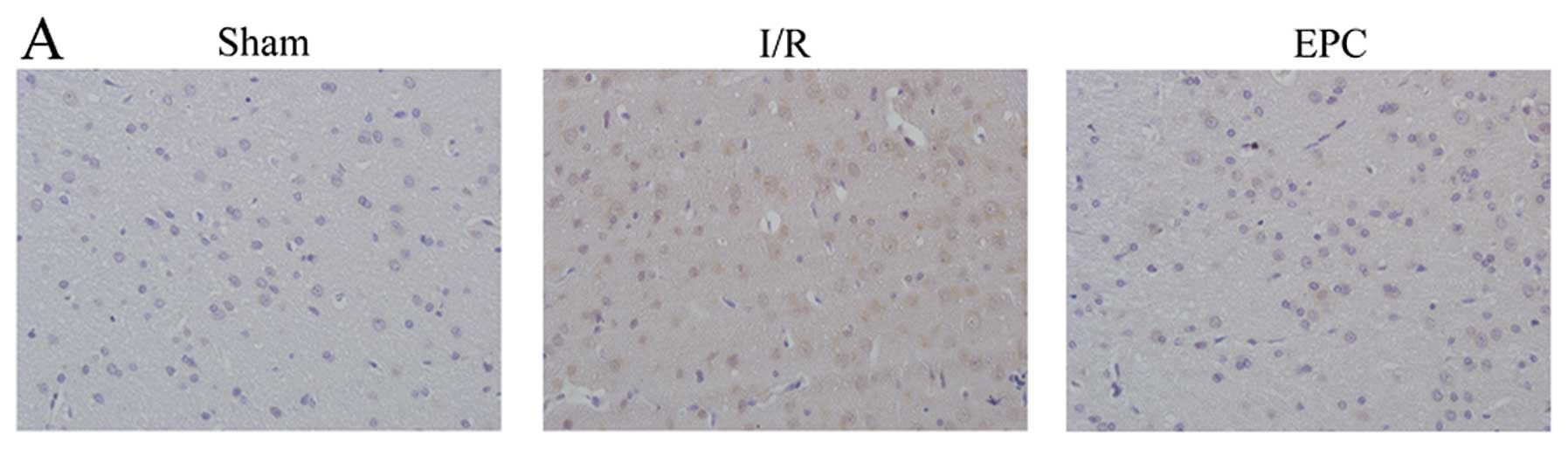
|
1
|
Feigin VL: Stroke epidemiology in the
developing world. Lancet. 365:2160–2161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Donnan GA, Fisher M, Macleod M and Davis
SM: Stroke. Lancet. 371:1612–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feigin VL, Lawes CM, Bennett DA and
Anderson CS: Stroke epidemiology: a review of population-based
studies of incidence, prevalence, and case-fatality in the late
20th century. Lancet Neurol. 2:43–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Durukan A and Tatlisumak T: Acute ischemic
stroke: overview of major experimental rodent models,
pathophysiology, and therapy of focal cerebral ischemia. Pharmacol
Biochem Behav. 87:179–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwiatkowski TG, Libman RB, Frankel M, et
al: Effects of tissue plasminogen activator for acute ischemic
stroke at one year. National Institute of Neurological Disorders
and Stroke Recombinant Tissue Plasminogen Activator Stroke Study
Group. N Engl J Med. 340:1781–1787. 1999.
|
|
6
|
Ginsberg MD: Neuroprotection for ischemic
stroke: past, present and future. Neuropharmacology. 55:363–389.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rafii S and Lyden D: Therapeutic stem and
progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Allegra A, Coppolino G, Bolignano D, et
al: Endothelial progenitor cells: pathogenetic role and therapeutic
perspectives. J Nephrol. 22:463–475. 2009.PubMed/NCBI
|
|
10
|
Sen S, McDonald SP, Coates PT and Bonder
CS: Endothelial progenitor cells: novel biomarker and promising
cell therapy for cardiovascular disease. Clin Sci (Lond).
120:263–283. 2011.PubMed/NCBI
|
|
11
|
Grisar JC, Haddad F, Gomari FA and Wu JC:
Endothelial progenitor cells in cardiovascular disease and chronic
inflammation: from biomarker to therapeutic agent. Biomark Med.
5:731–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohta T, Kikuta K, Imamura H, et al:
Administration of ex vivo-expanded bone marrow-derived endothelial
progenitor cells attenuates focal cerebral ischemia-reperfusion
injury in rats. Neurosurgery. 59:679–686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rouhl RP, van Oostenbrugge RJ, Damoiseaux
J, Tervaert JW and Lodder J: Endothelial progenitor cell research
in stroke: a potential shift in pathophysiological and
therapeutical concepts. Stroke. 39:2158–2165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song YS, Narasimhan P, Kim GS, Jung JE,
Park EH and Chan PH: The role of Akt signaling in oxidative stress
mediates NF-kappaB activation in mild transient focal cerebral
ischemia. J Cereb Blood Flow Metab. 28:1917–1926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Denk A, Wirth T and Baumann B: NF-kappaB
transcription factors: critical regulators of hematopoiesis and
neuronal survival. Cytokine Growth Factor Rev. 11:303–320. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schneider A, Martin-Villalba A, Weih F,
Vogel J, Wirth T and Schwaninger M: NF-kappaB is activated and
promotes cell death in focal cerebral ischemia. Nat Med. 5:554–559.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Desai A, Singh N and Raghubir R:
Neuroprotective potential of the NF-kappaB inhibitor peptide
IKK-NBD in cerebral ischemia-reperfusion injury. Neurochem Int.
57:876–883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang SJ, Zhang H, Hou M, et al: Is it
possible to obtain ‘true endothelial progenitor cells’ by in vitro
culture of bone marrow mononuclear cells? Stem Cells Dev.
16:683–690. 2007.
|
|
20
|
Timmermans F, Plum J, Yoder MC, Ingram DA,
Vandekerckhove B and Case J: Endothelial progenitor cells: identity
defined? J Cell Mol Med. 13:87–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Belayev L, Busto R, Zhao W, Fernandez G
and Ginsberg MD: Middle cerebral artery occlusion in the mouse by
intraluminal suture coated with poly-L-lysine: neurological and
histological validation. Brain Res. 833:181–190. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirschi KK, Ingram DA and Yoder MC:
Assessing identity, phenotype, and fate of endothelial progenitor
cells. Arterioscler Thromb Vasc Biol. 28:1584–1595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Devanesan AJ, Laughlan KA, Girn HR and
Homer-Vanniasinkam S: Endothelial progenitor cells as a therapeutic
option in peripheral arterial disease. Eur J Vasc Endovasc Surg.
38:475–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Q: Progenitor cells in vascular repair.
Curr Opin Lipidol. 18:534–539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Han B, Ma X and Qi S: The effects of
propofol on hippocampal caspase-3 and Bcl-2 expression following
forebrain ischemia-reperfusion in rats. Brain Res. 1356:11–23.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeong HS, Choi HY, Choi TW, et al:
Differential regulation of the antiapoptotic action of B-cell
lymphoma 2 (Bcl-2) and B-cell lymphoma extra long (Bcl-xL) by c-Jun
N-terminal protein kinase (JNK) 1-involved pathway in neuroglioma
cells. Biol Pharm Bull. 31:1686–1690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kinnaird T, Stabile E, Burnett MS, et al:
Marrow-derived stromal cells express genes encoding a broad
spectrum of arteriogenic cytokines and promote in vitro and in vivo
arteriogenesis through paracrine mechanisms. Circ Res. 94:678–685.
2004. View Article : Google Scholar
|
|
29
|
Urbich C, Aicher A, Heeschen C, et al:
Soluble factors released by endothelial progenitor cells promote
migration of endothelial cells and cardiac resident progenitor
cells. J Mol Cell Cardiol. 39:733–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sen S, Merchan J, Dean J, et al:
Autologous transplantation of endothelial progenitor cells
genetically modified by adeno-associated viral vector delivering
insulin-like growth factor-1 gene after myocardial infarction. Hum
Gene Ther. 21:1327–1334. 2010. View Article : Google Scholar
|
|
31
|
Cheng Y, Guo S, Liu G, et al:
Transplantation of bone marrow-derived endothelial progenitor cells
attenuates myocardial interstitial fibrosis and cardiac dysfunction
in streptozotocin-induced diabetic rats. Int J Mol Med. 30:870–876.
2012.
|
|
32
|
Allen CL and Bayraktutan U: Oxidative
stress and its role in the pathogenesis of ischaemic stroke. Int J
Stroke. 4:461–470. 2009. View Article : Google Scholar : PubMed/NCBI
|