Introduction
Tumor vaccines are currently very common in terms of
development of active immunotherapy. A major question is whether or
not a vaccine that can break immune tolerance and is widely
applicable to many patients really exists. A few recent studies
have shown that human embryonic stem cells (hESCs) have the ability
to become the new vaccine of choice. Li et al (1) demonstrated the capacity of hESCs to
effectively immunize against murine colon cancer for the first
time. This was further supported by an additional study (2).
However, results on colon and lung cancer only have
been reported, and only in mouse models. Furthermore, the embryonic
stem cells (ESCs) and the animals were the same species in the lung
cancer model. Whether antitumor effects of ESCs are occasional or
universal, whether their antitumor effects are present in some
specific types of cancer or in a broad spectrum of different types
of cancer, and whether hESCs can overcome species obstacles and
induce the same antitumor effects in different animal species and
different original cancer tissue, remains unknown. In this study,
we chose hESCs and mouse ESCs (mESCs), and administered them as
vaccines in ovarian cancer in a mouse and rat model. We
hypothesized that undifferentiated human stem cells could be a
proper vaccine for ovarian cancer and immunize mice and rats to
generate an immune response. hESCs were indeed able to induce a
broad spectrum of both immune responses and clinical responses
against ovarian carcinoma without evidence of inducing autoimmune
diseases or side-effects.
Materials and methods
Cell lines
The hESC line H9 was provided by Professor Ming-Xiao
Ding (School of Life Sciences, Peking University). These cells were
grown in hESC medium. The mESC line IVP-ES1 was a generous gift
from Qi Zhou (Institute of Zoology of Chinese Academy of Sciences).
Cells were cultured in mESC medium (Beijing Huasen Biotechnology).
The ID8 cell line (a mouse ovarian epithelial papillary serous
adenocarcinoma cell line) was a generous gift from K.F. Roby
(Center for Reproductive Sciences, University of Kansas Medical
Center, Kansas City, KS, USA). ID8 cells were cultured in DMEM
supplemented with 5% fetal bovine serum. NuTu-19 cells, a Fischer
344 rat-derived epithelial ovarian carcinoma cell line, was grown
in RPMI-1640 medium containing 10% newborn calf serum (NBCS). H9
and IVP-ES1 cells were irradiated with 15 Gy gamma-ray before
vaccination. ID8 and NuTu-19 cells were incubated with 10 μg/ml
mitomycin C for 3 h at 37°C. Additionally, one plate of H9 cells
was processed for paraffin embedding, 3 μm sections were prepared,
and immunohistochemistry was performed for several tumor antigens
and genes and examined with a microscope.
Animals
Specific pathogen-free C57BL/6 female mice (20–25 g)
and Fischer 344 female rats (100–125 g) were obtained from the
Academy of Military Medical Sciences (Beijing, China) and housed in
a pathogen-free animal facility at Peking University People’s
Hospital, Beijing, China. Treatment and care of the animals were in
accordance with Institutional Guidelines and the Animal Welfare
Assurance Act. The experimental protocol for the use of the animals
for these studies was approved by the Institutional Laboratory
Animal Care and Use Committee at Peking University People’s
Hospital (81072141).
Mouse immunization protocol
C57BL/6 mice were randomly divided into six groups,
each group containing nine mice. Groups 1, 2, 3 and 4 received
subcutaneous vaccination in the right flank with pre-inactivated
IVP-ES1 (5×106) or H9 (5×106) or ID8
(5×106) or phosphate-buffered saline (PBS),
respectively, repeated three times at a one-week interval, and
alive ID8 cells (5×106) were inoculated subcutaneously
in the left flank one week after the final vaccination. Groups 5
and 6 received subcutaneous vaccination with pre-irradiated H9
(5×106) or IVP-ES1 (5×106) three times at a
one-week interval, two weeks after the final vaccination. Orbital
venous blood was collected to obtain the serum as the primary
antibodies for western blotting (Fig.
1A).
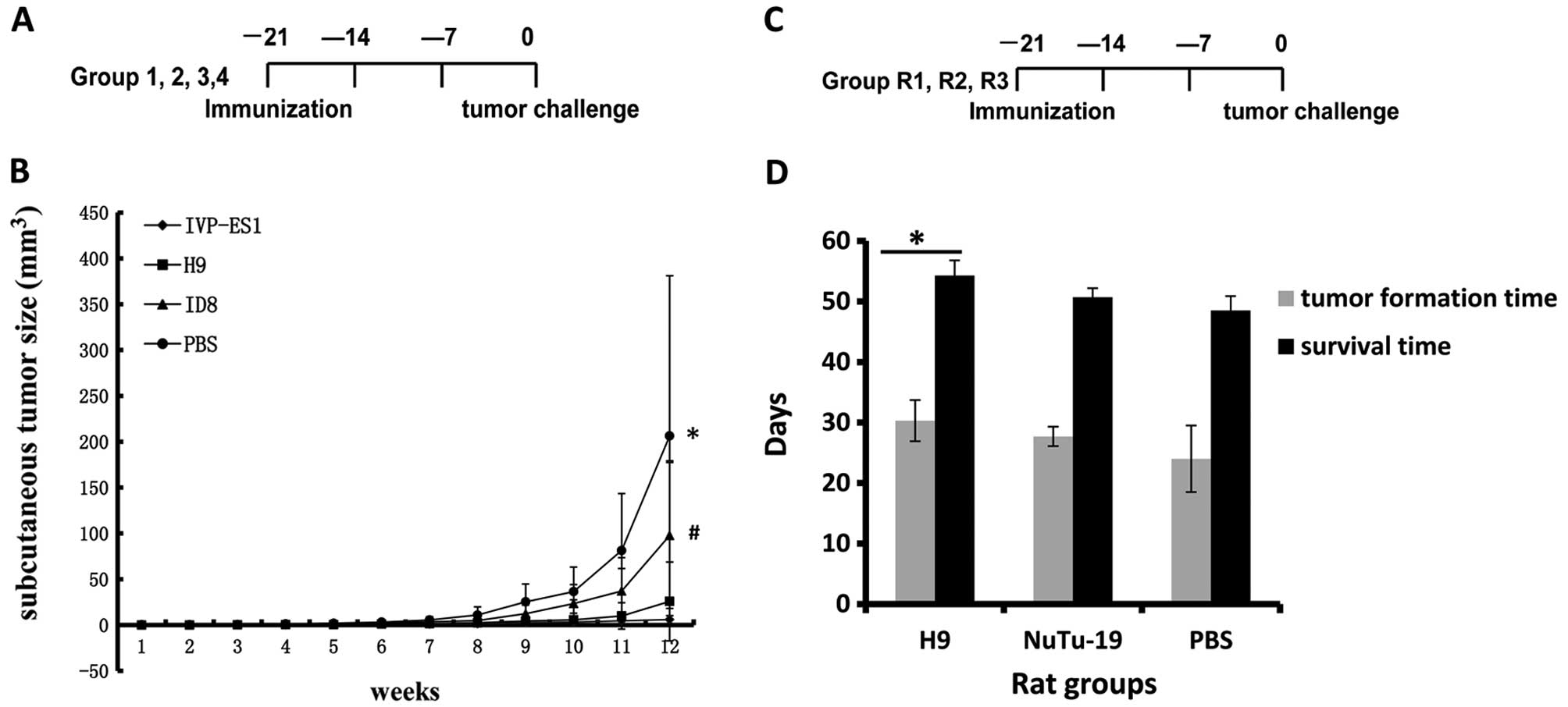 | Figure 1Undifferentiated ESCs exhibit potent
antitumor activity in mouse and rat ovarian cancer models. (A) Mice
in Groups 1, 2, 3 and 4 received subcutaneous vaccination
5×106 inactivated H9, IVP-ES1, ID8 and PBS,
respectively, three times at a one-week interval, and then alive
ID8 cells (5×106) were challenged one week after the
final vaccination. (B) Tumor formation time and growth rates in
mESCs (IVP-ES1, Group 1) and hESCs (H9, Group 2) were longer than
that in Group 3 (ID8) and in Group 4 (PBS). The sizes of the
subcutaneous tumors were 5.9±4.2, 25.7±43.0, 97.6±79.7, 206.3±174.8
mm3 (*,#P<0.05), respectively, at the end
of week 12. Furthermore, there was no statistically significant
difference in the antitumor effects between IVP-ES1 and H9 cells.
(C) Rats in Groups R1, R2 and R3 received 1×107
inactivated H9, NuTu-19 and PBS vaccination subcutaneously three
times at one-week intervals, and one week after the final
vaccination; 1×106 alive NuTu-19 cells were
intraperitoneally challenged. (D) hESC vaccination prolonged
survival in the rat model. Tumor formation times in H9, NuTu-19 and
PBS were 30.3±3.4, 27.7±1.6 and 22.0±5.5 days
(*P<0.05) respectively; the survival times were
54.3±2.5, 50.7±1.5 and 48.5±2.4 days (*P<0.05),
respectively. H9 vaccination resulted in a markedly longer survival
time compared with the control groups. |
Tumor growth was monitored every week using digital
calipers to measure both the longitudinal (L, mm) and transverse
(W, mm) diameters. Tumor volume (LxW2/2, mm3)
was plotted. Mice were also monitored for the following general
health indicators: overall behavior, feeding, neuromuscular tone,
body weight and appearance of fur, particularly after immunization.
The endpoint for this study was one diameter of tumor ≥10 mm, at
which point mice were euthanized. The primary tumor was also
excised and weighed after the mice were sacrificed.
Rat immunization protocol
Fischer 344 rats were randomly divided into four
groups with each group containing six rats. Group R1, R2, R3
received subcutaneous vaccination with pre-inactivated H9
(1×107) or NuTu-19 (1×107) or PBS,
respectively, repeated three times at one-week intervals, and alive
NuTu-19 cells (1×106) were intraperitoneally inoculated
one week after the final vaccination; Group R4 received
subcutaneous vaccination with pre-irradiated H9 (1×107)
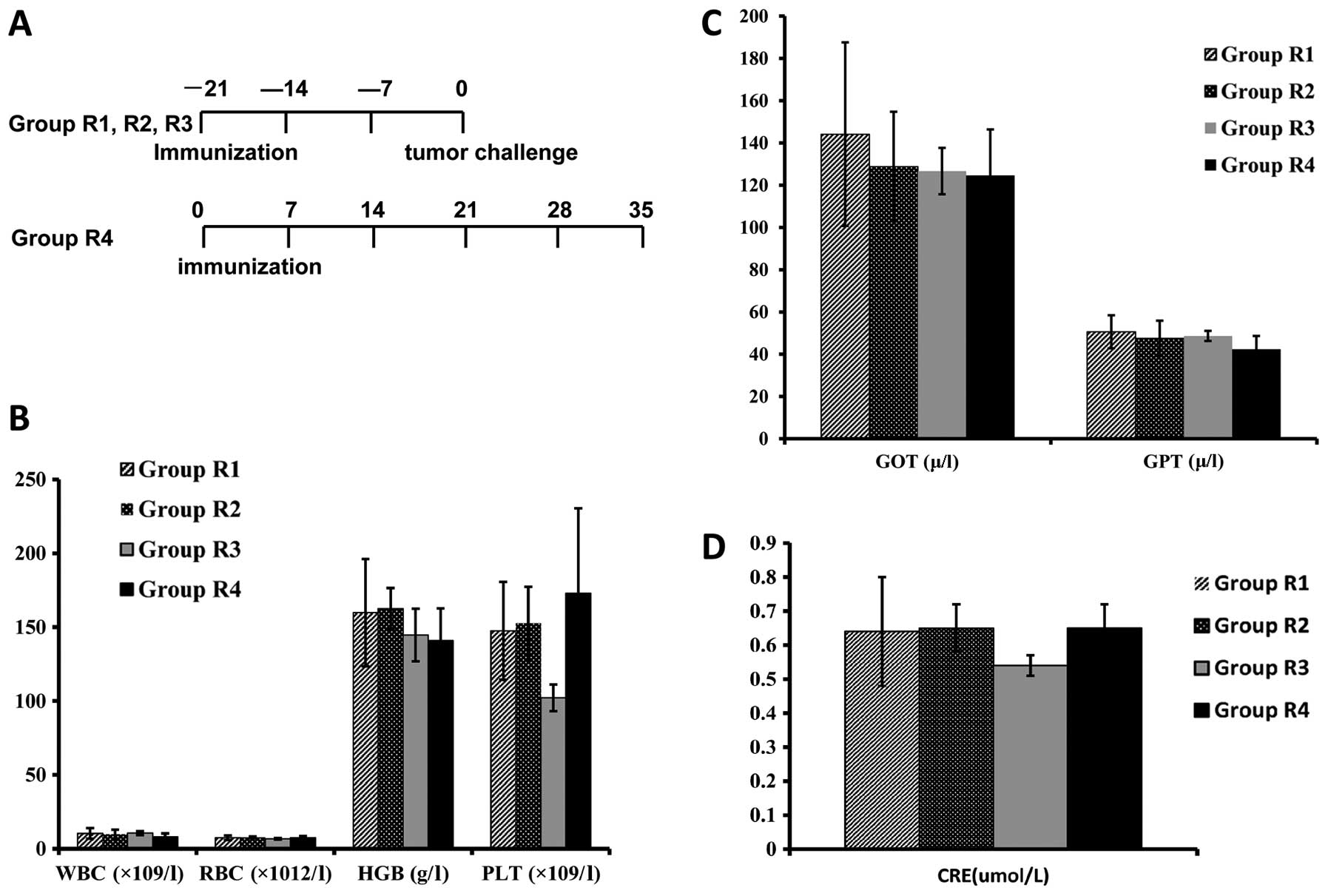
only, repeated six times at one-week intervals. Side-effects were
observed in this group, including dynamic complete blood count
(CBC) tests, liver and kidney function, including
glutamic-oxalacetic transaminase (GOT); glutamic-pyruvic
transaminase (GPT); creatinine (CRE) (Peking University Health
Science Center, PUHSC). Figs. 1C
and 3A show the scheme of ESC
immunization and tumor inoculation.
All animal health conditions were monitored daily.
Tumor formation and survival times were observed in Groups R1, R2
and R3. Tumor metastasis was recorded by counting the numbers and
sizes of tumor foci on each organ.
Th1/Th2/Th17 cytokines
Fresh blood from all mouse groups was obtained
before sacrificing by anesthesia. The serum levels of interleukin-2
(IL-2), IL-4, IL-6, IL-10, IL-17, IFN-γ, tumor necrosis factor-α
(TNF-α) were tested by cytometric bead array (CBA) kits
(Becton-Dickinson) in accordance with the instructions and analyzed
by BD Cytometric Bead Array Analysis.
Western blotting
H9, IVP-ES1 and ID8 cells were rinsed with PBS and
lysed in 100 μl Laemmli sample buffer. The samples were separated
by electrophoresis on a 10% denaturing and reducing
SDS-polyacrylamide gel. They were then transferred onto an
Immobilon-polyvinylidene fluoride membrane (Millipore). Membranes
were blocked in 5% skim milk for 1 h and then incubated with the
indicated primary antibodies at 1:50 dilution of sera from either
naïve or different cell-immunized mice overnight. This was followed
by incubation with the appropriate secondary antibodies, goat
anti-mice (1:3,000 dilution) for 1 h 30 min. Specific proteins were
detected using enhanced chemiluminescence.
Side-effects on the blood system, liver
and kidney function
Rat orbital venous blood collection was performed
after the first vaccination. The following punctures were performed
at the interval of vaccination twice at a two week interval in
Groups R1, R2, R3 and three times at a two week interval in Group
R4. Both whole blood and serum underwent CBC analysis, including
white blood count (WBC), red blood cell (RBC), hemoglobin (HGB),
and platelets (PLT), and assay of biochemical function indicator of
liver and kidney, including GOT, GPT, CRE (Peking University Health
Science Center, PUHSC).
Statistical analysis
Time to death (or euthanization) was completed using
Kaplan-Meier estimates of survival time. Equality of survival was
determined using the two-sided log-rank test. Median survival times
with 95% confidence limits for each treatment group were also
determined. Statistical significance of differences in tumor growth
rates was determined by ANOVA test analysis of variance using SPSS
20.0. Most data are presented as the means ± SD, and a P-value
<0.05 was considered to indicate statistically significant
differences.
Results
Inhibition of tumor formation in the
mouse model
We used a well-established C57BL/6 mice epithelial
ovarian cancer model (3). After
challenging with alive ID8 cells, the kinetics of tumor growth were
closely monitored. Tumor formation times and growth rates in mESCs
(IVP-ES1, Group 1) and hESCs (H9, Group 2) were longer than that in
Group 3 (ID8, positive control) and in Group 4 (PBS, negative
control). At the end point, the sizes of the subcutaneous tumors
were 5.9±4.2, 25.7±43.0, 97.6±79.7, 206.3±174.8 mm3
(P<0.05), respectively, (Fig.
1B). We concluded that immunization with undifferentiated human
or mouse ESCs can generate effective antitumor activity.
Prolonged survival and inhibition of
tumor metastasis in the rat model
In the Fischer 344 rat epithelial ovarian cancer
model (4), the tumor formation
time in hESCs (H9, Group R1) was longer than that in the two
control groups. Tumor formation times in Groups R1, R2, R3 were
30.3±3.4, 27.7±1.6 and 22.0±5.5 days (P<0.05) respectively; the
survival times were 54.3±2.5, 50.7±1.5 and 48.5±2.4 days
(P<0.05) respectively. H9 vaccination resulted in a markedly
longer survival time compared with the control groups (P<0.05)
(Fig. 1D). Furthermore,
vaccination with hESCs resulted in a retardation of tumor growth in
average tumor size compared to the control group (Table I). Upon gross visual inspection of
the peritoneum, numerous tumors were observed in control rats.
However, there were fewer tumors in the H9 vaccinated rats
(Table I). The results suggested
that the H9 vaccination group induced obvious antitumor immunity
and rejected tumor masses from proliferation and development
compared to the control groups. In the R1 group, metastatic lesions
were found in the diaphragm, peritoneal wall, intestine, mesentery
and omentum. Moreover, the metastatic tumor size ≥0.5
mm3 was only found in one rat; however, in Groups R2 and
R3 the metastasis included those in R1, and transferred to the
kidney, liver surface parenchyma and lung, the metastatic tumor
size ≥0.5 mm3 was found in five rats in these two
groups. Furthermore, lung and liver parenchyma metastases were
found in eight rats in Groups R2 and R3, however, no case developed
such distant metastasis as in Group R1.
 | Table IHuman ESC vaccination inhibits tumor
metastasis in a rat model. |
Table I
Human ESC vaccination inhibits tumor
metastasis in a rat model.
| Group R1 (H9) | Group R2
(NuTu-19) | Group R3 (PBS) |
|---|
|
|
|
|
|---|
| Metastatic
organs | Diaphragm, peritoneal
wall, intestine, mesentery, omentum | Diaphragm, peritoneal
wall, intestine, mesentery, omentum | Diaphragm, peritoneal
wall, intestine, mesentery, omentum, kidney, liver surface and
parenchyma lung |
|---|
| Liver and parenchyma
metastasis | 0% (0/6) | 16.7% (1/6) | 33.3% (2/6) |
| Lung metastasis | 0% (0/6) | 33.3% (2/6) | 50.0% (3/6) |
| Metastatic tumor size
>0.5 mm3 | 16.7% (1/6) | 66.7% (4/6) | 83.3% (5/6) |
Comparison of the immunogenicity between
undifferentiated hESCs and mESCs in the murine model
We compared hESCs and mESCs in the same ovarian
cancer mouse model. The primary antitumor responses of H9 and
IVP-ES1 were evaluated according to the same immunization
procedure. We demonstrated that immunization with undifferentiated
H9 and IVP-ES1 cells could inhibit tumor growth compared to two
control groups. Furthermore, there was no statistically significant
difference in the antitumor effects between IVP-ES1 and H9 cells
(Fig. 1B). These results strongly
suggest that the immunogenicity of hESCs is similar to mESCs to a
large extent, and hESCs can induce the same antitumor effects as
mESCs.
Vaccination with ESCs induces antibody
response against murine ovarian cancer
Sera from non-immunized naïve mice did not react
with H9, IVP-ES1 or ID8. However, we demonstrated that sera from
ID8 immunized rats were able to recognize multiple proteins in ID8,
H9 as well as IVP-ES1 lysates by western blot analysis (Fig. 2A and B). Moreover, prominent 135,
95, 72, 52, 30 kDa molecules were recognized by sera from
H9-immunized, IVP-ES1-immunized mice and detected in ID8 ovarian
cancer cells (Fig. 2A and B).
This suggested that an antitumor antibody response was produced
after H9, IVP-ES1 immunization and that there existed shared
antigens among ID8 and undifferentiated H9, IVP-ES1 cells.
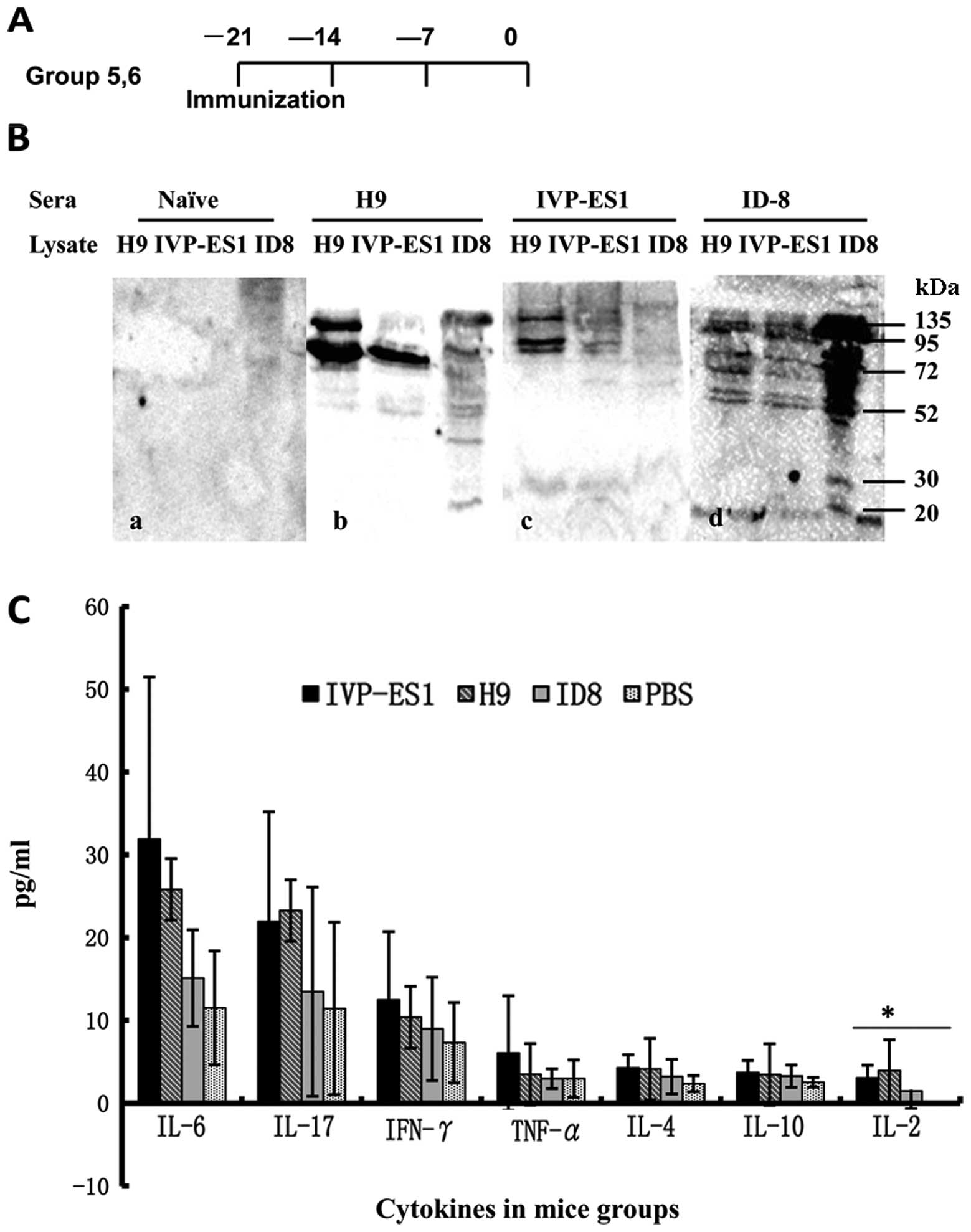 | Figure 2Vaccination with hESCs and mESCs
induced both T-cell and antibody response against ovarian cancer.
(A) Groups 5 and 6 received subcutaneous vaccination of inactivated
H9 and IVP-ES1, respectively, three times at a one-week interval,
two weeks after the final vaccination; orbital venous blood was
collected. (B) Immunization with H9 and IVP-ES1 generated
cross-reactive antibody against ID8 ovarian cancer. Western blot
analysis was performed with sera from immunized mice against total
cell lysate of ID8 and undifferentiated H9, IVP-ES1 cells. Numbers
indicate the molecular weight marker (kDa). (C) Mice were
sacrificed after the final immunization for the enumeration of the
frequency of IL-6, IL-17, IFN-γ, TNF-α, IL-4, IL-10 and IL-2.
Irradiated ID8 tumor cells served as target cells, along with PBS
as a negative control and ESCs as a positive control. There were
nine mice/group. IL-2, *P<0.05. |
Vaccination with ESCs induces cell-based
immunity
We next examined whether hESC and mESC immunization
induced cell-based antitumor immune response against ID8. We used
the Th1/Th2/Th17 CBA analysis kit (Becton-Dickinson) to test and
compare cytokine levels in each group, including IL-2, IL-4, IL-6,
IL-10, IL-17, TNF-α and IFN-γ. We found there was an increasing
trend of Th1/Th2/Th17 cytokine levels in the hESC and mESC
vaccinated groups compared with the control groups. We did not find
any difference of IL-4, IL-6, IL-10, IL-17, TNF-α, IFN-γ
(P>0.05) levels among the four groups except for IL-2. The IL-2
levels in each group were 3.9±1.7, 3.1±1.5, 1.5±2.1, 0 (pg/ml),
separately (P<0.05) (Fig. 2A and
C). It may be the case that cell-mediated immunity is so
complicated that other mechanisms and factors may be involved
(5).
Immunization with hESCs does not result
in significant hematologic toxicity or side-effects
An important consideration for stem cell-based
vaccines is the possibility of breaking immune tolerance against
self-antigens, such as cross-reactive antibodies against the
hematologic system and side-effects in the liver and kidney. This
question also has bearing on the application of stem cells for
regenerative medicine in an immunocompetent host. As an index for
inhibition in the hematologic system and side-effects in important
organs, we performed dynamic CBC tests, and the levels of several
blood serum enzymes in the sera of rats that were immunized with
PBS, H9 or NuTu-19 cells were determined, as side-effects are
easier to be observed and more blood is available in rats than in
mice. We used the vaccine Group R4 as the positive control, since
this group underwent inoculation with H9 cells only (Fig. 3A). CBC tests were in the normal
range and showed no difference among rats immunized with PBS, H9 or
NuTu-19 cells (Fig. 3B).
Creatinine and serum enzyme levels were normal in control and H9
cell-immunized rats (Fig. 3C and
D). However, four cases showed extraordinarily elevated levels
>100 fold compared to controls. Three rats treated with PBS and
all rats treated with pre-inactivated mitotic NuTu-19 cells were in
a late state and showed signs of severe cachexia and liver and
kidney toxicities, including metastases to both organs. More
importantly, we did not observe any abnormalities in the weight,
hair, joint swelling and neuromuscular tension of the animals.
hESCs express some non-specific ovarian
tumor antigens
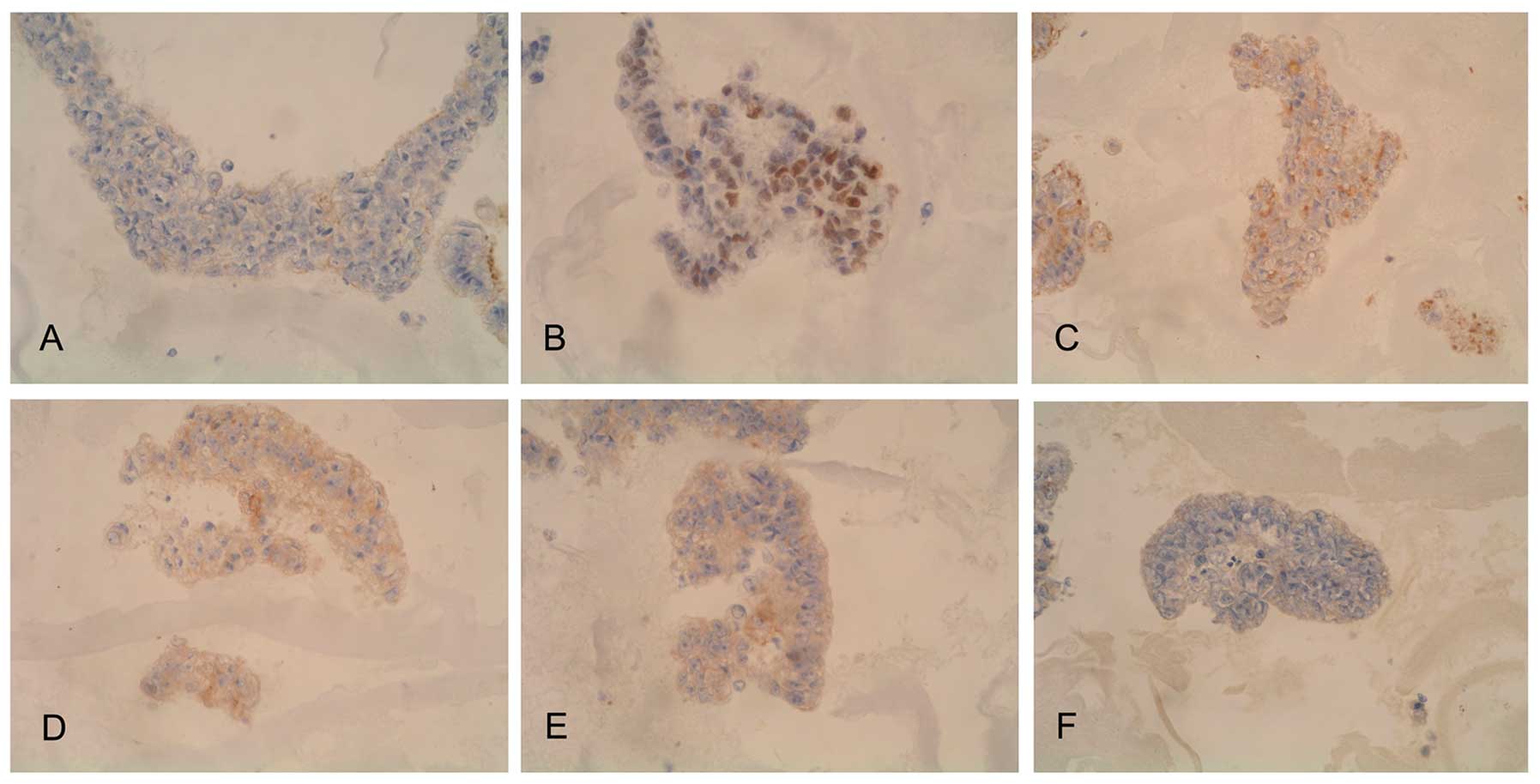
Using immunohistochemical methods to screen tumor
markers, we found that several oncogenes, tumor suppressor genes,
and metastasis-related genes had high expression in hESCs (H9).
Such markers included HER-2 (+), C-myc (++), p53 (++), nm23 (+++).
We also identified some tumor markers, such as NSE (+), PTEN (+),
PLAP (±), HPL (±), CK (++), MBP (++), and Vimentin (+++) (Fig. 4). hESCs express a broad spectrum
of tumor antigens, markers, and genes related to tumor growth and
metastasis.
Results were interpreted by two pathologists
independently, and a mean percentage of positive cells was
determined in at least five areas at ×400 and assigned to one of
four categories: (−), <5%; (+), 5–25%; (++) 25–50%; and (+++),
>50%.
Discussion
Embryonic stem cells have obvious immune antitumor
effects in mouse colon and lung cancer, a prospect that is
encouraging. Li et al (1)
demonstrated the capacity of human embryonic stem cells (hESCs) to
effectively immunize against murine colon cancer for the first
time. This was further supported by an additional study in which a
combination of carbon nanotubes and embryonic stem cells (ESCs)
successfully provided activation of antitumor immunity, leading to
an impressive suppression of proliferation and development of
malignant colon tumors (2).
Furthermore, Dong et al (6) demonstrated that administration of
ESCs could prevent and control the proliferation and development of
lung cancer. However, these studies only examined colon and lung
cancer, while Li et al (1)
reported only devised human iPS cells (induced pluripotent stem
cells) as a control group without mouse ESCs (mESCs). Dong et
al (6) reported administering
mESCs in mouse lung cancer without hESCs as a control group,
therefore it remains uncertain whether hESCs can overcome species
obstacles and induce the same antitumor effects in different animal
species and different original cancer tissue.
In this study, we used both a mouse and a rat
ovarian cancer animal model, particularly the rat model, since rats
have more similarities to human disease and make it is easier to
observe side-effects. Furthermore, we devised hESCs and mESCs in
the same mouse model, and compared the antitumor effects of human
and mouse ESCs. In this study, we proved that undifferentiated
hESCs could be a novel and safe vaccine for ovarian cancer both in
the mouse and the rat model. Additionally, this study also
demonstrated the potential concerns associated with cross-species
obstacles.
In our study, we found mESCs (IVP-ES1) were able to
immunize naïve mice against challenge with live murine ovarian
carcinoma cells, while hESCs (H9) were able to immunize naïve mice
and rats against challenge with a lethal dose of live ovarian
carcinoma cells. We also discovered administration of both hESCs
and mESCs could generate effective antitumor effects and protect
animals from tumor proliferation and/or further development.
Moreover, in the mouse ovarian cancer model, antitumor effects of
human and mice ESCs were quite similar. We also found that the
survival times in the vaccine group in rats were much longer than
those in the control groups. We speculated that hESCs could
suppress tumor proliferation and prolong the survival time.
More importantly, rats are a relatively bigger
animal model compared to mice, making it easier to find the
side-effects of therapy compared with mice; the dynamic blood
sampling is also more feasible in rats, so we performed dynamic CBC
tests, and the levels of several blood serum enzymes in the sera of
rats in all groups. We did not find marrow suppression or any
damage to liver and kidney function. Additionally, we did not
observe any abnormalities in the weight, hair, joint swelling and
neuromuscular tension of the animals. We did not observe any
significant side-effects in the hESC-immunized rats and mice.
Immunized rats and mice were generally healthy without clinical
evidence of autoimmune diseases.
We examined the potential mechanism of tumor
rejection by stem cell-immunized mice. We observed both humoral and
cell-based immunity. However, it is difficult to attribute
responsibility for tumor rejection to a single mechanism. It is
thus not surprising that not all H9 and IVP-ES1 cells induced
significant numbers of IL-4, IL-6, IL-10, IL-17A, TNF-α and IFN-γ,
however, there is an increasing trend in ES vaccinated mouse serum.
Furthermore, IL-2 levels in serum in H9 and IVP-ES1 vaccinated mice
was higher than in the control groups, which showed statistically
significant differences. IL-2 and IL-4 produced splenocytes and
TNF-α against ID8. hESCs induced growth and metastasis suppression
in rat ovarian cancer cell lines. However, such inhibition does not
appear to be mediated only by mouse cytokines IL-2, IL-4, IL-6,
IL-10, IL-17A, TNF-α and IFN-γ. There may still be other dynamic
immunological factors implicated in the administration of the hESC
vaccine processes. The unique properties of hESCs may provide a
novel approach for therapeutic options for the management of
cancer.
Previous studies support the hypothesis that
tumor-embryonic antigens (oncofetal antigens) are expressed in
cancer cells and in embryonic material (1–3).
Thus, anti-embryonic antigens play a role in the antitumor immune
response through cross-immune reactivity (7). The exact antigens shared by hESCs,
mESCs and ID8 ovarian carcinoma cells remain to be identified. It
is possible that the antigens that were reactive with the H9,
IVP-ES1 and ID8 immune sera were oncofetal antigens present in
these cells. Stem cell immunization might trigger an immune
response against these gene products that are also expressed by
tumor cells. Additionally, the immune response against H9, IVP-ES1
could lead to antigenic spread to induce protective immunity
against ID8 unique tumor antigens, a concept akin to that proposed
to explain the efficiency of xenogeneic antigen immunization
(8). We found cross-reactive
proteins among H9, IVP-ES1 and ID8 by western blotting. The ability
to separate and purify these proteins in order to find the exact
antigen and to explore the antitumor mechanism of ESCs are both
questions worthy of further exploration.
We also screened tumor-embryonic antigens and
several genes related to tumors by immunohistochemical methods. We
found several genes or markers related to tumorigenesis, tumor
growth and metastasis. Many of these were involved in critical
tumor signal transduction pathways. For example, nm23 and HER-2
were negatively correlated with tumor metastasis and prognosis.
Notably, HER-2 has been exploited as a promising candidate for
peptide-based cancer vaccines (9–12).
PTEN, p53, and c-myc, which are all well known to play important
roles in carcinogenesis, have also been shown to be associated with
prognosis (13–18). These results show that hESCs do
express a broad spectrum of tumor markers, many of which are also
shared by ovarian cancer. This provides us with a basis for
examining tumor markers for tumor immunotherapy.
However, additional follow-up studies are needed
before hESC-based cancer vaccines move into clinical testing. In a
broad context, our study has raised a number of intriguing
questions that deserve further research. For example, with further
optimization, could an hESC-based vaccination strategy be effective
against pre-established cancer? ESCs have been proven to be
effective vaccines in colon, lung, and, here, in ovarian cancer.
Animal models used for these studies include mice and rats,
suggesting that cross-species obstacles may not be particularly
problematic and that hESCs may produce a broad spectrum of
antitumor effects. Despite our progress, there is still much that
remains unknown, such as the exact mechanism of the antitumor
effects of ESCs. Although we detected some cross-reactivity of
antigens between ESCs and tumor cells, a major question is how to
identify and purify these cross antigens, what they are exactly, if
they are involved in tumor metastasis, and whether cancer stem
cells can be used as vaccines since they might share more
cross-antigens with the cancer cells of origin.
In summary, we demonstrated the capacity of hESCs to
effectively immunize against mouse and rat ovarian cancer, and no
side-effects were observed. hESC vaccines can induce similar
antitumor effects in different species and in different original
cancer tissue, which indicates that the activity of the vaccine is
universal. The unique properties of ES cells may provide a novel
approach for therapeutic options for the management of ovarian
cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81072141, to Y.L.), the
Research and Development foundation in Peking University People’s
Hospital (grant no. RDB2010-09, to Y.L.). The authors thank
Professor Katherine F. Roby for providing the ID8 cells, University
of Kansas Medical Center. The authors also thank Professor
Ming-Xiao Ding, Dr Dong-Hui Zhang and Dr Hai-Song Liu, School of
Life Sciences, Peking University, for their kind assistance in the
processing of human embryonic stem cell culture.
Abbreviations:
|
IL-6
|
interleukin-6
|
|
IFN-γ
|
interferon-γ
|
|
TNF-α
|
tumor necrosis factor-α
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Li Y, Zeng H, Xu RH, Liu B and Li Z:
Vaccination with human pluripotent stem cells generates a broad
spectrum of immunological and clinical responses against colon
cancer. Stem Cells. 27:3103–3111. 2009.PubMed/NCBI
|
|
2
|
Mocan T and Iancu C: Effective colon
cancer prophylaxis in mice using embryonic stem cells and carbon
nanotubes. Int J Nanomed. 6:1945–1954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roby KF, Taylor CC, Sweetwood JP, et al:
Development of a syngeneic mouse model for events related to
ovarian cancer. Carcinogenesis. 21:585–591. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rose GS, Tocco LM, Granger GA, et al:
Development and characterization of a clinically useful animal
model of epithelial ovarian cancer in the Fischer 344 rat. Am J
Obstet Gynecol. 175:593–599. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nolz JC, Starbeck-Miller GR and Harty JT:
Naive, effector and memory CD8 T-cell trafficking: parallels and
distinctions. Immunotherapy. 3:1223–1233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong W, Du J, Shen H, et al:
Administration of embryonic stem cells generates effective
antitumor immunity in mice with minor and heavy tumor load. Cancer
Immunol Immunother. 59:1697–1705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brewer BG, Mitchell RA, Harandi A and
Eaton JW: Embryonic vaccines against cancer: an early history. Exp
Mol Pathol. 86:192–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huebener N, Fest S, Hilt K, et al:
Xenogeneic immunization with human tyrosine hydroxylase DNA
vaccines suppresses growth of established neuroblastoma. Mol Cancer
Ther. 8:2392–2401. 2009. View Article : Google Scholar
|
|
9
|
Niitsu N, Nakamine H and Okamoto M:
Expression of nm23-H1 is associated with poor prognosis in
peripheral T-cell lymphoma, not otherwise specified. Clin Cancer
Res. 17:2893–2899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang PH, Yi YC, Tsai HT, et al:
Significant association of genetic polymorphism of human
nonmetastatic clone 23 type 1 gene with an increased risk of
endometrial cancer. Gynecol Oncol. 119:70–75. 2010. View Article : Google Scholar
|
|
11
|
Kedrin D, Wyckoff J, Boimel PJ, et al:
ERBB1 and ERBB2 have distinct functions in tumor cell invasion and
intravasation. Clin Cancer Res. 15:3733–3739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lekka E, Gritzapis AD, Perez SA, et al:
Identification and characterization of a HER-2/neu epitope as a
potential target for cancer immunotherapy. Cancer Immunol
Immunother. 59:715–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schade B, Rao T, Dourdin N, et al: PTEN
deficiency in a luminal ErbB-2 mouse model results in dramatic
acceleration of mammary tumorigenesis and metastasis. J Biol Chem.
284:19018–19026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodriguez OC, Lai EW, Vissapragada S, et
al: A reduction in Pten tumor suppressor activity promotes
ErbB-2-induced mouse prostate adenocarcinoma formation through the
activation of signaling cascades downstream of PDK1. Am J Pathol.
174:2051–2060. 2009. View Article : Google Scholar
|
|
15
|
Huang S, Benavente S, Armstrong EA, Li C,
Wheeler DL and Harari PM: p53 modulates acquired resistance to EGFR
inhibitors and radiation. Cancer Res. 71:7071–7079. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasaki Y, Negishi H, Idogawa M, et al: p53
negatively regulates the hepatoma growth factor HDGF. Cancer Res.
71:7038–7047. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasojima H, Shimomura A, Naoi Y, et al:
Association between c-myc amplification and pathological complete
response to neoadjuvant chemotherapy in breast cancer. Eur J
Cancer. 47:1779–1788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Xu J, Borowicz S, Collins C, Huo D
and Olopade OI: c-Myc activates BRCA1 gene expression through
distal promoter elements in breast cancer cells. BMC Cancer.
11:2462011. View Article : Google Scholar : PubMed/NCBI
|