with the changes of the
methylation level at CHD5 promoter. These results showed that the
level of CHD5 can be regained by genistein.
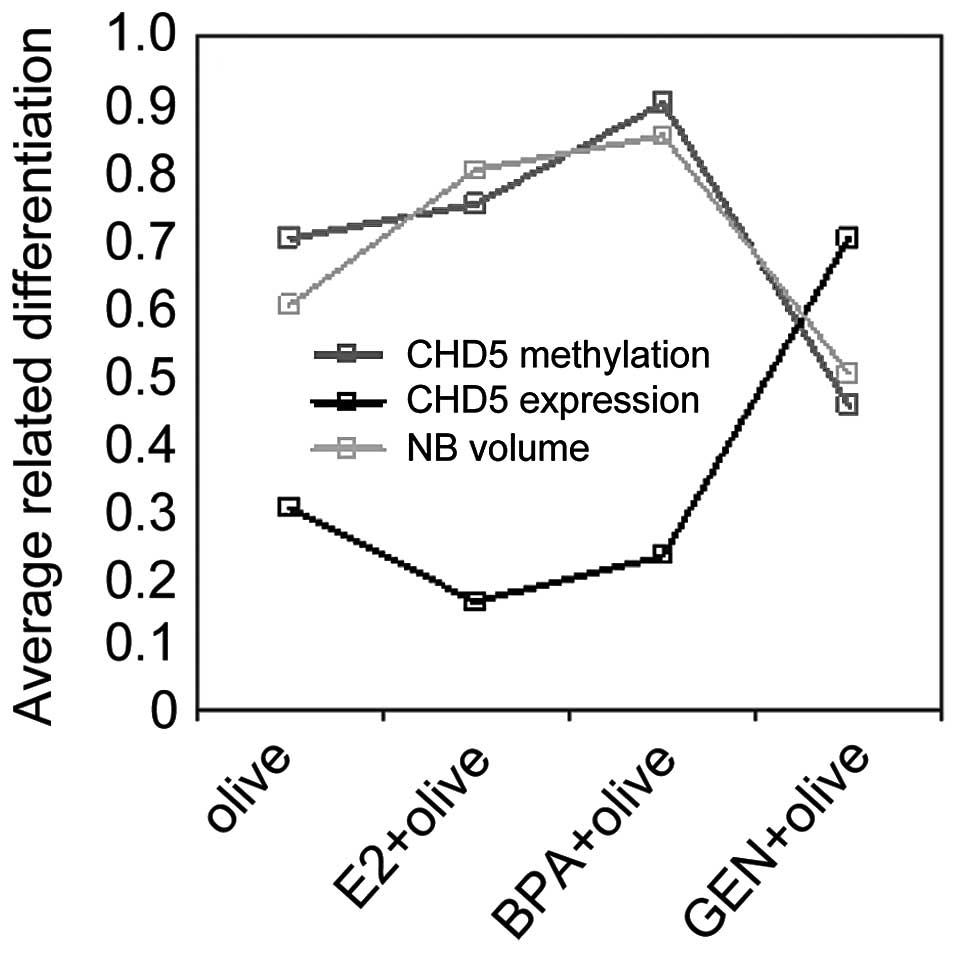
Effect of demethylation of CHD5 by
genistein on NB proliferation
CHD5 methylation not only influences its expression
level but also determines the NB growth in vivo. As shown in
Fig. 6, genistein increased the
protein expression level of CHD5 via reducing its gene methylation
level, thereby inhibiting NB growth in vivo. These results
suggested that epigenetic regulation of CHD5 plays a vital role in
NB proliferation.
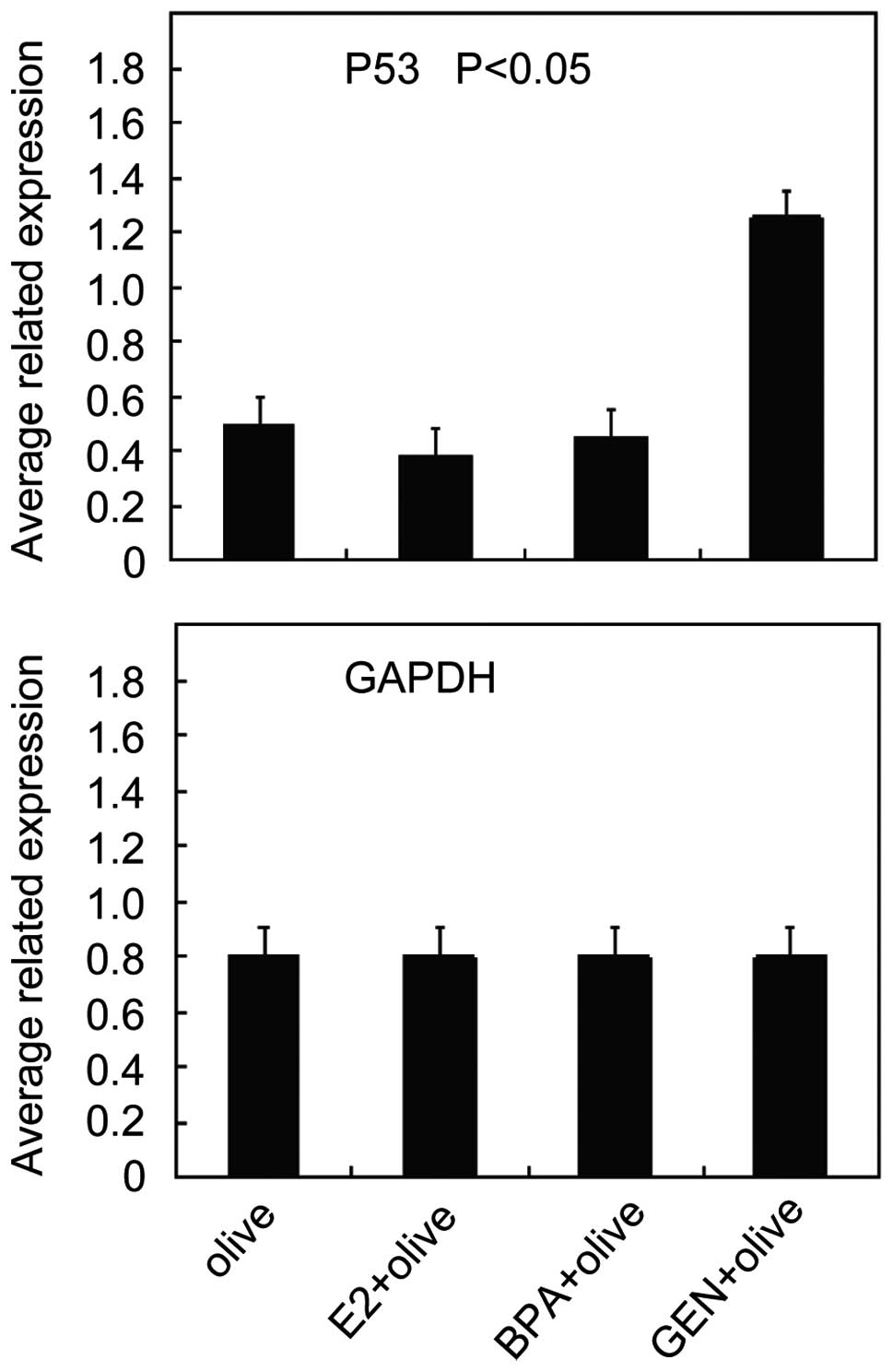
Effect of genistein on P53
expression
P53 is widely recognized as a tumor suppressor gene,
which inhibits tumor growth and induces cell apoptosis. However, it
is also a control target of CHD5. Our results showed that the
expression of P53 was increased after genistein treatment (Fig. 7).
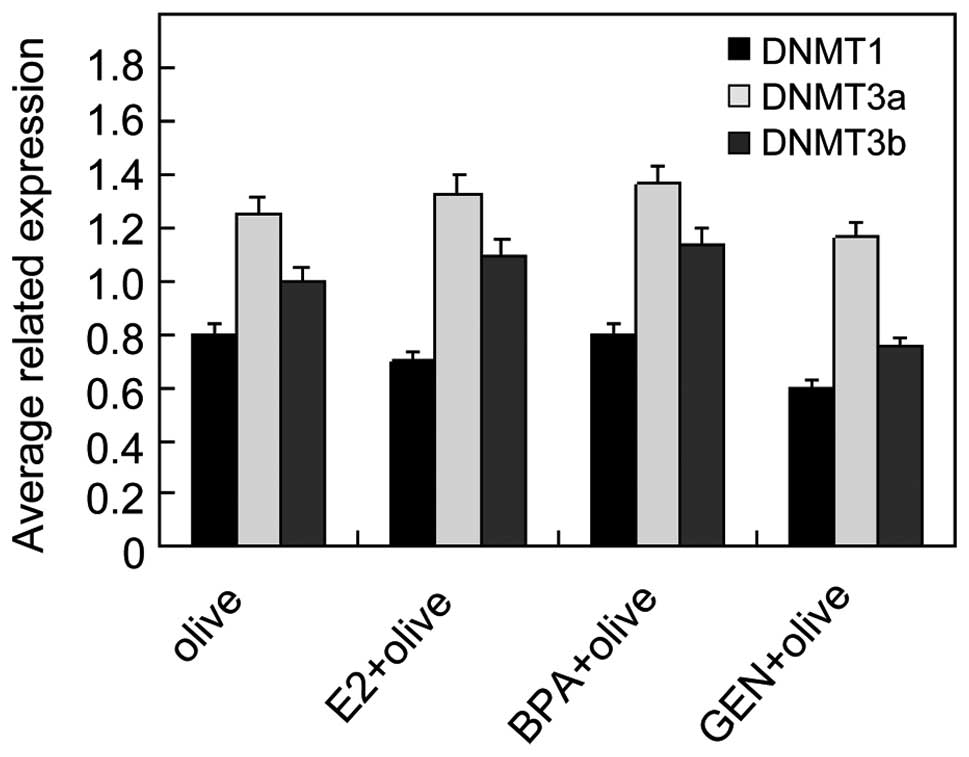
Effect of genistein on DNMT isoforms
expression
The expression of DNMT3b decreased significantly in
genistein treatment group, while no obvious changes were observed
in the isoforms DNMT1 and DNMT3a, as shown in Fig. 8, suggesting that genistein acts as
a DNMT inhibitor and involves epigenetic modifications. The
reduction of DNMT3b implies that DNMT3b may be implicated in DNA
demethylation in tumorigenesis of NB.
Discussion
The environmental estrogen-like contaminants that
are collectively called environmental endocrine disruptors (EEDs),
xenoestrogens, or environmental estrogens, are derived from a
variety of relatively common and abundant sources, such as
pesticides, plastics, combustion by-products, plants and
agricultural products (16–20). These contaminants can enter the
body by ingestion, adsorption or inhalation. They bind to the
estrogen receptor (ER), mimic or interfere with the action of
natural estrogens in animals and humans (21,22). Bisphenol A (BPA), a monomer
component of polycarbonate plastics and epoxy resins, is widely
used in many consumer products. It has been verified that BPA can
promote the proliferation of SK-N-SH cells, and the estrogen
receptor pathway may be involved in this effect (23). How to resist the hurt of
estrogen-like contaminants? Soy products were found to play a
critical role in reducing cardiovascular disease and carcinogenesis
and increasing evidence showed that soy phytoestrogens are at least
partially responsible for this effect (24–26). The causal relationship and the
mechanisms of phytoestrogen action have yet to be determined
(27). Genistein, one of the many
phytoestrogens contained in soy, has been shown to inhibit the
proliferation of both breast and prostate cancer cells (28).
In this study, we explored the effect of genistein
on NB cell growth in vivo. We established nude mice model by
subcutaneous injection of SK-N-SH cells, then performed oral
ingestion with BPA, E2, and genistein, respectively, since SK-N-SH
tumor formed in these nude mice. Compared with E2 and BPA group, in
the genistein group, tumors shrank. Hemangiogenesis and
lymphangiogenesis are associated with progressed tumor stages, in
GEN group, however, the microvessel density (MVD) also reduced,
suggesting that the tumor grade of the malignancy was reduced,
indicating that genistein had anti-cancer ability against NB.
We aimed to explore the mechanisms of genistein
remitting the process of NB. NB is a childhood cancer that is
characterized as having genomic deletions at chromosome 1p. Tumor
suppressor CHD5 localize in this region and it has been reported
that the CHD5 expression was low in NB, and partially because the
CHD5 promoter was highly methylated (13). Okawa et al (29) found that CHD5 had virtually absent
expression in 30 NB cell lines, and Fujita et al (13) verified CHD5 was a tumor suppressor
gene against NB, and its expression may be inhibited by promoter
methylation. In our study, CHD5 promoter in E2 and BPA group was
highly methylated, and almost all 12 CpG sites were methylated in
BPA group, which is consistent with Fujita et al (13). However, genistein reverses the
process, demethylation of CDH5 gene was found in genistein group,
enhancing the mRNA and protein levels of CHD5, exhibiting its
antitumor effect synergistically with p53. The degree of p53
expression had positive correlation with that of CHD5
demethylation, suggesting that genistein not only influenced CHD5,
but also restarted the normal function of other anti-cancer genes,
such as P53. p53, a key gene associated with cancer proliferation
and apoptosis, and an important suppressor of WNT signaling
(30). We hypothesized that
genistein can erase hypermethylation level at promoter of CHD5, and
improve the expression level of CHD5 as well as p53, thereby
cooperating with p53 to mediate cancer development and apoptosis
possibly through the WNT signaling pathway.
DNA methylation, as a main epigenetic modifier, has
been shown to be correlated to tumorigenesis (31). Interestingly, in our study, we
found that genistein could regulate DNA methylation level of CHD5
which is consistent with previous studies (32,33). DNA methylation and demethylation
on CpG dinucleotides are regulated by DNMT commonly regarded as a
candidate of epigenetic target (34,35). Therefore, we wished to verify
whether genistein regulates methylation level of CHD5 via
modulating DNMT, and the results of our study showed that genistein
indeed to some extent inhibited the expression of DNMTs.
In conclusion, genistein can erase the methylation
of CHD5 promoter and improve the expression level of CHD5 as well
as p53, thereby cooperating with p53 to inhibit NB proliferation
in vivo possibly through the WNT signaling pathway. This
process may be achieved by inhibiting DNMT. As a natural source
from soy, genistein has a very broad prospect for future therapy of
NB patients.
References
|
1.
|
M BerdascoS RoperoF SetienEpigenetic
inactivation of the Sotos overgrowth syndrome gene histone
methyltransferase NSD1 in human neuroblastoma and gliomaProc Natl
Acad Sci USA1062183021835200910.1073/pnas.090683110620018718
|
|
2.
|
A DecockM OngenaertJ VandesompeleF
SpelemanNeuroblastoma epigenetics: from candidate gene approaches
to genome-wide
screeningsEpigenetics6962970201110.4161/epi.6.8.1651621725203
|
|
3.
|
TD HowardSM HoL ZhangEpigenetic changes
with dietary soy in cynomolgus monkeysPloS
One6e26791201110.1371/journal.pone.002679122046358
|
|
4.
|
Y ZhangH ChenGenistein, an epigenome
modifier during cancer
preventionEpigenetics6888891201110.4161/epi.6.7.1631521610327
|
|
5.
|
SI KhanP AumsuwanIA KhanLA WalkerAK
DasmahapatraEpigenetic events associated with breast cancer and
their prevention by dietary components targeting the epigenomeChem
Res Toxicol256173201110.1021/tx200378c21992498
|
|
6.
|
H MatsukuraK AisakiK IgarashiGenistein
promotes DNA demethylation of the steroidogenic factor 1 (SF-1)
promoter in endometrial stromal cellsBiochem Biophys Res
Commun412366372201110.1016/j.bbrc.2011.07.10421821006
|
|
7.
|
N SatoN YamakawaM MasudaK SudoI HatadaM
MuramatsuGenome-wide DNA methylation analysis reveals phytoestrogen
modification of promoter methylation patterns during embryonic stem
cell differentiationPloS
One6e19278201110.1371/journal.pone.001927821559447
|
|
8.
|
S MajidAA DarV ShahryariGenistein reverses
hyper-methylation and induces active histone modifications in tumor
suppressor gene B-Cell translocation gene 3 in prostate
cancerCancer1166676201019885928
|
|
9.
|
M EstellerCpG island hypermethylation and
tumor suppressor genes: a booming present, a brighter
futureOncogene2154275440200210.1038/sj.onc.120560012154405
|
|
10.
|
MZ FangD ChenY SunZ JinJK ChristmanCS
YangReversal of hypermethylation and reactivation of p16INK4a,
RARbeta, and MGMT genes by genistein and other isoflavones from
soyClin Cancer
Res1170337041200510.1158/1078-0432.CCR-05-040616203797
|
|
11.
|
MZ FangZ JinY WangPromoter
hypermethylation and inactivation of O(6)-methylguanine-DNA methyltransferase in
esophageal squamous cell carcinomas and its reactivation in cell
linesInt J Oncol26615622200515703815
|
|
12.
|
A BagchiC PapazogluY WuCHD5 is a tumor
suppressor at human
1p36Cell128459475200710.1016/j.cell.2006.11.05217289567
|
|
13.
|
T FujitaJ IgarashiER OkawaCHD5, a tumor
suppressor gene deleted from 1p36.31 in neuroblastomasJ Natl Cancer
Inst100940949200810.1093/jnci/djn17618577749
|
|
14.
|
DH HanMS DenisonH TachibanaK
YamadaRelationship between estrogen receptor-binding and estrogenic
activities of environmental estrogens and suppression by
flavonoidsBiosci Biotechnol
Biochem6614791487200210.1271/bbb.66.147912224631
|
|
15.
|
HC ZhaoR QinXX ChenMicrovessel density is
a prognostic marker of human gastric cancerWorld J
Gastroenterol1275987603200617171787
|
|
16.
|
B HammondBS KatzenellenbogenN KrauthammerJ
McConnellEstrogenic activity of the insecticide chlordecone
(Kepone) and interaction with uterine estrogen receptorsProc Natl
Acad Sci USA7666416645197910.1073/pnas.76.12.664193289
|
|
17.
|
JA McLachlanKS KorachRR NewboldGH
DegenDiethylstilbestrol and other estrogens in the
environmentFundam Appl
Toxicol4686691198410.1016/0272-0590(84)90089-76510599
|
|
18.
|
CL Hughes JrPhytochemical mimicry of
reproductive hormones and modulation of herbivore fertility by
phytoestrogensEnviron Health
Perspect78171174198810.1289/ehp.88781713203635
|
|
19.
|
T ColbornFS vom SaalAM SotoDevelopmental
effects of endocrine-disrupting chemicals in wildlife and
humansEnviron Health
Perspect101378384199310.1289/ehp.931013788080506
|
|
20.
|
SC NagelFS vom SaalKA ThayerMG DharM
BoechlerWV WelshonsRelative binding affinity-serum modified access
(RBA-SMA) assay predicts the relative in vivo bioactivity of the
xenoestrogens bisphenol A and octylphenolEnviron Health
Perspect1057076199710.1289/ehp.97105709074884
|
|
21.
|
WW LeavittDM MeismerSexual development
altered by non-steroidal
oestrogensNature218181182196810.1038/218181a05689495
|
|
22.
|
AM SotoC SonnenscheinKL ChungMF FernandezN
OleaFO SerranoThe E-SCREEN assay as a tool to identify estrogens:
an update on estrogenic environmental pollutantsEnviron Health
Perspect103Suppl 7113122199510.1289/ehp.95103s71138593856
|
|
23.
|
J ZhengX XiaoJ LiuS ZhengQ YinY
YuGrowth-promoting effect of environmental endocrine disruptors on
human neuroblastoma SK-N-SH cellsEnviron Toxicol
Pharmacol24189193200710.1016/j.etap.2007.05.00321783809
|
|
24.
|
H AdlercreutzPhytoestrogens: epidemiology
and a possible role in cancer protectionEnviron Health
Perspect103Suppl 7103112199510.1289/ehp.95103s71038593855
|
|
25.
|
KD SetchellA CassidyDietary isoflavones:
biological effects and relevance to human healthJ
Nutr129S758S767199910082786
|
|
26.
|
T FotsisM PepperH AdlercreutzGenistein, a
dietary-derived inhibitor of in vitro angiogenesisProc Natl Acad
Sci USA9026902694199310.1073/pnas.90.7.26907681986
|
|
27.
|
L StraussR SanttiN SaarinenT StrengS
JoshiS MakelaDietary phytoestrogens and their role in hormonally
dependent diseaseToxicol Lett102–103349354199810022277
|
|
28.
|
MA MoyadSoy, disease prevention, and
prostate cancerSemin Urol Oncol1797102199910332923
|
|
29.
|
ER OkawaT GotohJ ManneExpression and
sequence analysis of candidates for the 1p36.31 tumor suppressor
gene deleted in
neuroblastomasOncogene27803810200810.1038/sj.onc.121067517667943
|
|
30.
|
NH KimHS KimNG Kimp53 and microRNA-34 are
suppressors of canonical Wnt signalingSci
Signal4ra71201122045851
|
|
31.
|
J TostDNA methylation: an introduction to
the biology and the disease-associated changes of a promising
biomarkerMol Biotechnol447181201010.1007/s12033-009-9216-2
|
|
32.
|
M FangD ChenCS YangDietary polyphenols may
affect DNA methylationJ Nutr137S223S2282007
|
|
33.
|
Z WangH ChenGenistein increases gene
expression by demethylation of WNT5a promoter in colon cancer cell
line SW1116Anticancer Res3045374545201021115903
|
|
34.
|
SH SongSW HanYJ BangEpigenetic-based
therapies in cancer: progress to
dateDrugs7123912403201110.2165/11596690-000000000-0000022141383
|
|
35.
|
JM FoulksKM ParnellRN NixEpigenetic drug
discovery: targeting DNA methyltransferasesJ Biomolecular
Screen17217201110.1177/1087057111421212
|