Contents
Introduction
Irritable bowel syndrome
Inflammatory bowel disease
Chronic idiopathic slow transit constipation
Celiac disease
Diabetes gastroenteropathy
Colorectal carcinoma
Neuromuscular and system diseases
Surgical resection of stomach and intestine
Conclusion
Introduction
Peptide YY (PYY) was originally isolated from
porcine gut (1–3). PYY has close molecular similarities
to neuropeptide Y (NPY) and pancreatic polypeptide (PP) (2–4),
which has led to the suggestion that they be grouped in a family:
the ‘PP-related peptides’ family (5). In humans, PYY has been found
localized in endocrine cells in the colon (6). Further studies on humans have shown
that PYY immunoreactive cells occur in the ileum, colon, and
rectum, with the highest density in the rectum (7). Furthermore, PYY immunoreactivity has
been localized ultrastructurally in large intestinal H(L)-cells,
whose secretory product was previously unknown (7). PYY endocrine cells occur in the
gastrointestinal mucosa of representatives of all the vertebrate
classes, i.e. cartilaginous and bony fish, amphibians, reptiles,
birds and mammals (8–15). The topo-graphic distribution of
PYY in the gastrointestinal tract differs, however, in different
animals. Thus, in primates, PYY cells occur in the ileum and large
intestine with the highest concentration in the rectum, whereas in
rats, PYY cells occur in all parts of the small and large
intestine, and in fish, reptiles, and amphibians, in the stomach
and upper part of the small intestine. Ontological studies have
shown PYY cells appear early in the gastrointestinal tract of the
embryo (10,12,16). Although PYY cell density is not
affected by aging (16), it is
abnormal in several gastrointestinal diseases and disorders
(17).
The release of PYY from intestinal endocrine cells
is stimulated by intraluminal nutrients, lipids, short-chain fatty
acids, glucose, amino-acids, and bile salts. PYY release can also
be mediated via a neural reflex involving the vagus nerve, as well
as by other gut neuroendocrine peptides such as vasoactive
intestinal peptide (VIP), cholecystokinin (CCK), gastrin, and
glucagon-like peptide-1 (GLP-1) (18).
PYY is one of the major anorexigenic
gastrointestinal neuroendocrine peptides (19). Upon release, PYY is metabolized by
dipeptidyl peptidase-IV (DPP-IV) to PYY (3–36),
which crosses the blood-brain barrier. There, it binds to Y2
receptors on NPY neurons in the arcuate nucleus of the
hypothalamus. Thus, it eliminates the tonic inhibition on
proopiomelanocortin (POMC) neurons with subsequent satiation
(20–22). PYY plays an important role in
regulating gastrointestinal motility and absorption of water and
electrolytes (23–25). These functions regulated by PYY
are disturbed in several gastrointestinal diseases and disorders
(17). It is not surprising,
therefore, that abnormalities in PYY have been reported in
gastrointestinal diseases and disorders. The aim of the present
review was to provide an overview of the changes in PYY in some
gastrointestinal diseases and disorders, and their possible
clinical implications.
Irritable bowel syndrome
Irritable bowel syndrome (IBS) is a chronic common
syndrome affecting 5–20% of the world’s population. IBS symptoms
include diarrhea, constipation, or a combination of the two, and
abdominal pain or discomfort as well as abdominal distension. IBS
is not known to be associated with the development of serious
disease or with excess mortality. IBS, however, reduces
considerably the quality of life. IBS patients are a substantial
concern in both primary and secondary care, and the annual cost in
the USA, both direct and indirect, for the management of patients
with IBS is estimated at 15–30 billion USD (26). The pathogenesis of IBS is not
completely known, but it appears to be multifactorial. Evidence
shows that the following factors play a central role in the
pathogenesis of IBS: heritability and genetics, environment and
social learning, dietary or intestinal microbiota, low-grade
inflammation, and disturbances in the neuroendocrine system (NES)
of the gut (26). A subset of IBS
patients, with no previous gastrointestinal complaints, have a
sudden onset of IBS symptoms following gastroenteritis. This subset
is called post-infectious IBS (PI-IBS). PI-IBS, however, has also
been reported following non-gastrointestinal infections such as
respiratory, urinary tract and skin infections (26).
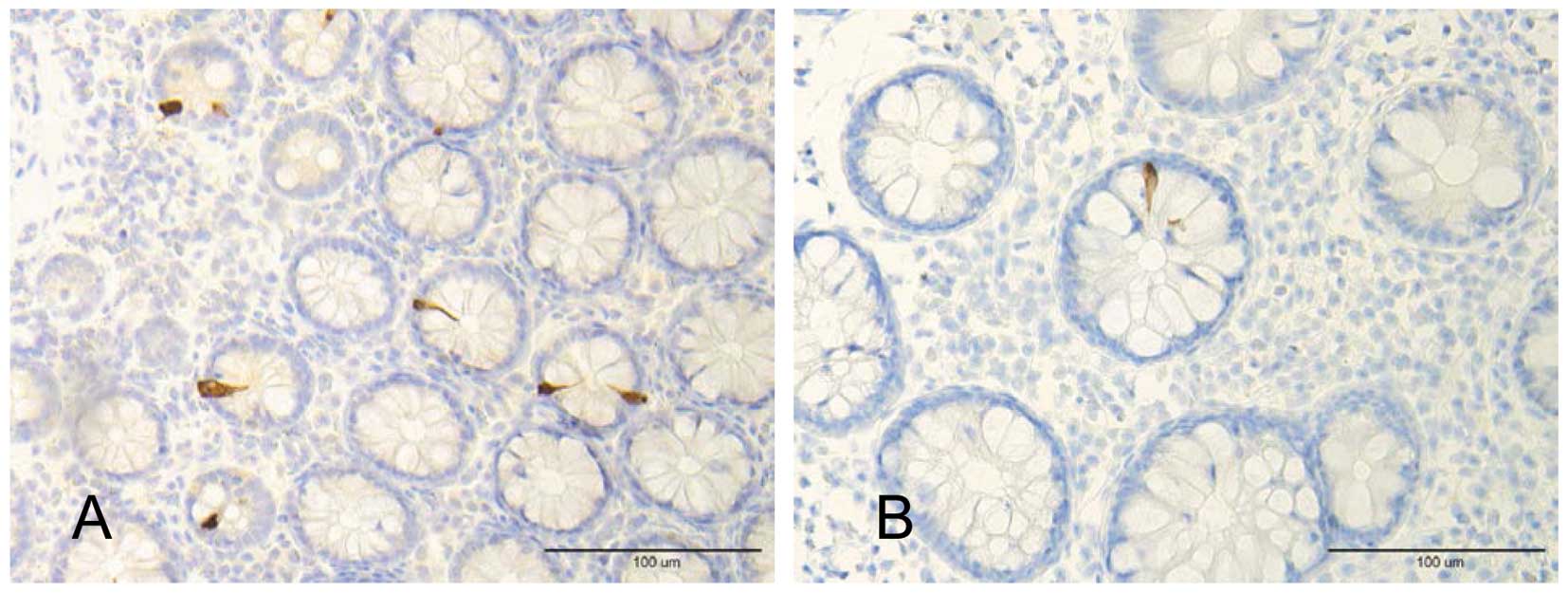
In the large intestine of sporadic IBS patients, PYY
cell density was found to be low in both IBS-constipation and
IBS-diarrhea patients (Fig. 1)
(27). In the large intestine of
the same patients, serotonin cell density was reduced and the
mucosal 5-HT concentration was also reported to be low (28). In the duodenum of IBS patients,
the number of CCK cells was also low (29). Serotonin acts on 5-HT1p receptors,
which are located on a subset of inhibitory motor neurons of the
myenteric plexus and relax the stomach via a nitrergic pathway,
delaying gastric emptying (26).
The primary targets of serotonin are the mucosal projections of
primary afferent neurons, which transmit the sensation of nausea
and discomfort to the central nervous system, and the mucosal
projections of intrinsic primary afferent neurons, which initiate
peristaltic and secretory reflexes (30–35). Serotonin also stimulates the
secretion of chloride and water from the small intestine by acting
through 5-HT3 and 5-HT4 receptors (36–40). The low density of serotonin cells
is likely to reduce motility and secretion of chloride and water in
the colons of patients with IBS. As compensation for this, PYY is
reduced. As previously mentioned, CCK stimulates the release of
PYY. Moreover, low CCK would result in low bile salts, which are
stimulatory for PYY secretion. Thus, a low CCK could contribute to
the low density of large intestinal PYY cells in IBS patients. The
findings of genetic in transmission pathways of serotonin and CCK
(41–44) support the assumption that the
change in colonic PYY cells is secondary to changes in serotonin
and CCK.
In contrast to sporadic IBS, PYY cell numbers are
reported to be increased in the large intestine of PI-IBS patients
(45). Serotonin and CCK cell
densities are also increased in these patients (45–50). As low-grade inflammation has been
reported in PI-IBS, the increase in cell density of CCK and
serotonin cells appears to be a result of an interaction with
immune cells, as described below.
Therefore, PYY is affected in IBS and may play a
role in its symptomology. These changes, however, seem to be
secondary to the changes in CCK and serotonin.
Inflammatory bowel disease
Inflammatory bowel disease (IBD) comprises two
distinct disorders with independent clinicopathologies of unknown
etiology. These diseases, ulcerative colitis (UC) and Crohn’s
disease (CD), are fairly distinct in their organ specificity and
their histopathological characteristics. The onset of IBD occurs
most often at the age of 20–30 years. Thus, IBD represents an
important public health problem as it affects young individuals,
interfering with the patient’s education, working abilities, social
life, and quality of life. These diseases are chronic conditions
whose clinical courses vary considerably, with frequent relapses or
chronic active disease in some patients, whereas some have years of
virtually complete remission. In addition to UC and CD, another
inflammatory bowel disease is included in this category,
microscopic colitis (MC). MC is also a chronic condition,
characterized by watery diarrhea with normal radiologic and
endoscopic findings. However, histopathological examinations of the
colon reveal abnormal histology (51). This abnormality is of two
distinctive types: lymphocytic colitis (LC) and collagenous colitis
(CC) (51).
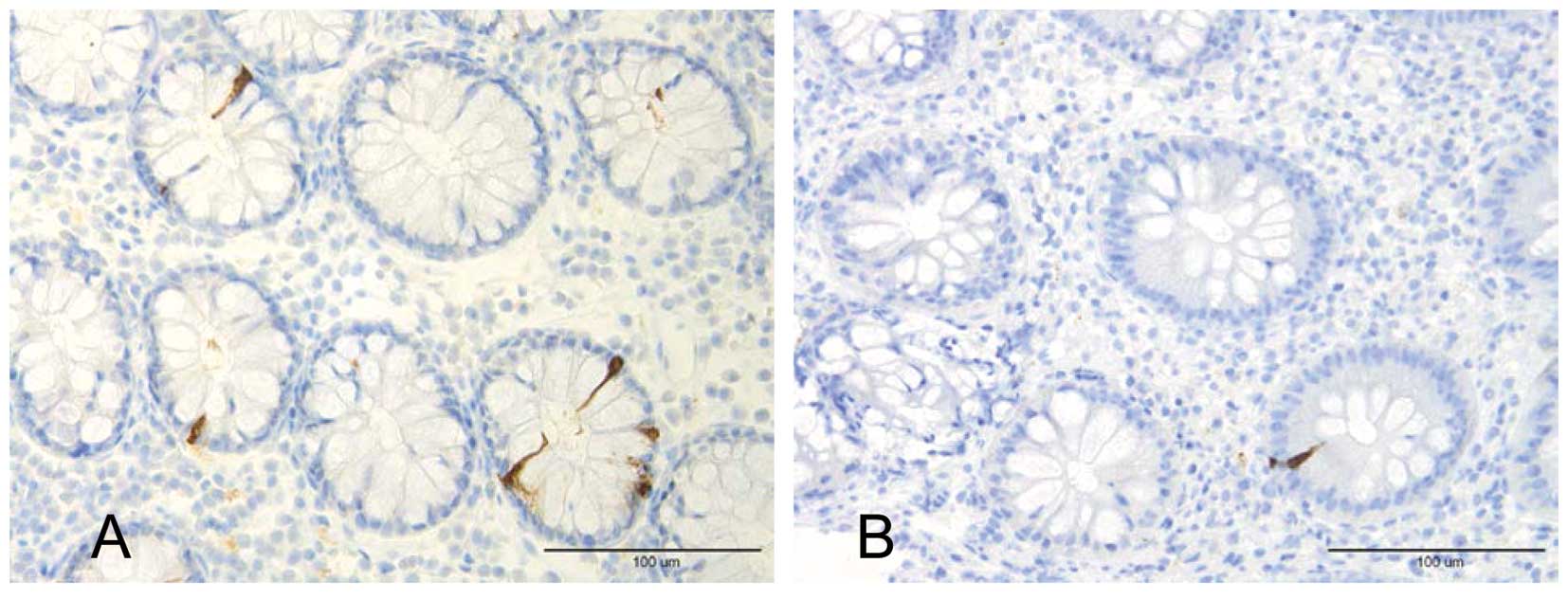
Colonic PYY cell area was found to be decreased in
patients with UC and CD (Fig. 2),
whereas those with serotonin and enteroglucagon immunoreactivities
were elevated (52). As
enteroglucagon and PYY are colocalized in the same colorectal
endocrine cell type (L-cells) (53–55), it appears that this cell increased
its expression of enteroglucagon, and reduced expression of PYY. In
the ileal mucosa of patients with CD, PYY cell density was
decreased, as was that of serotonin (56). In one study, serotonin cell
density in rectal biopsies from patients with UC was found to be
elevated (57), whereas another
study showed it to be decreased (28). In rectal biopsies from patients
with UC, mucosal serotonin, tryptophan hydroxylase 1 messenger RNA,
serotonin transporter messenger, and serotonin transporter were all
reduced (28). It has also been
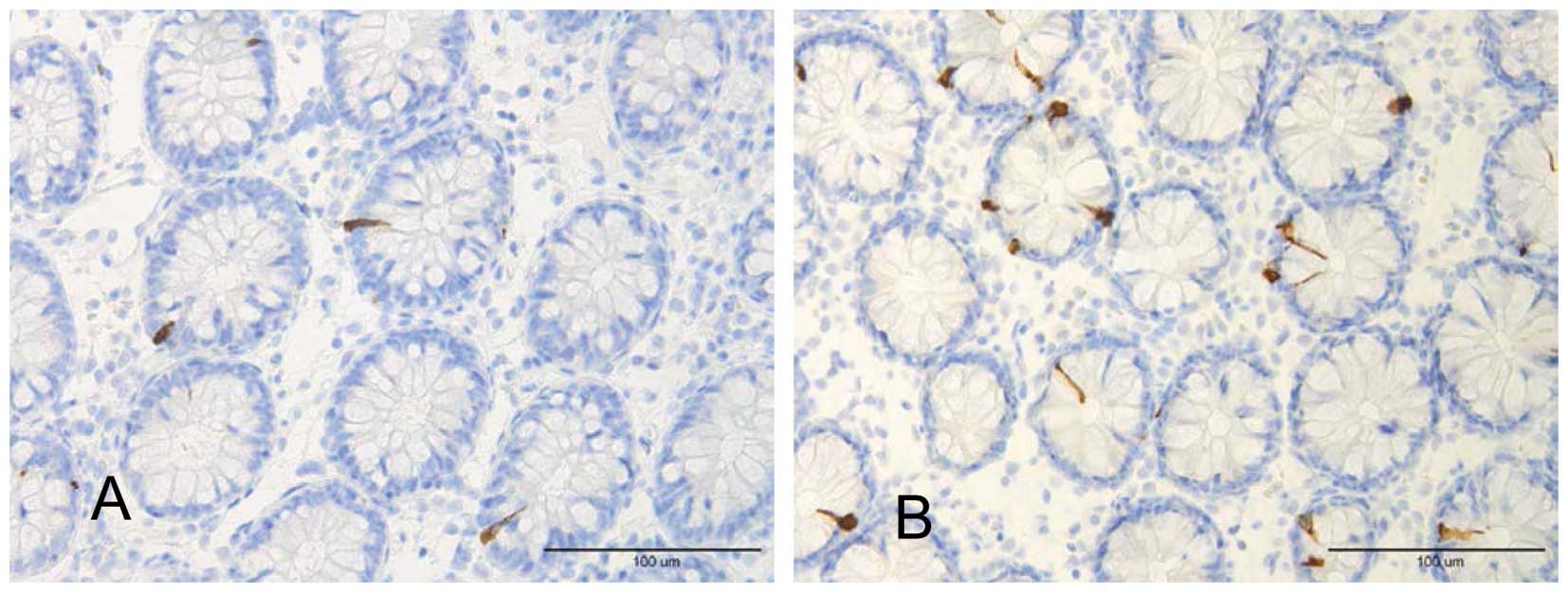
shown that patients with LC have high densities of colonic PYY and
serotonin cells (58) (Fig. 3).
In an experimental animal model of colitis (IL-2
knockout mice), PYY and serotonin cell densities were decreased in
mice with colitis, whereas enteroglucagon remained unchanged
(59). In another animal model
(rats treated with dextran sulfate sodium), the PYY density
decreased in both the small and large intestines (60).
Increasing evidence shows that inflammation and
immune cells interact with the NES of the gut, which controls and
regulates gastrointestinal motility and sensitivity (61). Thus, serotonin secretion by
enterochromaffin (EC) cells can be enhanced or attenuated by
secretory products of immune cells such as CD4+ T
lymphocytes (62). Furthermore,
serotonin modulates the immune response (62). The EC cells are in contact with or
very close to CD3+ and CD20+ lymphocytes, and several
serotonergic receptors have been characterized in lymphocytes,
monocytes, macrophages, and dendritic cells (63). Moreover, immune cells in the small
and large intestines show receptors for substance P and VIP
(59).
Based on the above-mentioned interaction between
immune and serotonin cells, it may be assumed that the changes in
serotonin cells are caused by the inflammatory process. It further
seems that the changes in PYY cells in IBD are secondary to changes
in serotonin cells.
Chronic idiopathic slow transit
constipation
Chronic idiopathic slow transit constipation (CST)
is a common clinical problem. This condition is characterized by
chronic severe constipation, which is not alleviated by bulking
agents, prokinetic drugs or other laxative treatments. These
patients require enema for defecation (64,65). Histopathological examination fails
to identify any abnormality in the colon of these patients
(64,65). However, slow colonic transit and
motility disorders of the colon and rectum have been found in these
patients (66–70).
PYY cells have been reported to be increased
compared to controls in the ascending colon of patients with CST
(71). In another study from our
laboratory, however, the number of colonic PYY cells was unaffected
(72). The concentration of PYY
in colonic tissue extracts from patients with CST has been reported
to be high (73), but basal and
peak plasma PYY levels have been reported to be unaffected
(74). These results initially
appear to be contradictory. However, taking CST patients as
individuals instead of as a group, and analyzing the neuroendocrine
peptide profile in the colon of each individual, revealed that a
disturbance in the neuroendocrine system is the most probable cause
for the disease, and that this disturbance affects different
neuroendocrine peptides in different patients (73). This seems to explain the
contradiction found in different studies.
The increase in the number of colonic PYY cells and
PYY synthesis seems to be primary and may be one of the etiologic
factors for CST. Consequently, this increase would increase
absorption and decrease secretion of water and electrolytes,
strengthening the ileal brake and inhibiting intestinal motility,
which leads to constipation.
Celiac disease
Celiac disease is associated with derangement of the
architecture of the small intestinal mucosa in the form of villus
atrophy, increased crypt length, and increased volume of lamina
propria (75). Several changes in
the small intestinal endocrine cells have been reported (75). Basal and postprandial plasma
levels of PYY are elevated in patients with celiac disease
(76,77). PYY levels have been found to be
inversely correlated with the concentration of serum folate acid
(77). These elevated levels of
PYY have been reported to normalize within 8 months on a
gluten-free diet (77).
The changes in the endocrine cells of the small
intestine in patients with celiac disease are considered to be a
selective process to meet the new demands exerted by the marked
decrease in intestinal absorptive area; these changes contribute to
the manifestation of the symptoms seen in patients with celiac
disease, such as diarrhea and steatorrhea (75). This could be caused by incomplete
digestion of ingested food and its rapid elimination from the
intestine (75). The elevated
levels of circulating PYY in patients with celiac disease appear to
be involved in this hormonal process, and are probably a response
to diarrhea and steatorrhea, an attempt to slow down the intestinal
transit time and increase intestinal absorption. The finding that
patients with diarrhea due to other causes, such as chronic
destructive pancreatitis and infective gastroenteritis, also have
high plasma levels of PYY support this assumption (78).
Diabetes gastroenteropathy
Gastrointestinal symptoms, such as nausea and
vomiting, diarrhea, constipation, and abdominal pain, are common in
patients with diabetes mellitus (79–85). These symptoms are considered to be
caused by gastrointestinal dysmotility and secretion/absorption
disturbances (81). Abnormalities
in PYY have been reported in patients with diabetes type 1, and in
animal models of human diabetes.
Rectal PYY cells were investigated in patients with
a long duration (13–48 years) of diabetes type 1, with organ
complications and gastrointestinal symptoms, such as nausea,
vomiting and diarrhea; they also had slow gastric emptying. The
number of rectal PYY cells in these patients was found to be
significantly higher than that in healthy volunteers (86).
In animal models of human diabetes type 1, i.e.
non-obese diabetic (NOD) mice, the number of colonic PYY cells was
reduced in diabetic, but not in pre-diabetic NOD mice (83–86). Radioimmunoassay of tissue extracts
showed, however, low concentrations of colonic PYY in both
pre-diabetic and diabetic mice (87). It seems that the synthesis of PYY
decreased prior to the onset of diabetes, although the number of
PYY cells was unaffected. Once the diabetic state is established,
even the number of PYY cells declines. This animal model exhibits
slow gastric emptying, fast gastrointestinal transit, and diarrhea
(88–90).
The studies of PYY in animal models of human
diabetes type 1 and patients with diabetes appear to yield
contradictory results. Thus, whereas the number of PYY cells and
the concentration of PYY in the large intestine of NOD mice was
low, the number of rectal PYY cells was high in patients with
diabetes type 1. This discrepancy may be due to the NOD mouse model
not being completely similar to human diabetes type 1, or to the
difference in the segment of large intestine being studied (the
colon in NOD mice and the rectum in diabetic patients). It is most
likely, however, that the difference was caused by the difference
in the duration of the diabetic state. Thus, whereas the NOD mice
were investigated shortly after the onset of diabetes, patients
were studied after a long duration of diabetes.
PYY has been studied in two animal models of human
diabetes type 2, namely ob/ob and db/db obese diabetic mice. In
ob/ob mice, the number of colonic PYY cells and the tissue
concentrations of PYY were found to be lower than those of controls
(83–86,90). By contrast, the number of colonic
PYY cells in db/db mice were reported to be higher than in controls
(91). This disagreement in the
results was explained by the difference in the duration of diabetes
in ob/ob mice (83–86).
As already mentioned, PYY inhibits the secretion of
fluid and electrolytes, while stimulating their absorption in the
intestine. It is also a potent mediator of ileal brake, which
inhibits gastric emptying and delays intestinal transit. It is
possible that at the onset of diabetes type 1, hyperglycemia, as
well as other factors, inhibit gastric motility and, consequently,
gastric emptying. As PYY inhibits gastric emptying, the number of
PYY cells and their synthesis is decreased in an attempt to
compensate for the slow gastric emptying. Subsequently, when fast
intestinal transit and diarrhea developed in these patients, PYY
cells and, possibly, PYY synthesis, increased in response to these
changes. This increase would, however, worsen the gastric emptying.
In diabetes type 2, at least in animal models, PYY cells and
synthesis may be decreased to compensate for the slow intestinal
transit and constipation.
Colorectal carcinoma
The number of PYY cells in the colons of rats with
chemically induced adenocarcinoma has been reported to be high
(92). The difference in
concentration of PYY in colon tissue extracts from these animals
was not statistically significant, although it was higher than in
controls (93). In patients with
colorectal carcinoma, neither the number of PYY cells nor the
concentration of PYY in the colon is affected (94–96). PYY receptors have been
demonstrated in colonic adenocarcinoma cell lines; however, PYY
exerts no direct growth regulatory effect on colon cancer cell
lines (97–99). Collectively, these findings show
that it is unlikely that PYY is involved in the development and
growth of colorectal carcinoma.
Neuromuscular and system diseases
PYY cells have been studied in the large intestine
in two hereditary diseases that affect either the nervous system or
muscles, i.e. familial amyloidotic polyneuropathy (FAP) and
myotonic dystrophy (MD) (100–103). FAP is caused by amyloid deposits
of mutated transthyretin in the nervous system and other organs.
Several gastrointestinal symptoms, such as constipation, nausea,
vomiting, and diarrhea, are invariably present in FAP patients
during the course of the disease (104–106). MD is a disease caused by a
genetic defect in chromosome 19. Gastrointestinal symptoms, such as
abdominal pain, nausea and diarrhea, are often encountered in MD
patients (103). The
gastrointestinal symptoms in both diseases are believed to be
caused by gastrointestinal dysmotility (100,103). Despite this gastrointestinal
dysmotility, PYY cells in the large intestine of FAP patients, both
in Sweden and Japan, as well as of MD patients, were unaffected
(100,102,103). It seems, therefore, that PYY
does not play a significant role in these categories of
patients.
Plasma levels of PYY in patients with systemic
sclerosis have been found to be elevated (107). Fat malabsorption has also been
found to be more common among patients with increased levels of
plasma PYY. This increase in circulating PYY in patients with
systemic sclerosis seems to be secondary to the diarrhea, rather
than a primary cause, and is probably an attempt to slow down
gastrointestinal motility.
Surgical resection of stomach and
intestine
Surgical resection of the stomach and intestine is
frequently used as primary treatment, or when medical therapy has
failed, in several gastrointestinal diseases, such as gastric
carcinoma, colorectal carcinoma, inflammatory bowel disease and
CST.
Gastric resection is associated with several
problems, such as dumping syndrome, reflux esophagitis, and
malabsorption. The serum levels of several neuroendocrine hormones
have been investigated in patients with partial distal gastrectomy
or total gastrectomy. The levels of circulating PYY in these
patients were elevated (108).
This elevation may be an adaptation to compensate for the rapid
gastric transit, and an attempt to slow it.
Basal and postprandial levels of PYY increased in
the intestine adjacent to the anastomatic site after a massive
(75%) small bowel resection in dog (76). This increase was observed one
month after the small bowel resection and remained high throughout
the six-month experiment. Circulating PYY concentrations have also
been investigated in patients subjected to small bowel resection,
mainly due to Crohn’s disease. Thus, in patients receiving
treatment with home parental nutrition following near-total
enterectomy, basal and postprandial circulating PYY have been found
to be high (109). Similarly,
elevated fasting serum PYY levels have been reported in patients
with Crohn’s disease who had previous resections of >48 cm of
the ileum (109). Circulating
PYY levels have also been measured (110,111). Basal and postprandial plasma
levels of PYY in patients who have undergone resection of the colon
increased after the construction of a pelvic reservoir (110). The combination of an infusion of
oleic acid in the ileal pouch and a meal increased PYY plasma
levels, slowed gastrointestinal transit, and delayed defecation
(111). Massive small bowel
resection in 4-week-old piglets also resulted in an increase in
colonic PYY cells (112).
Following resection of a considerable part of either
the small or large intestine, PYY synthesis and release increased
as an adaptive response. This response is an attempt to slow the
rapid gastrointestinal transit caused by the intestinal
resection.
Conclusion
PYY changes in several gastrointestinal disorders.
These changes seem to be adaptive responses to the
pathophysiological alterations caused by the disease. However, in
some gastrointestinal disorders, such as chronic idiopathic slow
transit constipation (CST), the abnormality in PYY seems to be
primary and may, at least in part, be one of the causes of the
disease. These abnormalities in PYY seem to contribute to the
development of symptoms seen in gastrointestinal diseases/disorders
such as gastroenteropathy in long-standing diabetes, inflammatory
bowel disease and CST. The changes in PYY could, however, be
favorable in some gastrointestinal disorders such as celiac
disease, systemic sclerosis, and post-intestinal resection
state.
The accumulated data regarding the changes in PYY in
gastrointestinal disorders could be beneficial in clinical
practice. Thus, a receptor agonist or antagonist can be used as a
drug depending on the condition. Infusion of PYY in dogs increases
colonic absorption of water, Na and Cl ions (113), and intraluminal administration
of a synthetic analog, BIM-34004, has the same effect (114). It has been suggested that PYY or
its analog can be used as clinical agents in intestinal
malabsorption disorders or after bowel resection (113,114). In clinical trials, nausea and
fullness are the most common side-effects of PYY (115). Similar to other neuroendocrine
peptides/amines of the gut, PYY has broad
physiological/pharmacological effects: it can bind to and activate
several receptors with independent actions. Thus, in order to use
PYY as a drug, receptor-specific agonists or antagonists need to be
developed.
Acknowledgements
The authors’ studies cited in this
review were supported by grants from Helse-Fonna, Helse-Vest in
Norway. The Swedish Medical Research Council, Bengt Ihre’s
Foundation, Sahlberg’s Foundation, the Faculty of Medicine, Umeå
University Research Funds, Åke Wiberg’s Foundation, Bergvalls
Foundation, Lions Cancer Research Foundation, Umeå, and the
Familial Amyloidosis Association (FAMY) in Sweden.
References
|
1
|
Tatemoto K and Mutt V: Isolation and
characterization of two novel candidate hormones using a chemical
method for finding naturally occurring polypeptides. Nature.
285:417–418. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tatemoto K: Isolation and characterization
of peptide YY (PYY), a candidate gut hormone that inhibits
pancreatic exocrine secretion. Proc Natl Acad Sci USA.
79:2514–2518. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tatemoto K: Neuropeptide Y: the complete
amino acid sequence of the brain peptide. Proc Natl Acad Sci USA.
79:5485–5489. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tatemoto K, Carlquist M and Mutt V:
Neuropeptide Y - a novel brain peptide with structural similarities
to peptide YY and pancreatic polypeptide. Nature. 296:659–660.
1982. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sundler F, Moghimazadeh E, Håkanson R, et
al: Nerve fibers in the gut and pancrease of the rat displaying
neuropeptide Y immunoreactivity. Intrinsic and extrinsic origin.
Cell Tissue Res. 230:487–493. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lundberg JM, Tatemoto K, Terenius L,
Hellerström PM, Mutt V, Hökfelt PM and Hemberger B: Localization of
polypeptide YY (PYY) in gastrointestinal endocrine cells and
effects on intestinal blood flow and motility. Proc Natl Acad Sci
USA. 79:4471–4475. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Salhy M, Grimelius L, Wilander E,
Ryberg B, Lundberg JM, Terenius L and Tatemoto K:
Immunocytochemical identification of polypeptide YY (PYY) cells in
the human gastrointestinal tract. Histochemistry. 77:15–23. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Salhy M: Immunocytochemical
investigation of the gastroentero-pancreatic (GEP) neurohormonal
peptides in the pancrease and gastrointestinal tract of a
cartilaginous fish, the dogfish Squalus achanthias.
Histochemistry. 80:193–205. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El-Salhy M: The occurrence of polypeptide
YY (PYY) and pancreatic polypeptide (PP) in the gastrointestinal
tract of bony fish. Biomed Res. 5:441–444. 1984.
|
|
10
|
El-Salhy M, Grimelius L, Lundberg JM,
Tatemoto K and Terenius L: Immunocytochemical evidence for the
occurrence of PYY, a newly isolated gut polypeptide, in endocrine
cells in the gut of amphibians and reptiles. Biomed Res. 3:303–306.
1982.
|
|
11
|
El-Salhy M, Wilander E, Abu-Sinna G,
Shabaka H, Abu-Sinna G, Lundberg JM and Tatemoto K: On the ontogeny
of polypeptide YY (PYY) in the chickens. Biomed Res. 3:680–682.
1982.
|
|
12
|
El-Salhy M, Grimelius L, Wilander E,
Lundberg JM, Tatemoto K and Terenius L: The distribution of
polypeptide YY (PYY) and pancreatic polypeptide (PP)-immunoreactive
cells in the domestic fowl. Histochemistry. 75:25–30. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El-Salhy M, Wilander E, Juntti-Berggren L
and Grimelius L: The distribution and ontogeny of polypeptide YY
(PYY) and polypetide (PP)-immunoreactive cells in the
gastrointestinal tract of rat. Histochemistry. 78:53–60. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Salhy M and Lundqvist M:
Immunocytochemical investigation of polypeptide YY (PYY) and
pancreatic polypeptide (PP) in the gastrointestinal tract of three
rodents. Biomed Res. 5:401–404. 1984.
|
|
15
|
El-Salhy M and Grimelius L:
Immunocytochemical demonstration of polypeptide YY (PYY) in the
gastrointestinal tract of the monkey, Macaca rhesus. Biomed
Res. 4:289–294. 1983.
|
|
16
|
Sandström O and El-Salhy M: Ontogeny and
the effect of aging on pancreatic polypeptide and peptide YY.
Peptides. 23:263–267. 2002.PubMed/NCBI
|
|
17
|
El-Salhy M, Suhr O and Danielsson A:
Peptide YY in gastrointestinal disorders. Peptides. 23:397–402.
2002. View Article : Google Scholar
|
|
18
|
Ballantyne GH: Peptide YY (1–36) and
peptide YY (3–36): Part I. Distribution, release and actions. Obes
Surg. 16:651–658. 2006.
|
|
19
|
Grudell ABM and Camilleri M: The role of
peptide YY in integrative gut physiology and potent role in
obesity. Curr Opin Endocrinol Diabetes Obes. 14:52–57. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batterham RL, Crowley MA, Small CJ, Herzog
H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone
RD and Bloom SR: Gut hormone PYY (3–36) physiologically inhibits
food intake. Nature. 418:650–654. 2002.
|
|
21
|
Broberger C: Brain regulation of food
intake and appetite: molecules and networks. J Intern Med.
258:301–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McGowan BM and Bloom SR: Peptide YY and
appetite control. Curr Opin Pharmacol. 4:583–588. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mannon P and Taylor I: The pancreatic
polypeptide family. Gut Peptides: Biochemistry and Physiology.
Walsh B and Dockary G: Raven Press; New York: pp. 351–358. 1994
|
|
24
|
Spiller RC, Trotman IF, Higgins BE, Ghati
MI, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ and Silk DBA: The
ileal brake-inhibition of jujunal motility after ileal fat
perfusion in man. Gut. 25:365–374. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spiller RC, Trotma IF, Adrian TE, Bloom
SR, Misiewicz JJ and Silk DB: Further characterisation of the
‘ileal brake’ reflex in man-effect of ileal infusion of partial
digests of fat, protein, and starch on jejunal motility and release
of neurotensin, enteroglucagon, and peptride YY. Gut. 29:1042–1051.
1988.
|
|
26
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Diagnosis, Pathogenesis and Treatment Options. Nova
Science Publishers; New York: 2012
|
|
27
|
El-Salhy M, Gundersen D, Østgaard H,
Lomholt-Beck B, Hatlebakk JG and Hausken T: Low densities of
serotonin and peptide YY cells in the colon of patients with
irritable bowel syndrome. Dig Dis Sci. 57:873–878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Coates MD, Mahoney CR, Linden DR, Sampson
JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Greshon MD, Mawe GM
and Moses PL: Molecular defects in mucosal serotonin content and
decreased serotonin reuptake transporter in ulcerative colitis and
irritable bowel syndrome. Gastroenterology. 126:1657–1664. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El-Salhy M, Vaali K, Dizdar V and Hausken
T: Abnormal small-intestinal endocrine cells in patients with
irritable bowel syndrome. Dig Dig Sci. 55:3508–3513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blackshaw LA and Grundy D: Effects of
5-hydroxytryptamine on discharge of vagal mucosal afferent fibres
from the upper gastrointestinal tract of the ferret. J Auton Nerv
Syst. 45:41–50. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kirchgessner AL, Tamir H and Gershon MD:
Identification and stimulation by serotonin of intrinsic sensory
neurons of the submucosal plexus of the guinea pig gut:
activity-induced expression of Fos immunoreactivity. J Neurosci.
12:235–249. 1992.PubMed/NCBI
|
|
32
|
Kirchgessner AL, Liu MT and Gershon MD: In
situ identification and visualization of neurons that mediate
enteric and enteropancreatic reflexes. J Comp Neurol. 371:270–286.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hillsley K and Grundy D: Sensitivity to
5-hydroxytryptamine in different afferent subpopulations within
mesenteric nerves supplying the rat jejunum. J Physiol.
509:717–727. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grundy D, Blackshaw LA and Hillsley K:
Role of 5-hydroxytryptamine in gastrointestinal chemosensitivity.
Dig Dis Sci. 3(Suppl 12): S44–S47. 1994. View Article : Google Scholar
|
|
35
|
Pan H and Gershon MD: Activation of
intrinsic afferent pathways in submucosal ganglia of the guinea pig
small intestine. J Neurosci. 20:3295–3309. 2000.PubMed/NCBI
|
|
36
|
Sidhu M and Cooke HJ: Role for 5-HT and
ACh in submucosal reflexes mediating colonic secretion. Am J
Physiol. 269:G346–G351. 1995.PubMed/NCBI
|
|
37
|
Cooke HJ, Sidhu M and Wang YZ: 5-HT
activates neural reflexes regulating secretion in the guinea-pig
colon. Neurogastroenterol Motil. 9:181–186. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim M, Cooke HJ, Javed NH, Carey HV,
Christofi F and Raybould HE: D-glucose releases 5-hydroxytryptamine
from human BON cells as a model of enterochromaffin cells.
Gastroenterology. 121:1400–1406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gershon MD: Review article: serotonin
receptors and transporters-roles in normal and abnormal
gastrointestinal motility. Aliment Pharmacol Ther. 20(Suppl 7):
S3–S14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bearcroft CP, Andre EA and Farthing MJ: In
vivo effects of the 5-HT3 antagonist alosetron on basal and
cholerat toxin-induced secretion in the human jejunum: a segmental
perfusion study. Aliment Pharmacol Ther. 11:1109–1114. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Camilleri M: Is there a SERT-ain
association with IBS. Gut. 53:1396–1398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yeo A, Boyd P, Lumsden S, Saunders T,
Handley A, Stubbins M, Knaggs A, Asquith S, Taylor I, Bahari B,
Crocker N, Rallan R, Varsani S, Montgomery D, Alpers DH, Dukes GE,
Purvis I and Hicks GA: Association between a functional
polymorphism in the serotonin transporter gene and diarrhea
predominant irritable bowel syndrome in women. Gut. 53:1452–1458.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Y, Nie Y, Xie J, Tang W, Liang P, Sha
W, Yang H and Zhou Y: The association of serotonin transporter
genetic polymorphisms and irritable bowel syndrome and its
influence on tegaserod treatment in Chinese patients. Dig Dis Sci.
52:2942–2949. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park SY, Rew JS, Lee SM, Ki HS, Lee KR,
Cheo JH, Kim HI, Noh DY, Joo YE, Kim HS and Choi SK: Association of
CCK(1) receptor gene polymorphisms and irritable bowel syndrome in
Korean. J Neurogastroenterol Motil. 16:71–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang LH, Fang XC and Pan GZ: Bacillary
dysentery as a causative factor for irritable bowel syndrome and
its pathogenesis. Gut. 53:1096–1101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spiller RC, Jenkins D, Thornely JP, Hebden
JM, Wright T, Skinner M and Neal KR: Increased rectal mucosal
enteroendocrine cells, T lymphocytes, and increased gut
permeability following acute Campylobacter enteritis and in
post-dysenteric irritable bowel syndrome. Gut. 47:804–811. 2000.
View Article : Google Scholar
|
|
47
|
Dunlop SP, Jenkins D, Neal KR and Spiller
RC: Relative importance of enterochromaffin cell hyperplasia,
anxiety, and depression in postinfectious IBS. Gastroenterology.
125:1651–1659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee KJ, Kim YB, Kwon HC, Kim DK and Cho
SW: The alteration of enterochromaffin cell, mast cell and lamina
propria T lymphocyte numbers in irritable bowel syndrome and its
relationship with psychological factors. J Gastroenterol Hepatol.
3:1689–1694. 2008.PubMed/NCBI
|
|
49
|
Kim HS, Lim JH, Park H and Lee SI:
Increased immunoreactive cells in intestinal mucosa of
postinfectious irritable bowel syndrome patients 3 years after
acute Shigella infection - an observation in a small case control
study. Yonsei Med. 51:45–51. 2009.
|
|
50
|
Dizdar V, Spiller R, Hanevik K, Gilja OH,
El-Salhy M and Hausken T: Relative importance of abnormalities of
CCK and 5-HT (serotonin) in Giardia-induced post-infectious
irritable bowel syndrome and functional dyspepsia. Aliment
Pharmacol Ther. 31:883–891. 2010.PubMed/NCBI
|
|
51
|
Liszka L, Woszczyk D and Pajak J:
Histopathological diagnosis of microscopic colitis. J Gastroenterol
Hepatol. 21:792–797. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
El-Salhy M, Danielsson A, Stenling R and
Grimelius L: Colonic endocrine cells in inflammatory bowel disease.
J Intern Med. 242:413–419. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Böttcher G, Alumets J, Håkanson R and
Sundler F: Co-existence of glicentin and peptide YY in colorectal
L-cells in cat and man. An electron microscopic study. Regul Pept.
13:283–291. 1986.PubMed/NCBI
|
|
54
|
Ali-Rachedi A, Varndell MI, Adrian TE,
Gapp DA, van Noorden S, Bloom SR and Polak JM: Peptide YY (PYY)
immunoreactivity is co-stored with glucagon-related immunoreactants
in endocrine cells of the gut and pancreas. Histochemistry.
80:487–491. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nilsson O, Bilchik AJ, Goldenring JR,
Ballantyne GH, Adrian TE and Modlin IM: Distribution and
immunocytochemical colocalization of peptide YY and enteroglucagon
in endocrine cells of the rabbit colon. Endocrinology. 129:139–148.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lu SJ, Liu YQ, Lin JS, Wu HJ, Sun YH and
Tan YB: VIP immunoreactive nerve and somatostatin and serotonin
containing cells in Crohn’s disease. World J Gastroenterol.
5:541–543. 1999.PubMed/NCBI
|
|
57
|
Stoyanova II and Gulubova MV: Mast cells
and inflammatory mediators in chronic ulcerative colitis. Acta
Histochem. 104:185–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: High densities of serotonin and peptide YY cells in the
colon of patients with lymphocytic colitis. World J Gastroenterol.
18:6070–6075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Qian BF, El-Salhy M, Melgar S, Hammarström
ML and Danielsson A: Neuroendocrine changes in colon of mice with a
disrupted IL-2 gene. Clin Exp Immunol. 120:424–433. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hirotani Y, Mikajiri K, Ikeda K, Myotoku M
and Kurokawa N: Changes in the peptide YY levels in the intestinal
tissue of rats with experimental colitis following oral
administration of mesalazine and prednisolone. Yakugaku Zasshi.
128:1347–1353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Spiller R: Serotonin, inflammation, and
IBS: fitting the jigsaw together? J Pediatr Gastroenterol Nutr.
45(Suppl 2): S115–S119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Khan WI and Ghia JE: Gut hormones:
emerging role in immune activation and inflammation. Clin Exp
Immunol. 161:19–27. 2010.PubMed/NCBI
|
|
63
|
Yang GB and Lackner AA: Proximity between
5HT secreting enteroendocrine cells and lymphocytes in gut mucosa
of rhesus macaques (Macaca mulatta) is suggestive of a role
for enterochromaffin cell 5HT in mucosal immunity. J Neuroimmunol.
146:46–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
MacDonald A, Baxter JN and Finlay IG:
Idiopathic slow-transit constipation. Br J Surg. 80:1107–1111.
1993. View Article : Google Scholar
|
|
65
|
El-Salhy M: Chronic idiopathic slow
transit constipation: pathophysiology and management. Colorectal
Dis. 5:288–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bassotti G, Gaburri M, Imbimbo BP, Rossi
L, Faroni F, Pelli MA and Morelli A: Colonic mass movement in
idiopathic chronic constipation. Gut. 29:1173–1178. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bassotti G, Betti C, Pelli MA and Morelli
A: Prolonged (24-hour) manometeric recording of rectal contractile
activity in patients with slow transit constipation. Digestion.
49:72–77. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bassotti G, Chiarioni G, Imbimbo BP, Betti
C, Bonfante F, Vantini I, Morelli A and Whitehead WE: Impaired
colonic motor response to cholinergic stimulation in patients with
severe chronic idiopathic (slow transit type) constipation. Dig Dis
Sci. 38:1040–1045. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bazocchi IG, Ellis J, Meyer J, Mena I,
Reddy SN and Morento-Osset E: Colonic scintigraphy and manometry in
constipation, diarrhea and inflammatory bowel disease.
Gastroenterology. 94:A291988.
|
|
70
|
Klauser AG, Peyerl C, Schindbeck NE and
Müller-Lissner SA: Nutrition and physical activity in chronic
constipation. Eur J Gastroenterol Hepatol. 4:227–233. 1992.
|
|
71
|
Sjölund K, Fasth S, Ekman R, Hulten L,
Jiborn H, Nordgren S and Sundler F: Neuropeptides in idiopathic
chronic (slow transit constipation). Neurogastroenterol Motil.
9:143–150. 1997.
|
|
72
|
El-Salhy M, Norrgård Ö and Spinnell S:
Abnormal colonic endocrine cells in patients with chronic
idiopathic slow-transit constipation. Scand J Gastroenterol.
34:1007–1011. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
El-Salhy M and Norrgård Ö: Colonic
neuroendocrine peptide levels in patients with chronic idiopathic
slow transit constipation. Ups J Med Sci. 103:223–230. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tough IR and Cox HM: Selective inhibition
of neuropeptide Y Y1, receptors by B1BP3226 in rat and human
epithelial preparations. Eur J Pharmacol. 310:55–60. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
El-Salhy M: The nature and implication of
intestina1 endocrine cell changes in coeliac disease. Histol
Histopathol. 13:1069–1075. 1998.PubMed/NCBI
|
|
76
|
Adrian TE, Savage AP, Bacarese-Hamilton
AJ, Wolfe K, Besterman HS and Bloom SR: Peptide YY abnormalities in
gastrointestinal diseases. Gastroenterology. 90:379–384.
1986.PubMed/NCBI
|
|
77
|
Sjölund K and Ekman R: Increased plasma
level of peptide YY in coeliac disease. Scand J Gastroenterol.
23:297–300. 1988.PubMed/NCBI
|
|
78
|
Adrian TE, Thompson JS and Quigley EM:
Time course of adaptive regulatory peptide changes following
massive small bowel resection in the dog. Dig Dis Sci.
41:1194–1203. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Enck P, Rathmann W, Spiekermann M, Czener
D, Tschöpe D, Ziegler D, Strohmeyer G and Gries FA: Prevalence of
gastrointestinal symptoms in diabetic patients and non-diabetic
subjects. Z Gastroenterol. 32:637–641. 1996.PubMed/NCBI
|
|
80
|
Feldman M and Schiller LR: Disorders of
gastrointestina1 motility associated with diabetes mellitus. Ann
Intern Med. 98:378–384. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Locke GR III: Epidemiology of
gastrointestinal complications of diabetes mellitus. Eur J
Gastroenterol Hepatol. 7:711–716. 1995.PubMed/NCBI
|
|
82
|
Schwartz E, Palmér M, Ingberg CM, Åman J
and Berne C: Increased prevalence of gastrointestinal symptoms in
long-term type 1 diabetes mellitus. Diabet Med. 13:478–781. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
El-Salhy M: The possible role of the gut
neuroendocrine system in diabetes gastroenteropathy. Histol
Histopathol. 17:1153–1161. 2002.PubMed/NCBI
|
|
84
|
El-Salhy M: Overview of diabetic
gastroenteropathy. Geriatric Times. 4:15–16. 2003.
|
|
85
|
El-Salhy M: Gut neuroendocrine system in
diabetes gastroenteropathy: possible role in pathophysiology and
clinical implications. Focus on Diabetes Research. Ashley M: Nova
Science Publishers; New York: pp. 79–102. 2006
|
|
86
|
El-Salhy M and Sitohy B: Abnormal
gastrointestinal endocrine cells in patients with diabetes type 1:
relationship to gastric emptying and myoelectrical activity. Scand
J Gastroenterol. 36:1162–1169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
El-Salhy M: Neuroendocrine peptides in
stomach and colon of an animal model for human diabetes type 1. J
Diabetes Complications. 13:170–173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
El-Salhy M: Gastrointestinal transit in
non-obese diabetic mouse: an animal model of human diabetes type l.
J Diabetes Complications. 15:277–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
El-Salhy M: Gastric emptying in an animal
model of human diabetes type 1: relation to endocrine cells. Acta
Diabetol. 38:139–144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
El-Salhy M: Neuroendocrine peptides of the
gastrointestinal tract of an animal model of human type 2 diabetes
mellitus. Acta Diabetol. 35:194–198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Portela-Gomes GM, Wilander E, Grimelius L,
Bergström R and Ruhn G: The enterochromaffin cells in the mouse
gastrointestinal tract after streptozotocin treatment. Pathol Res
Pract. 186:260–264. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sitohy B and El-Salhy M: Colonic endocrine
cells in rats with chemically induced colon carcinoma. Histol
Histopathol. 16:833–838. 2001.PubMed/NCBI
|
|
93
|
El-Salhy M and Wilander E: Colonic
neuroendocrine peptides in rats with chemically-induced colon
carcinoma. GI Cancer. 3:73–78. 1999.
|
|
94
|
El-Salhy M, Norrgård O and Boström A: Low
levels of colonic somatostatin and galanin in patients with colon
carcinoma. GI Cancer. 2:221–225. 1998.
|
|
95
|
El-Salhy M, Mahdavi J and Norrgård Ö:
Colonic endocrine cells in patients with carcinoma of the colon.
Eur J Gastroenterol Hepatol. 10:517–522. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
El-Salhy M, Norrgård Ö, Franzén L and
Forsgren S: Colonic endocrine cells in patients with carcinoma of
the rectum with special regard to preoperative irradiation. GI
Cancer. 2:285–292. 1998.
|
|
97
|
Baldwin GS and Whitehead RH: Gut hormones,
growth and malignancy. Baillieres Clin Endocrinol Metab. 8:185–214.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Björk J, Nilsson R, Hultcranz R and
Johansson C: Growth regulatory effects of sensory neuropeptides,
epidermal growth factor, insulin, and somatostatin on
non-transformed intestinal epithelial cell line IEC-6, and colon
cancer cell line HT 29. Scand J Gastroenterol. 28:879–884.
1993.
|
|
99
|
El-Salhy M: Triple treatment with
octreotide, galanin and serotonin is a promising therapy for
colorectal cancer. Curr Pharma Des. 11:2107–2117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Anan I: The neuroendocrine system in
patients with familial amyloidotic polyneuropathy. (unpublished PhD
thesis) Umeå University Medical Dissertations. 2000
|
|
101
|
Anan I, El-Salhy M, Ando Y, Nyhlin N,
Terazaki H, Sakashita N and Suhr O: Colonic endocrine cells in
patients with familial amyloidotic polyneuropathy. J Intern Med.
245:469–473. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
El-Salhy M and Suhr O: Enocrine cells in
rectal biopsy specimens from patients with familial amyloidotic
polyneuropathy. Scand J Gastroenterol. 31:68–73. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rönnblom A, Danielsson Å and EI-Salhy M:
Intestinal endocrine cells in myotonic dystrophy: an
immunohistochemical and computed image ana1ytical study. J Intern
Med. 245:91–97. 1999.
|
|
104
|
Andersson R: Familial amyloidosis with
polyneuropathy. A clinical study based on patients living in
northern Sweden. Acta Med Scand Suppl. 590:1–64. 1976.PubMed/NCBI
|
|
105
|
Steen L and Ek B: Familial amyloidosis
with polyneuropathy. A long term follow-up of 21 patients with
reference to gastrointestinal symptoms. Acta Med Scand.
214:387–397. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Suhr O, Danielsson Å and Steen L: Bile
acid malabsorption caused by gastrointestinal motility dysfunction?
An investigation of gastrointestinal disturbances in familial
amyloidosis with polyneuropathy. Scand J Gastroenterol. 27:201–207.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Akesson A and Ekman R: Gastrointestinal
regulatory peptides in systemic sclerosis. Arthritis Rheum.
36:698–703. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yamashita Y, Toge T and Adrian TH:
Gastrointestinal hormone in dumping syndrome and reflux esophigitis
after gastric surgery. J Smooth Muscle Res. 33:37–48. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Andrews NJ and Irving MH: Human gut
hormone profiles in patients with short bowel syndrome. Dig Dis
Sci. 37:729–732. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Pietroletti R, Slores FJ, Mariani P, Leard
IS, Simi M and Brummelkamp WH: Enteroglucagon and peptide YY
response after construction of pelvic reservoir in humans. Dis
Colon Rectum. 33:966–969. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Soper NJ, Chapman NJ, Kelly K, Brown ML,
Phillips SF and Go VL: The ‘ileal brake’ after ileal pouch-anal
anastomosis. Gastroenterology. 98:111–116. 1990.
|
|
112
|
Healey KL, Bines JE, Thomas SL, Wilson G,
Taylor RG, Sourial M and Pereira-Fantini PM: Morphological and
functional changes in the colon after massive small bowel
resection. J Pediatr Surg. 45:1581–1590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu CD, Aloia T, Adrian TE, Newton TR,
Bilchik AJ, Zinner MJ, Ashley SW and McFadden DW: Peptide YY: a
potential proabsorptive hormone for the treatment of malabsorption
disorders. Am Surg. 62:232–236. 1996.PubMed/NCBI
|
|
114
|
Liu CD, Hines OJ, Whang EE,
Balasubramanian A, Newton TR, Zinner MJ, Ashley SW and McFadden DW:
A novel synthetic analog of peptide YY, BIM-43004, given
intraluminally, is proabsorptive. J Surg Res. 59:80–84. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Degen L, Oesch S, Casanova M, Graf S,
Ketterer S, Drewe J and Beglinger C: Effect of peptide YY3-36 on
food intake in humans. Gastroenterology. 129:1430–1436. 2005.
View Article : Google Scholar : PubMed/NCBI
|