Introduction
It is well known that the majority of cancer-related
deaths are not due to the primary tumor itself but to the
dissemination of tumor cells to secondary sites by a series of
events collectively known as the metastatic cascade (1). Cancer metastasis, the spread of
cancer cells from the primary neoplasm to distant sites and their
growth there, is the major cause of mortality in patients with
various types of cancer (2).
Tumor invasion and metastasis involve multiple processes and
various cytophysiological changes, including changed adhesion
capability between cells and the extracellular matrix and damaged
intercellular interactions (3).
Most cancer treatments such as surgery, radiation therapy, or
chemotherapy, usually attack not only cancer cells but normal cells
as well, causing harmful side-effects (4). In recent years, a number of
botanical compounds isolated from food or natural herbal medicines
have been found to inhibit proliferation, induce apoptosis,
suppress angiogenesis, retard metastasis and enhance chemotherapy,
exhibiting anti-cancer potential in vitro and in vivo
(5). While they possess
anti-cancer properties, toxicity to normal tissues is rare
(6).
Colocasia esculenta Linn. (C.
esculenta) (family, Araceae) is an annual herbaceous plant with
a long history of use in traditional medicine worldwide,
particularly in tropical and subtropical regions. The edible corm
of C. esculenta is commonly termed Taro, it is found
throughout India and is also cultivated worldwide (7). Taro has been known since ancient
times for its curative properties and has been utilized for the
treatment of various ailments such as asthma, arthritis, diarrhea,
internal hemorrhaging, as well as neurological and skin disorders
(8). Brown et al (9) reported that the soluble extracts of
a starchy paste made from Taro showed anti-proliferative activity
against the rat YYT colon cancer cell line and activated the
lymphocytes from splenocytes. However, they only used the soluble
extracts of the starchy paste, without knowing the identity of the
active compound. Therefore, the purpose of this study was to
identify the active compound with immunostimulating activity in the
edible corm of C. esculenta and elucidate the mechanisms by
which it stimulates the immune system.
Materials and methods
Plant material
The edible corm of C. esculenta (Taro), which
was cultivated in Gyeongbuk, Korea in 2009, was purchased from a
commercial market. A voucher specimen was deposited at the Graduate
School of Food Biotechnology, Kyonggi University, Gyeonggi,
Korea.
Isolation and purification of the
polysaccharides from Taro
Taro (1 kg) was sliced and mixed with three volumes
of distilled water and stirred at 4°C overnight. After
centrifugation at 6,000 rpm for 30 min, the supernatant was
precipitated with four volumes of ethanol, dialyzed and
lyophilized. Finally, 10.4 g (1.04%) of crude polysaccharides were
obtained. The crude polysaccharides were purified by ion exchange
chromatography on a DEAE-Sepharose FF (Cl− form) column
(GE Healthcare, Uppsala, Sweden) with a stepwise gradient of NaCl
(0, 0.05–2 M NaCl). Each fraction was collected, dialyzed against
tap water and freeze-dried. The active fraction, Taro-4, was eluted
with 0.2 M NaCl and was further purified by size-exclusion
chromatography on a Sephadex G-100 column (GE Healthcare) using 50
mM ammonium formate buffer (pH 5.5). High-performance
size-exclusion chromatography (HPSEC) of Taro-4-I was performed on
an high-performance liquid chromatography (HPLC)-9500 instrument
(Young-Lin Co., Gyeonggi, Korea) equipped with a Superdex 75 GL
column (GE Healthcare). A total of 10 μl of each
polysaccharide solution were analyzed using an isocratic mobile
phase (50 mM ammonium formate buffer, pH 5.5) at a flow rate of 0.5
ml/min at room temperature. The molecular weights of the purified
polysaccharides were estimated from a calibration curve constructed
with standard pullulans (P-800, 400, 200, 100, 50, 20, 10 and 5;
Showa Denko Co., Ltd., Tokyo, Japan).
General analytical methods
Total carbohydrate, uronic acid and protein were
determined using phenol-H2SO4 (10), m-hydroxydiphenyl (11) and the Bradford method (12) with a protein assay kit, using
galactose, galacturonic acid and bovine serum albumin as the
respective standards. The sugar composition of the polysaccharide
samples was determined by gas chromatography (GC) analysis of their
alditol acetates. The samples were then hydrolyzed with 2 M
trifluoroacetic acid for 1.5 h at 121°C, converted into the
corresponding alditol acetates (13) and analyzed by GC at 60°C for 1
min, 60→220°C (30°C/min), 220°C for 12 min, 220→250°C (8°C/min),
and 250°C for 15 min, using a GC (GC 6000 series; Young-Lin Co.)
equipped with an SP-2380 (Supelco, Bellefonte, PA, USA) capillary
column. The molar ratios were calculated from the peak areas and
response factors using a flame ionization detector.
Anti-complementary activity assay
Anti-complementary activity was measured by the
complement fixation test based on complement consumption and the
degree of red blood cell lysis by residual complement (14). Normal human serum (NHS) was
obtained from volunteer adults. A total of 50 μl aliquots of
exopolysaccharide of various concentrations (100, 500 and 1,000
μg/ml) were mixed with equal volumes of NHS and gelatin
veronal-buffered saline (GVB2+, pH 7.4) containing 500
mM Mg2+ and 150 mM Ca2+, respectively. The
mixtures were pre-incubated at 37°C for 30 min and the residual
total hemolytic complement (TCH50) was determined using
IgM hemolysin-sensitized sheep erythrocytes (EA cells,
1×108 cells/ml). The NHS was incubated with water and
GVB2+ to provide a control. The anti-complementary
activity of the isolated polysaccharides is expressed as the
percent inhibition of the control TCH50 polysaccharide K
(PSK) (15) from Coriolus
versicolor. TCH50 (%) = TCH50 (control) −
TCH50 (treated with sample)/TCH50
(control).
Immunoelectrophoresis
Alternative activation of the C3 protein was
examined using standard one- and two-dimensional immuno
electrophoresis methods. NHS was incubated with Taro-4-I and an
equal volume of one of the following three solutions: i)
GVB2+, ii) 10 mM
ethylene-glycol-bis-(β-aminoethylether)-N,N,N′,N′-tetraacetic acid
(EGTA) solution containing 2 mM MgCl2 in
GVB2+ (Mg2+-EGTA-GVB), or iii) 10 mM EDTA
solution in GVB2+ (EDTA-GVB). The incubations were
carried out at 37°C for 30 min. The serum was then subjected to
crossed immunoelectrophoresis to observe the C3 cleavage products
(16). Shortly after the first
run in barbital buffer (pH 8.6; ionic strength, 0.025 with 1%
agarose), the second run was performed on a gel plate (layer
thickness, 1.5 mm) containing 0.5% anti-human C3 serum (Sigma
Chemical Co, St. Louis, MO, USA) which recognizes both C3a and C3b,
at a potential gradient of 15 mA/plate for 15 h. After
electrophoresis, the plate was fixed and stained with 0.2%
bromophenol blue in MeOH:water:acetic acid (5:4:1) (17).
Animals
Specific pathogen-free (SPF), 6-week-old female
BALB/c mice were purchased from G-Bio Animal, Inc. (Seoul, Korea).
The mice were maintained in a clean rack in an SPF room at Kyonggi
University. Water and a diet of pellets were supplied ad
libitum. All animals experiments were carried out according to
the instructions of the Ethics Committee for Use of Experimental
Animals at Kyonggi University (2011-003).
Macrophage proliferation and cytokine
production
Peritoneal macrophages were harvested from
thioglycollate-treated 6-week-old BALB/c mice as described
previously (18). The cells
(1×106/well) were suspended in complete RPMI-1640 medium
and plated in 96-well culture plates. After 2 h of incubation in a
5% humidified CO2 incubator, non-adherent cells were
removed by washing with PBS and the adherent macrophages were
incubated with the indicated doses of Taro-4-I for 24 h. Macrophage
proliferation was assayed using the Cell Counting kit-8 (Dojindo
Molecular Technologies, Gaithersburg, MD, USA) (19) and the concentrations of various
cytokines in the medium were determined by enzyme-linked
immunosorbent assay kits (Becton-Dickinson and Co., Franklin Lakes,
NJ, USA) according to the manufacturer’s instructions.
Natural killer (NK)-mediated cytotoxicity
assay
Yac-1 is a Moloney murine leukemia virus-induced
lymphoma that lacks the expression of MHC-I and is sensitive to
lysis by NK cells (20).
Therefore, NK-mediated cytotoxicity was determined in Yac-1 and
primary cultured splenocytes from sample-treated animals (21). Briefly, three BALB/c mice/group
were administered Taro-4-I intravenously (i.v.) (5, 50 and 500
μg/mouse) and their splenocytes were harvested three days
after treatment. Single-cell suspensions of splenocytes were added
to the Yac-1 cells (1×105 cells/ml) to obtain
effector-to-target (E/T)cell ratios of 100:1, 50:1 and 25:1 in
U-bottomed nine-well plates, after which the cultures were
incubated for 6 h. Following incubation, the culture supernatants
(100 μl/well) were mixed with lactate dehydrogenase (LDH)
solution (Promega Co., Madison, WI, USA) and the absorbance value
of each well was measured at 490 nm. The percentage of NK cellular
cytotoxicity was calculated using the following formula:
cytotoxicity (%) = [(experimental release-spontaneous
release)/(maximum release-spontaneous release)] ×100.
Anti-metastatic activity in vivo
Experimental lung metastasis was assessed by
i.v. inoculation of B16BL6 melanoma cells
(2.7×104 cell/mouse) into syngeneic BALB/c mice
(22). Treatment with various
Taro-4-I doses was carried out two days prior to or one day after
i.v. inoculation with B16BL6 melanoma cells. The mice were
sacrificed 14 days following tumor inoculation and their lungs were
fixed in Bouin’s solution. Lung tumor colonies were counted under a
dissecting microscope.
Statistical analysis
All statistical analyses were performed using the
Statistical Package for Social Sciences (SPSS) version 12.0 (SPSS
Inc., Chicago, IL, USA). Differences among groups were evaluated by
a one-way Analysis of variance (ANOVA) and Duncan’s multiple range
test. All data are presented as the means ± standard deviation
(SD).
Results
Purification of an active compound from
C. esculenta with anti-complementary activity
The edible corm of C. esculenta was extracted
with cold water (4°C) and 10.44 g (1.04%) of the crude
polysaccharide (Taro-0) was obtained. The polysaccharide was then
applied to column chromatography using DEAE-Sepharose FF.
Anti-complementary activities of subfractions from DEAE-Sepharose
FF were detected in the following order: fraction eluted with 0.4 M
NaCl (Taro-6) and fraction eluted with 0.3 M NaCl (Taro-5) >
fraction eluted with 0.5 M NaCl (Taro-7) > fraction eluted with
0.2 M NaCl (Taro-4) > fraction eluted with 1.0 M NaCl (Taro-3).
The yield of Taro-4 (30.5%) was higher than that of Taro-6 (3.1%),
Taro-5 (3.9%) and Taro-7 (0.1%). Therefore, we selected the Taro-4
fraction for the following purification step. When Taro-4 was
applied to a Sephadex G-100 column, it was divided into two
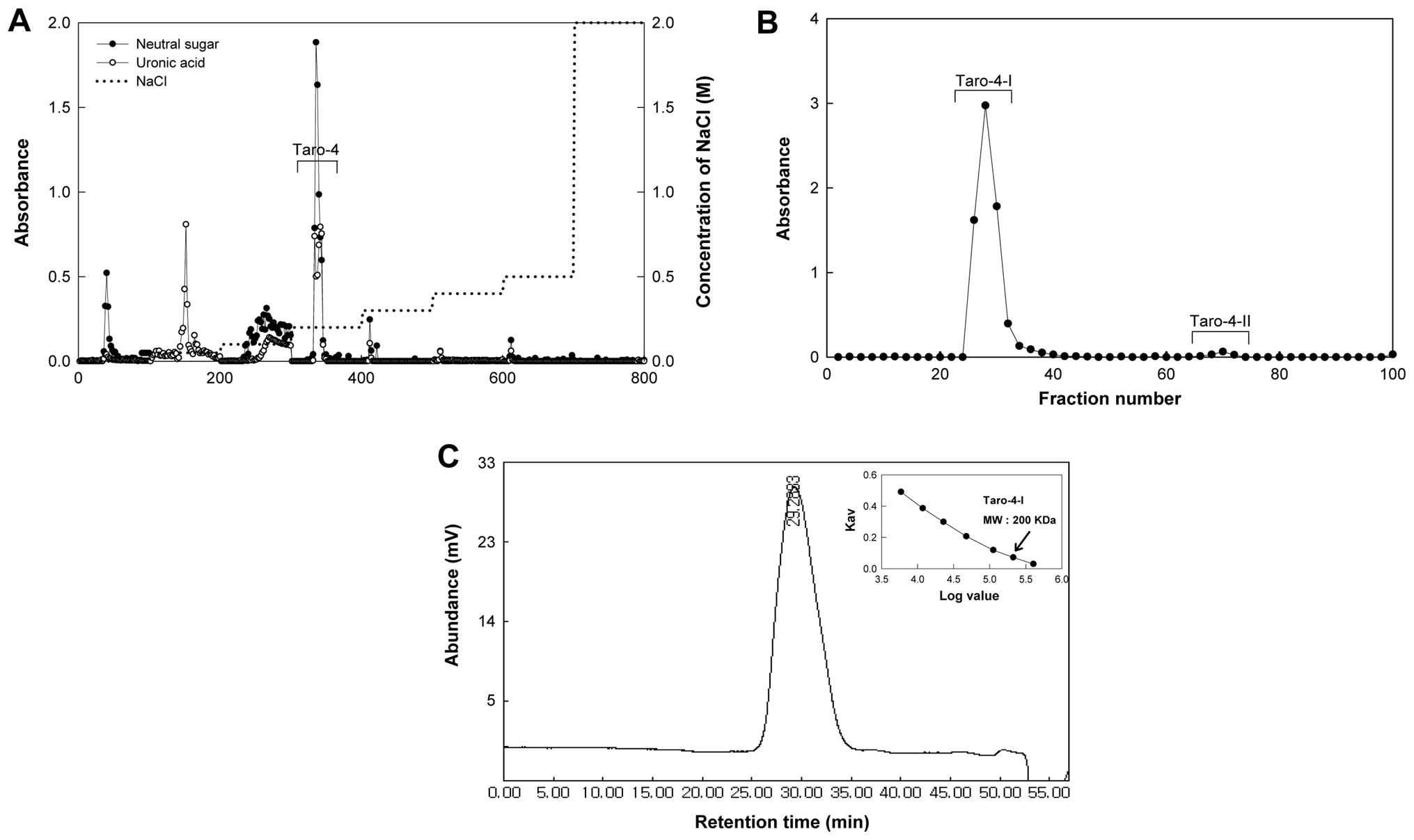
subfractions, Taro-4-I and Taro-4-II (Fig. 1B) and the Taro-4-I subfraction was
found to exhibit higher anti-complementary activity, as shown in
Table I. Taro-4-I showed a single
peak on HPLC, indicating that this fraction was highly purified
(Fig. 1C).
 | Table I.Purification procedure and yield of
each fraction. |
Table I.
Purification procedure and yield of
each fraction.
| Purification
step | Recovery | Yield (%) | Anti-complementary
activity ITCH50 (%)a |
|---|
| Colocasia
esculenta | 1.0 kg | | |
| Cold water
extract | 10.4 g | 100.0 |
41.3±0.8d |
| DEAE Sepharose
FF | | | |
| 0.00 M NaCl | 637 mg | 6.1 |
30.8±3.4e |
| 0.05 M NaCl | 1,936 mg | 18.5 |
34.0±1.1e |
| 0.10 M NaCl | 1,617 mg | 15.5 |
43.1±1.0d |
| 0.2 M NaCl
(Taro-4) | 3,188 mg | 30.5 |
55.8±3.1c |
| 0.3 M NaCl | 410 mg | 3.9 | 65.1±1.3a |
| 0.4 M NaCl | 319 mg | 3.1 | 66.4±0.4a |
| 0.5 M NaCl | Trace | 0.1 |
61.7±1.6b |
| 2 M NaCl | 273 mg | 2.6 |
34.1±2.0e |
| Sephadex G-100 | | | |
| Taro-4-I | 2,060 mg | 19.7 |
57.3±4.5c |
| Taro-4-II | Trace | | |
Characterization of purified compound
having anti-complementary activity
The anti-complementary activity of Taro-4-I
(57.3±4.5%) was similar to that of PSK, which was used as the
positive control (Table I). The
molecular weight of Taro-4-I was 200 kDa (Fig. 1C). Taro-4-I was a polysaccharide
composed of 64.4% neutral sugars and 35.6% uronic acid. Taro-4-I
mainly comprised of galactose (38.9 mole %), mannose (19.2 mole %)
and glucose (4.2 mole %) in the neutral sugar portion (Table II).
 | Table II.Chemical properties of the purified
compound (Taro-4-I) from Colocasia esculenta. |
Table II.
Chemical properties of the purified
compound (Taro-4-I) from Colocasia esculenta.
|
Composition/component | (%) |
|---|
| Chemical
compositiona | |
| Neutral
sugar | 64.4 |
| Uronic acid | 35.6 |
| Protein | 0.0 |
| Sugar
componentb | (Mole %)c |
| Rhamnose | 0.1 |
| Fucose | 0.0 |
| Arabinose | 1.7 |
| Xylose | 0.3 |
| Mannose | 19.2 |
| Galactose | 38.9 |
| Glucose | 4.2 |
| Galacturonic acid
+ glucuronic acid | 35.3 |
Activation mode of the complement
system
The most important effector of the complement
system, C3, is present in the human plasma in large quantities
(800–1,800 μg/ml) and it is converted to C3a and C3b by
cleavage, which is the major reaction in complement activation
(23). Both Mg2+ and
Ca2+ are required to activate the classical pathway, but
only Mg2+ is required to activate the alternative
pathway. Taro-4-I was used in different buffer systems to evaluate
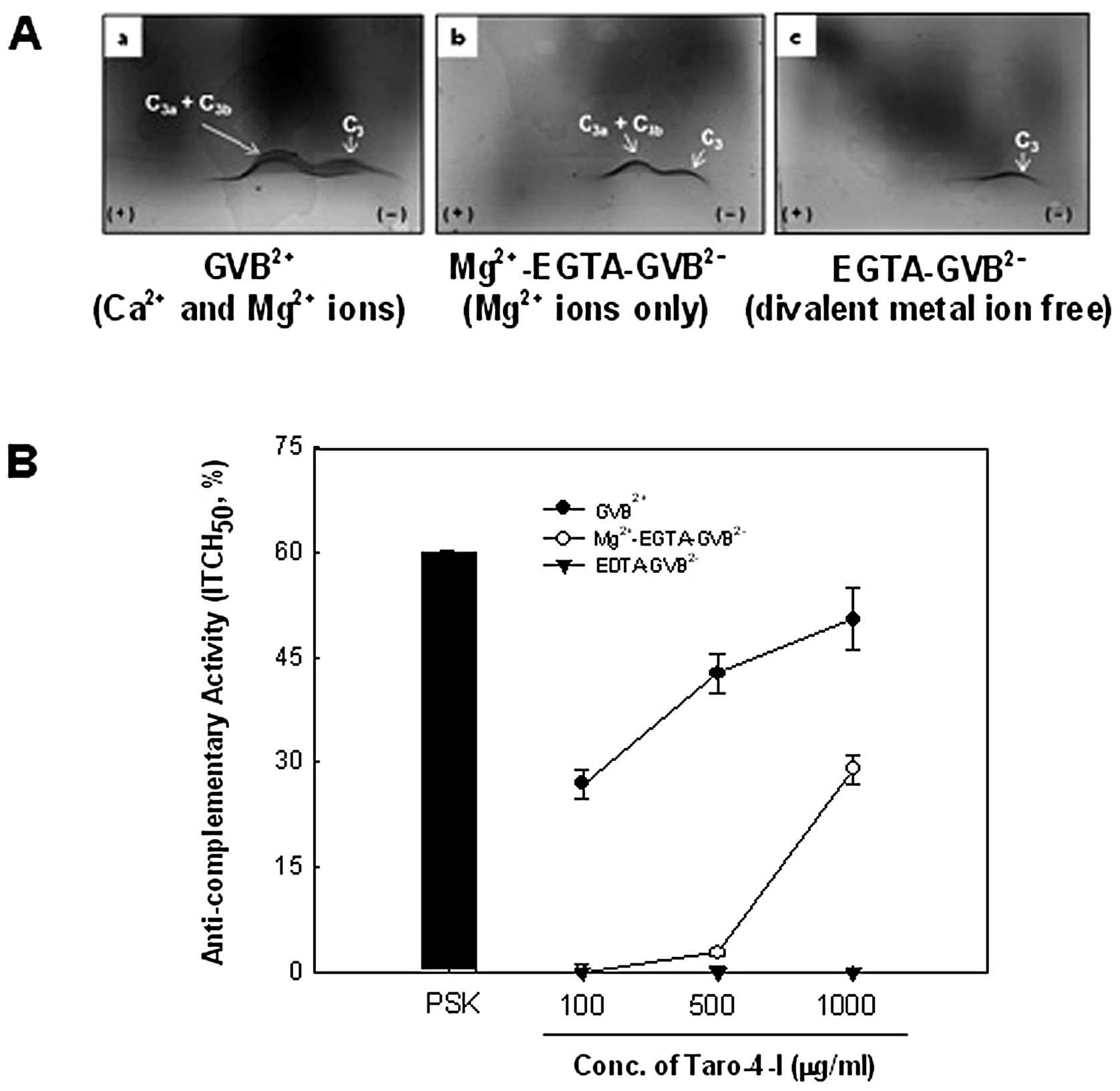
the complement activation pathway. Under the GVB2+
experimental condition containing Mg2+ and
Ca2+ ions, Taro-4-I cleaved C3 and exhibited a clear
second precipitin line (C3a and C3b protein) (Fig. 2Aa). Additionally, under the
GVB2+ experimental condition containing Mg2+,
Taro-4-I exhibited a clear second precipitin line. Under the
GVB2+ experimental condition containing Mg2+
and Ca2+, the detected anti-complementary activity was
∼50.5±4.5% at 1,000 μg/ml (Fig. 2B), which was the outcome of
participation in both complement-activated pathways leading to
cellular lysis. Furthermore, when anti-complementary activity was
determined under the Ca2+-depleted experimental
condition (GVB2+ containing Mg2+) (Fig. 2Ab), which only acts on the
alternative pathway, Taro-4-I cleaved C3 and exhibited a clear
second precipitin line and the detected anti-complementary activity
was ∼29.0±2.0% of the activity at 1,000 μg/ml (Fig. 2B). These results indicated that
the mode of complement activation by Taro-4-I was via not only the
classical, but also the alternative pathway, although to a lesser
extent.
Effect of Taro-4-I on macrophage
activation
We examined the Taro-4-I toxicity on primary
cultured peritoneal macrophages by incubating the cells with doses
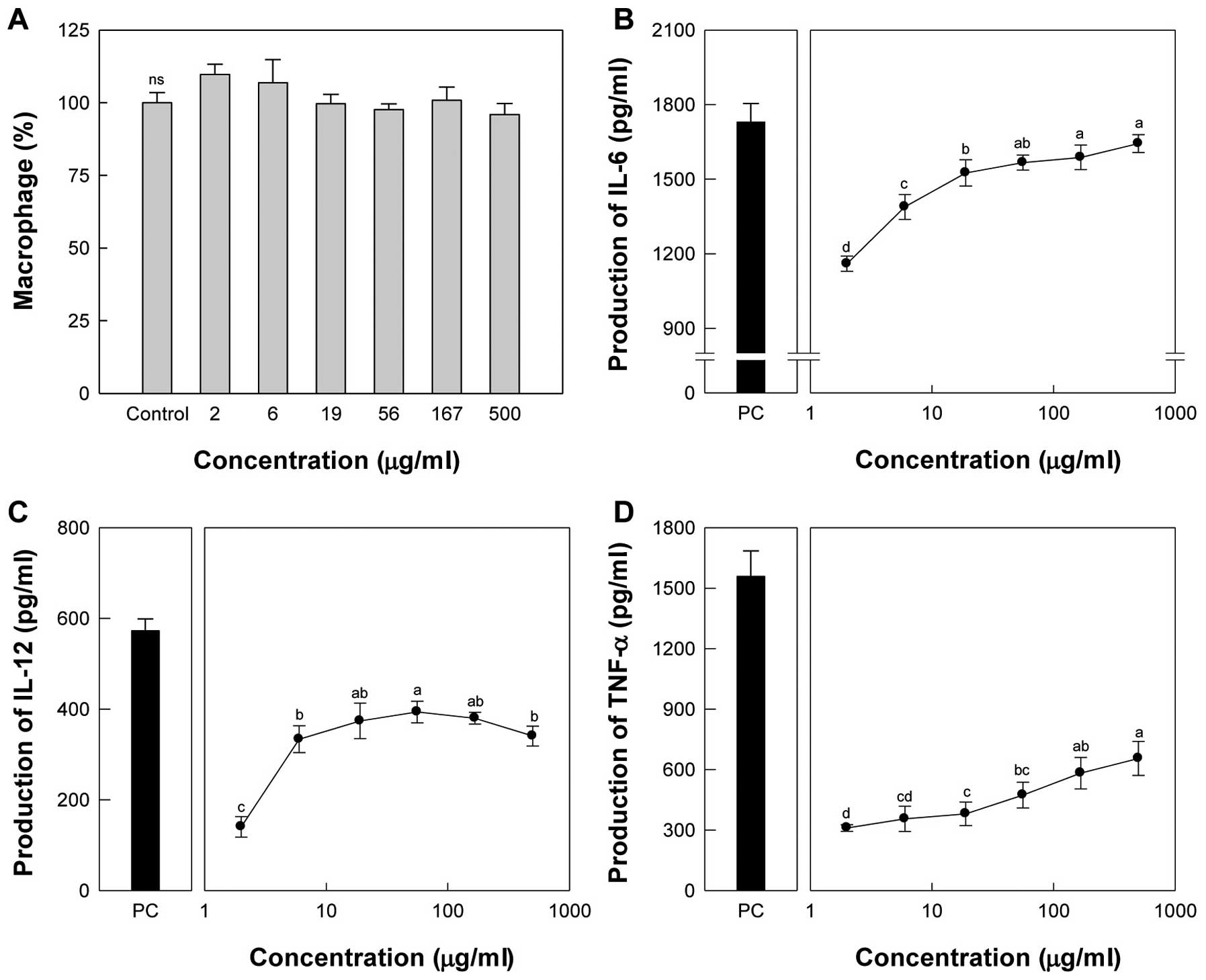
up to 500 μg/ml. Taro-4-I at the maximum dose did not affect
cell viability compared to the control (Fig. 3A). Subsequently, the effect of
Taro-4-I on various cytokines, such as interleukin (IL)-6, IL-12
and tumor necrosis factor (TNF)-α, was assessed by incubating
peritoneal macrophages with doses up to 500 μg/ml. The
treatment of peritoneal macrophages with Taro-4-I significantly
increased the production of IL-6 (Fig. 3B) and TNF-α (Fig. 3D) in a dose-dependent manner. The
production of IL-12 showed maximal activity at 56 μg/ml,
after which it declined (Fig.
3C).
Effect of Taro-4-I on NK cell
activity
The effect of Taro-4-I on NK cell activity was
estimated by the cytotoxic activity against Yac-1 cells, a
NK-sensitive mouse lymphoma cell line, using an LDH release assay.
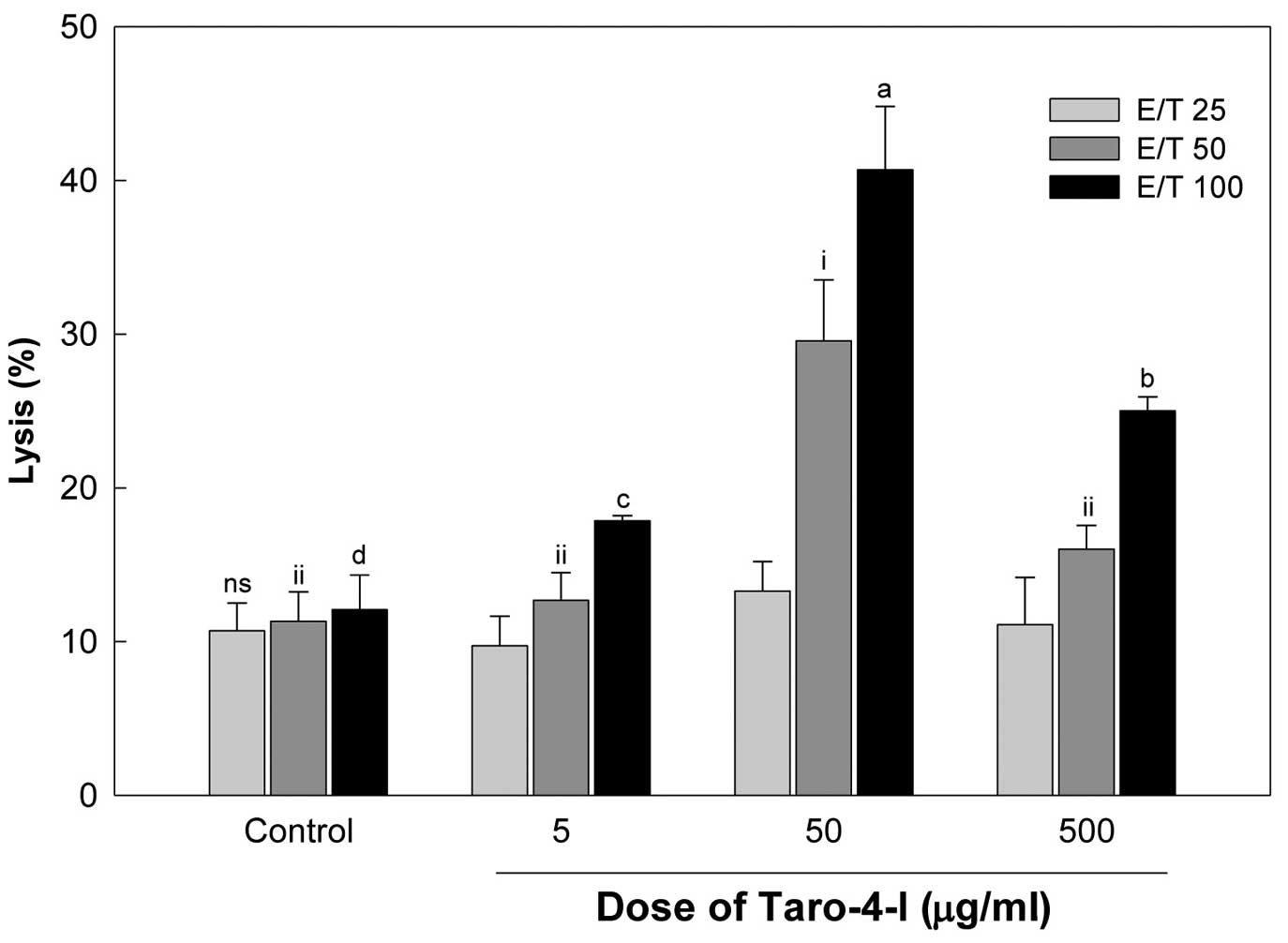
Splenocytes obtained from mice administered with Taro-4-I showed a
higher toxicity to Yac-1 cells compared to those obtained from
untreated mice in a E/T ratio-dependent manner (Fig. 4). The group treated with 50
μg/ml Taro-4-I-showed a significantly higher toxicity to
Yac-1 cells than the group treated with 500 μg/ml
Taro-4-I.
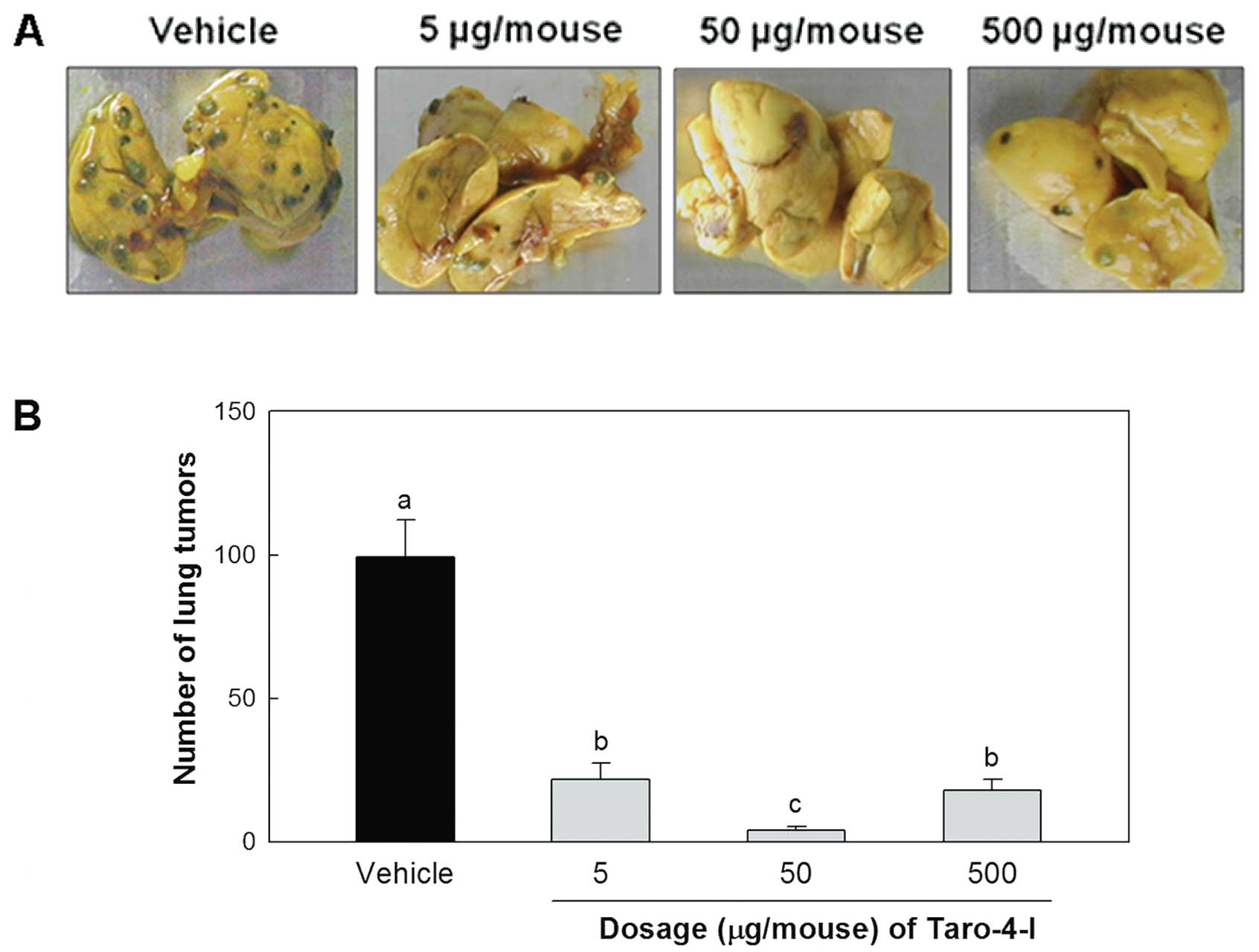
Inhibitory effect of Taro-4-I on lung
metastasis
We examined the effect of Taro-4-I on the
experimental lung metastasis produced by B16BL6 melanoma cells. The
administration of Taro-4-I significantly inhibited the lung
metastasis of B16BL6 melanoma cells (Fig. 5). However, the group treated with
50 μg/mouse Taro-4-I had a significantly lower number of
tumors than the group treated with 500 μg/mouse
Taro-4-I.
Discussion
The innate immune system is phylogenetically older
than the acquired (adaptive) or specific immune system and provides
rapid but incomplete host defense until the slower, more
definitive, acquired immune response develops (24). The complement system is an
essential component of innate immunity and also plays an important
role in modulating adaptive immunity (25). The complement system is a key
component of the innate immune system, playing a central role in
host defense against pathogens or cancer (26).
Research of food-derived bioactive components for
cancer prevention and cancer therapy is expanding due to the
relatively low or undetected toxicity (27) and better bioavailability of these
components. Over the last three decades, polysaccharides isolated
from botanical sources (mushrooms, algae, lichens and higher
plants) have also attracted a great deal of attention in the
biomedical arena, due to their broad-spectrum therapeutic
properties (28) and relatively
low toxicity. The most promising biopharmacological activities of
these biopolymers are their immunomodulatory and anti-cancer
effects (29). Three anti-tumor
mushroom polysaccharides, lentinan, schizophyllan and protein-bound
PSK (Krestin), isolated from Lentinus edodes,
Schizophyllum commune and Coriolus versicolor,
respectively, have become major commercial items in Japan (29). Although the mechanism of their
antitumor action is not yet completely understood, these
polysaccharides and polysaccharide-protein complexes have been
shown to enhance cell-mediated immune responses in vivo and
in vitro and act as biological response modifiers (BRMs).
BRMs are considered a useful tool in tumor growth suppression and
inhibition of metastasis.
In this study, we identified and purified a
potentially novel therapeutic compound (Taro-4-I), derived from
Taro. This compound is a polysaccharide containing 35.6% uronic
acid and is mainly comprised of galactose (38.9 mole%), mannose
(19.2 mole%) and glucose (4.2 mole%) with a molecular weight of 200
kDa (Fig. 1C). The molecular
weights of most immunostimulating polysaccharides are in the range
of 6–1,000 kDa (30) and Taro-4-I
falls within this range.
Anti-complementary activity is measured by the
complement fixation test (14)
and is expressed as the percentage inhibition of the control
TCH50 PSK (15) from
Coriolus versicolor as a positive control. The antitumor
activity of PSK has been evaluated in Japan for the prevention of
esophageal, gastric and lung cancers in humans with promising
results and is even sold as a drug (29). The polysaccharide has been found
to be well-tolerated and compatible with chemotherapy and radiation
therapy. However, the mechanism of action of PSK is not yet
completely understood. Animals administered PSK have shown
increased neutrophil levels with concomitant toxicity of target
cells and a marked decrease in size and number of metastatic lung
foci (31). Torisu et al
(32) evaluated the clinical
efficacy and the mechanism of action of PSK using a randomized
double-blind trial in 111 patients who underwent surgery for
colorectal cancer. They reported that the survival rate of patients
was significantly higher (P<0.05) in the PSK group than in the
control group and the polymorphonuclear leukocytes from PSK-treated
patients showed remarkable enhancement in their activities, such as
random and/or chemotactic locomotion and phagocytic activity, when
compared with those in the control group. In this study, the
anti-complementary activity of Taro-4-I (57.3±4.5%) was found to be
similar to that of PSK (60.0±0.0%) (Table I). Taro-4-I activated the
complement system via the classical and alternative pathways
(Fig. 2). The complement system
plays an important role in host defense, inflammation and allergic
reactions and is activated via the classical and alternative
pathways. The classical pathway is activated by an immune complex
containing IgM and IgG antibodies, the acute phase protein,
C-reactive protein and RNA tumor viruses. The alternative pathway
does not require antibodies and is directly activated by
polysaccharides, certain immunoglobulins, viruses, fungi, bacteria,
certain animal cells and parasites.
Macrophages are ancient and phylogenetically
conserved cells found in all multicellular organisms and they,
together with neutrophils, represent the first line of host defense
after the epithelial barrier. Macrophages participate both in
non-specific defense (innate immunity) and in the initiation of
specific defense mechanisms (adaptive immunity) in vertebrate
animals. The production of IL-12 in a co-incubation system of
peritoneal macrophages with Taro-4-I showed maximal activity at 56
μg/ml (Fig. 3C). The group
treated with 50 μg/ml Taro-4-I showed a significantly higher
toxicity to Yac-1 cells, a NK-sensitive mouse lymphoma cell line,
compared to the group treated with 500 μg/ml Taro-4-I
(Fig. 4). The group treated with
50 μg/mouse Taro-4-I had a significantly lower number of
tumors compared to the group treated with 500 μg/mouse
Taro-4-I (Fig. 5). IL-12 is
produced mainly by macrophages and is a NK cell stimulatory factor.
NK cell cytotoxicity may represent a way to eliminate
overstimulated macrophages. NK cells are lymphocytes of the innate
immune system that are involved in early defense against both
allogeneic (non-self) cells and autologous cells undergoing various
forms of stress, such as infection (with viruses, bacteria, or
parasites) and malignant transformation (33). In vitro studies using cells
from humans and several other mammalian species, as well as in
vivo studies using mice and rats, have suggested that tumor
cells are recognized as NK cell targets (34). It is thus speculated that Taro-4-I
stimulates the complementary system and induces the secretion of
various cytokines, such as IL-6, IL-12 and TNF-α from macrophages.
The NK cells activated by IL-12 inhibit tumor metastasis.
The anti-cancer activity of the polysaccharides from
Taro has previously been reported by Brown et al (9). However, this is the first study to
demonstrate the anti-metastatic activity of the active compound
isolated from Taro. Our data provide a scientific foundation for
the anti-cancer and anti-metastatic activity of Taro-4-I,
demonstrating that it exerts its effects through immunostimulation.
The group treated with 50 μg/mouse Taro-4-I showed
remarkable preventive activity (96.2±1.3%).
Based on our data, the administration of ∼162.5
mg/day of Taro-4-I or 824.85 mg/day of the cold water extract would
be expected to have an anti-metastastic effect in humans (65 kg
body weight) as calculated by the FDA dose calculator program.
However, the anti-metastatic activity of Taro-4-I showed a
bell-shaped profile. Its molecular weight is approximately 200 kDa.
It is therefore questionable whether the whole structure is
required for its anti-metastatic effects. Therefore, in future
studies, we aim to further explore the optimal range for clinical
trials and identify the essential structure.
Acknowledgements
This study was supported by the
Technology Development Program of Ministry of Food, Agriculture,
Forestry and Fisheries, Republic of Korea.
References
|
1.
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Weiss L: Metastatic inefficiency. Adv
Cancer Res. 54:159–211. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Han SS, Cho CK, Lee YW and Yoo HS:
Antimetastatic and immunomodulating effect of water extracts from
various mushrooms. J Acupunct Meridian Stud. 2:218–227. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tan W, Lu J, Huang M, et al: Anti-cancer
natural products isolated from Chinese medicinal herbs. Chin Med.
6:27–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Harada M, Seta K, Ito O, et al:
Concomitant immunity against tumor development is enhanced by the
oral administration of a kampo medicine, Hochu-ekki-to (TJ-41:
Bu-Zhong-Yi-Qi-Tang). Immunopharmacol Immunotoxicol. 17:687–703.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sheth AK: The Herbs of Ayurveda. A.K.
Sheth Publishers; Ahmedabad: 2005
|
|
8.
|
Prajapati R, Kalariya M, Umbarkar R, et
al: Colocasia esculenta: a potent indigenous plant. Int J
Nutr Pharmacol Neurol Dis. 1:90–96. 2011. View Article : Google Scholar
|
|
9.
|
Brown AC, Reitzenstein JE, Liu J, et al:
The anti-cancer effects of poi (Colocasia esculenta) on
colonic adenocarcinoma cells in vitro. Phytother Res. 19:767–771.
2005.PubMed/NCBI
|
|
10.
|
Dubois M, Gilles K, Hamilton JK, et al: A
colorimetric method for the determination of sugars. Nature.
168:1671951. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Blumenkrantz N and Asboe-Hansen G: New
method for quantitative determination of uronic acids. Anal
Biochem. 54:484–489. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jones TM and Albersheim P: A gas
chromatographic method for the determination of aldose and uronic
acid constituents of plant cell wall polysaccharides. Plant
Physiol. 49:926–936. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kabat EA and Mayer MM: Complement and
complement fixation. Experimental Immunochemistry. 2nd edition.
Charles C. Thomas; Springfield, IL: pp. 133–240. 1971
|
|
15.
|
Saito H, Tomioka H and Sato K: PSK, a
polysaccharide from Coriolus vesicolor, enhances oxygen
metabolism of murine peritoneal macrophages and the host resistance
to listerial infection. J Gen Microbiol. 134:1029–1035.
1988.PubMed/NCBI
|
|
16.
|
Shimura K, Ito H and Hibasami H: Screening
of host-mediated antitumor polysaccharides by crossed
immunoelectrophoresis using fresh human serum. Jpn J Pharmacol.
33:403–408. 1983. View Article : Google Scholar
|
|
17.
|
Cyong JC, Witkin SS, Rieger B, et al:
Antibody-independent complement activation by myelin via the
classical complement pathway. J Exp Med. 155:587–598. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Saiki I, Saito S, Fujita C, et al:
Induction of tumoricidal macrophages and production of cytokines by
synthetic muramyl dipeptide analogues. Vaccine. 6:238–244. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kim YH, Park JH, Lee M, et al:
Polyethylenimine with acid-labile linkages as a biodegradable gene
carrier. J Control Release. 103:209–219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kiessling R, Klein E and Wigzell H:
Natural killer cells in the mouse. I. Cytotoxic cells with
specificity for mouse Moloney leukemia cells Specificity and
distribution according to genotype. Eur J Immunol. 5:112–117. 1975.
View Article : Google Scholar
|
|
21.
|
Yoon TJ, Yoo YC, Kang TB, et al:
Prophylactic effect of Korean mistletoe (Viscum album
coloratum) extract on tumor metastasis is mediated by
enhancement of NK cell activity. Int J Immunopharmacol. 20:163–172.
1998.
|
|
22.
|
Yoo YC, Saiki I, Sato K and Azuma I:
MDP-Lys(L18), a lipophilic derivative of muramyl dipeptide,
inhibits the metastasis of haematogenous and non-haematogenous
tumours in mice. Vaccine. 12:175–180. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Platts-Mills TA and Ishizaka K: Activation
of the alternative pathway of human complement by rabbit cells. J
Immunol. 113:348–358. 1974.
|
|
24.
|
Fearon DT and Locksley RM: The instructive
role of innate immunity in the acquired immune response. Science.
272:50–53. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Zhu H, Di H, Zhang Y, Zhang J, et al: A
protein-bound polysaccharide from the stem bark of Eucommia
ulmoides and its anti-complementary effect. Carbohydr Res.
344:1319–1324. 2009. View Article : Google Scholar
|
|
26.
|
Frank MM and Fries LF: The role of
complement in inflammation and phagocytosis. Immunol Today.
12:322–326. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Wasser SP: Medicinal mushrooms as a source
of antitumor and immunomodulating polysaccharides. Appl Microbiol
Biotechnol. 60:258–274. 2002.PubMed/NCBI
|
|
28.
|
Paulsen BS: Plant polysaccharides with
immunostimulatory activities. Curr Org Chem. 5:939–950. 2001.
View Article : Google Scholar
|
|
29.
|
Ooi VE and Liu F: Immunomodulation and
anti-cancer activity of polysaccharide-protein complexes. Curr Med
Chem. 7:715–729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kweon MH, Hwang HJ and Sung HC: Isolation
and characterization of anticomplementary beta-glucans from the
shoots of bamboo Phyllostachys edulis. Planta Med. 69:56–62.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Tzianabos AO: Polysaccharide
immunomudulators as therapeutic agents: structural aspects and
biologic function. Clin Microbiol Rev. 13:523–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Torisu M, Hayashi Y, Ishimitsu T, et al:
Significant prolongation of disease-free period gained by oral
polysaccharide K (PSK) administration after curative surgical
operation of colorectal cancer. Cancer Immunol Immunother.
31:261–268. 1990. View Article : Google Scholar
|
|
33.
|
Vivier E, Nunes JA and Vely F: Natural
killer cell signaling pathways. Science. 306:1517–1519. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
van Dommelen SLH, Sumaria N, Schreiber RD,
et al: Perforin and granzymes have stinct roles in defensive
immunity and immunopathology. Immunity. 25:835–848. 2006.PubMed/NCBI
|