Introduction
Oxidized low-density lipoproteins (LDL) uptake is a
key initial event in atherogenesis. Foam cell formation plays a
critical role in the development of atherosclerosis. Oxidized LDL
is taken up by several receptors, such as scavenger receptor (SR)
class A (SR-A), SR-B1 (CD36), and macrophage CD68 (1). Macrophage SR-B1 is the membrane
receptors responsible for oxidized LDL uptake and facilitates the
intracellular lipid accumulation (2). After oxidized LDL is taken up by
macrophages, oxidized LDL cholesterol is degraded to oxysterols or
free cholesterols in the lysosomes (3). The resulted oxysterols influence the
formation of atherosclerotic plaques and are thought to have a
central role in promoting atherogenesis (4). It has been speculated that
oxysterols may represent the most toxic form of oxidized lipids in
LDL (5). Thus, potential agents
blunting oxidized LDL uptake leading to production of oxysterols
are considered anti-atherogenic. However, the underlying mechanisms
by which the agents antagonize oxidized LDL uptake and oxysterol
formation by diverse stimulators remain to be elucidated.
The removal of oxidized lipids from artery wall is
arbitrated by reverse cholesterol transport, the process involving
the membrane proteins of ATP-binding membrane cassette transport
protein A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1)
(6,7). ABCA1 is involved in the exporting of
cholesterol and phospholipids from peripheral cells to lipid-poor
apolipoprotein A1 (apoA1) to generate precursors for HDL particles
(6,8). ABCG1 mediates oxysterol efflux from
oxidized LDL-loaded macrophages, and the exported oxysterol by
ABCG1 pathway can be selectively taken up by hepatocytes (7). Accordingly, ABCA1 and ABCG1 play a
pivotal role in encumbering atherogenesis by promoting the efflux
of cholesterol and oxysterols. Macrophages secrete high levels of
apoE and cholesterol efflux from macrophages to apoE has been shown
to decrease foam cell formation and prevent atherosclerosis
(9). Furthermore, a recent study
has shown a novel function of apoE as a significant determinant of
cholesterol efflux in macrophages (10). ApoE associated with ApoB-carrying
lipoproteins plays an upregulatory role on ABCA1 expression via
induction of Sp1.
Sage weed (Salvia plebeia R. Br.) is an
annual or biennial, hairy herb broadly distributed in many
countries including China, India, Iran and Australia. Sage weed has
long been used as a folk medicine in Asia and its medicinal
properties are being more seriously investigated (11). Crude extract of Salvia
plebeia exhibited antioxidant activity, and antioxidant
compounds were isolated and identified as royleanonic acid,
hispidulin and eupatorin (11).
The ethanol extract of Salvia plebeia possess
anti-inflammatory and anti-angiogenic, anti-nociceptive and
antioxidant activities (12).
Antioxidative activity and nitrite scavenging ability were observed
in methanol extract from Salvia plebeia (13). However, there are no reports
demonstrating anti-atherogenic activity of sage weed extract and
its constituents.
Based on literature evidence that manipulating
cellular cholesterol flux of peripheral cells and macrophages can
be anti-atherogenic, this study hypothesized that sage weed
methanol extract (SWE) improved macrophage cholesterol handling in
oxidized LDL-loaded macrophages. To test this hypothesis, this
study elucidated oxidized LDL uptake and cholesterol efflux in
J774A1 murine macrophages treated with submicromolar SWE and its
components of homoplantaginin and hispidulin. The aims of this
study were to investigate the pathways mediating the removal of
oxysterols and cholesterol from oxidized LDL-loaded macrophages.
SR-B1 induction was measured for oxidized LDL uptake, and ABCA1 and
ABCG1 expression was determined for apoE-mediated cholesterol
efflux in a lipid-laden macrophages.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM)chemicals,
fatty acid-free bovine serum albumin (BSA) and Oil red O were
provided by Sigma-Aldrich Chemical (St. Louis, MO), as were all
other reagents, unless specifically stated otherwise. Fetal bovine
serum and penicillin-streptomycin were obtained from Lonza
(Walkersville, MD). SR-B1 antibody was supplied by Santa Cruz
Biotechnology (Santa Cruz, CA). ABCA1 and ABCG1 antibodies were
purchased from Novus Biologicals (Littleton, CO). Apolipoprotein
(apo) E antibody was obtained from Abcam (Cambridge, UK). β-actin
antibody was provided from Sigma Chemicals. Horseradish
peroxidase-conjugated goat anti-rabbit IgG and rabbit anti-mouse
IgG were supplied by Jackson Immuno Research Laboratory (West
Grove, PA). Homoplantaginin and hispidulin were supplied by
Shanghai Tauto Biotech Co., Ltd. (Shanghai, China).
Preparation of SWE
Sage weeds (Salvia plebeia R.Br.) were
purchased from Dae Kwang Herb (Chuncheon, Korea). The voucher
specimen (RIC-22) was deposited at Regional Innovation Center
(Hallym University, Chuncheon, Korea). The dried whole parts of
sage weeds were pulverized and extracted with 100% methanol for 4 h
at 65°C (5L, 3 times). The liquid extract was evaporated in
vacuo to give crude SWE. In the experiments, SWE,
homoplantaginin and hispidulin were dissolved in dimethyl sulfoxide
(DMSO) for live culture with cells; its final culture concentration
was ≤0.1%.
Preparation and oxidation of human plasma
LDL
Human plasma LDL was prepared by a discontinuous
density gradient ultra-centrifugation as previously described
(14). Pooled human
normolipidemic plasma LDL fraction was dialyzed overnight against
0.154 mM NaCl and 0.01% EDTA (pH 7.4) at 4°C and used within 4
weeks. Protein concentration of the plasma LDL fraction was
measured by the Lowry method (15), and the concentrations of
triacylglycerol, total cholesterol and phospholipids were
determined using diagnostic kits (Asan Pharmaceuticals, Hwasung,
Korea).
Oxidized LDL was prepared by incubating LDL fraction
with 10 μM CuSO4 (Cu2+) in F-10 medium at
37°C for 24 h. The LDL oxidation was routinely checked using TBARS
concentration and eletrophoretic mobility assay (16). Aliquots of oxidized LDL were run
on a 0.8% agarose eletrophoresis gel in barbital buffer (pH 8.6) to
measure eletrophoretic mobility. Gel photographs were obtained
using a Polaroid film (Polaroid, Wayland, MA).
Cell culture
The macrophage-like cell line J774A1 (mouse
histocytic lymphoma cells) were grown in DMEM supplemented with 10%
FBS at 37°C in a humidified atmosphere of 5% CO2 in air.
Macrophages were pre-treated with 1–20 μg/ml SWE, 10 μM
homoplantaginin, or 10 μM hispidulin and exposed to 50 μg/ml
cholesterol-oxidized LDL for various times. For the lipid uptake,
macrophages were incubated in DMEM supplemented with 0.4% fatty
acid-free BSA.
Lipid uptake
Oil red O staining was performed to detect foam
cells of macrophages. Oil red O is a fat-soluble diazo dye staining
neutral triglycerides and some lipoproteins (2). J774A1 cells cultured with 50 μg/ml
oxidized LDL were washed with phosphate buffered saline (PBS)
containing 0.05% Tween-20, and fixed in 4% ice-cold formaldehyde
for 1 h. Subsequently, 0.5% oil red O dissolved in 60% 2-propanol
was added to cells for 1 h. After mounting with aqueous mounting
medium, images were obtained by using an optical microscope. Oil
red O staining was quantified by dissolving stained cells in 100%
isopropanol with a spectrophotometer at λ/nm = 490.
Western blot analysis
Western blot analysis was performed using whole cell
extracts from J774A1 macrophage as previously described (17). Cells were lysed in a lysis buffer
containing 1% β-mercaptoethanol, 1 M β-glycerophosphate, 0.1 M
Na3VO4, 0.5 M NaF and protease inhibitor
cocktail. Equal protein amounts of cell lysates and equal volumes
of culture media were electrophoresed on 6–12% SDS-PAGE and
transferred onto a nitrocellulose membrane. After blocking
non-specific binding with 5% skim milk for 3 h, the membrane was
incubated overnight at 4°C with polyclonal rabbit antibodies of
SR-B1, ABCA1, ABCG1 and apoE. After three washes with Tris-buffered
saline-Tween-20, the membrane was incubated for 1 h with a goat
anti-rabbit IgG or a rabbit anti-mouse IgG conjugated to
horseradish peroxidase (HRP). The individual protein level was
determined using immobilon western chemiluminescent horseradish
peroxidase substrate (Millipore Corp., Billerica, MA) and Agfa
X-ray film (Agfa-Gevaert, Belgium). Incubation with monoclonal
mouse β-actin antibody was also performed for comparative
controls.
Quantitative RT-PCR analysis
Following culture protocols, total RNA was isolated
from J774A1 macrophages using a commercially available TRIzol
reagent kit (Molecular Research Center, Cincinnati, OH). The RNA (5
μg) was reverse transcribed with 200 U of reverse transcriptase
(Promega Corp., Madison, WI) and 0.5 g/l oligo-(dT)15
primer (Bioneer, Korea). RT-PCR analysis was also performed for
semi-quantifying the levels of mRNA transcripts of SR-B1, ABCA1 and
ABCG1. The PCR conditions for SR-B1 [5′-ATG GGC CAG CGT GCT TTT ATG
A-3′ (forward), 5′-AAC CAC AGC AAC GGC AGA ACT A-3′ (reverse, 752
bp)] and ABCA1 [5′-AGC TGC CCC ATC ATG TAA AG-3′ (forward), 5′-GGG
AGA AGA GCG TGC TAA TG-3′ (reverse, 580 bp) were 94°C (3 min), and
30 cycles at 94°C (30 sec), 60°C (45 sec) and 72°C (45 sec).
Moreover, the condition for ABCG1 [5′-CCA AGT GGT GTC TCT GAT GA-3′
(forward), 5′-CTG AGG AAG GTC CTC TTG AA-3′ (reverse, 450 bp)] was
94°C (3 min), and 28 cycles at 94°C (30 sec), 55°C (45 sec) and
72°C (45 sec). The housekeeping gene GAPDH [5′-AAC TTT GGC ATT GTG
GAA GGG-3′ (forward), 5′-GAC ACA TTG GGG GTA GGA ACA C-3′ (reverse,
224 bp)] was used for an internal normalization for the
co-amplification with the respective gene.
Cholesterol efflux assay
J774A1 macrophages were treated with 1–20 μg/ml SWE
for 12 h and then equilibrated with 1 μg/ml
3-dodecanoyl-NBD-labeled cholesterol (Cayman Chemical, Ann Arbor,
MI) for additional 6 h. Cells exposed to NBD-labeled cholesterol
were washed with PBS and incubated in DMEM for 6 h. The
fluorescence-labeled cholesterol released from cells into medium
for 6 h was detected at wavelength range λ = 485–538 nm by using a
fluorometer. Cholesterol efflux was expressed as percent
fluorescence in medium relative to total fluorescence.
Enzyme-linked immunosorbent assay
(ELISA)
Following culture protocols, culture media were
collected to measure HDL formation by using sandwich ELISA kits
(Uscn Life Science Inc., Wuhan, China). After reacting to collected
media on microtiter plate, wells pre-coated with a biotinconjugated
antibody specific to HDL, avidin-conjugated to HRP was added to
microplate wells and incubated. The TMB substrate solution was
added to wells for detecting color change and the enzyme-substrate
reaction was terminated by the addition of 3 N sulphuric acid
solution. The color change was measured spectrophotometrically at λ
= 450 nm.
Data analysis
The results are presented as mean ± SEM. Statistical
analyses were conducted using the Statistical Analysis Statistical
software package version 6.12 (SAS institute, Cary, NC). One-way
ANOVA was used to determine the inhibitory effects of SWE on the
cholesterol handling of macrophages. Differences among the
treatment groups were analyzed with Duncan’s multiple range test
and were considered to be significant at P≤0.05.
Results
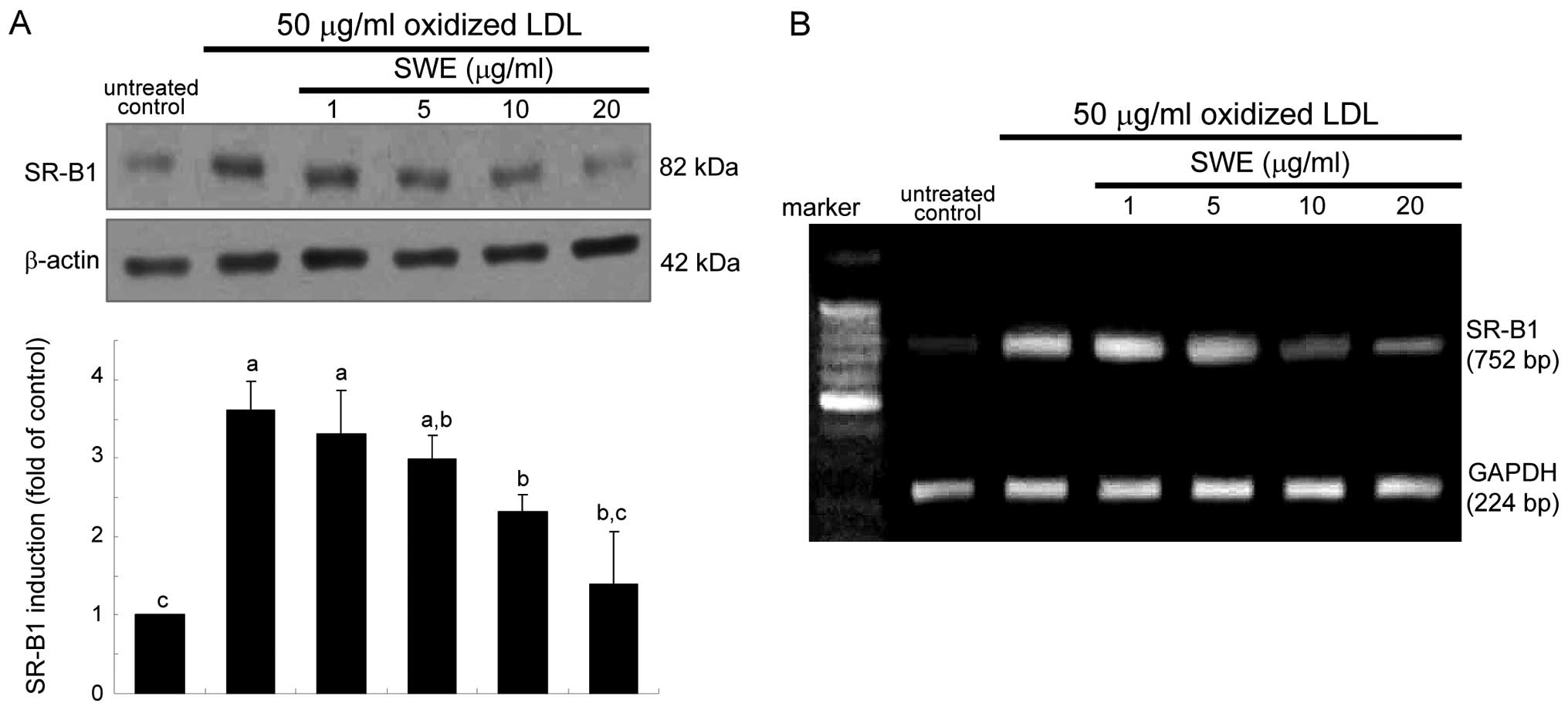
Suppression of SR-B1 expression by
SWE
It has been shown that the uptake of oxidized LDL
require SR induction in macrophages (1,2).
As expected, oxidized LDL hastened SR-B1 expression rapidly within
2 h, which was sustained up to 8 h (data not shown). When oxidized
LDL was treated for 6 h, the SR-B1 expression diminished in a
dose-dependent manner due to the presence of 1–20 μg/ml SWE
(Fig. 1A). This study
investigated whether the suppression of SR-B1 induction by SWE was
achieved at its transcriptional level. Quantitative RT-PCR data
revealed that ≥10 μg/ml SWE lowered oxidized LDL-elevated SR-B1
mRNA level of macrophages (Fig.
1B). It should be noted that treating macrophages with oxidized
LDL markedly elevated their SR-B1 mRNA level within 2 h (data not
shown).
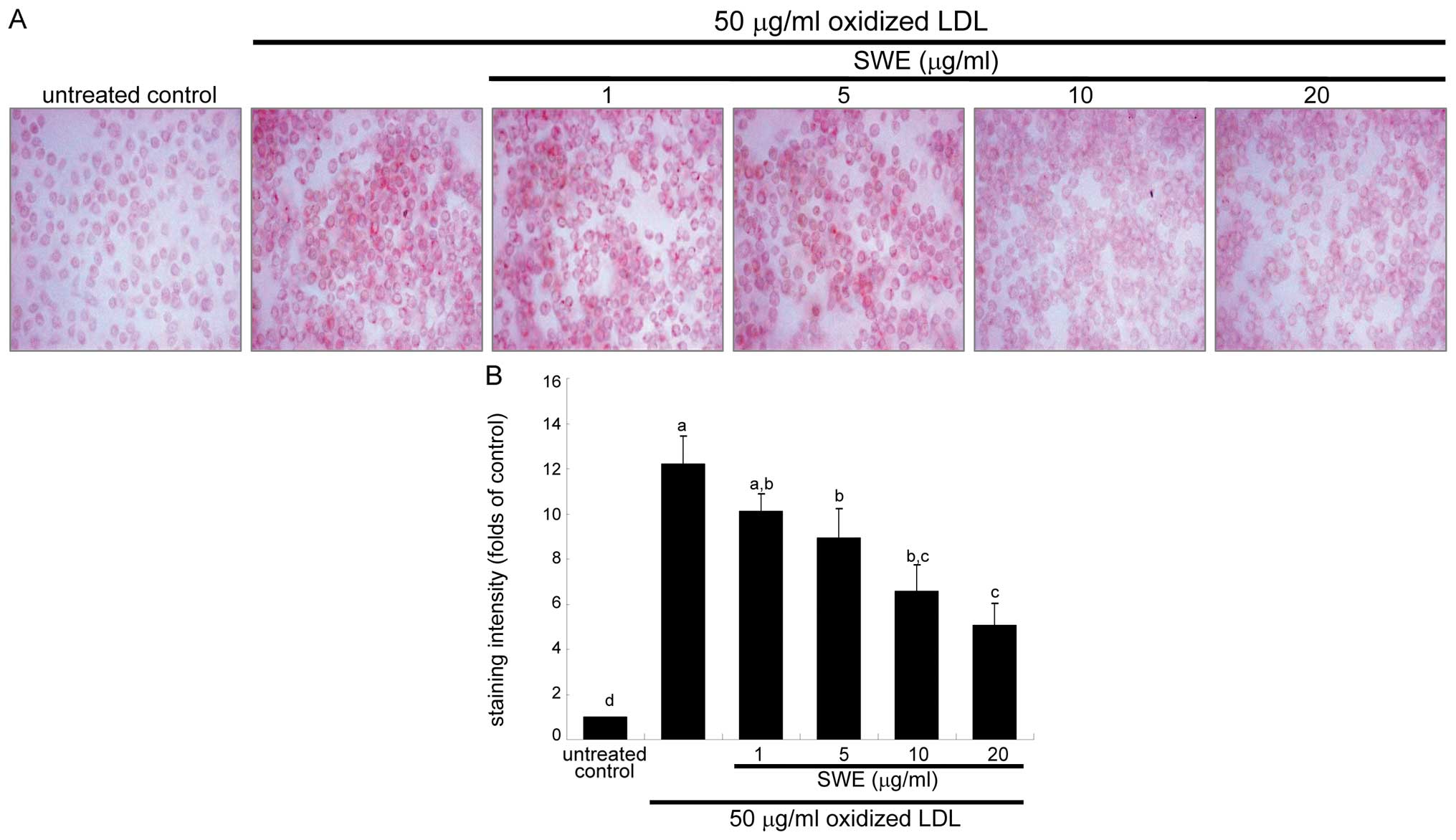
Effects of SWE on reduction of foam cell
formation
This study examined intracellular lipid accumulation
in macrophages by using oil red O staining. There was strong
reddish staining observed in macrophages exposed to 50 μg/ml
oxidized LDL for 18 h. This indicates cellular accumulation of
lipids through the upregulated SR-B1. When ≥5 μg/ml SWE was applied
to macrophages for 18 h, reddish lipid droplets disappeared
(Fig. 2). Accordingly, SWE
delayed foam cell formation in macrophages exposed to 50 μg/ml
oxidized LDL.
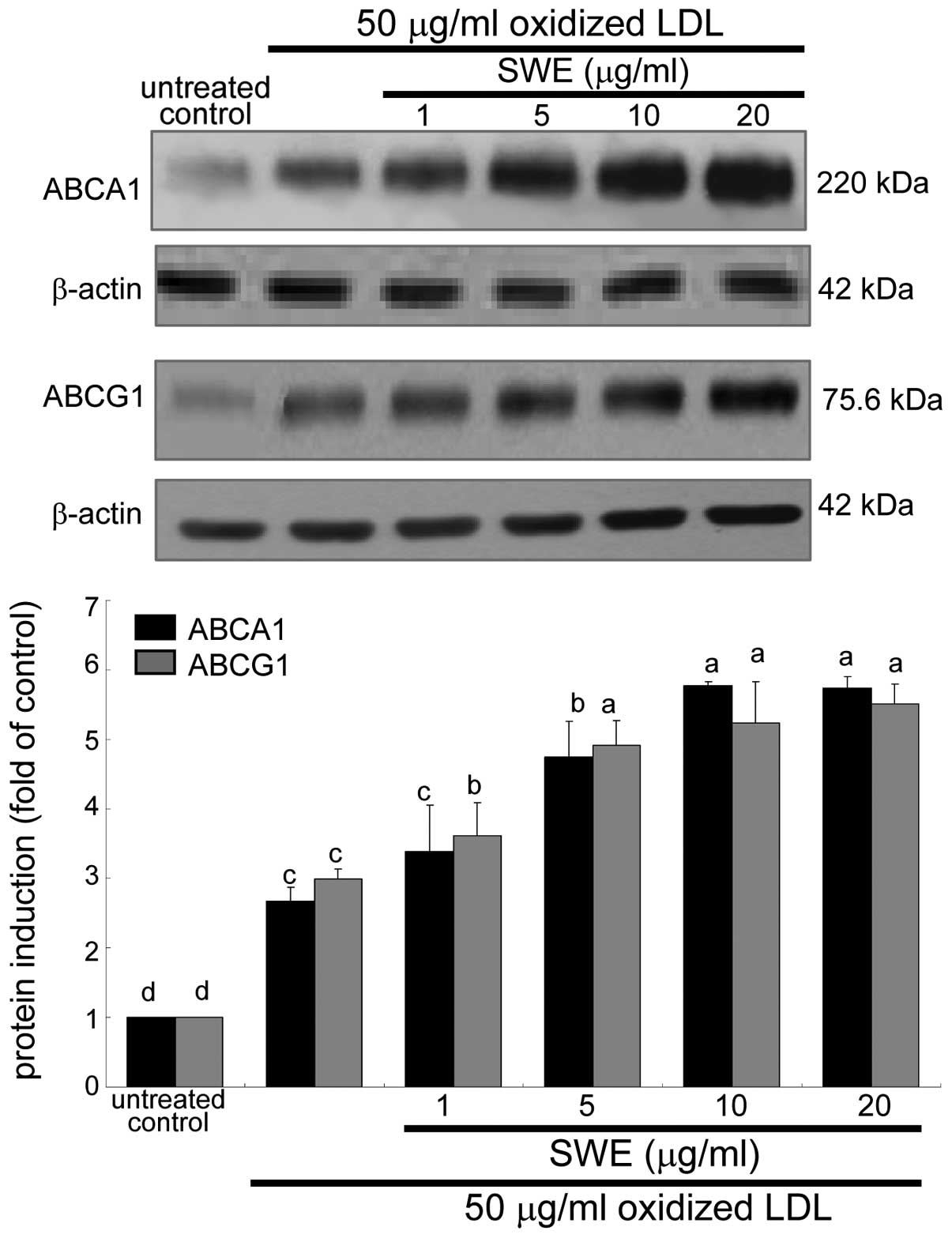
Enhancement of ABCA1 and ABCG1 expression
by SWE
The membrane proteins of ABCA1 and ABCG1 are
responsible for cholesterol efflux from lipid-laden macrophages
(6,7). The application of 50 μg/ml oxidized
LDL to macrophages promoted the expression of ABCA1 and ABCG1 8 h
after its treatment (data not shown). When ≥5 μg/ml SWE was applied
to macrophages exposed to oxidized LDL, the induction of these
proteins was further upregulated (Fig. 3). Accordingly, ≥5 μg/ml SWE may
promote cholesterol efflux from lipid-laden foam cells. In
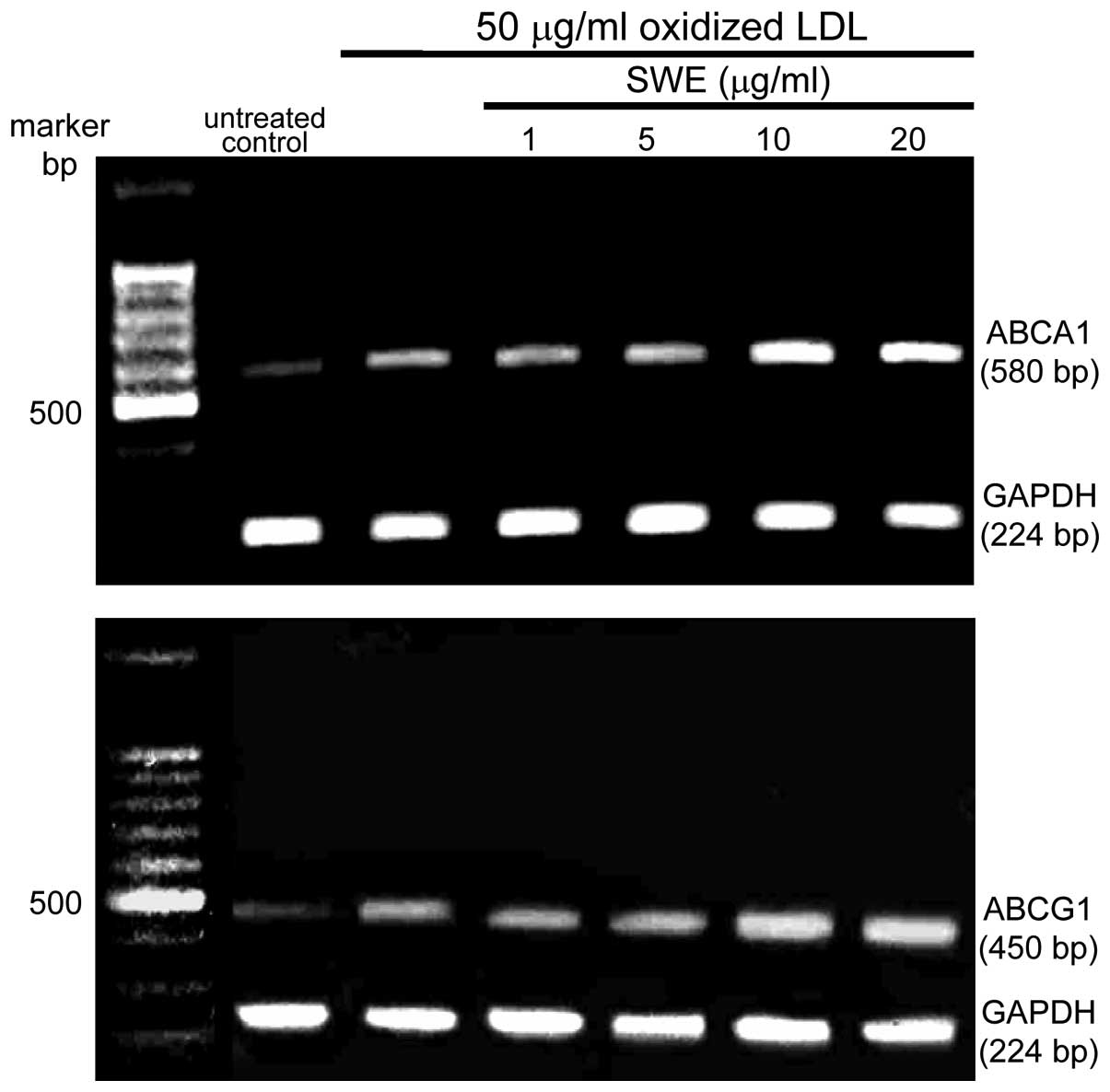
addition, the ABCA1 and ABCG1 mRNA levels were elevated within 2 h
in macrophages exposed to 50 μg/ml oxidized LDL, as determined by
quantitative RT-PCR assay. The transcription of ABCA1 and ABCG1 was
further enhanced in lipid-laden macrophages supplemented with ≥10
μg/ml SWE (Fig. 4).
Acceleration of cholesterol efflux by
SWE
It has been shown that cholesterol efflux from
macrophages to apoE decreases foam cell formation and prevents
atherosclerosis (8,10). This study showed that apoE was
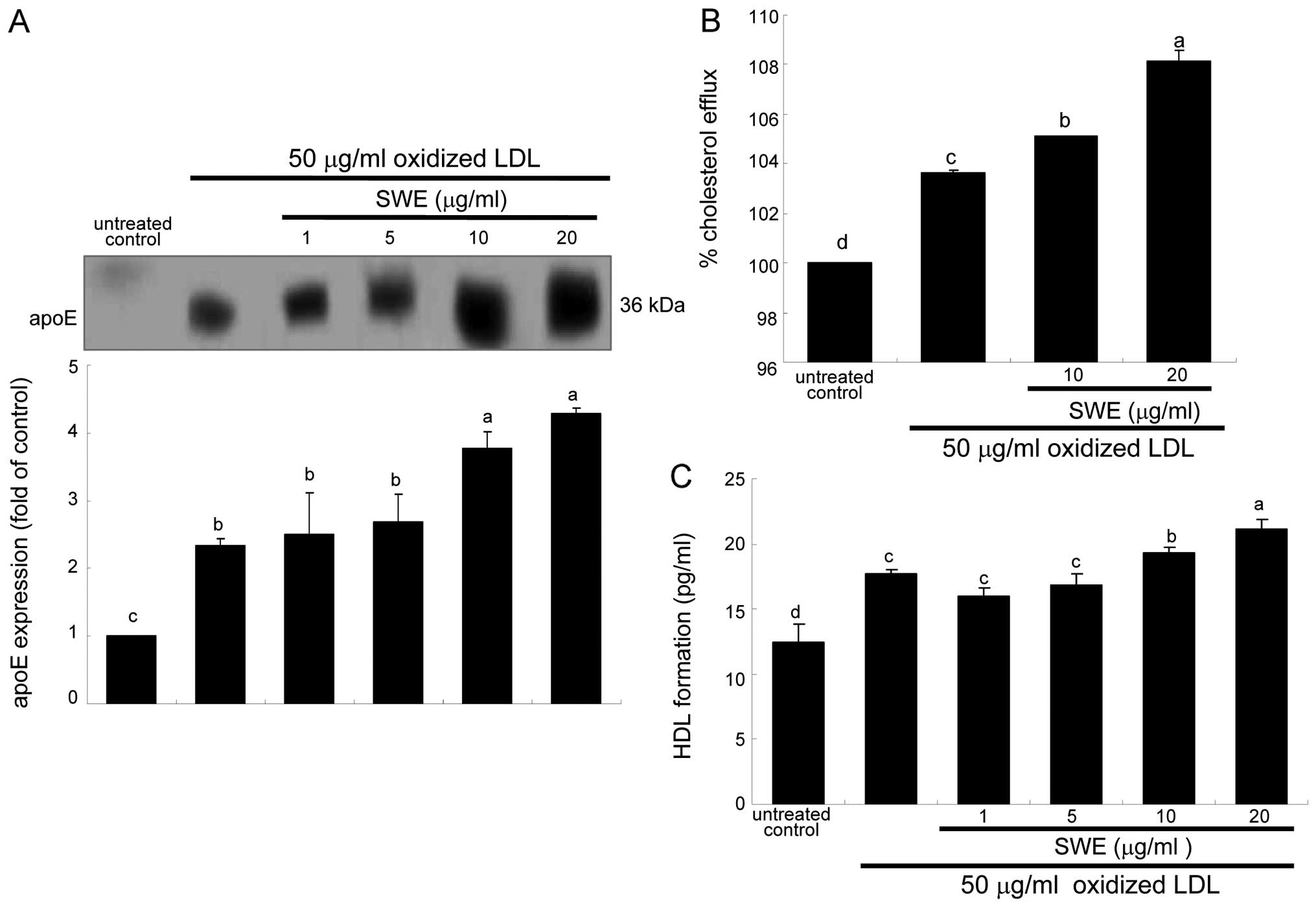
secreted from 50 μg/ml oxidized LDL-added macrophages (Fig. 5A). In lipid-laden macrophages
supplemented with ≥10 μg/ml SWE, apoE secretion was enhanced at
protein levels (Fig. 5A). Thus,
it is deemed that ≥10 μg/ml SWE promoted cholesterol efflux through
ABCA1 and ABCG1 upregulated by apoE (10).
SWE boosted the induction of ABCA1 and ABCG1
proteins at the transcriptional levels (Fig. 3). Consistently, cholesterol efflux
was enhanced in oxidized LDL-treated macrophages (Fig. 5B). The efflux was further
accelerated in macrophages exposed to 50 μg/ml oxidized LDL and
treated with ≥10 μg/ml SWE (Fig.
5B). In addition, the HDL formation was enhanced at levels of
protein in lipid-laden macrophages treated with oxidized LDL, which
was further highly enhanced by 20 μg/ml SWE (Fig. 5C).
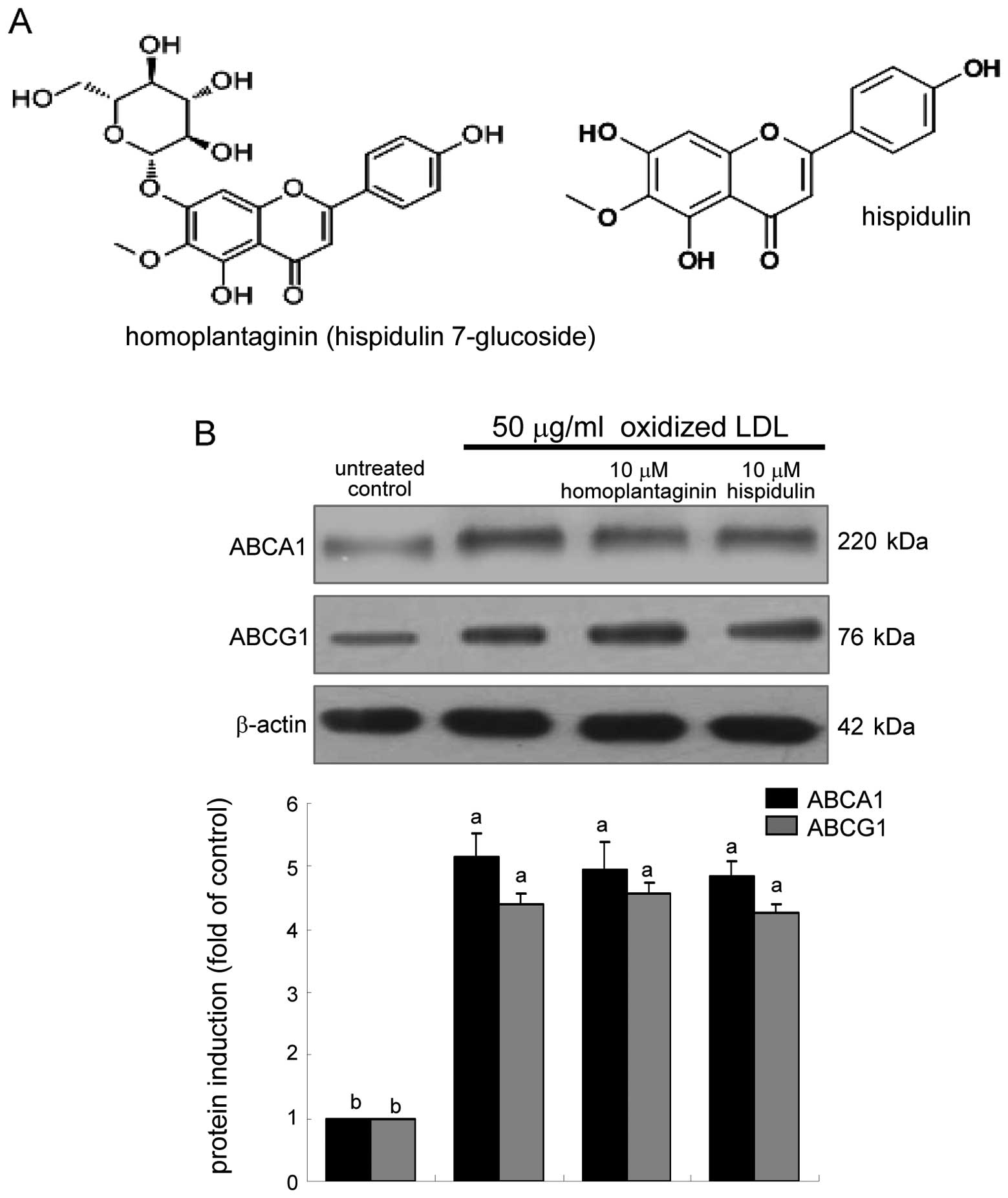
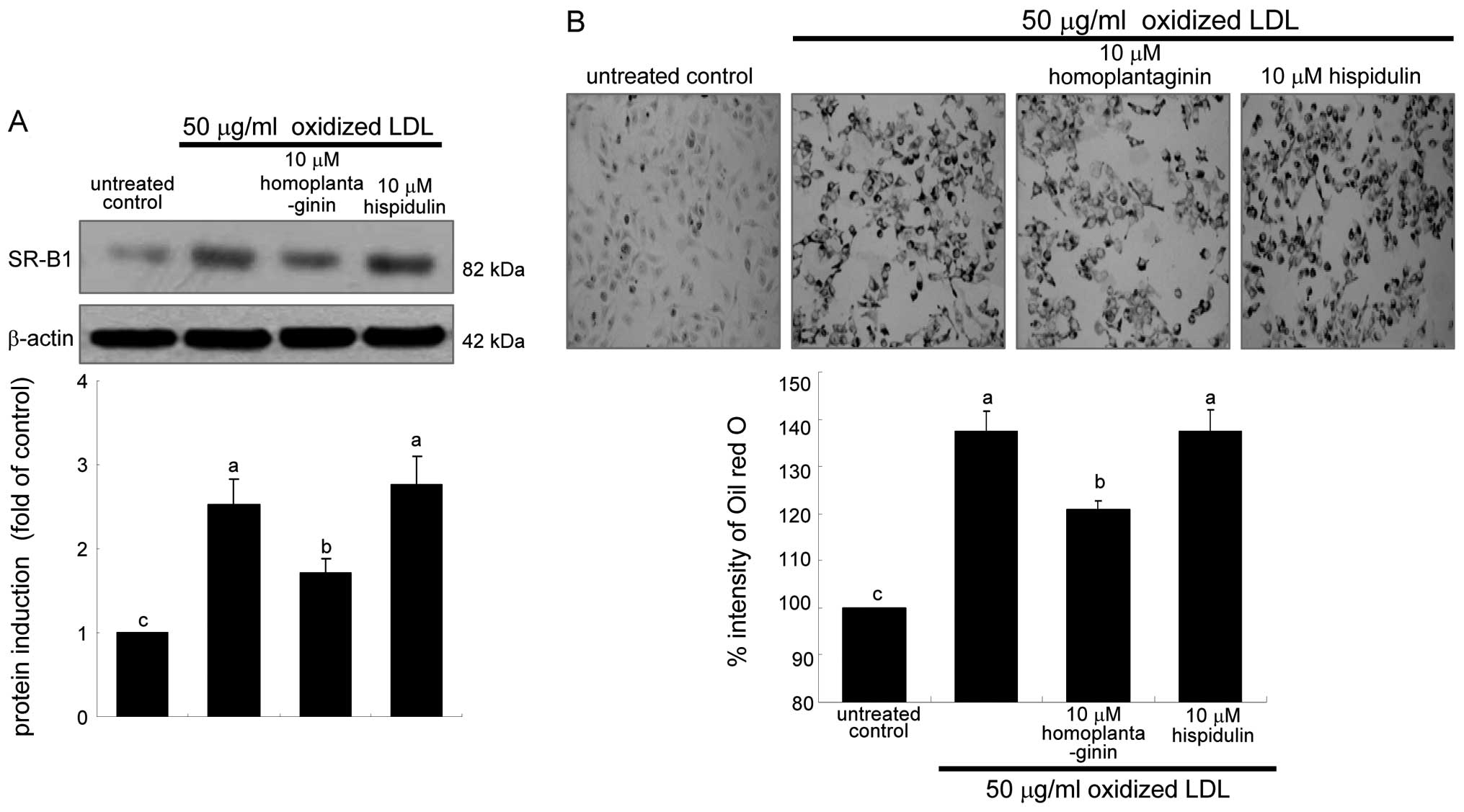
Inhibitory effects of homoplantaginin on
uptake of oxidized LDL
The SWE application to oxidized LDL-treated
macrophages upregulated the cellular expression of ABCA1 and ABCG1
(Fig. 3). This study elucidated
whether homoplantaginin and hispidulin, the single compounds
present in sage weeds (Fig. 6A),
influenced cholesterol efflux from lipid-laden foam cells.
Homoplantaginin and hispidulin did not modulate the cellular
expression of ABCA1 and ABCG1 upregulated in oxidized LDL-exposed
macrophages (Fig. 6B). In
contrast, 10 μM homoplantaginin, but not hispidulin inhibited SR-B1
induction and consequent uptake of oxidized LDL in J774A.1
macrophages (Fig. 7). These
results indicate that SWE containing homoplantaginin retarded foam
cell formation as an anti-atherogenic agent.
Discussion
There are five major observations extracted from
this study. i) SWE at ≥10 μg/ml inhibited SR-B1 expression
accounting for oxidized LDL uptake and cellular lipid accumulation
in J774A1 murine macrophages. ii) Oxidized LDL enhanced ABCA1 and
ABCG1 expression, which was further promoted at transcriptional and
protein levels by ≥5 μg/ml SWE. iii) SWE accelerated apoE secretion
from lipid-laden macrophages. iv) SWE promoted cholesterol efflux
and HDL formation through inducing ABCA1 and ABCG1 expression. v)
Homoplantaginin but not hispidulin, the single components of sage
weeds, alleviated SR-B1 induction and foam cell formation.
Accordingly, it is deemed that SWE antagonized foam cell formation
in the vascular wall and promoted the export of cholesterol from
lipid-laden macrophages to the reverse cholesterol transport.
Therefore, SWE may be a therapeutic anti-atherogenic agent in the
development of atherosclerosis.
Oxidized LDL uptake, is a key initial event in
atherogenesis, steering foam cell formation toward the development
of atherosclerosis. Modified LDL is taken up mainly by SR-A and
SR-B1 of macrophages (1).
Especially the macrophage SR-B1 is responsible for oxidized LDL
uptake and facilitates the intracellular accumulation of lipid
droplets (2,18). The present study revealed that SWE
dampened foam cell formation augmented within 18 h after treating
oxidized LDL. This inhibition was mediated most likely through
downregulating SR-B1 induction stimulated by oxidized LDL.
Intracellular lipids in the cytoplasm of macrophages are shown to
be degraded to phospholipids, oxysterols and free cholesterol in
the lysosome (3,19). Cytotoxic oxysterols induce
macrophage apoptosis and play a central role in promoting
atherogenesis with the build-up of atherosclerotic plaques
(4,5). Accordingly, SWE is a potential
anti-atherogenic agent blunting oxidized LDL uptake leading to
production of oxysterols. It should be noted that homoplantaginin
may play a role in antagonizing foam cell formation by SWE. The
antagonizing activity appeared to require the glucose moiety of
homoplataginin. However, the underlying mechanisms by which SWE
interrupted oxidized LDL uptake remain to be elucidated. The
inhibition of oxidized LDL-induced SR-B1 induction by SWE was
achieved at its transcriptional level. Similarly, soy pinitol has
inhibitory effects on foam cell formation by reducing lipid
accumulation and macrophage SR expression via its insulin-like
action (20).
Sage weed as a folk medicine has been investigated
for its medicinal properties (11). The ethanol extract of Salvia
plebeia possessed anti-inflammatory and anti-angiogenic,
anti-nociceptive and antioxidant activities (12). Numerous antioxidant compounds such
as royleanonic acid, hispidulin and eupatorin were isolated and
identified in crude extract of sage weed (11). Chemical fingerprint and
quantitative analysis showed that seven bioactive compounds of
homoplantaginin, hispidulin, luteolin, nepetin, caffeic acid,
luteolin-7-glucoside, and nepetin-7-glucoside contained as
constituents in Salvia plebeia (21). The major compound of Salvia
plebeia, homoplantaginin, has a protective and therapeutic
effect on hepatocyte injury, which might be associated with its
antioxidant activity (21,22).
Unfortunately, the present study did not identify the constituents
of SWE. However, this study found that homoplataginin, a major
constituent in Salvia plebeia, inhibited cellular SR-B1
induction and oxidized LDL uptake of lipid-laden macrophages. There
have been no reports demonstrating anti-atherogenic activity of SWE
and homoplantaginin. It can be speculated that homoplataginin
present in sage weeds may exert anti-atherogenic activity dampening
SR-B1 induction.
The export of cholesterol and lipids from
macrophages is arbitrated by reverse cholesterol transport, the
process involving ABCA1 and ABCG1 (6,7).
These proteins mediate the movement of cholesterol between cells
and extracellular acceptors (23). ABCA1 is responsible for the export
of cholesterol and phospholipids to generate precursors for HDL
particles (6,8). In addition, ABCG1 mediates oxysterol
efflux from oxidized LDL-loaded macrophages (7). Accordingly, the upregulation of
ABCA1 and ABCG1 is considered anti-atherogenic. This study showed
that SWE further upregulated ABCA1 and ABCG1 of lipid-laden foam
cells and elevated cholesterol efflux. Thus, SWE may be a
therapeutically athero-protective agent against the development of
atherosclerosis. Several studies show that hispidulin, another
compound present in Salvia plebeia, exerts anticancer
effects and has the potential to be a chemopreventive and
therapeutic agent (24,25). Nevertheless, this compound is
unknown in the anti-atherogenic activity upregulating cholesterol
efflux. This study failed to show promoting effects of
homoplantaginin and hispidulin, on ABCA1 expression. However,
caffeic acid, one of Salvia plebeia constituents, enhanced
cholesterol efflux from THP-1 macrophages mediated by HDL, and
induced expression of ABCG1 and SR-BI but not ABCA1 (26). Thus, other unknown components
appeared to be responsibe for increasing cholesterol efflux and HDL
formation by SWE.
ApoE is essential for the normal catabolism of
triglyceride-rich lipoprotein constituents. There are numerous
studies in regard of apoE-mediated cholesterol efflux. Cholesterol
efflux from macrophages to apoE diminishes foam cell formation and
prevents atherosclerosis (9,10).
However, very little is known about the apoE activity related to
cholesterol transporters. Several membrane transporters related to
cholesterol efflux include ABCA1, ABCG1, SR-B1 and caveolin-1
(27). A recent study showed that
apoE is involved in cholesterol efflux in macrophages by
upregulating ABCA1 expression (10). This study showed that SWE was an
apoE promoter enhancing its secretion in oxidized LDL-loaded
macrophages. This may be an athero-protective mechanism by which
SWE boosted ABCA1 expression and cholesterol efflux. Conversely,
epigallocatechin-3-gallate attenuated downregulated ABCA1 and
decreased cholesterol efflux to apoA1 in TNF-α-exposed macrophage
foam cells (28).
In conclusion, this study demonstrated that SWE
dampened oxidized LDL uptake through reducing SR-B1 expression at a
transcriptional level and attenuated foam cell formation full of
cholesterol in the cytoplasm of macrophages. Homoplantaginin but
not hispidulin, major components of SWE, alleviated foam cell
formation by downregulating of SR-B1 induction. In addition, SWE
enhanced cholesterol efflux in oxidized LDL-loaded macrophages
through further inducing ABCA1 and ABCG1 expression and apoE
secretion. Other unknown components in SWE, but not homoplantaginin
and hispidulin, appeared to be involved in HDL formation.
Therefore, SWE may be a protective agent against atherosclerosis by
inhibiting foam cell formation and boosting cholesterol efflux from
lipid-laden macrophages.
Abbreviations:
|
ABCA1
|
ATP-binding cassette transporter
A1
|
|
ABCG1
|
ATP-binding cassette transporter
G1
|
|
apoE
|
apolipoprotein E
|
|
LDL
|
low-density lipoproteins
|
|
SR
|
scavenger receptor
|
|
SWE
|
sage weed methanol extract
|
Acknowledgements
This study was financially supported
by Ministry for Food, Agriculture, Forestry and Fisheries
(112085-3), National Research Foundation of Korea (2012012946), and
by National Research Foundation of Korea through the Human Resource
Training Project for Regional Innovation
(2012-01-A-05-003-12-010100).
References
|
1.
|
VV KunjathoorM FebbraioEA PodrezKJ MooreL
AnderssonS KoehnJS RheeR SilversteinHF HoffMW FreemanScavenger
receptors class A-I/II and CD36 are the principal receptors
responsible for the uptake of modified low density lipoprotein
leading to lipid loading in macrophagesJ Biol
Chem774998249988200210.1074/jbc.M209649200
|
|
2.
|
JS ChoiJY BaeDS KimJ LiJL KimYJ LeeYH
KangDietary compound quercitrin dampens VEGF Induction and PPARγ
activation in oxidized LDL-exposed murine macrophages: Association
with scavenger receptor CD36J Agric Food
Chem5813331341201019928818
|
|
3.
|
Y WenDS LeakeLow density lipoprotein
undergoes oxidation within lysosomes in cellsCirc
Res10013371343200710.1161/CIRCRESAHA.107.15170417446432
|
|
4.
|
N ShibataCK GlassMacrophages, oxysterols
and atherosclerosisCirc
J7420452051201010.1253/circj.CJ-10-086020838002
|
|
5.
|
NE FreemanAE RusinolM LintonDL HacheyS
FazioMS SinenskyD ThewkeAcyl-coenzyme A: cholesterol
acyltransferase promotes oxidized LDL/oxysterol-induced apoptosis
in macrophagesJ Lipid
Res4619331943200510.1194/jlr.M500101-JLR20015995174
|
|
6.
|
A ChawlaWA BoisvertCH LeeBA LaffitteY
BarakSB JosephD LiaoL NagyPA EdwardsLK CurtissRM EvansP TontonozA
PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol
efflux and atherogenesisMol Cell71611712001
|
|
7.
|
M XuH ZhouKC TanR GuoSW ShiuY WongABCG1
mediated oxidized LDL-derived oxysterol efflux from
macrophagesBiochem Biophys Res
Commun39013491354200910.1016/j.bbrc.2009.10.15219895785
|
|
8.
|
TE AkiyamaS SakaiG LambertCJ NicolK
MatsusueS PimpraleYH LeeM RicoteCK GlassHB Brewer JrFJ
GonzalezConditional disruption of the peroxisome
proliferator-activated receptor gamma gene in mice results in
lowered expression of ABCA1, ABCG1, and apoE in macrophages and
reduced cholesterol effluxMol Cell
Biol2226072619200210.1128/MCB.22.8.2607-2619.2002
|
|
9.
|
DE DoveMF LintonS FazioApoE-mediated
cholesterol efflux from macrophages: separation of autocrine and
paracrine effectsAm J Physiol Cell
Physiol288C586C592200510.1152/ajpcell.00210.200415509658
|
|
10.
|
Y ZhaoX ChenH YangL ZhouEU OkoroZ GuoA
novel function of apolipoprotein E: upregulation of ATP-binding
cassette transporter A1 expressionPLoS
One6e21453201110.1371/journal.pone.002145321779326
|
|
11.
|
L GuX WengAntioxidant activity and
components of Salvia plebeia R.Br. - a Chinese herbFood
Chem73299305200110.1016/S0308-8146(00)00300-9
|
|
12.
|
HJ JungYS SongCJ LimEH
ParkAnti-inflammatory, anti-angiogenic and anti-nociceptive
activities of an ethanol extract of Salvia plebeia R. BrownJ
Ethnopharmacol126335360200919715750
|
|
13.
|
JA LimBW YunSH BaekAntioxidative activity
and nitrite scavenging ability of methanol extract from Salvia
plebeia R. BrKorean J Med Crop Sci151831882007
|
|
14.
|
JS ChoiSW KangJ LiJL KimJY BaeDS KimSY
ShinJG JunMH WangYH KangBlockade of oxidized LDL-triggered
endothelial apoptosis by quercetin and rutin through differential
signaling pathways involving JAK2J Agric Food
Chem5720792086200910.1021/jf803390m19196000
|
|
15.
|
OH LowryNJ RosebroughAL FarrRJ
RandallProtein measurement with the Folin phenol reagentJ Biol
Chem193265275195114907713
|
|
16.
|
YJ JeongYJ ChoiJS ChoiHM KwonSW KangJY
BaeSS LeeJS KangSJ HanYH KangAttenuation of monocyte adhesion and
oxidised LDL uptake in luteolin-treated human endothelial cells
exposed to oxidised LDLBr J
Nutr97447457200710.1017/S000711450765789417313705
|
|
17.
|
SW KangJS ChoiJY BaeJ LiDS KimJL KimSY
ShinHJ YouHS ParkGE JiYH KangBlockade of vascular angiogenesis by
Aspergillus usamii var. shirousamii-transformed Angelicae
Gigantis Radix and Zizyphus jujubaNutr Res
Pract338200920016695
|
|
18.
|
G EndemannLW StantonKS MaddenCM BryantRT
WhiteAA ProtterCD36 is a receptor for oxidized low density
lipoproteinJ Biol Chem268118111181619937685021
|
|
19.
|
W LiXM YuanAG OlssonUT BrunkUptake of
oxidized LDL by macrophages results in partial lysosomal enzyme
inactivation and relocationArterioscler Thromb Vasc
Biol18177184199810.1161/01.ATV.18.2.1779484981
|
|
20.
|
MS ChoiWH LeeEY KwonMA KangMK LeeYB ParkSM
JeonEffects of soy pinitol on the pro-inflammatory cytokines and
scavenger receptors in oxidized low-density lipoprotein-treated
THP-1 macrophagesJ Med
Food10594601200710.1089/jmf.2006.22018158828
|
|
21.
|
XF JinYH LuDZ WeiZT WangChemical
fingerprint and quantitative analysis of Salvia plebeia
R.Br. by high-performance liquid chromatographyJ Pharm Biomed
Anal481001042008
|
|
22.
|
XJ QuX XiaYS WangMJ SongLL LiuYY XieYN
ChengXJ LiuLL QiuL XiangProtective effects of Salvia plebeia
compound homoplantaginin on hepatocyte injuryFood Chem
Toxicol47171017152009
|
|
23.
|
M OquraM AyaoriY TeraoT HisadaM IizukaS
TakiquchiH Uto-KondoE YakushijiK NakayaM SasakiProteasomal
inhibition promotes ATP-binding cassette transporter A1 (ABCA1) and
ABCG1 expression and cholesterol efflux from macrophages in vitro
and in vivoArterioscler Thromb Vasc
Biol3119801987201110.1161/ATVBAHA.111.22847821817095
|
|
24.
|
YC LinCM HungJC TsaiJC LeeYL ChenCW WeiJY
KaoTD WayHispidulin potently inhibits human glioblastoma multiforme
cells through activation of AMP-activated protein kinase (AMPK)J
Agric Food Chem5895119517201010.1021/jf101953320698539
|
|
25.
|
L HeY WuL LinJ WangY WuY ChenZ YiM LiuX
PangHispidulin, a small flavonoid molecule, suppresses the
angiogenesis and growth of human pancreatic cancer by targeting
vascular endothelial growth factor receptor 2-mediated
PI3K/Akt/mTOR signaling pathwayCancer
Sci102219225201110.1111/j.1349-7006.2010.01778.x
|
|
26.
|
H Uto-KondoM AyaoriM OguraK NakayaM ItoA
SuzukiS TakiguchiE YakushijiY TeraoH OzasaCoffee consumption
enhances high-density lipoprotein-mediated cholesterol efflux in
macrophagesCirc
Res106779787201010.1161/CIRCRESAHA.109.20661520075335
|
|
27.
|
AR TallCholesterol efflux pathways and
other potential mechanisms involved in the athero-protective effect
of high density lipoproteinsJ Intern
Med263256273200810.1111/j.1365-2796.2007.01898.x18271871
|
|
28.
|
J JiangZC MoK YinGJ ZhaoYC LvXP OuyangZS
JiangY FuCK TangEpigallocatechin-3-gallate prevents TNF-α-induced
NF-κB activation thereby upregulating ABCA1 via the Nrf2/Keap1
pathway in macrophage foam cellsInt J Mol Med299469562012
|