Introduction
To achieve the optimal effects of gene therapy, it
is crucial to develop a strategy to conditionally express the
therapeutic gene of interest and reduce any unexpected side-effects
resulting from excessive expression of the exogenous gene (1,2).
Previous studies have suggested that multiple copies of the hypoxia
response element (HRE) can regulate the expression of therapeutic
genes in response to hypoxia (3–6).
The hypoxia inducible factor-1 (HIF-1) is a transcription factor
that plays a key role in the regulation of gene expression under
hypoxic conditions (7). HIF-1 is
composed of 2 subunits, HIF-1α and HIF-1β. Under normoxic
conditions, HIF-1α is rapidly degraded by the ubiquitin-proteasome
pathway. However, under hypoxic conditions, HIF-1α becomes
stabilized and forms an active HIF-1 heterodimer with HIF-1β.
Through its binding to the HRE located in the regulatory domain of
target genes, HIF-1α can upregulate the expression of several
hypoxia-related genes in response to hypoxia (8). Thus, HRE can regulate downstream
gene expression according to the activity of HIF-1, which is
affected by the oxygen concentration.
Neurotrophin-3 (NT-3) not only activates its own
specific orphan receptor, TrkC, but also activates TrkA and TrkB to
mediate almost all of its neuron survival and
differentiation-promoting activities in the central and peripheral
nervous system (9). In
vitro, exogenous NT-3 promotes the proliferation and survival
of neural stem cells and increases the percentage of neural stem
cells that differentiate into neurons (10–12). In vivo, NT-3 facilitates
angiogenesis and neurogenesis by which functional recovery can be
promoted after stroke (13–15). These results suggest that NT-3 may
be an optimal gene to promote neuroprotection against a number of
brain injuries including cerebral ischemia.
In the present study, we generated a recombinant
retroviral vector expressing NT-3 under the control of a cassette
constructed by 5 copies of the HRE and the simian virus 40 minimal
promoter (5HRE-SV40mp). The hypoxia-regulated NT-3 expression was
confirmed, and the protective effects of hypoxia-regulated NT-3
against apoptosis were demonstrated in PC12 cells. Moreover, the
activities of p38 and caspase-3, which may be involved in these
effects, were investigated.
Materials and methods
Cell culture and hypoxia treatment
PT67 cells (American Type Culture Collection) were
cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum
(Gibco), 100 U/ml penicillin and 100 U/ml streptomycin (Sigma) at
37°C in a humidified incubator containing 95% air and 5%
CO2. PC12 cells were maintained in DMEM supplemented
with 10% horse serum (HyClone), 5% fetal bovine serum, 100 U/ml
penicillin and 100 U/ml streptomycin. For immunocytochemistry and
in situ cell death detection, the cells were plated onto
poly-L-lysine-coated coverslips.
On day 2 after plating, the cells were placed in an
anaerobic workstation (Bugbox; Ruskinn Technology) and perfused
with a gas mixture of 5% CO2, 0.3% O2 and
94.7% N2 to induce hypoxia. For HIF-1α detection, the
cells were exposed to hypoxia for 3, 6, 12, 24 and 48 h. For
hypoxia-regulated NT-3 expression experiments and the apoptosis
assay, the cells were exposed to hypoxia for 48 h.
Construction of recombinant retroviral
vector
The full length coding sequences of human NT-3, 5
copies of the vascular endothelial growth factor (VEGF) HRE
consensus sequence (CCACAGTGCATACGTGGGCTCCAACAGGTCCTCTT), SV40mp
and enhanced green fluorescent protein (EGFP) were cloned into the
retroviral vector, pLNCX (Clontech), between 2 long terminal
repeats to generate pLNC-5HRE-SV40-EGFP, pLNC-SV40-NT3 and
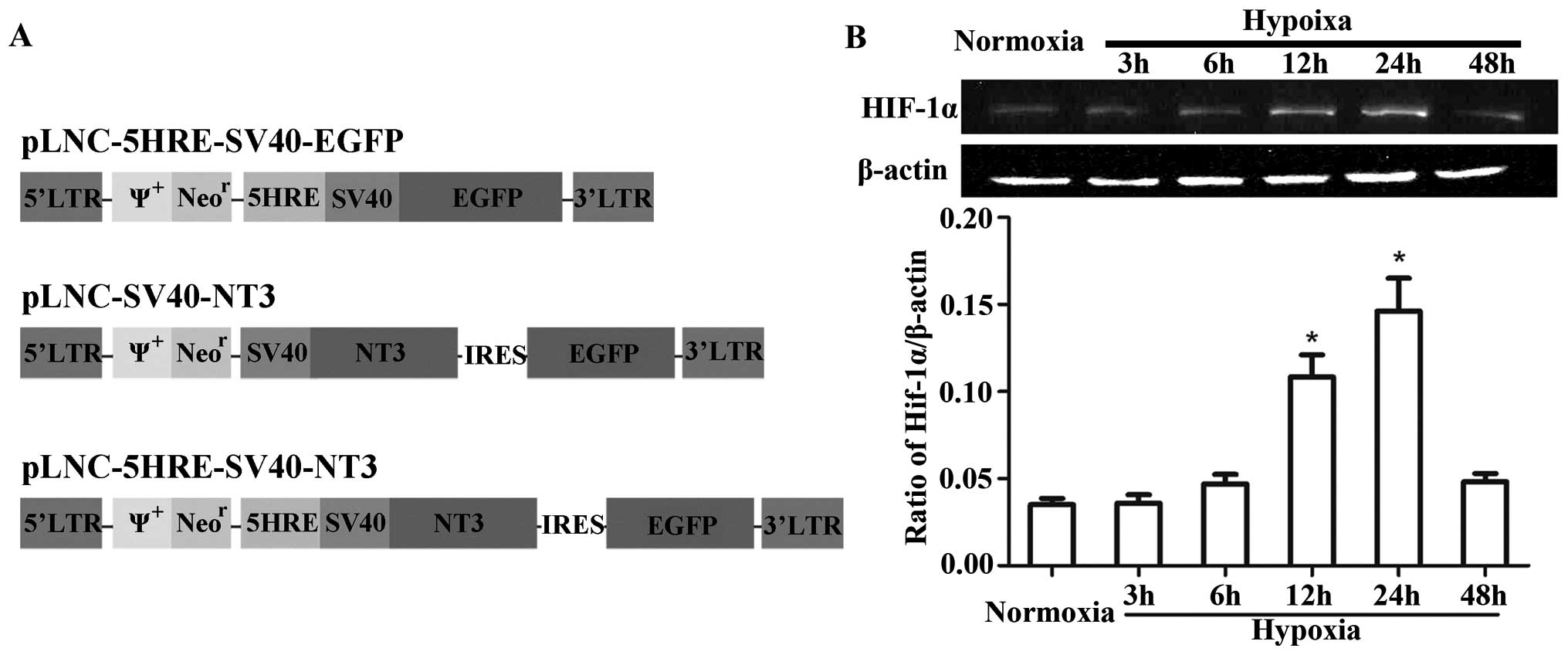
pLNC-5HRE-SV40-NT3 (Fig. 1A).
Generation of transgenic PC12 cells
To generate retroviruses that can mediate 5HRE-EGFP,
NT-3 or 5HRE-NT3 gene transfer, the PT67 packaging cells were
transfected with the constructed plasmids using Lipofectamine 2000
(Invitrogen). After 2 days, the virus-containing supernatants were
filtered through a 0.45-μm cellulose acetate filter and added to
PC12 cells (American Type Culture Collection) for 24 h. The medium
was then replaced with DMEM containing 10% horse serum and 5% fetal
bovine serum. After 24 h, G418 (Invitrogen) was added to the medium
to screen and select for individual colonies. The colonies were
identified using reverse transcription PCR (RT-PCR),
immunocytochemistry and western blot analysis. The transgenic PC12
cells were designated as PC12-5HRE-EGFP, PC12-NT3 and
PC12-5HRE-NT3.
RT-PCR
Total RNA was isolated from cells using the TRIzol
LS reagent (Invitrogen), and first-strand cDNA was synthetized
using the PrimeScript™ RT reagent kit (Takara Bio, Inc.). PCR
reactions were performed using the Premix Taq Version 2.0 (Takara
Bio, Inc.). To detect the exogenous genes, β-actin mRNA was used as
the internal control and the following primers were used: β-actin,
5′-GGAGATTACTGCCCTGGCTCCTA-3′ (forward) and
5′-GACTCATCGTACTCCTGCTTGCTG-3′ (reverse); SV40mp,
5′-CGGGATCCGTTAACTCTGCGATCTGC-3′ (forward) and 5′-CGGTGAAGATCTCT
GCAGAATTCGAAGC-3′ (reverse); NT-3, 5′-GGCAGATCTGGTGATGTCCATCTTG-3′
(forward) and 5′-GCCGAGCTCTCATGTTCTTCCGATT-3′ (reverse); and EGFP,
5′-CGGGATCCAGATCTCGCCACCATG-3′ (forward) and
5′-CCATCGATGGTTACTTGTACAGCTCGTCC-3′ (reverse). The reactions were
performed in a total volume of 20 μl with an initial cycle at 94°C
for 2 min, followed by 30 cycles as follows: 94°C for 30 sec, 60°C
for 30 sec and 72°C for 1 min. The amplified PCR products were
separated on 1.5% agarose gels and subsequently imaged. For a
semiquantitative analysis of NT-3 mRNA, the band intensities were
measured using the ImageJ software (version 1.43u, National
Institutes of Health), and the data were normalized to the level of
β-actin in the same sample.
Immunocytochemistry
The cells were fixed with 4% paraformaldehyde for 20
min, blocked with 5% normal goat serum for 1 h and labeled with
rabbit monoclonal anti-NT-3 primary antibody (1:100; Santa Cruz
Biotechnology, Inc.) and Cy3-conjugated goat anti-rabbit IgG
secondary antibody (1:500; Vector Laboratories). After
counterstaining with DAPI (Sigma), the cells were imaged using a
fluorescence microscope (Olympus BX51).
Western blot analysis
The cells were harvested and lysed in RIPA lysis
buffer (Pierce Biotechnology, Inc.) supplemented with a protease
inhibitor cocktail (Roche Diagnostics). The lysate was centrifuged
at 12,000 rpm for 10 min at 4°C, and the protein concentration of
the supernatant was determined using a BCA protein assay kit
(Pierce Biotechnology, Inc.). Equal amounts of protein from each
lysate were separated using 12% SDS-PAGE, and transferred onto a
nitrocellulose membrane. The membrane was blocked with 5% non-fat
milk in TBS-T (50 mM Tris, 150 mM NaCl, pH 7.6, 0.1% Tween-20) for
4 h. The following primary antibodies were incubated with the
membrane overnight at 4°C: mouse monoclonal anti-HIF-1α (1:2000;
Abcam), rabbit monoclonal anti-NT-3 (1:200, Santa Cruz
Biotechnology, Inc.), rabbit polyclonal anti-active caspase-3
(1:1000; Abcam), rabbit monoclonal anti-P-p38 (1:1000; Cell
Signaling Technology, Inc.), rabbit monoclonal anti-p38 (1:2000;
Cell Signaling Technology, Inc.) and mouse monoclonal anti-β-actin
(1:5000; Santa Cruz Biotechnology, Inc.). The immunoreactive bands
were visualized by enhanced chemiluminescence (Pierce
Biotechnology, Inc.) using a horseradish peroxidase-labeled
secondary anti-rabbit/mouse antibody (1:2000; Santa Cruz
Biotechnology, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
The cells were incubated for 48 h under normoxic or
hypoxic conditions. The NT-3 levels in the PC12-NT3 and
PC12-5HRE-NT3-conditioned medium were determined using an ELISA kit
for human NT-3 (Promega) according to the manufacturer’s
instructions.
In situ cell death detection
Terminal dUTP nick end-labeling (TUNEL) assay was
performed to determine apoptosis using an In situ Cell Death
Detection kit (Roche Diagnostics). The cells were fixed with 4%
paraformaldehyde for 1 h, permeabilized with 0.1% Triton X-100 in
0.1% sodium citrate for 2 min on ice, incubated with 50 μl TUNEL
reaction mixtures for 1 h at 37°C and counterstained with DAPI
(Sigma) for 5 min. Three microscopic fields (x20 magnification) of
TUNEL-positive cells on 3 separate coverslips were selected and
imaged. The number of TUNEL-positive cells was then counted and
normalized to the total number of cells in the images captured from
these areas. The percentage of positive cells was calculated as the
mean of the percentages obtained from 9 images.
Statistical analysis
The data are presented as the means ± SD, and the
statistical analysis was performed using ANOVA followed by Fisher’s
least significant difference test. A probability value of <0.05
was considered statistically significant.
Results
Detection of HIF-1α expression after
hypoxic treatment in PC12 cells
Western blot analyses showed that HIF-1α was
expressed at low levels under normoxic conditions and increased
after hypoxic treatment in PC12 cells (Fig. 1B). Although HIF-1α expression had
not significantly increased after hypoxic treatment in the 3- or
6-h groups, compared with the normoxic group, it did significantly
increase after hypoxia in the 12- and 24-h groups (P<0.05), and
peaked at 24 h. These data demonstrate the feasibility of using HRE
to regulate NT-3 expression in response to hypoxia.
Identification of transgenic PC12
cells
In this study, we successfully constructed
recombinant retroviral vectors and generated transgenic PC12 cells.
By combining fluorescence with light microphotographs (Fig. 2A), we revealed that all of the
transgenic cells were EGFP-positive, whereas no EGFP was detected
in the PC12 cells without gene transfer. The RT-PCR results
(Fig. 2B) showed mRNA expression
of SV40mp and EGFP in all of the transgenic cells, and NT-3 mRNA
expression was detected in the PC12-NT3 and PC12-5HRE-NT3 cells but
not in the PC12-5HRE-EGFP or PC12 cells without gene transfer.
Immunocytochemistry demonstrated that NT-3 and EGFP were co-labeled
in PC12-NT3 and PC12-5HRE-NT3 cells, but no NT-3-positive staining
could be detected in PC12-5HRE-EGFP or PC12 cells without gene
transfer (Fig. 2C), which was
consistent with our RT-PCR results.
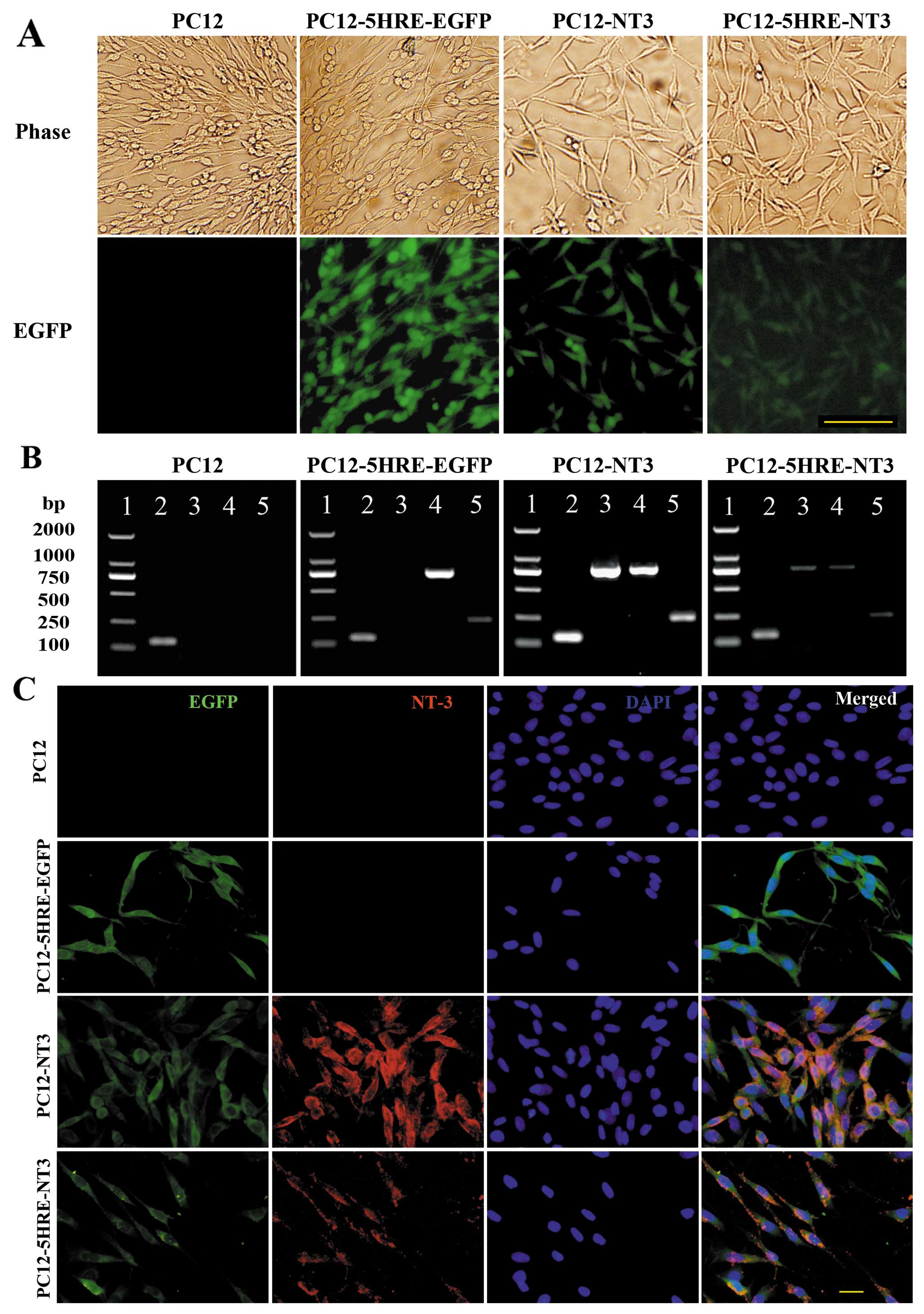 | Figure 2.(A) Identification of transgenic PC12
cells. EGFP fluorescence was observed in PC12-5HRE-EGFP, PC12-NT3
and PC12-5HRE-NT3 cells. Green shows EGFP; scale bar, 100 μm. (B)
Simultaneously, the expression of the exogenous genes in the PC12
and transgenic PC12 cells was detected by RT-PCR. Lane 1, DNA
molecular weight marker; lane 2, β-actin PCR product; lane 3, NT-3
PCR product; lane 4, EGFP PCR product; lane 5, SV40mp PCR product.
(C) Immunocytochemistry with an antibody against NT-3. NT-3 (red)
was detected in PC12-NT3 and PC12-5HRE-NT3 cells. Green shows EGFP;
blue shows DAPI; merged, EGFP/NT-3/DAPI. Scale bar, 20 μm. |
Verification of hypoxia-regulated NT-3
expression in PC12-5HRE-NT3 cells
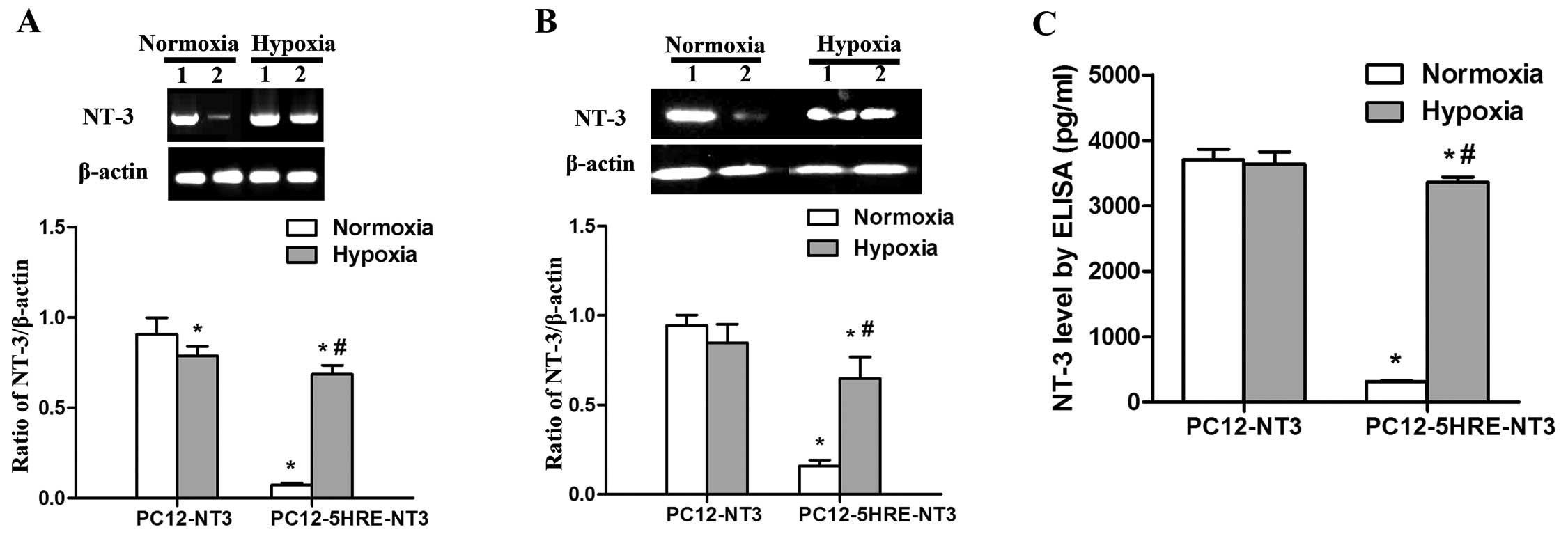
To determine whether 5 copies of HRE can
conditionally control NT-3 gene expression, we performed RT-PCR,
western blot analysis and an ELISA assay to examine the mRNA and
protein expression of NT-3. Under normoxic conditions, a very low
level of NT-3 mRNA expression was observed in PC12-5HRE-NT3 cells,
and this level was significantly lower than that in the PC12-NT3
group (P<0.05) (Fig. 3A).
However, NT-3 mRNA expression was significantly increased in
PC12-5HRE-NT3 cells after hypoxic treatment compared with the
normoxic group (P<0.05). By contrast, NT-3 mRNA expression was
decreased in PC12-NT3 cells after hypoxic treatment compared with
the normoxic group (P<0.05). The western blot analysis results
(Fig. 3B) revealed that the NT-3
protein was also expressed at an extremely low level in
PC12-5HRE-NT3 cells, which was significantly lower than that in the
PC12-NT3 group under normoxic conditions (P<0.05). After hypoxic
treatment, NT-3 protein expression was significantly increased in
PC12-5HRE-NT3 cells compared with the normoxic group (P<0.05).
We found no significant difference in the level of NT-3 protein
expression between the normoxic and hypoxic group in PC12-NT3
cells.
As a secreted protein, NT-3 can be detected in
conditional medium with the use of an ELISA assay (Fig. 3C). Our results showed that the
NT-3 protein in PC12-5HRE-NT3 cells was detected at 315.79±10.94
pg/ml, which was significantly lower than the level in PC12-NT3
cells (3702.28±163.48, P<0.05) under normoxic conditions.
Following hypoxic treatment, NT-3 protein expression significantly
increased to a high level of 3361.26±77.86 pg/ml, which was
approximately 10-fold greater than that in the normoxic group
(P<0.05). Taken together, these data suggest that NT-3
expression was effectively increased by 5HRE in PC12-5HRE-NT3 cells
in response to hypoxia.
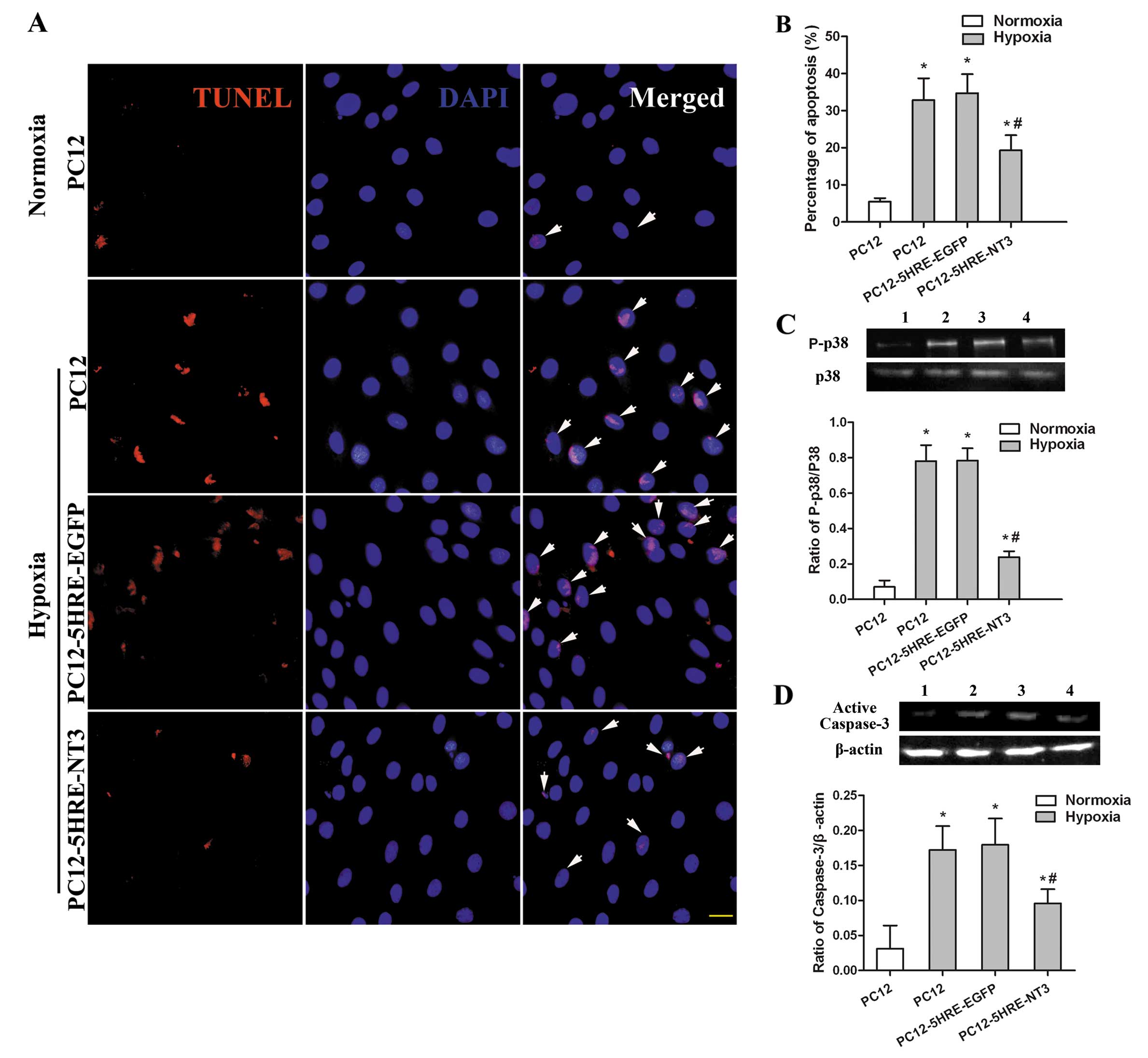
Reduced apoptosis in 5HRE-NT3 transgenic
PC12 cells after hypoxia
Although only a few cells were TUNEL-positive under
normoxic conditions (Fig. 4A and
B), the percentage of TUNEL-positive cells had significantly
increased after 48 h of hypoxic treatment in the PC12 cell group
(P<0.05). However, the percentage of TUNEL-positive cells in the
PC12-5HRE-NT3 group was significantly reduced compared to the PC12
cell group under hypoxic conditions (P<0.05), whereas the
percentage of TUNEL-positive cells was not significantly different
between the PC12-5HRE-EGFP and PC12 groups under hypoxic
conditions.
Suppressed activation of p38 and
caspase-3 is accompanied with a decrease in PC12 cell
apoptosis
The phosphorylation of p38 and activation of
caspase-3 were detected by western blot analysis to determine
whether these proteins were involved in the reduction of apoptosis
mediated by gene transfer in PC12 cells. Compared with the PC12
cells in the normoxic group, the phosphorylation of p38 (P<0.05)
(Fig. 4C) and activation of
caspase-3 (P<0.05) (Fig. 4D)
were significantly increased in PC12 cells after 48 h of hypoxia.
However, the phosphorylation of p38 and activation of caspase-3
were remarkably decreased in the PC12-5HRE-NT3 group compared with
the PC12 cells under hypoxic conditions (P<0.05). There was no
significant difference in the phosphorylation of p38 and the
activation of caspase-3 between the PC12-5HRE-EGFP and PC12 cell
groups under hypoxic conditions. These data indicate that both p38
and caspase-3 may participate in the protective effects of 5HRE-NT3
gene transfer against apoptosis induced by hypoxia in PC12
cells.
Discussion
Hypoxia-specific regulatory systems have been
developed to regulate transgene expression in hypoxic tissues
(3–8), in which gene expression is
upregulated in a tissue-specific manner and reduced in normal
tissues. Various copies of HRE have been developed to control
therapeutic gene expression and all of these copies of HRE can
upregulate the expression of downstream genes in response to
hypoxia. Some studies have demonstrated that 5 copies of HRE
derived from the human VEGF gene were optimal to mediate response
to hypoxia (5,16). However, other studies have found
that 9 copies of the HRE from the human EPO gene were more
effective than 3 or 6 copies (17). This discrepancy may be attributed
to the different promoters used (6,18).
There are 2 main promoters used in hypoxia-specific regulatory
systems, the minimal cytomegalovirus promoter (CMVmp) and SV40mp.
Taking into account the results from our previous study (3), we selected 5 copies of the HRE from
the human VEGF gene and SV40mp to construct a cassette to
conditionally regulate NT-3 expression in response to hypoxia in
this study. As expected, under the control of 5HRE, both the mRNA
and protein expression of NT-3 significantly increased after
hypoxia in PC12-5HRE-NT3 cells. An ELISA analysis revealed that the
concentration of NT-3 protein in the conditioned medium of
PC12-5HRE-NT3 cells in the hypoxic group increased by almost
10-fold compared to the normoxic group. These findings indicate
that the cassette composed of 5HRE and SV40mp is an available
‘modulator’ to regulate therapeutic gene expression in
hypoxia-related diseases.
Different copies of HRE can decrease the basal
promoter activity under normoxic conditions. As shown in a previous
study, the 5HRE-3NRSE regulator cassette decreased the basal CMVmp
activity by approximately 50–97% in comparison with that of the CMV
construct in cultured cells under normoxic conditions (19). Moreover, almost no humanized
Renilla GFP (hrGFP) expression occurred in the PC12 cells infected
by Ad-5HRE-hrGFP under normoxic conditions (20). In vivo, Shen et al
(4) found that the lacZ gene was
not expressed in the normal areas of the AAVH9-lacZ-transduced
mouse brain. Consistent with these data, we found that under
normoxic conditions, the 5HRE-SV40mp construct had a significantly
lower promoter activity compared with the SV40mp construct, as the
basal SV40mp promoter activity was reduced by 92% from NT-3 mRNA
expression, 83% from NT-3 protein expression and 91% from secreted
NT-3 protein expression. Taken together, these data indicate that
5HRE is an ideal enhancer to perform hypoxia-specific gene
expression. Therefore, 5HRE can effectively upregulate downstream
gene expression under hypoxic conditions and only small or
unexpected side-effects on the physiological process occur through
suppression of the promoter under normal conditions.
The PC12 cell line has been extensively used as a
model for the study of neurosecretion, neuronal signaling pathways,
neuronal differentiation, neuroprotective effects of neurotrophin
and ischemic tolerance (21). A
number of studies have demonstrated that NT-3 exerts various
effects on PC12 cells that have been subjected to various types of
treatment, including hypoxia (21). Therefore, the PC12 cell line,
which possesses neuron-like properties, is an ideal cell line to
use to determine the effects of NT-3 and the bioactive functions of
conditionally expressed NT-3.
Hypoxic injury in the PC12 cell model was induced
with treatment at an oxygen concentration of 0.3% to mimic cerebral
ischemic injury (22). Although
HIF-1α expression increased and peaked after hypoxia at 24 h in
PC12 cells, the expression of downstream genes was induced in a
time-dependent manner (20).
Therefore, hypoxia for 48 h was used as a model of hypoxia-induced
injury in PC12 cells, in which clear apoptotic responses could be
induced and hypoxia-regulated NT-3 expression could be thoroughly
induced to display the protective effects against apoptosis.
Caspase-3 plays a critical role in apoptotic
execution (23) and hypoxia
induces the cleavage of caspase-3 through the p38/mitogen-activated
protein kinase (MAPK)-dependent pathway (24). It has previously been reported
that NT-3 can promote extracellular signal-regulated protein
kinase/MAPK and phosphatidylinositol 3-kinase/Akt phosphorylation,
where NT-3 can exert its anti-apoptotic effects (25). In the present study, the decreased
activation of p38 and caspase-3 in the PC12-5HRE-NT3 cell group
under hypoxic conditions indicated that both p38 and caspase-3 were
involved in the reduction of apoptosis induced by hypoxia in PC12
cells, which was mediated by 5HRE-NT3 gene transfer.
In conclusion, in this study, we successfully
constructed a retroviral vector carrying 5HRE-NT3 and transferred
it into PC12 cells (PC12-5HRE-NT3). Under hypoxic conditions, NT-3
expression was significantly upregulated by 5HRE in PC12-5HRE-NT3
cells. Conditionally expressed NT-3 reduced apoptosis induced by
hypoxia in PC12 cells, which may be due to the depressed activation
of p38 and caspase-3. These results suggest that the 5HRE-NT3
vector may be useful for the therapy of ischemic diseases, while
avoiding side-effects.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (Grant no. 81070998)
and a fund from the State Key Laboratory of Medical Neurobiology,
Shanghai (no. 10-03). The authors would like to thank Professors
Julie Y.H. Chan and Samuel H.H. Chan from the Center for
Translational Research in Biomedical Sciences, Chang Gung Memorial
Hospital-Kaohsiung Medical Center, Taiwan for their invaluable
comments and editorial support.
References
|
1.
|
A BeenkenM MohammadiThe FGF family:
biology, pathophysiology and therapyNat Rev Drug
Discov8235253200910.1038/nrd279219247306
|
|
2.
|
RJ LeeML SpringerWE Blanco-BoseR ShawPC
UrsellHM BlauVEGF gene delivery to myocardium: deleterious effects
of unregulated
expressionCirculation102898901200010.1161/01.CIR.102.8.89810952959
|
|
3.
|
Q ShiP ZhangJ ZhangAdenovirus-mediated
brain-derived neurotrophic factor expression regulated by hypoxia
response element protects brain from injury of transient middle
cerebral artery occlusion in miceNeurosci
Lett465220225200910.1016/j.neulet.2009.08.049
|
|
4.
|
F ShenH SuY FanAdeno-associated
viral-vector-mediated hypoxia-inducible vascular endothelial growth
factor gene expression attenuates ischemic brain injury after focal
cerebral ischemia in
miceStroke3726012606200610.1161/01.STR.0000240407.14765.e8
|
|
5.
|
T ShibataAJ GiacciaJM BrownDevelopment of
a hypoxia-responsive vector for tumor-specific gene therapyGene
Ther7493498200010.1038/sj.gt.330112410757022
|
|
6.
|
H SuJ Arakawa-HoytYW KanAdeno-associated
viral vector-mediated hypoxia response element-regulated gene
expression in mouse ischemic heart modelProc Natl Acad Sci
USA9994809485200210.1073/pnas.13227529912084814
|
|
7.
|
GL SemenzaTargeting HIF-1 for cancer
therapyNat Rev Cancer3721732200310.1038/nrc1187
|
|
8.
|
NV IyerLE KotchF AganiCellular and
developmental control of O2 homeostasis by
hypoxia-inducible factor 1 alphaGenes Dev1214916219989436976
|
|
9.
|
A PatapoutianLF ReichardtTrk receptors:
mediators of neurotrophin actionCurr Opin
Neurobiol11272280200110.1016/S0959-4388(00)00208-711399424
|
|
10.
|
X LiZ YangA ZhangThe effect of
neurotrophin-3/chitosan carriers on the proliferation and
differentiation of neural stem
cellsBiomaterials3049784985200910.1016/j.biomaterials.2009.05.04719539985
|
|
11.
|
H LuM LiT SongRetrovirus delivered
neurotrophin-3 promotes survival, proliferation and neuronal
differentiation of human fetal neural stem cells in vitroBrain Res
Bull77158164200810.1016/j.brainresbull.2008.02.03719875351
|
|
12.
|
HX LuZM HaoQ JiaoNeurotrophin-3 gene
transduction of mouse neural stem cells promotes proliferation and
neuronal differentiation in organotypic hippocampal slice
culturesMed Sci Monit17BR305BR311201122037732
|
|
13.
|
ZH ZhangRZ WangGL LiTransplantation of
neural stem cells modified by human neurotrophin-3 promotes
functional recovery after transient focal cerebral ischemia in
ratsNeurosci Lett444227230200810.1016/j.neulet.2008.08.049
|
|
14.
|
B CristofaroOA StoneA
CaporaliNeurotrophin-3 is a novel angiogenic factor capable of
therapeutic neovascularization in a mouse model of limb
ischemiaArterioscler Thromb Vasc
Biol3011431150201010.1161/ATVBAHA.109.20546820360537
|
|
15.
|
K ShimazuM ZhaoK SakataNT-3 facilitates
hippo-campal plasticity and learning and memory by regulating
neurogenesisLearn Mem13307315200610.1101/lm.7600616705139
|
|
16.
|
T ShibataAJ GiacciaJM
BrownHypoxia-inducible regulation of a prodrug-activating enzyme
for tumor-specific gene
therapyNeoplasia44048200210.1038/sj.neo.790018911922390
|
|
17.
|
H RuanH SuL HuKR LambornYW KanDF DeenA
hypoxia-regulated adeno-associated virus vector for cancer-specific
gene therapyNeoplasia3255263200110.1038/sj.neo.790015711494119
|
|
18.
|
N MoriR SteinO SigmundDJ AndersonA cell
type-preferred silencer element that controls the neural-specific
expression of the SCG10
geneNeuron4583594199010.1016/0896-6273(90)90116-W2322462
|
|
19.
|
D HuangA DesboisST HouA novel adenoviral
vector which mediates hypoxia-inducible gene expression selectively
in neuronsGene Ther1213691376200510.1038/sj.gt.3302538
|
|
20.
|
HW HuXK LiRY ZhengJ XiaoJQ ZengST HoubFGF
expression mediated by a hypoxia-regulated adenoviral vector
protects PC12 cell death induced by serum deprivationBiochem
Biophys Res
Commun390115120200910.1016/j.bbrc.2009.09.07719782044
|
|
21.
|
JA HillionK TakahashiD MaricC RuetzlerJL
BarkerJM HallenbeckDevelopment of an ischemic tolerance model in a
PC12 cell lineJ Cereb Blood Flow
Metab25154162200510.1038/sj.jcbfm.960000315647748
|
|
22.
|
I SilverM ErecinskaOxygen and ion
concentrations in normoxic and hypoxic brain cellsAdv Exp Med
Biol454716199810.1007/978-1-4615-4863-8_29889871
|
|
23.
|
SJ RiedlY ShiMolecular mechanisms of
caspase regulation during apoptosisNat Rev Mol Cell
Biol5897907200410.1038/nrm149615520809
|
|
24.
|
C MoriscoC MarroneV TrimarcoInsulin
resistance affects the cytoprotective effect of insulin in
cardiomyocytes through an impairment of MAPK phosphatase-1
expressionCardiovasc
Res76453464200710.1016/j.cardiores.2007.07.01217698050
|
|
25.
|
G LiotC GabrielM
CacquevelNeurotrophin-3-induced PI-3 kinase/Akt signaling rescues
cortical neurons from apoptosisExp
Neurol1873846200410.1016/j.expneurol.2004.01.00215081586
|